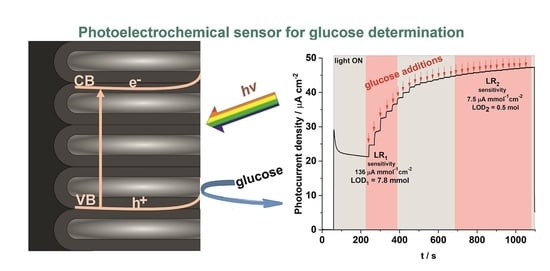
A Photoelectrochemical Sensor Based on Anodic TiO2 for Glucose Determination
Abstract
1. Introduction
2. Materials and Methods
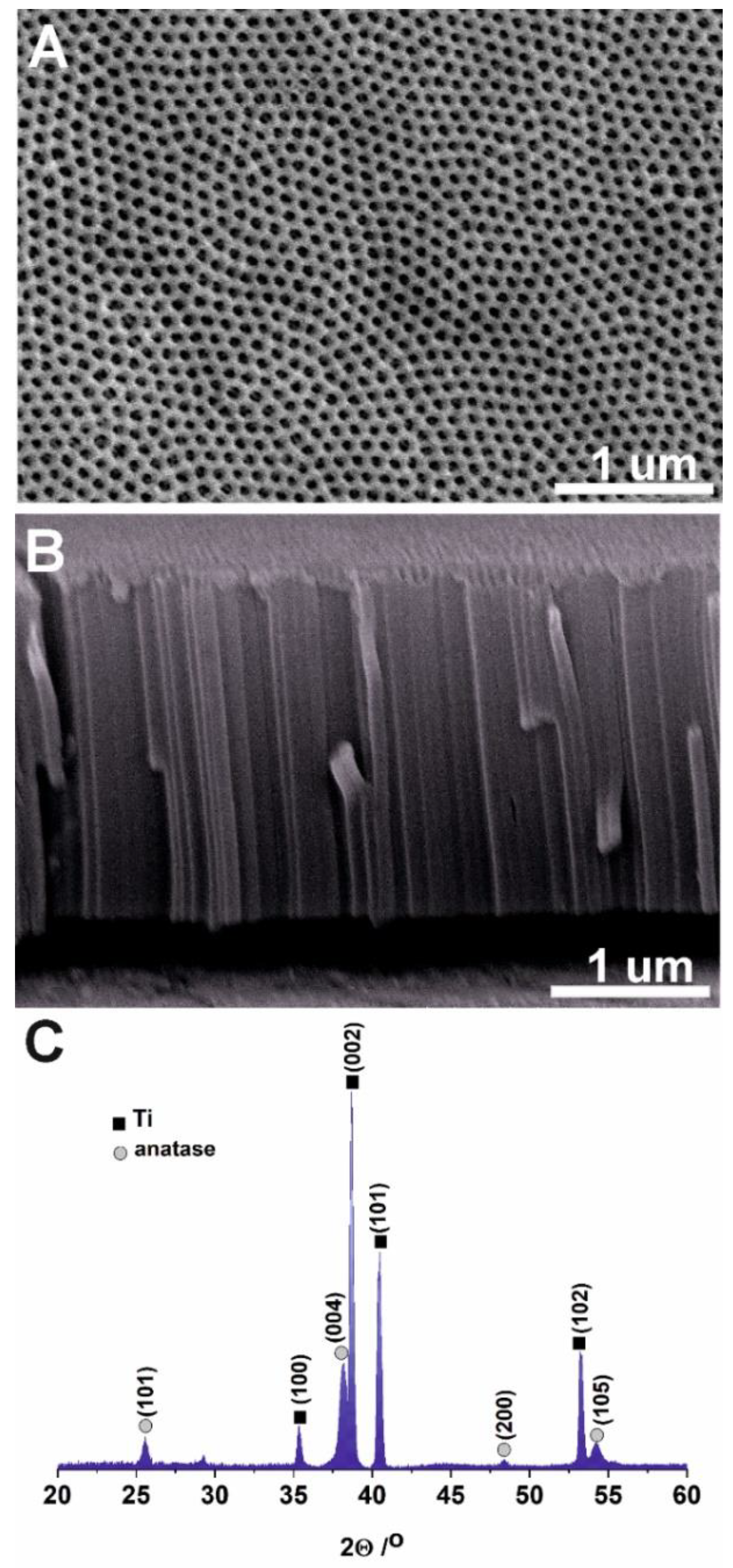
2.1. ATO Synthesis and Characterization
2.2. Photoelectrochemical Study
3. Results
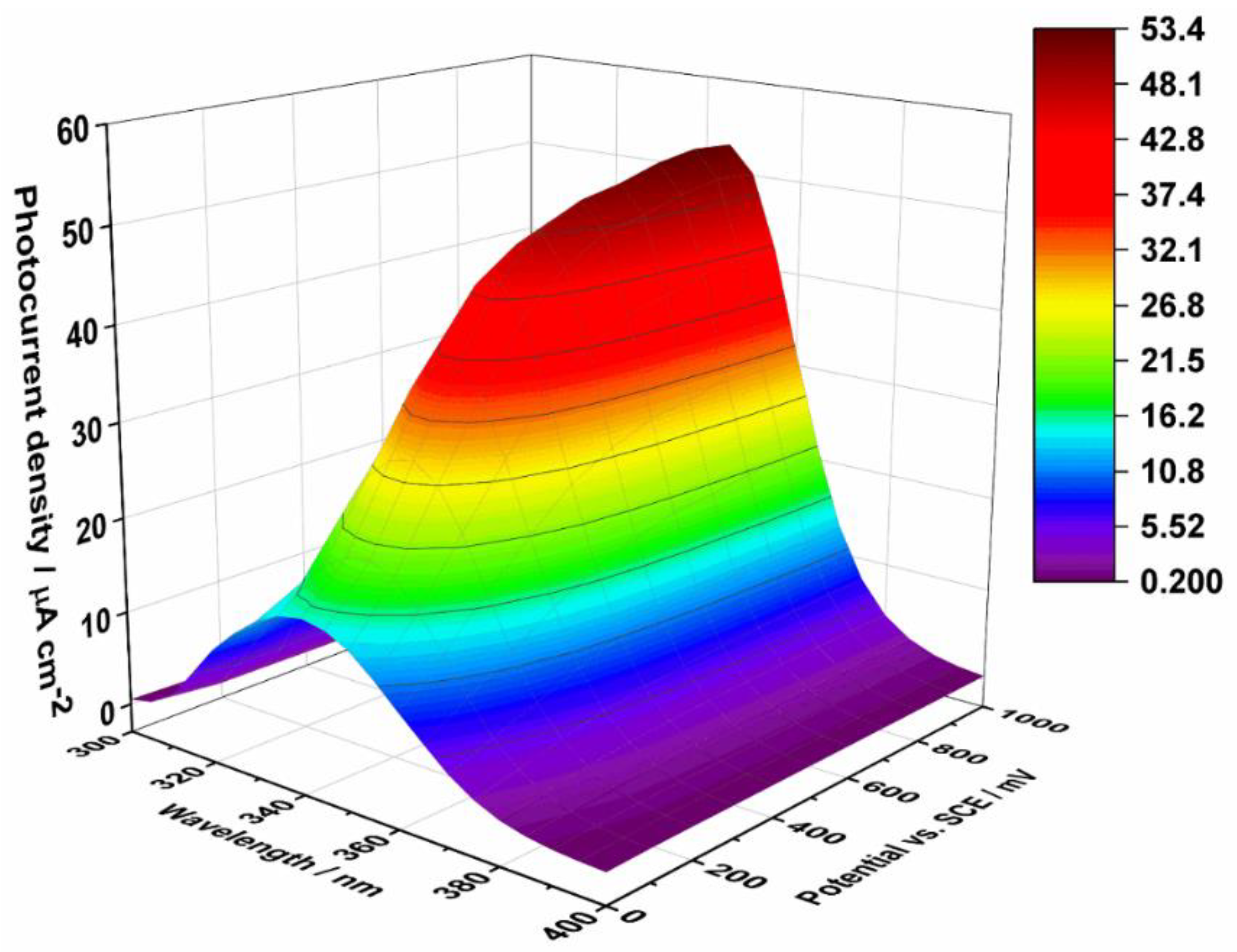
3.1. Photoelectrochemical Properties of ATO
3.2. PEC Sensing of Glucose
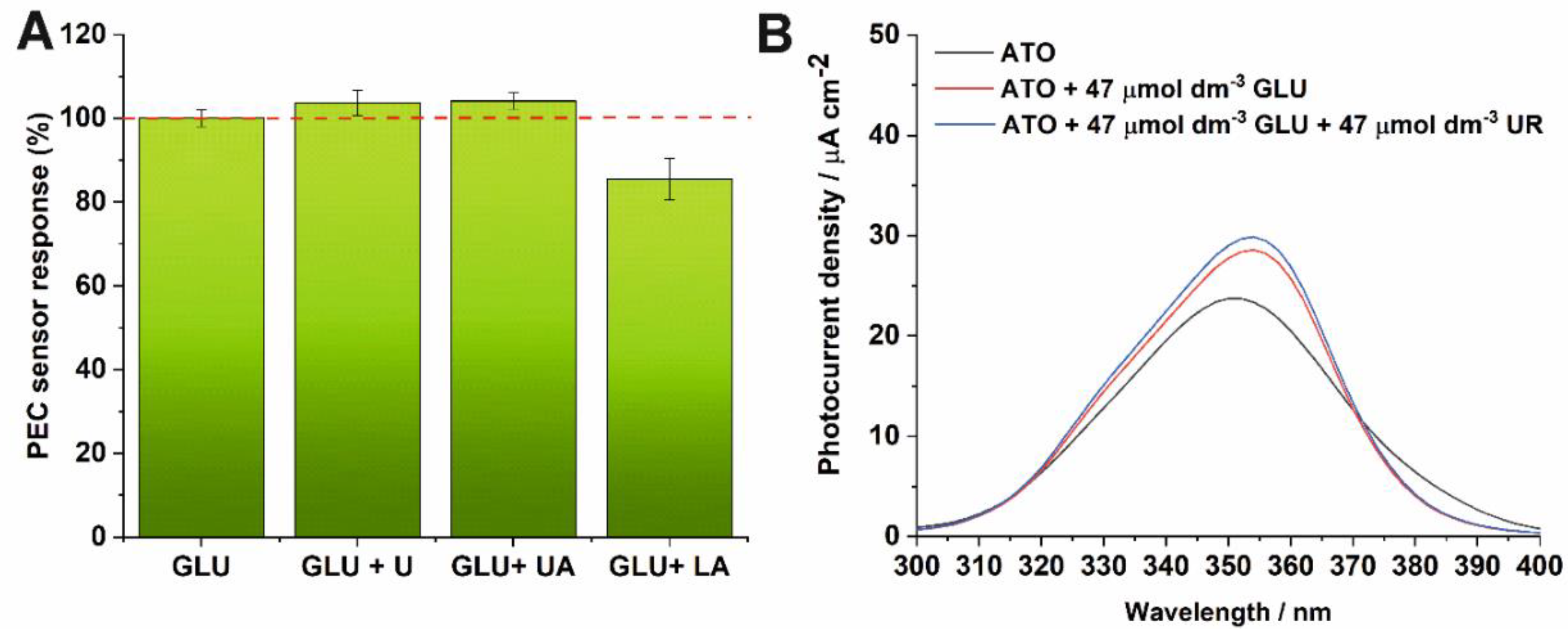
3.3. Interfering Substances
3.4. Real-Life Samples Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, C.W. Photocatalysis and photoelectrochemical properties of tungsten trioxide nanostructured films. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Y.; Li, J.; Da, P.; Geng, J.; Zheng, G. Sensitive enzymatic glucose detection by TiO2 nanowire photoelectrochemical biosensors. J. Mater. Chem. A 2014, 2, 6153–6157. [Google Scholar] [CrossRef]
- Xia, L.; Xu, L.; Song, J.; Xu, R.; Liu, D.; Dong, B.; Song, H. CdS quantum dots modified CuO inverse opal electrodes for ultrasensitive electrochemical and photoelectrochemical biosensor. Sci. Rep. 2015, 5, 10838. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.; Liu, Q.; Jin, Y.; Yan, J.; Cai, J. Ultrasensitive photoelectrochemical sensing of nicotinamide adenine dinucleotide based on grapheme-TiO2 nanohybrids under visible irradiation. Anal. Chim. Acta 2012, 745, 131–136. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry. Science 1980, 207, 139–144. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A Review of Photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Liu, M.; Zhang, S. Photoelectrochemically active species and photoelectrochemical biosensors. RSC Adv. 2013, 3, 2846–2857. [Google Scholar] [CrossRef]
- Wang, X.; Xia, X.; Zhang, X.; Meng, W.; Yuan, C.; Guo, M. Nonenzymatic glucose sensor based on Ag&Pt hollow nanoparticles supported on TiO2 nanotubes. Mater. Sci. Eng. C 2017, 80, 174–179. [Google Scholar]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical DNA biosensors. Chem. Rev. 2014, 114, 7421–7441. [Google Scholar] [CrossRef] [PubMed]
- Hun, X.; Wang, S.; Wang, S.; Zhao, J.; Luo, X. A photoelectrochemical sensor for ultrasensitive dopamine detection based on single-layer NanoMoS2 modified gold electrode. Sens. Actuat. B Chem. 2017, 249, 83–89. [Google Scholar] [CrossRef]
- Wang, J.; Thomas, D.F.; Chen, A. Nonenzymatic electrochemical glucose sensor based on nanoporous PtPb networks. Anal. Chem. 2008, 80, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Gao, Z.; Lee, K.; Schmuki, P. A self-cleaning nonenzymiatic glucose detection system based on titania nanotube arrays modified with platinum nanoparticles. Electrochem. Commun. 2011, 13, 1217–1220. [Google Scholar] [CrossRef]
- Liang, Y.; Kong, B.; Zhu, A.; Wang, Z.; Tian, Y. A facile and efficient strategy for photoelectrochemical detection of cadmium ions based on in situ electrodeposition of CdSe clusters on TiO2 nanotubes. Chem. Commun. 2012, 48, 245–247. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhi, Q.; Wang, Q.; Gittleson, F.S.; Lib, J.; Taylor, A.D. Enhanced photoelectrochemical and sensing performance of novel TiO2 arrays to H2O2 detection. Sens. Actuat. B Chem. 2015, 211, 111–115. [Google Scholar] [CrossRef]
- Xu, G.; Liu, H.; Wang, J.; Lv, J.; Zheng, Z.; Wu, Y. Photoelectrochemical performances and potential applications of TiO2 nanotube arrays modified with Ag and Pt nanoparticles. Electrochim. Acta 2014, 121, 194–202. [Google Scholar] [CrossRef]
- Feng, C.; Xu, G.; Liu, H.; Lv, J.; Zheng, Z.; Wu, Y. Glucose biosensors based on Ag nanoparticles modified TiO2 nanotube arrays. J. Solid State Electrochem. 2014, 18, 163–171. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ye, J.; Guo, M.; Chen, J.; Chen, W. Nonenzymatic glucose biosensors based on silver nanoparticles deposited on TiO2 nanotubes. J. Nanotech. 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, L.; Wang, Y.; Qin, D.; Shan, D.; Lu, X. Ternary nanocomposites of Au/Cu/TiO2 for an ultrasensitive photoelectrochemical non-enzymatic glucose sensor. Analyst 2018, 143, 1699–1704. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Zhang, G.; Hou, K.; Pan, H.; Du, M. Self-assembly of palladium nanoparticles on functional TiO2 nanotubes for a nonenzymatic glucose sensor. Mater. Sci. Eng. C 2016, 62, 323–328. [Google Scholar] [CrossRef]
- Chen, J.; Kang, Y.; Li, C.; Chen, H.; Sun, L.; Wang, Y.; Zhong, S. A Pt/TiO2 nanotube array electrode for glucose detection and its photoelectrocatalysis self-cleaning ability. J. Electrochem. Soc. 2017, 164, B66–B73. [Google Scholar] [CrossRef]
- Pang, X.; He, D.; Luo, S.; Cai, Q. An amperometric glucose biosensor fabricated with Pt nanoparticle-decorated carbon nanotubes/TiO2 nanotube arrays composite. Sensor Actuat. B Chem. 2009, 137, 134–138. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Xia, C.; Du, H.; Tian, F. Titanium dioxide nanotube arrays modified with a nanocomposite of silver nanoparticles and reduced graphene oxide for electrochemical sensing. Microchim. Acta 2014, 181, 1325–1331. [Google Scholar] [CrossRef]
- Han, X.; Zhu, Y.; Yang, X.; Li, C. Electrocatalytic activity of Pt doped TiO2 nanotubes catalysts for glucose determination. J. Alloys Compd. 2010, 500, 247–251. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Ge, M.; Iocozzia, J.; Lin, Z.; Zhang, K.-Q.; Lai, Y. Immobilization of Pt nanoparticles via rapid and reusable electropolymerization of dopamine on TiO2 nanotube arrays for reversible SERS substrates and nonenzymatic glucose sensors. Small 2017, 13, 160424. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Smith, Y.R.; Misra, M.; Mohanty, S.K. Electrochemical detection of methyl nicotinate biomarker using functionalized anodized titania nanotube arrays. Mater. Res. Exp. 2015, 2, 025002. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Xing, R.; Song, J.; Song, H.; Liu, D.; Zhou, J. Electrospun three-dimensional porous CuO/TiO2 hierarchical nanocomposites electrode for nonenzymatic glucose biosensing. Electrochem. Commun. 2012, 20, 75–78. [Google Scholar] [CrossRef]
- Long, M.; Tan, L.; Liu, H.; He, Z.; Tang, A. Novel helical TiO2 nanotube arrays modified by Cu2O for enzyme-free glucose oxidation. Biosens. Bioelectron. 2014, 59, 243–250. [Google Scholar] [CrossRef]
- Luo, S.; Su, F.; Liu, C.; Li, J.; Liu, R.; Xiao, Y.; Li, Y.; Liu, X.; Cai, Q. A new method for fabricating a CuO/TiO2 nanotube arrays electrode and its application as a sensitive nonenzymatic glucose sensor. Talanta 2011, 86, 157–163. [Google Scholar] [CrossRef]
- Thome-Duert, V.; Reach, G.; Gangnerau, M.N.; Lemonnier, F.; Klein, J.C.; Zhang, Y.; Hu, Y.; Wilson, G.S. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration priori to observation in blood. Anal. Chem. 1996, 68, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
- Kapusta-Kołodziej, J.; Syrek, K.; Pawlik, A.; Jarosz, M.; Tynkevych, O.; Sulka, G.D. Effects of anodizing potential and temperature on the growth of anodic TiO2 and its photoelectrochemical properties. Appl. Surf. Sci. 2017, 396, 1119–1129. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Chlebda, D.K.; Socha, R.P.; Sulka, G.D. Controlled synthesis of nanoporous tin oxide layers with various pore diameters and their photoelectrochemical properties. Electrochim. Acta 2017, 254, 238–245. [Google Scholar] [CrossRef]
- Jarosz, M.; Kapusta-Kołodziej, J.; Jaskuła, M.; Sulka, G.D. Effect of different polishing methods on anodic titanium dioxide formation. J. Nanomater. 2015. [Google Scholar] [CrossRef]
- Jarosz, M.; Syrek, K.; Kapusta-Kołodziej, J.; Mech, J.; Małek, K.; Hnida, K.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. Heat treatment effect on crystalline structure and photoelectrochemical properties of anodic TiO2 nanotube arrays formed in ethylene glycol and glycol based electrolytes. J. Phys. Chem. C 2015, 119, 24182–24191. [Google Scholar] [CrossRef]
- Syrek, K.; Kapusta-Kołodziej, J.; Jarosz, M.; Sulka, G.D. Effect of electrolyte agitation on anodic titanium dioxide (ATO) growth and its photoelectrochemical properties. Electrochim. Acta 2015, 180, 801–810. [Google Scholar] [CrossRef]
- Syrek, K.; Zych, M.; Zaraska, L.; Sulka, G.D. Influence of annealing conditions on anodic tungsten layers and their photoelectrochemical activity. Electrochim. Acta 2017, 231, 61–68. [Google Scholar] [CrossRef]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Fabrication on nanoporous TiO2 by electrochemical anodization. Electrochim. Acta 2010, 55, 4359–4367. [Google Scholar] [CrossRef]
- Li, S.; Qiu, J.; Ling, M.; Peng, F.; Wood, B.; Zhang, S. Photoelectrochemical characterization of hydrogenated TiO2 nanotubes as photoanodes for sensing applications. ACS Appl. Mater. Interfaces 2013, 5, 11129–11135. [Google Scholar] [CrossRef]
- Salvador, P. Kinetic approach to the photocurrent transients in water photoelectrolysis at n-TiO2 electrodes. 1. Analysis of the ratio of the instantaneous to steady-state photocurrent. J. Phys. Chem. 1985, 89, 3863–3869. [Google Scholar] [CrossRef]
- Corby, S.; Francàs, L.; Selim, S.; Sachs, M.; Blackman, C.; Kafizas, A.; Durrant, J.R. Water oxidation and electron extraction kinetics in nanostructured tungsten trioxide photoanodes. J. Am. Chem. Soc. 2018, 140, 16168–16177. [Google Scholar] [CrossRef] [PubMed]
- Le Formal, F.; Sivula, K.; Grätzel, M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. J. Phys. Chem. C 2012, 116, 26707–26720. [Google Scholar] [CrossRef]

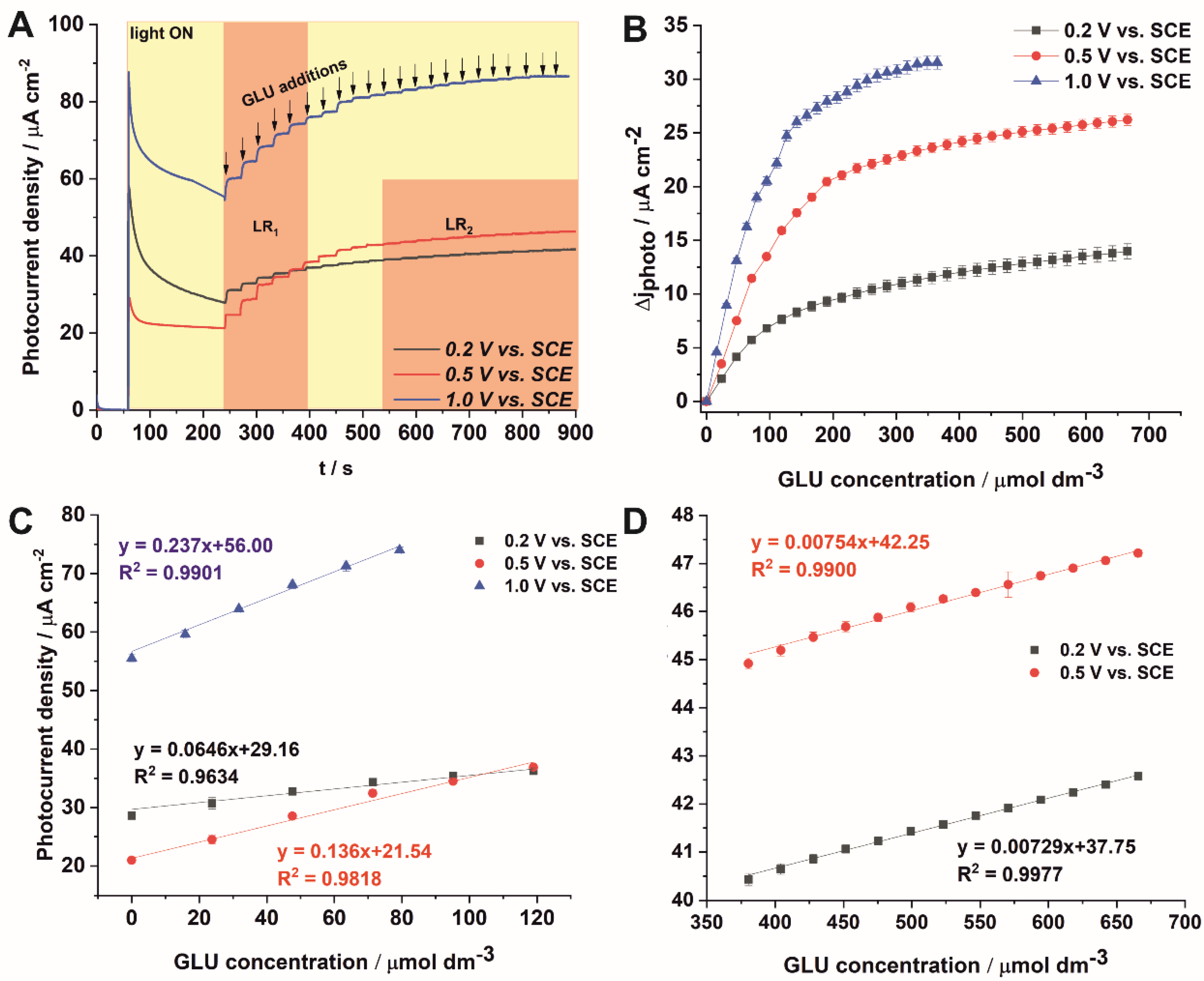

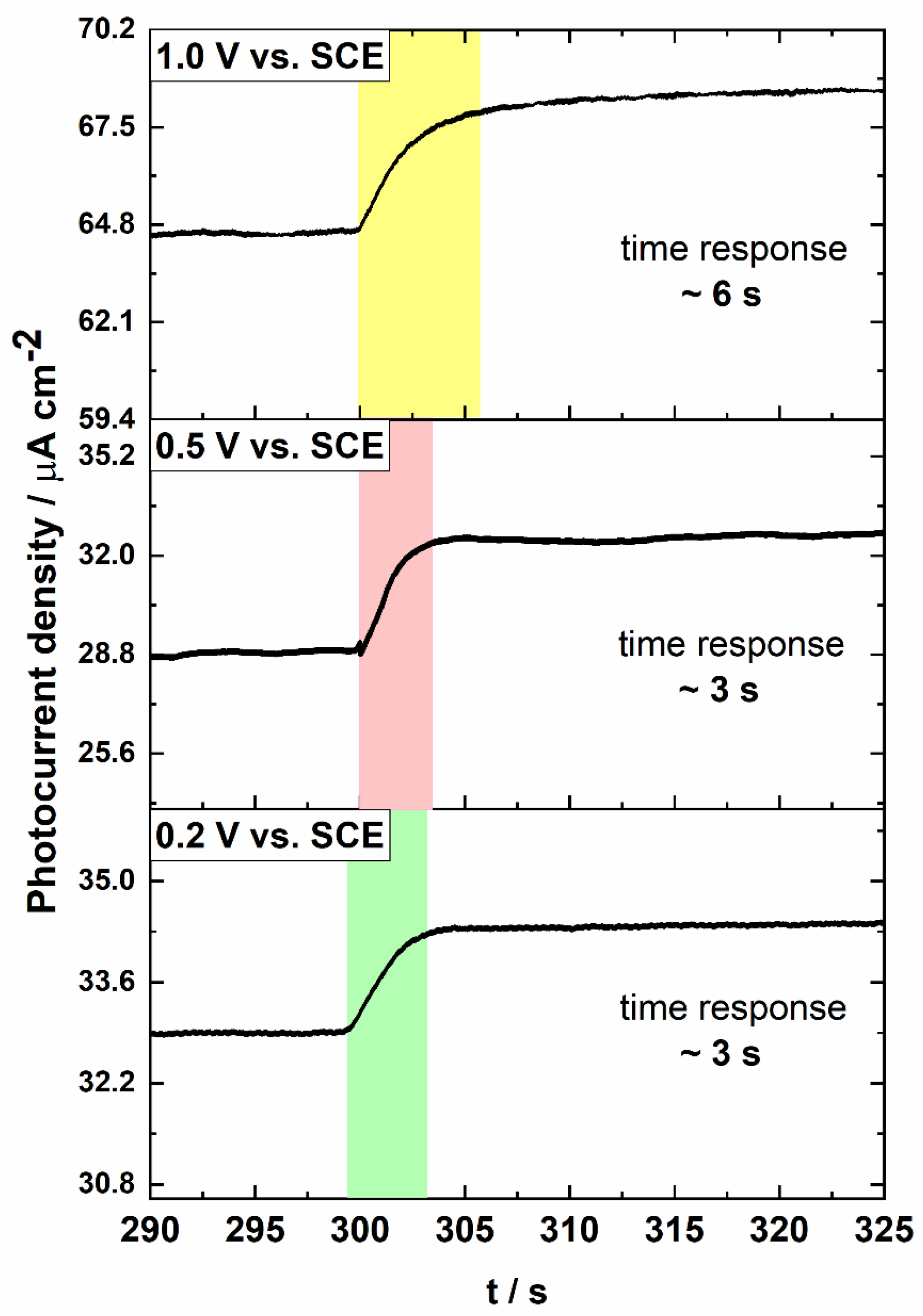

| Electrode | Applied Potential | Sensitivity [µA µmol−1 cm−2] | Linear Range [µmol dm−3] | LOD [µmol] | Response Time [s] | Reference |
|---|---|---|---|---|---|---|
| Anodic TiO2 NT GLU | 0.2 vs. Ag/AgCl (tested range: 0.2–1.0 V) | 0.14 | 10–1200 | 2.7 | ~56 s | [18] |
| Anodic TiO2 NT GLU | 0.2 V vs. Ag/AgCl | - | - | - | - | [14] |
| Anodic TiO2 NT GLU | 0.2 V vs. Ag/AgCl | 0.12 | 0–1000 | 6.49 | ~20 s | [17] |
| Anodic TiO2 NT + AgNPs GLU | 0.194 | 0–700 | 0.53 | |||
| Anodic TiO2 NT + PtNPs GLU | 0.076 | 0–650 | 13.5 |
| Δjphoto (µA cm−2) | GLU Concentration (µmol dm−3) | Recovery (%) | Repeatability (% RSD) | |
|---|---|---|---|---|
| Freeflex added | - | 47.6 | - | - |
| Freshly prepared ATO | 6.49 | 43.7 ± 1.3 | 92 | 2.97 |
| 1 month storage Self-cleaning with UV light | 6.25 | 43.0 ± 0.9 | 90 | 2.10 |
| 3 month storage Self-cleaning with UV light | 6.16 | 44.3 ± 0.8 | 93 | 2.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrek, K.; Skolarczyk, M.; Zych, M.; Sołtys-Mróz, M.; Sulka, G.D. A Photoelectrochemical Sensor Based on Anodic TiO2 for Glucose Determination. Sensors 2019, 19, 4981. https://doi.org/10.3390/s19224981
Syrek K, Skolarczyk M, Zych M, Sołtys-Mróz M, Sulka GD. A Photoelectrochemical Sensor Based on Anodic TiO2 for Glucose Determination. Sensors. 2019; 19(22):4981. https://doi.org/10.3390/s19224981
Chicago/Turabian StyleSyrek, Karolina, Maciej Skolarczyk, Marta Zych, Monika Sołtys-Mróz, and Grzegorz D. Sulka. 2019. "A Photoelectrochemical Sensor Based on Anodic TiO2 for Glucose Determination" Sensors 19, no. 22: 4981. https://doi.org/10.3390/s19224981
APA StyleSyrek, K., Skolarczyk, M., Zych, M., Sołtys-Mróz, M., & Sulka, G. D. (2019). A Photoelectrochemical Sensor Based on Anodic TiO2 for Glucose Determination. Sensors, 19(22), 4981. https://doi.org/10.3390/s19224981