Love Wave Surface Acoustic Wave Sensor with Laser-Deposited Nanoporous Gold Sensitive Layer
Abstract
1. Introduction
2. Materials and Methods
3. Results
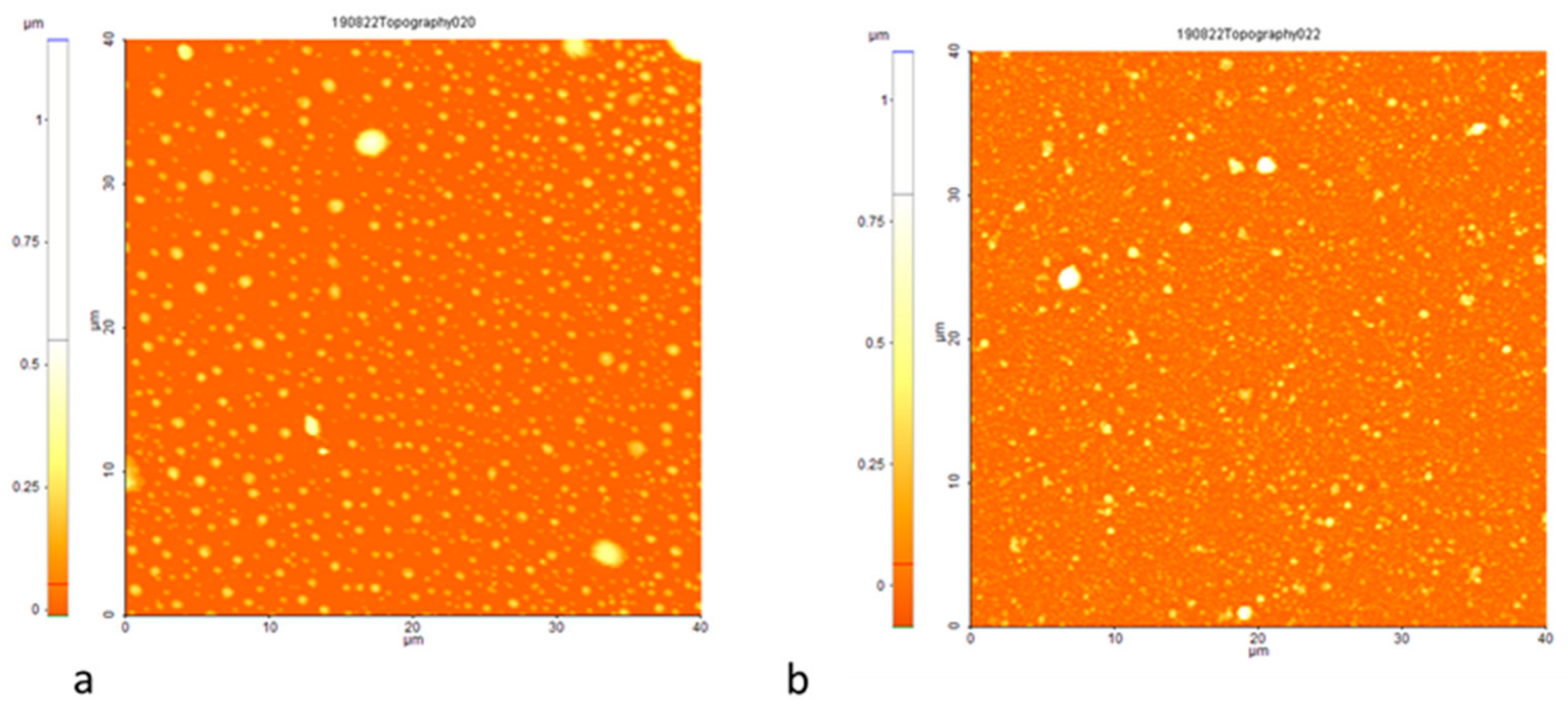
3.1. Morphology
3.2. Sensor Properties
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buryakov, I.A.; Buryakov, T.I.; Matsaev, V.T. Mass-Sensitive Micro- and Nanosensors for Detecting the Vapors of Explosives and Associated Substances. J. Anal. Chem. 2014, 69, 299–310. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Mahlotra, B.D.; Bhansali, S. Organic-Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Aliphatic Halogenated Hydrocarbons: Report and Analysis of Liver Injury in 60 Patients. J. Clin. Transl. Hepatol. 2018, 6, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Madeiros, L.D.; De Alencar, F.L.S.; Navoni, J.A.; De Aranjo, A.L.C.; Amaral, V.S.D. Toxicological aspects of trihalomethanes: A systematic review. Environ. Sci. Pollut. Res. 2019, 26, 5316–5332. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed]
- Preiss, E.M.; Rogge, T.; Krauss, A.; Seidel, H. Tin oxide based thin films prepared by pulsed laser deposition for gas sensing. Sens. Actuators B Chem. 2016, 236, 865–873. [Google Scholar] [CrossRef]
- Devkota, J.; Ohodnicki, P.R.; Greve, D.W. SAW Sensors for Chemical Vapors and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef]
- Ayaz, M.; Ammad-uddin, M.; Baig, I.; Aggoune, E.M. Wireless sensors civil applications, prototypes, and future integration possibilities: A review. IEEE Sens. J. 2018, 18, 4–30. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Mei, S.; Jia, Y.; Xue, X.; Yong, D. Development of a Pd/Cu nanowires coated SAW hydrogen gas sensor with fast response and recovery. Sens. Actuators B 2019, 287, 157–164. [Google Scholar] [CrossRef]
- Ballantine, D.S.; White, R.M.; Martin, S.I.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Acoustic Wave Sensors, Theory, Design and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Mujahid, A.; Dickert, F.L. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef]
- Laenge, K.; Rapp, B.E.; Rapp, M. Surface Acoustic Wave Biosensors: A Review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Gronewold, T.M.A. Surface Acoustic Wave Sensors in the bioanalytical field: Recent trends and challenges. Anal. Chem. Acta 2007, 603, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, Y.; An, C.; Ying, K.; Chen, X.; Wang, P. Sensitive detection of carcinoembryonic antigen in exhaled breath condensate using surface acoustic wave immunosensor. Sens. Actuators B 2015, 217, 100–106. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. Highly sensitive Love Wave acoustic biosensor for uric acid. Sens. Actuators B 2018, 261, 169–177. [Google Scholar] [CrossRef]
- Puiu, M.; Gurban, A.-M.; Rotariu, L.; Brajnicov, S.; Viespe, C.; Bala, C. Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats. Sensors 2015, 15, 10511–10525. [Google Scholar] [CrossRef]
- Pietrantonia, F.D.; Benetti, M.; Cannata, D.; Verona, E.; Girasole, M.; Fosca, M.; Dinarelli, S.; Staiano, M.; Marzullo, V.M.; Capo, A.; et al. A Shear Horizontal Surface Acoustic Wave biosensor for a rapid and specific detection of D-serine. Sens. Actuators B 2016, 226, 1–6. [Google Scholar] [CrossRef]
- Berkenpas, E.; Millard, P.; Da Cunha, M.P. Detection of Escherichia coli O157:H7 with langasite pure shear horizontal surface acoustic wave sensors. Biosens. Bioelectron. 2006, 21, 2255–2262. [Google Scholar] [CrossRef]
- Zhang, R.; Olin, H. Porous Gold Films—A Short Review on Recent Progress. Materials 2014, 7, 3834–3854. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef]
- Asai, N.; Terasawa, H.; Shimizu, T.; Shingubara, S.; Ito, T. Sensitized mass change detection using Au nanoporous electrode for biosensing. Jpn. J. Appl. Phys. 2017, 56, 06GG04. [Google Scholar] [CrossRef]
- Hieda, M.; Garcia, R.; Dixon, M.; Daniel, T.; Allara, D.; Chan, M.H.W. Ultrasensitive quartz crystal microbalance using porous gold electrodes. Appl. Phys. Lett. 2004, 84, 628–630. [Google Scholar] [CrossRef]
- Amani, E.; Khojier, K.; Zoriasatain, S. Improving the hydrogen gas sensitivity of WO3 thin films by modifying the deposition angle and thickness of different promoter layers. Int. J. Hydrogen Energy 2017, 42, 29620–29628. [Google Scholar] [CrossRef]
- Ippolito, S.J.; Kandasamy, S.; Kalantor-Zadeh, K.; Wlodarski, W. Layered SAW hydrogen sensor with modified tungsten trioxide selective layer. Sens. Actuators B 2005, 108, 553–557. [Google Scholar] [CrossRef]
- Hur, Y.; Hau, J.; Seon, J.; Pak, Y.E.; Roh, Y. Detection of an SH-SAW sensor for the detection of DNA hybridization. Sens. Actuators A 2005, 120, 462–467. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Surface Acoustic Wave Sensor with Pd/ZnO bilayer structure for Room Temperature Hydrogen Detection. Sensors 2017, 17, 1529. [Google Scholar] [CrossRef]
- Miu, D.; Birjega, R.; Viespe, C. Surface Acoustic Wave hydrogen sensors based on nanostructured Pd/WO3 bilayers. Sensors 2018, 18, 3636. [Google Scholar] [CrossRef]
- Ghidelli, M.; Mascaretti, L.; Bricchi, B.R.; Zapelli, A.; Russo, V.; Casari, C.S.; Li Bassi, A. Engineering plasmonic nanostructured surfaces by pulsed laser deposition. Appl. Surf. Sci. 2018, 434, 1064–1073. [Google Scholar] [CrossRef]
- Ashford, M.N.R.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2004, 33, 23–31. [Google Scholar] [CrossRef]
- Di Fonzo, F.; Tonini, D.; Li Bassi, A.; Casari, C.S.; Beghi, M.G.; Bottani, C.E.; Gastaldi, D.; Vena, P.; Contro, R. Growth regimes in pulsed laser deposition of aluminum oxide thin films. Appl. Phys. A 2008, 93, 765–769. [Google Scholar] [CrossRef]
- Verma, S.; TirumalaRao, B.; Rai, S.; Ganesan, V.; Kukreja, L.M. Influence of process parameters on surface plasmon resonance characteristics of densely packed gold nanoparticle films grown by pulsed laser deposition. Appl. Surf. Sci. 2012, 258, 4898–4905. [Google Scholar] [CrossRef]
- Irissou, E.; Le Drogoff, B.; Chaker, M.; Guay, D. Correlation between plasma expansion dynamics and gold-thin film structure during pulsed-laser deposition. Appl. Phys. Lett. 2002, 80, 1716–1718. [Google Scholar] [CrossRef]
- Vandeput, M.; Parsajoo, C.; Vanheuverzwijn, J.; Patris, S.; Yardim, Y.; Le Jeune, A.; Sarakbi, A.; Mertens, D.; Kauffmann, J.-M. Flow-through enzyme immobilized amperometric detector for the rapid screening of acetylcholinesterase inhibitors by flow injection analysis. J. Pharm. Biomed. Anal. 2015, 102, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.L.; Bolger, F.B.; Lowry, J.P. A microelectrochemical biosensor for real-time in vivo monitoring of brain extracellular choline. Analyst 2015, 140, 3738–3745. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef] [PubMed]
- El Harrad, L.; Bourais, I.; Mohammadi, H.; Amine, A. Recent Advances in Electrochemical Biosensors Based on Enzyme Inhibition for Clinical and Pharmaceutical Applications. Sensors 2018, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Dinca, V.; Viepse, C.; Brajnicov, S.; Constantinoiu, I.; Moldovan, A.; Bonciu, A.; Toader, C.N.; Ginghina, R.E.; Grigoriu, N.; Dinescu, M.; et al. MAPLE Assembled Acetylcholinesterase-Polyethylenimine Hybrid and Multailayered Interfaces for Toxic Gases Detection. Sensors 2018, 18, 4265. [Google Scholar] [CrossRef]
- Oh, H.; Fu, C.; Kin, K.; Lee, K. Wireless and Simultaneous Detections of Multiple Bio-Molecules in a Single Sensor Using Love Wave Biosensor. Sensors 2014, 14, 21660–21675. [Google Scholar] [CrossRef]
- Amarandei, G.; O’Dwyer, C.; Arshak, A.; Thiele, U.; Steiner, U.; Corcoran, D. Effect of Au nanoparticle spatial distribution on the stability of thin polymer films. Langmuir 2013, 29, 6706–6714. [Google Scholar] [CrossRef]
- Lackner, J.M.; Waldhauser, W.; Hartmann, P.; Miskovics, O.; Schmied, F.; Teichert, C.; Schoeberl, T. Self-assembling (nano-) wrinkling topography formation in low-temperature vacuum deposition on soft polymer surfaces. Thin Solid Films 2012, 520, 2833–2840. [Google Scholar] [CrossRef]
- Stetsenko, M.O.; Maksimenko, L.S.; Rudenko, S.P.; Krischchenko, I.M.; Korchovyi, A.A.; Kryvyi, S.B.; Kaganovich, E.B.; Serdega, B.K. Surface Plasmon’s Dispersion Properties of Porous Gold Films. Nanoscale Res. Lett. 2016, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Geohegan, D.B.; Puretzky, A.A. Laser ablation plume thermalization dynamics in background gases: Combined imaging, optical absorption and emission spectroscopy, and ion probe measurements. Appl. Surf. Sci. 1996, 96–98, 131–138. [Google Scholar] [CrossRef]
- Harilal, S.S.; O’Shay, B.; Tao, Y.; Tillack, M.S. Ambient gas effects on the dynamics of laser-produced tin plume expansion. J. Appl. Phys. 2006, 99, 083303. [Google Scholar] [CrossRef]
- Geohegan, D.B.; Puretzky, A.A.; Duscher, G.; Pennycook, S.J. Time-resolved imaging of gas phase nanoparticle synthesis by laser ablation. Appl. Phys. Lett. 1998, 72, 2987–2989. [Google Scholar] [CrossRef]
- Tillack, M.S.; Blair, D.W.; Harilal, S.S. The effect of ionization on cluster formation in laser ablation plumes. Nanotechnology 2004, 15, 390–403. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussmana, J.L. Acetylcholinesterase: From 3D Structure to Function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef]



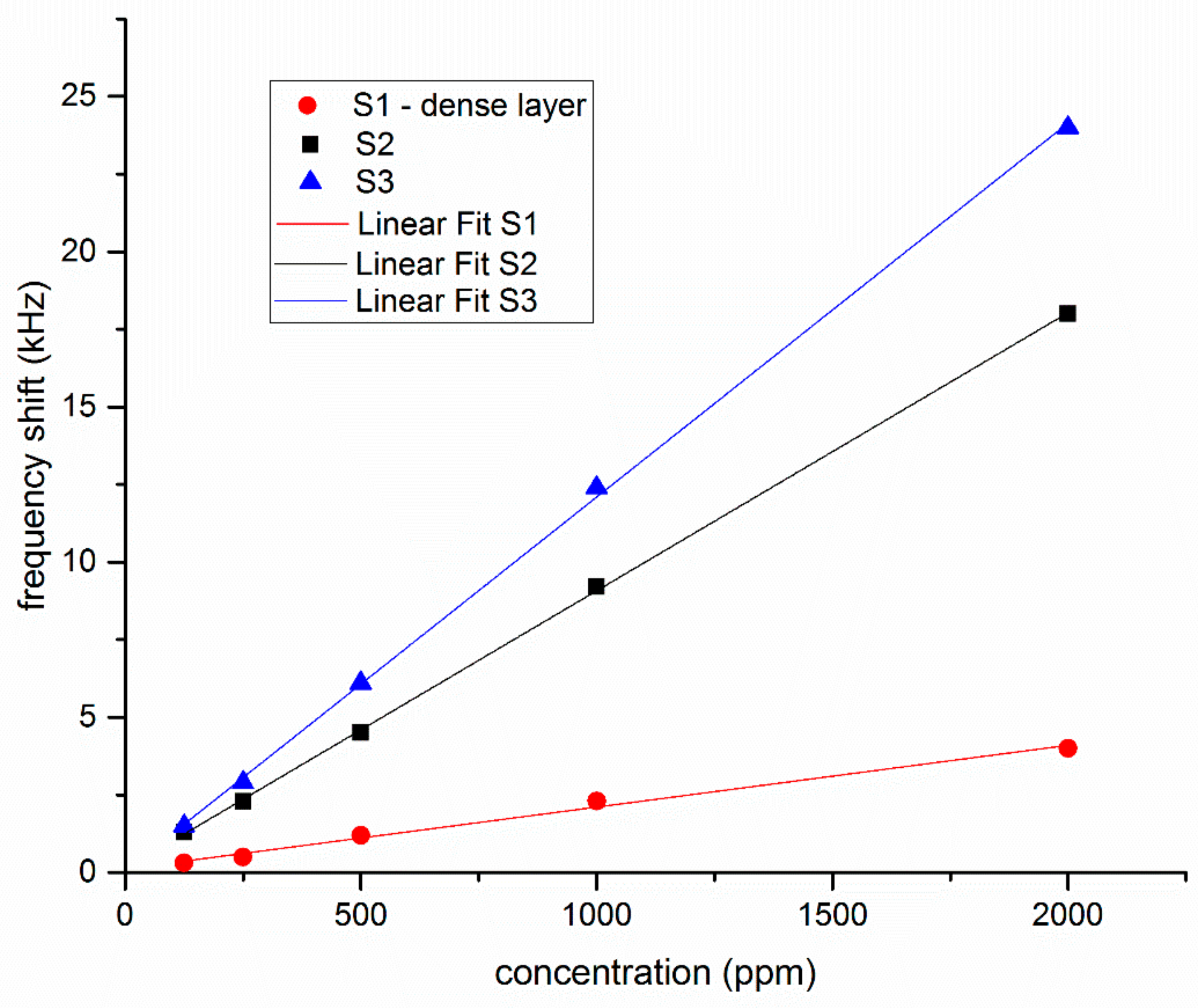

| LW-SAW Sensors | Sensitivity Δf/c Hz/ppm | LOD ppm |
|---|---|---|
| S1 | 2 | 41 |
| S2 | 9 | 10 |
| S3 | 12 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viespe, C.; Dinca, V.; Popescu-Pelin, G.; Miu, D. Love Wave Surface Acoustic Wave Sensor with Laser-Deposited Nanoporous Gold Sensitive Layer. Sensors 2019, 19, 4492. https://doi.org/10.3390/s19204492
Viespe C, Dinca V, Popescu-Pelin G, Miu D. Love Wave Surface Acoustic Wave Sensor with Laser-Deposited Nanoporous Gold Sensitive Layer. Sensors. 2019; 19(20):4492. https://doi.org/10.3390/s19204492
Chicago/Turabian StyleViespe, Cristian, Valentina Dinca, Gianina Popescu-Pelin, and Dana Miu. 2019. "Love Wave Surface Acoustic Wave Sensor with Laser-Deposited Nanoporous Gold Sensitive Layer" Sensors 19, no. 20: 4492. https://doi.org/10.3390/s19204492
APA StyleViespe, C., Dinca, V., Popescu-Pelin, G., & Miu, D. (2019). Love Wave Surface Acoustic Wave Sensor with Laser-Deposited Nanoporous Gold Sensitive Layer. Sensors, 19(20), 4492. https://doi.org/10.3390/s19204492







