Experimental-Numerical Design and Evaluation of a Vibration Bioreactor Using Piezoelectric Patches
Abstract
:1. Introduction
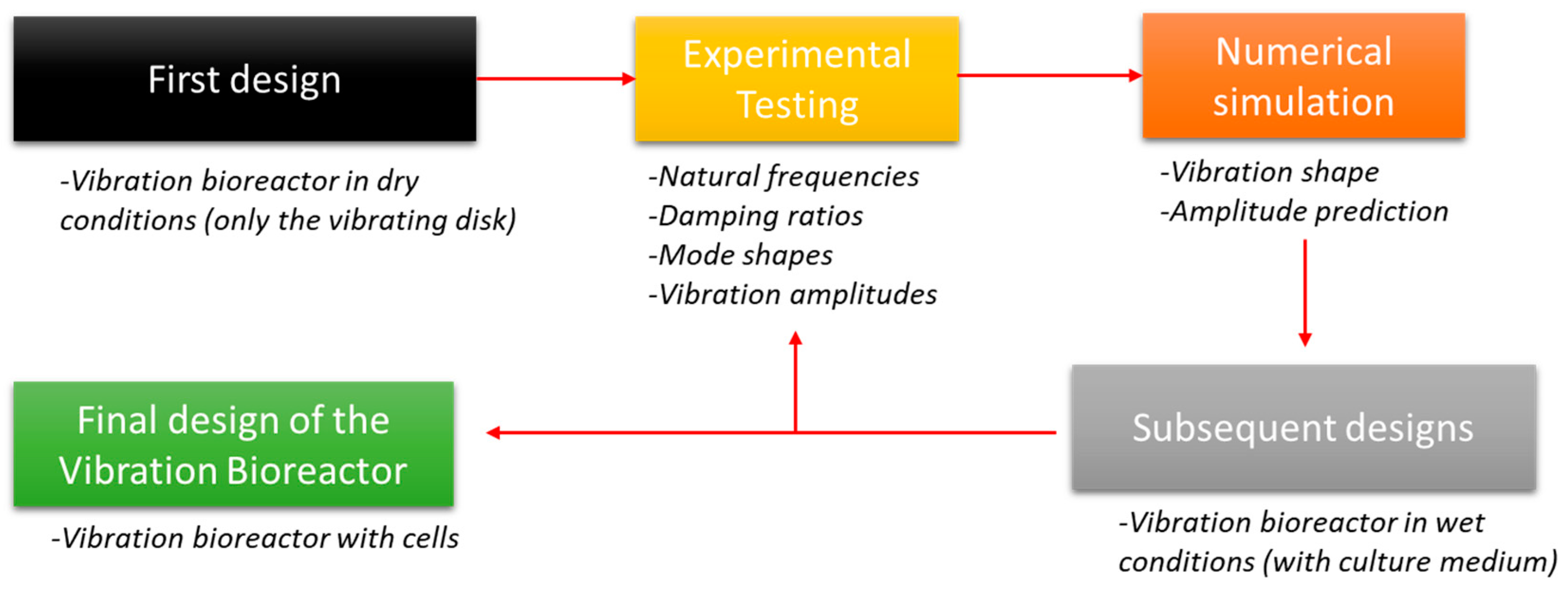
2. Methodology
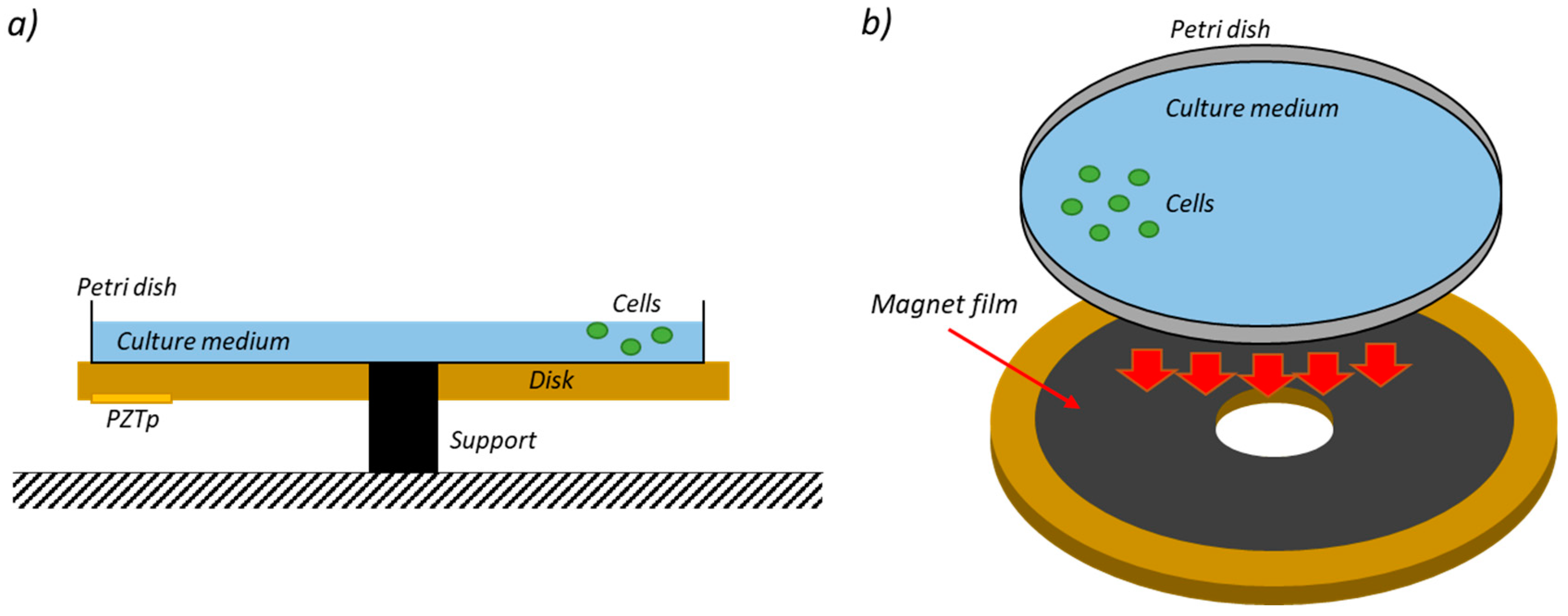
2.1. Experimental Investigation
2.1.1. Experiment Set-Up
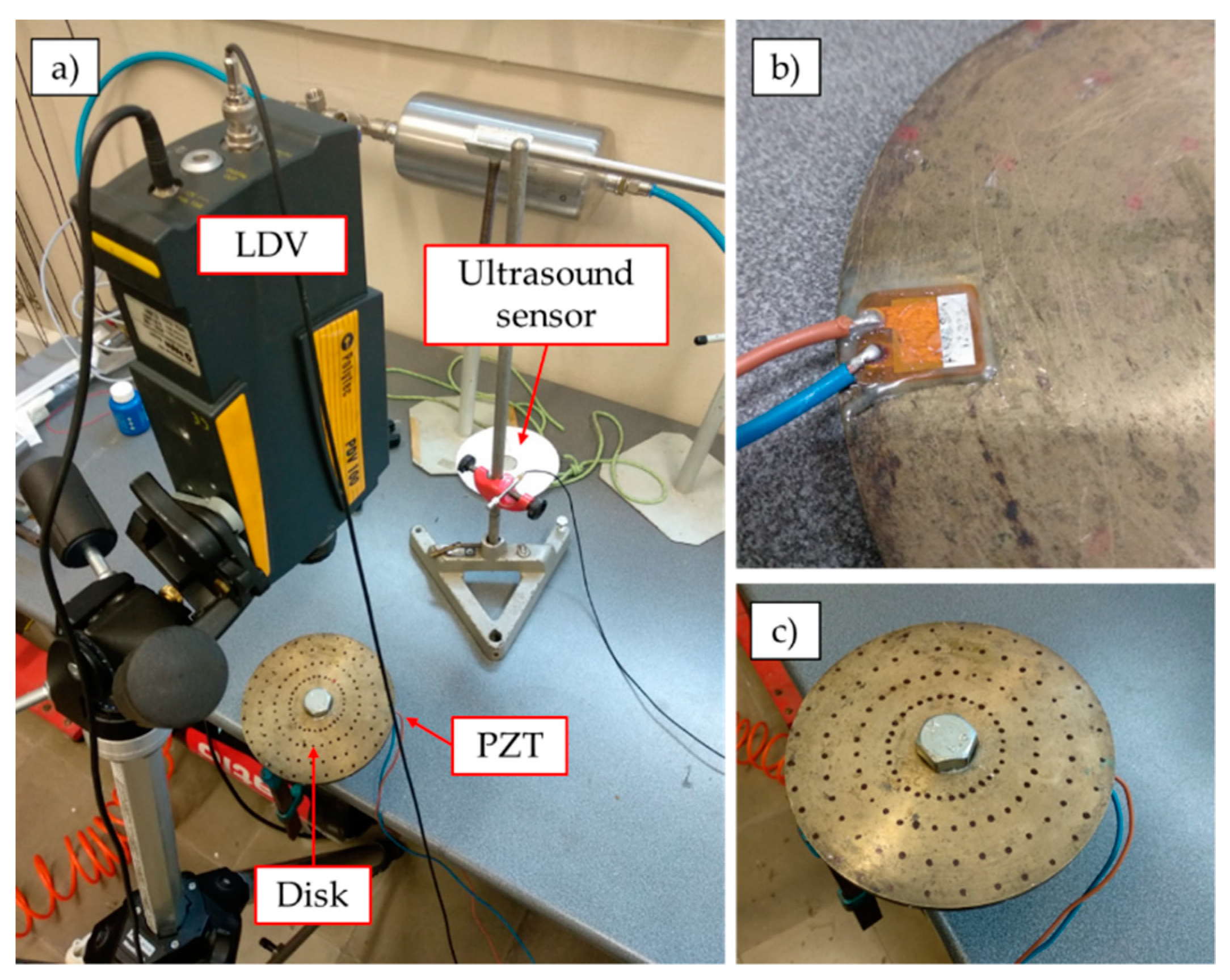
2.1.2. Instrumentation
2.1.3. Experimental Procedure
2.2. Numerical Simulation
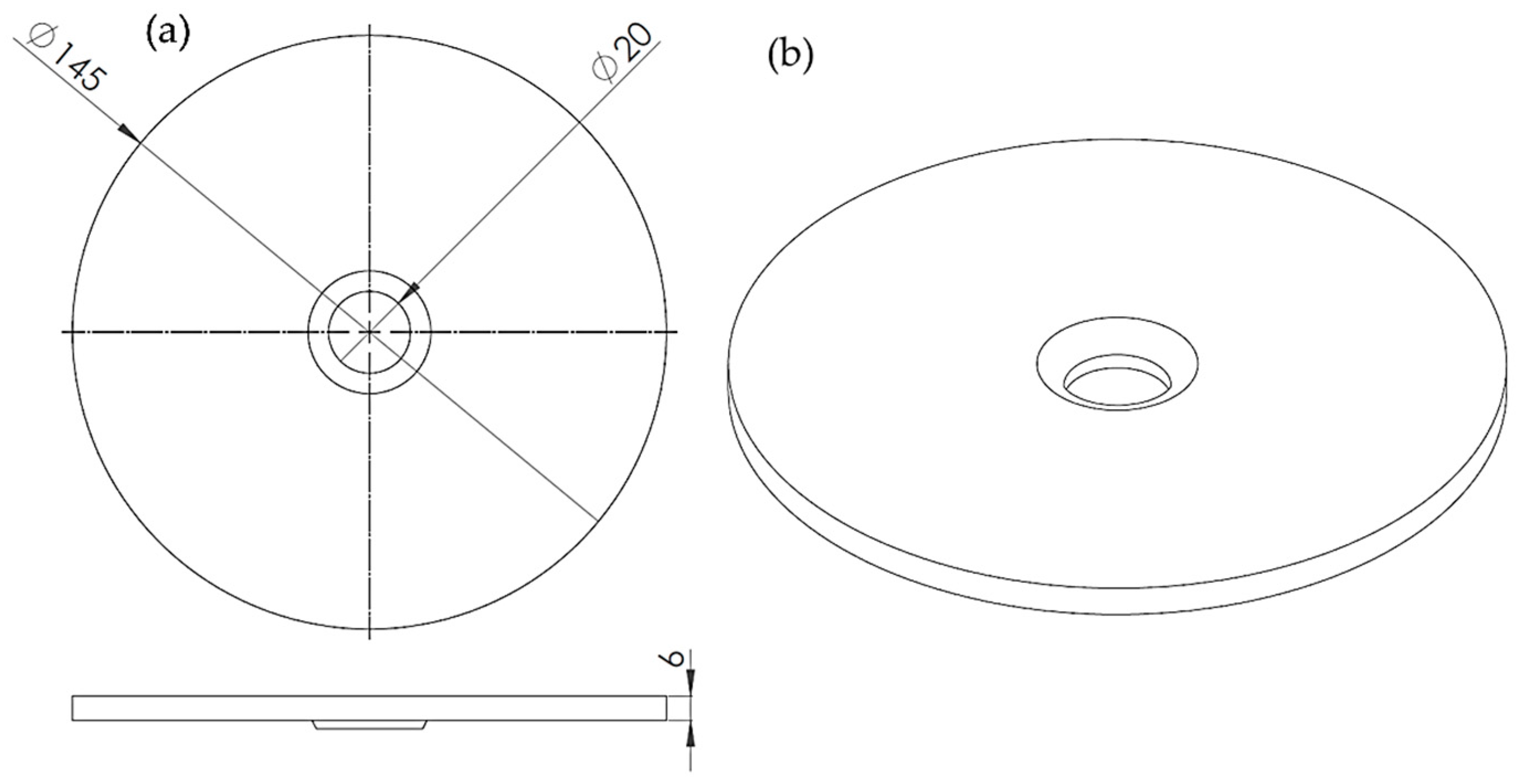
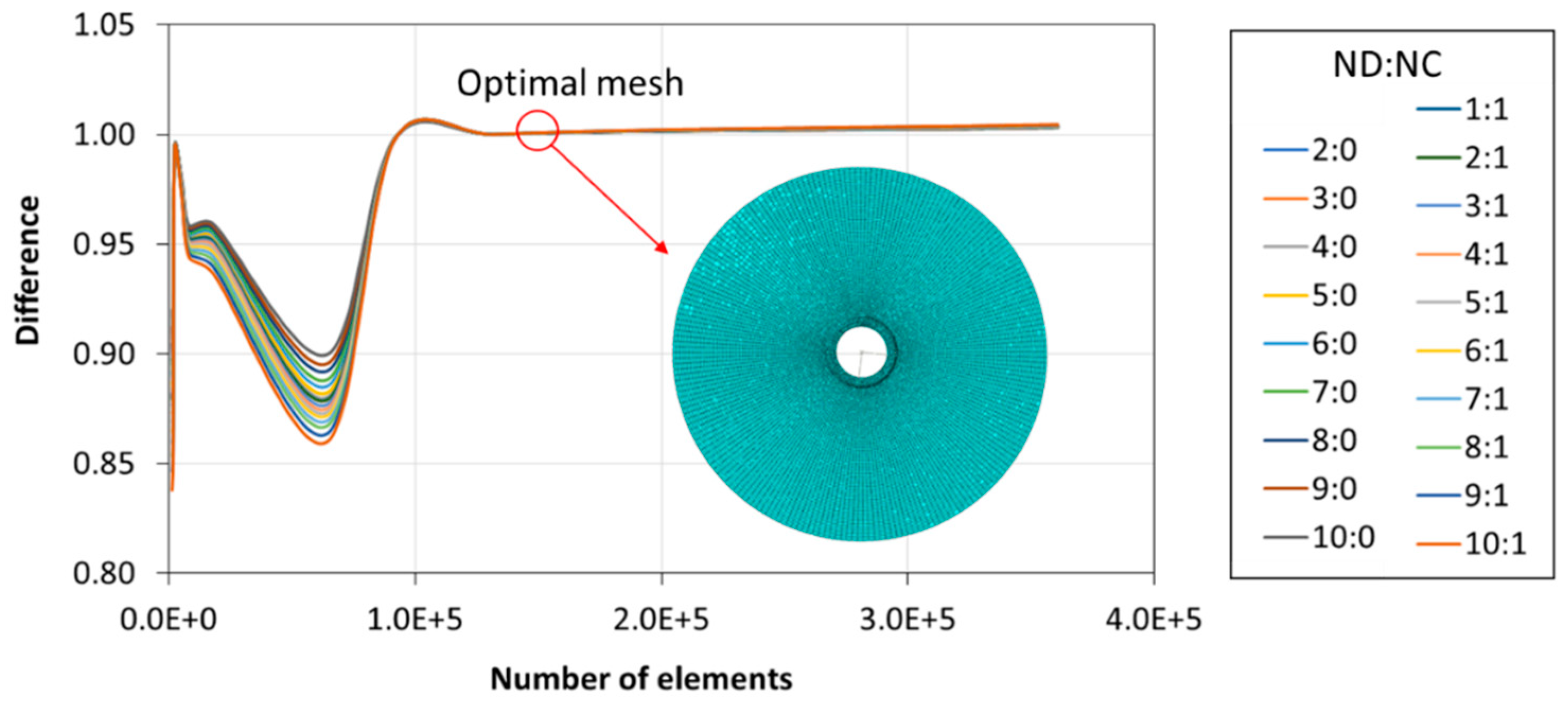
2.2.1. Finite Element Method (FEM) Model
2.2.2. Numerical Procedure
3. Results and Discussion
3.1. Experimental Results
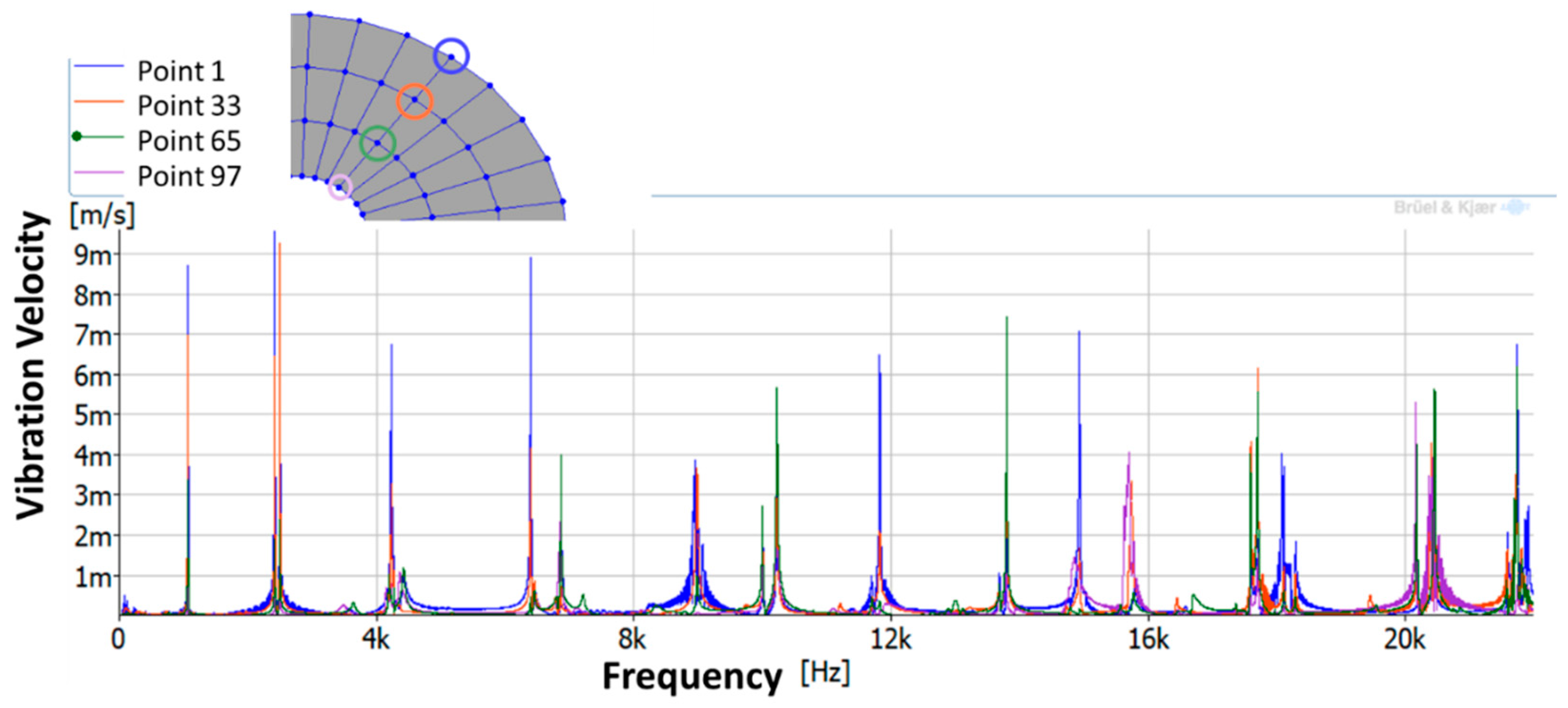
3.1.1. Experimental Modal Analysis
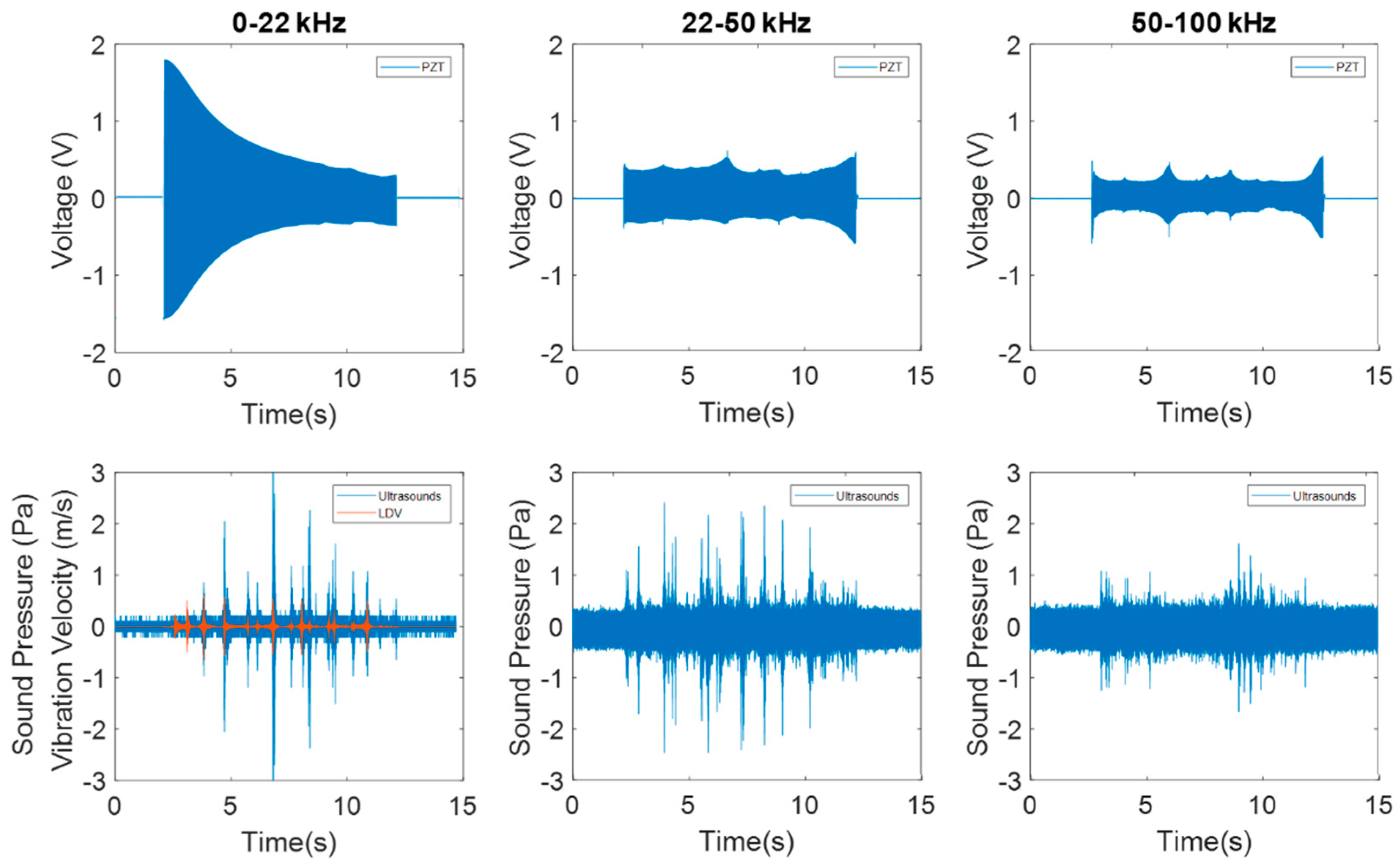
3.1.2. Dynamic Behavior at Higher Frequency Ranges
3.2. Numerical Results
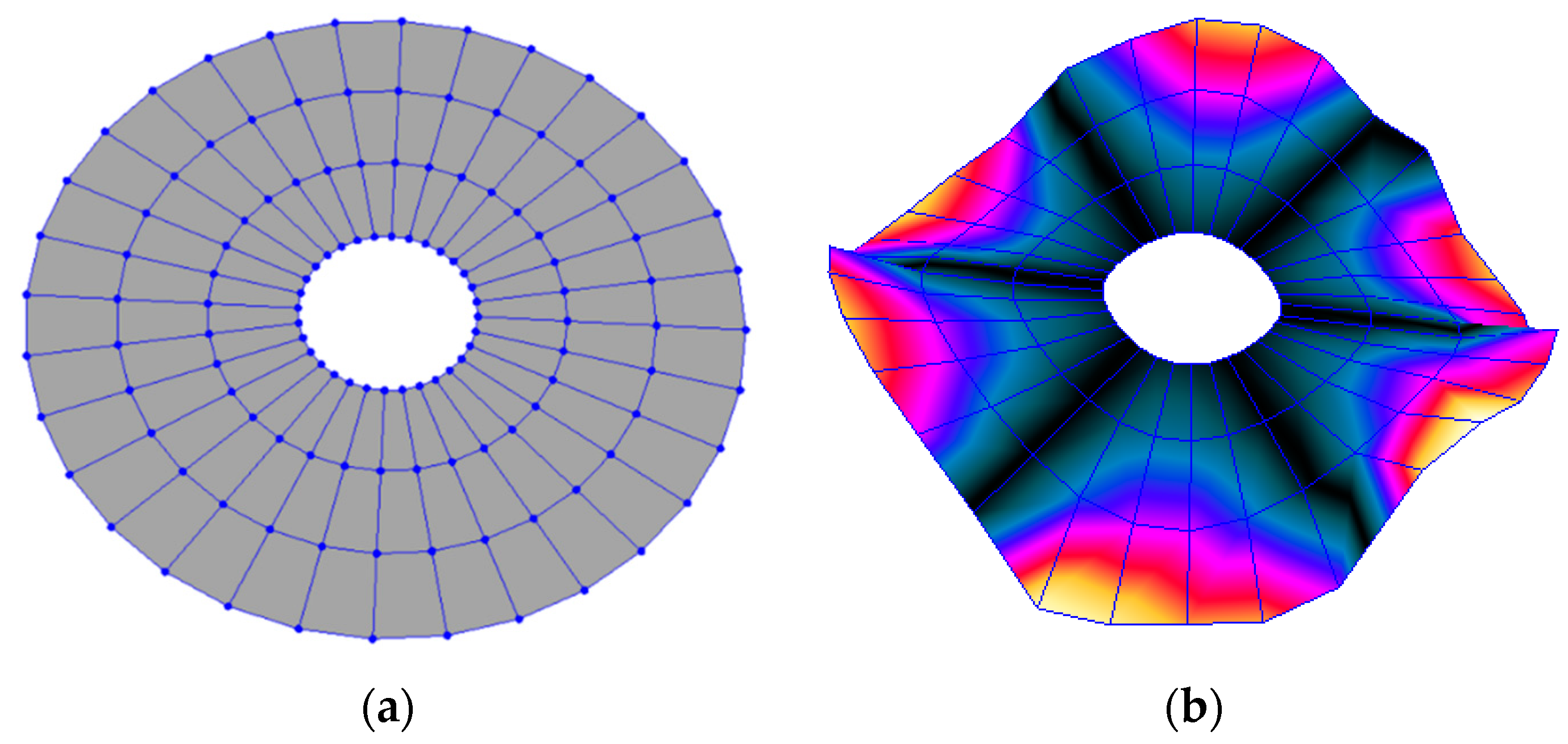
3.2.1. Modal Analysis
3.2.2. Harmonic Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valentin, D.; Charline, R.; Presas, A.; Heiss, C.; Wolfram, B. Biomechanical damage behaviour in cells under vibration. In Proceedings of the 2018 International Conference on Trauma Surgery Technology, Giessen, Germany, 16–18 November 2018. [Google Scholar]
- Ter Haar, G.; Coussios, C. High intensity focused ultrasound: Past, present and future. Int. J. Hyperth. 2007, 23, 85–87. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Wijlemans, J.W.; Bartels, L.W.; Deckers, R.; Ries, M.; Mali, W.P.T.M.; Moonen, C.T.W.; Van Den Bosch, M.A.A.J. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) ablation of liver tumours. Cancer Imaging 2012, 12, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Song, Y.M.; Lee, B.; Hwang, S.J. Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J. Dent. Res. 2012, 91, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, K.; Li, S.; Wang, Y.; Huang, T.S.; Zhang, A.; Cheng, Z.Y. In situ real-time detection of E. coli in water using antibody-coated magnetostrictive microcantilever. Sens. Actuators B Chem. 2010, 150, 220–225. [Google Scholar] [CrossRef]
- Ramos, D.; Tamayo, J.; Mertens, J.; Calleja, M.; Villanueva, L.G.; Zaballos, A. Detection of bacteria based on the thermomechanical noise of a nanomechanical resonator: Origin of the response and detection limits. Nanotechnology 2008, 19, 035503. [Google Scholar] [CrossRef] [PubMed]
- Ilic, B.; Czaplewski, D.; Zalalutdinov, M.; Craighead, H.G.; Neuzil, P.; Campagnolo, C.; Batt, C. Single cell detection with micromechanical oscillators. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2001, 19, 2825. [Google Scholar] [CrossRef]
- Carmen, J.C.; Roeder, B.L.; Nelson, J.L.; Robison Ogilvie, R.L.; Robison, R.A.; Schaalje, G.B.; Pitt, W.G. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 2005, 33, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Z.; Sagers, R.D.; Pitt, W.G. The effect of ultrasonic frequency upon enhanced killing of P. aeruginosa biofilms. Ann. Biomed. Eng. 1997, 25, 69–76. [Google Scholar] [CrossRef]
- Belyaev, I.Y.; Alipov, Y.D.; Polunin, V.A.; Shcheglov, V.S. Evidence for dependence of resonant frequency of millimeter wave interaction with escherichia coli k12 cells on haploid genome length. Electromagn. Biol. Med. 1993, 12, 39–49. [Google Scholar]
- Duka, M.; Balbekin, N.; Smolyanskaya, O.; Sobakinskaya, E.; Panin, A.; Vaks, V. Characterization of DNA absorption lines in the terahertz range. In Proceedings of the 2015 IEEE North West Russia Section Young Researchers in Electrical and Electronic Engineering Conference, ElConRusNW 2015, St. Petersburg, Russia, 2–4 February 2015; pp. 327–328. [Google Scholar]
- Fassina, L.; Saino, E.; Sbarra, M.S.; Visai, L.; De Angelis, M.G.C.; Mazzini, G.; Benazzo, F.; Magenes, G. Ultrasonic and Electromagnetic Enhancement of a Culture of Human SAOS-2 Osteoblasts Seeded onto a Titanium Plasma-Spray Surface. Tissue Eng. Part C 2009, 15, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Hauser, M.; Muhr, G.; Esenwein, S. Ultrasound-induced modifications of cytoskeletal components in osteoblast-like SAOS-2 cells. J. Orthop. Res. 2009, 27, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Fassina, L.; Saino, E.; Visai, L.; Magenes, G. Physically enhanced coating of titanium plasma-spray with human osteoblasts and bone matrix. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar]
- Mccormick, S.M.; Saini, V.; Yazicioglu, Y.; Demou, Z.N.; Royston, T.J. Interdependence of pulsed ultrasound and shear stress effects on cell morphology and gene expression. Ann. Biomed. Eng. 2006, 34, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Zou, J.Z.; Bai, J.; Zhu, H.; Li, K.Q.; Xie, F.L.; Jin, C.B.; Su, H.B.; et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med. Biol. 2004, 30, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.; Fan, W.; Huang, J.; Zhang, F.; Wu, P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010, 116, 3934–3942. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wu, P.; Zhang, L.; Fan, W.; Huang, J.; Zhang, F. Osteosarcoma: Limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound. Cancer Biol. Ther. 2009, 8, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Tang, L.; Lin, F.; Yao, Y.; Shen, Z.; Zhou, X. High-intensity focused ultrasound: Noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg. Oncol. 2015, 24, 9–15. [Google Scholar] [CrossRef]
- Ozcivici, E.; Kim Luu, Y.; Adler, B.; Qin, Y.-X.; Rubin, J.; Judex, S.; Rubin, C.T. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 2010, 6, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef]
- Bosbach, W.A. Mechanical bone growth stimulation by magnetic fibre networks obtained through a competent finite element technique. Sci. Rep. 2017, 7, 11109. [Google Scholar] [CrossRef]
- Bosbach, W.A. The Elastic Behaviour of Sintered Metallic Fibre Networks: A Finite Element Study by Beam Theory. PLoS ONE 2015, 10, e0143011. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.; Bussolari, S.; Gimbrone, M.; Davies, P. The Dynamic Response of Vascular Endothelial Cells to Fluid Shear Stress. J. Biomech. Eng. 1981, 103, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Takahashi, M.; Corson, M.; Berk, B. Fluid shear stress-mediated signal transduction: How do endothelial cells transduce mechanical force into biological responses? Ann. N. Y. Acad. Sci. 1997, 811, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Kim, J.; McPhedran, S.K.; Camp, A.J. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp. Brain Res. 2006, 175, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Young, E.D.; Fernández, C.; Goldberg, J.M. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977, 84, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Presas, A.; Luo, Y.; Wang, Z.; Valentin, D.; Egusquiza, M.; Presas, A.; Luo, Y.; Wang, Z.; Valentin, D.; Egusquiza, M. A Review of PZT Patches Applications in Submerged Systems. Sensors 2018, 18, 2251. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-G.; Jung, W.-S.; Kang, C.-Y.; Yoon, S.-J.; Kang, M.-G.; Jung, W.-S.; Kang, C.-Y.; Yoon, S.-J. Recent Progress on PZT Based Piezoelectric Energy Harvesting Technologies. Actuators 2016, 5, 5. [Google Scholar] [CrossRef]
- Elahi, H.; Eugeni, M.; Gaudenzi, P.; Elahi, H.; Eugeni, M.; Gaudenzi, P. A Review on Mechanisms for Piezoelectric-Based Energy Harvesters. Energies 2018, 11, 1850. [Google Scholar] [CrossRef]
- Curie, J. Développement par compression de l’électricité polaire dans les cristaux hémièdres à faces inclinées. Bull. Soc. Fr. Miner. 1880, 3, 90. [Google Scholar] [CrossRef]
- Presas, A.; Valentin, D.; Egusquiza, E.; Valero, C.; Egusquiza, M.; Bossio, M. Accurate determination of the frequency response function of submerged and confined structures by using PZT-patches. Sensors 2017, 17, 660. [Google Scholar] [CrossRef]
- Presas, A.; Egusquiza, E.; Valero, C.; Valentin, D.; Seidel, U. Feasibility of using PZT actuators to study the dynamic behavior of a rotating disk due to rotor-stator interaction. Sensors 2014, 14, 11919–11942. [Google Scholar] [CrossRef] [PubMed]
- Billah, K.Y.; Scanlan, R.H. Resonance, Tacoma Narrows bridge failure, and undergraduate physics textbooks. Am. J. Phys. 1991, 59, 118–124. [Google Scholar] [CrossRef]
- Green, D.; Unruh, W.G. The failure of the Tacoma Bridge: A physical model. Am. J. Phys. 2006, 74, 706–716. [Google Scholar] [CrossRef]
- Valentín, D.; Presas, A.; Egusquiza, E.; Valero, C.; Egusquiza, M. Experimental Study of a Vibrating Disk Submerged in a Fluid-Filled Tank and Confined with a Nonrigid Cover. J. Vib. Acoust. 2017, 139, 21005. [Google Scholar] [CrossRef]
- Valentín, D.; Presas, A.; Egusquiza, E.; Valero, C. Experimental study on the added mass and damping of a disk submerged in a partially fluid-filled tank with small radial confinement. J. Fluids Struct. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- Presas, A.; Valentin, D.; Egusquiza, E.; Valero, C.; Seidel, U. Dynamic response of a rotating disk submerged and confined. Influence of the axial gap. J. Fluids Struct. 2016, 62, 332–349. [Google Scholar] [CrossRef]
- Presas, A.; Valentin, D.; Egusquiza, E.; Valero, C.; Seidel, U. On the detection of natural frequencies and mode shapes of submerged rotating disk-like structures from the casing. Mech. Syst. Signal Process. 2015, 60, 547–570. [Google Scholar] [CrossRef] [Green Version]
- Blevins, R.D. Formulas for Natural Frequency and Mode Shape; Krieger Publishing Company: Malabar, FL, USA, 2001. [Google Scholar]
- Amabili, M.; Kwak, M.K. Free vibrations of circular plates coupled with liquids: Revising the lamb problem. J. Fluids Struct. 1996, 10, 743–761. [Google Scholar] [CrossRef]
- Amabili, M.; Dalpiaz, G. Vibration of base plates in annular cylindrical tanks: Theory and experiments. J. Sound Vib. 1998, 210, 329–350. [Google Scholar] [CrossRef]
- Valentín, D.; Presas, A.; Bossio, M.; Egusquiza, M.; Egusquiza, E.; Valero, C. Feasibility of Detecting Natural Frequencies of Hydraulic Turbines While in Operation, Using Strain Gauges. Sensors 2018, 18, 174. [Google Scholar] [CrossRef]
- Dassault Systèmes Abaqus 6.13 Online Documentation. Available online: http://dsk.ippt.pan.pl/docs/abaqus/v6.13/index.html (accessed on 15 January 2019).
- Binda, M.; Harčarik, T.; Šimčák, F. Modal parameters estimation by using PULSE Reflex modal analysis. In Proceedings of the 4th International Conference of Modelling of Mechanical and Mechatronic Systems; Faculty of Mechanical Engineering, Technical University of Košice, Košice, Slovakia, 20–22 September 2011; pp. 17–21. [Google Scholar]
- Papagiannopoulos, G.A.; Hatzigeorgiou, G.D. On the use of the half-power bandwidth method to estimate damping in building structures. Soil Dyn. Earthq. Eng. 2011, 31, 1075–1079. [Google Scholar] [CrossRef]
- Zhu, W.D.; Liu, J.M.; Xu, Y.F.; Ying, H.Q. A modal test method using sound pressure transducers based on vibro-acoustic reciprocity. J. Sound Vib. 2014, 333, 2728–2742. [Google Scholar] [CrossRef]
- Watson, M.; Sheldon, J.; Amin, S.; Lee, H.; Byington, C.; Begin, M. A comprehensive high frequency vibration monitoring system for incipient fault detection and isolation of gears, bearings and shafts/couplings in turbine engines and accessories. In Proceedings of the ASME Turbo Expo 2007: Power for Land, Sea, and Air, Montreal, QC, Canada, 14–17 May 2007; pp. 885–894. [Google Scholar]
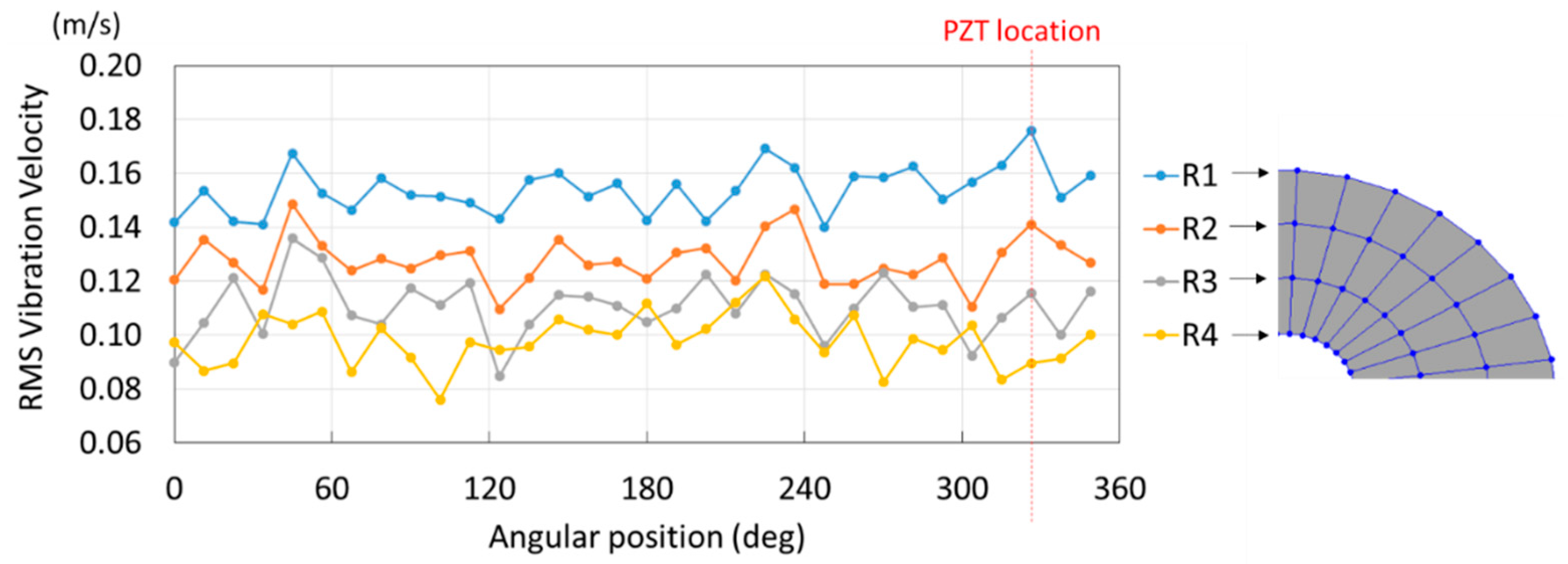
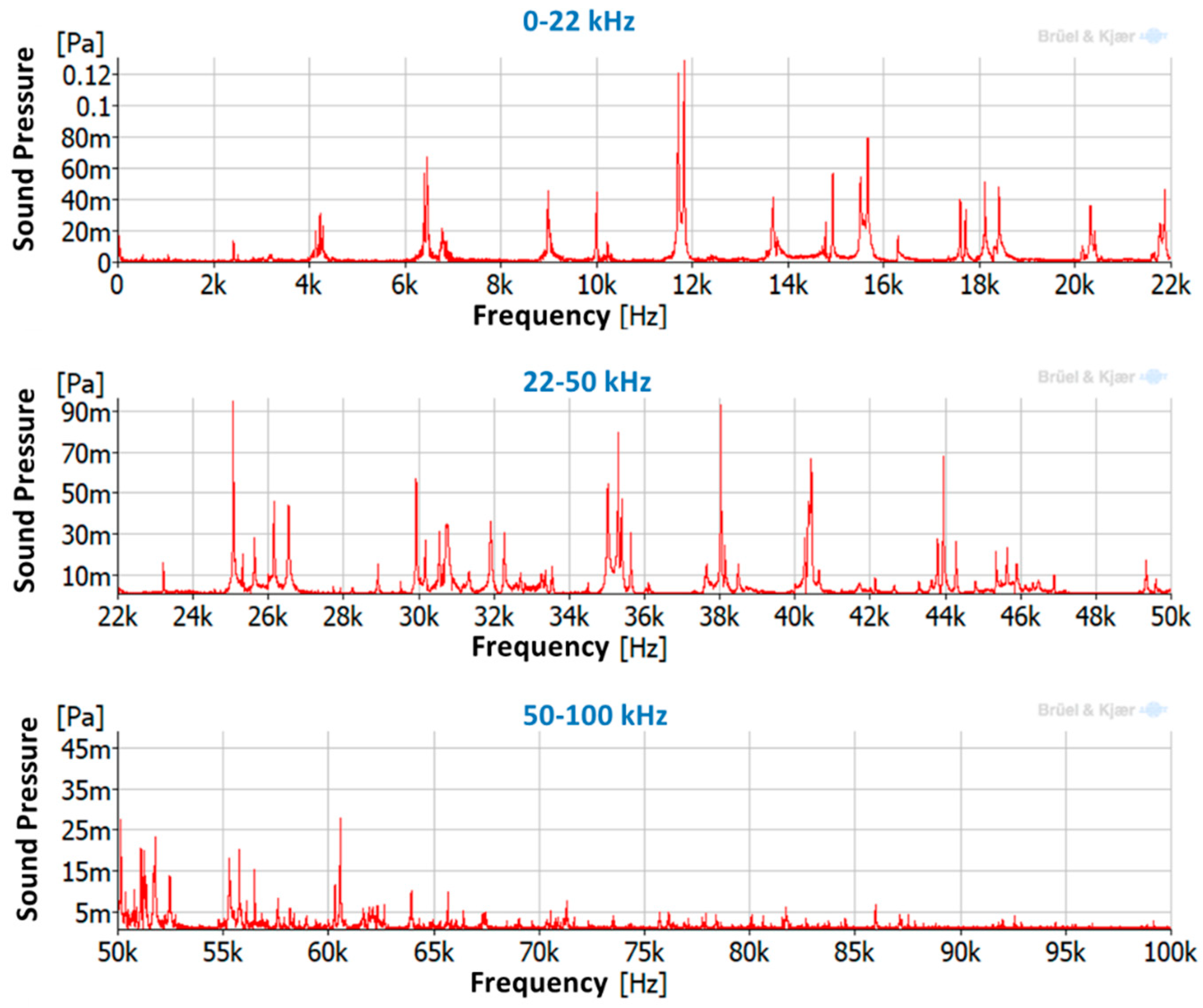
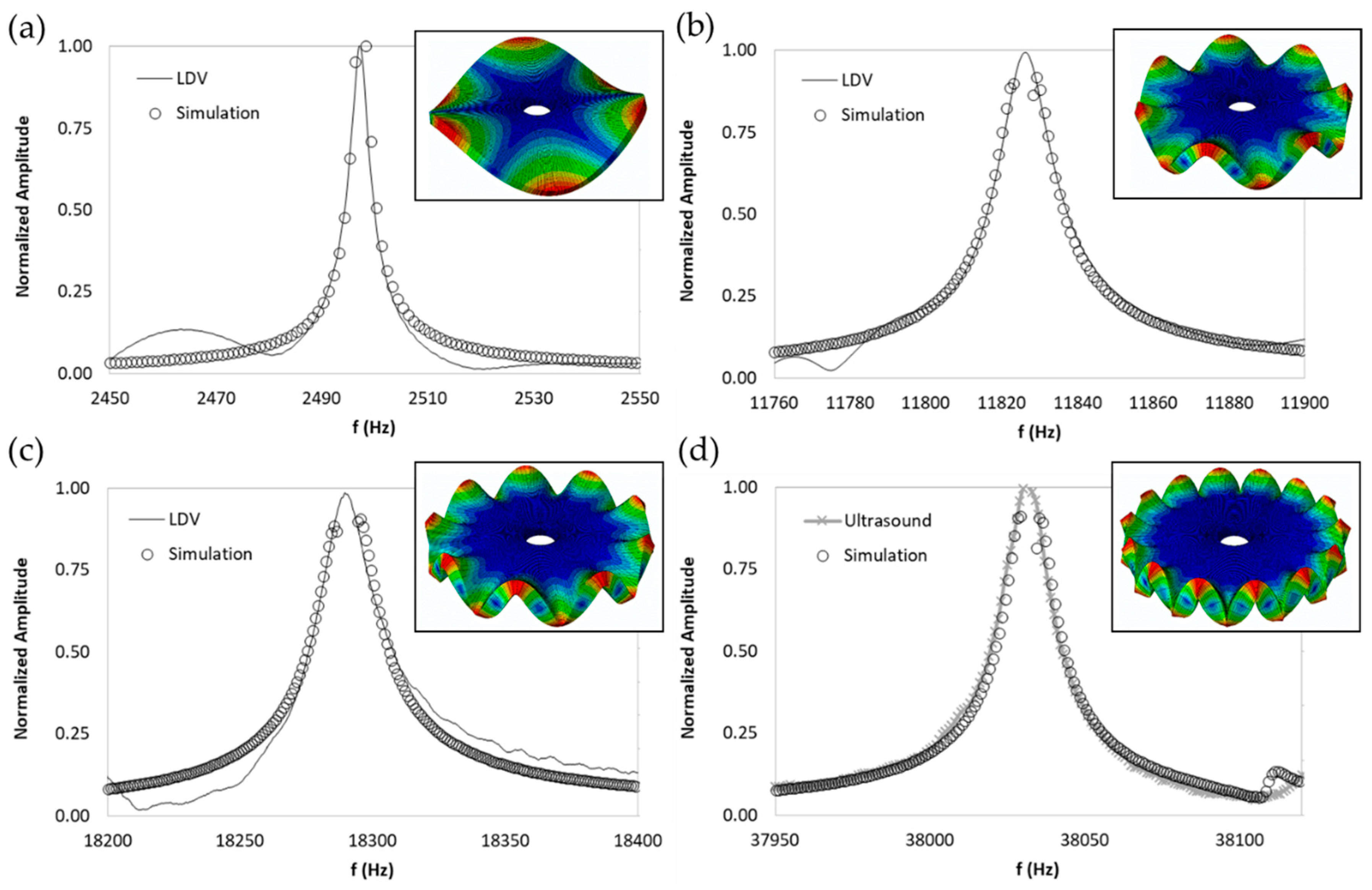
| Natural Frequency (Hz) | Damping Ratio (%) | Mode-Shape | |
|---|---|---|---|
| ND | NC | ||
| 1060 | 0.1520 | 2 | 0 |
| 2416 | 0.0660 | 3 | 0 |
| 4197 | 0.2240 | 4 | 0 |
| 6419 | 0.1610 | 5 | 0 |
| 8982 | 0.1050 | 6 | 0 |
| 11826 | 0.0540 | 7 | 0 |
| 14929 | 0.0440 | 8 | 0 |
| 18111 | 0.0530 | 9 | 0 |
| 21586 | 0.0470 | 10 | 0 |
| 6850 | 0.1370 | 2 | 1 |
| 10236 | 0.0710 | 3 | 1 |
| 13685 | 0.0490 | 4 | 1 |
| 17594 | 0.0230 | 5 | 1 |
| Exp (Hz) | Sim (Hz) | Diff % | Mode-Shape | |||
|---|---|---|---|---|---|---|
| ND | NC | Experiment | Simulation | |||
| 1060 | 1025 | −3.30 | 2 | 0 |  |  |
| 2416 | 2396 | −0.83 | 3 | 0 |  |  |
| 4197 | 4156 | −0.98 | 4 | 0 |  |  |
| 6419 | 6286 | −2.07 | 5 | 0 |  |  |
| 8982 | 8753 | −2.55 | 6 | 0 |  |  |
| 11826 | 11525 | −2.55 | 7 | 0 |  |  |
| 14929 | 14570 | −2.40 | 8 | 0 |  |  |
| 18111 | 17859 | −1.39 | 9 | 0 |  |  |
| 21586 | 21365 | −1.02 | 10 | 0 |  |  |
| 6850 | 6561 | −4.22 | 2 | 1 |  |  |
| 10236 | 9764 | −4.61 | 3 | 1 |  |  |
| 13685 | 13267 | −3.05 | 4 | 1 |  |  |
| 17594 | 17056 | −3.06 | 5 | 1 |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentín, D.; Roehr, C.; Presas, A.; Heiss, C.; Egusquiza, E.; Bosbach, W.A. Experimental-Numerical Design and Evaluation of a Vibration Bioreactor Using Piezoelectric Patches. Sensors 2019, 19, 436. https://doi.org/10.3390/s19020436
Valentín D, Roehr C, Presas A, Heiss C, Egusquiza E, Bosbach WA. Experimental-Numerical Design and Evaluation of a Vibration Bioreactor Using Piezoelectric Patches. Sensors. 2019; 19(2):436. https://doi.org/10.3390/s19020436
Chicago/Turabian StyleValentín, David, Charline Roehr, Alexandre Presas, Christian Heiss, Eduard Egusquiza, and Wolfram A. Bosbach. 2019. "Experimental-Numerical Design and Evaluation of a Vibration Bioreactor Using Piezoelectric Patches" Sensors 19, no. 2: 436. https://doi.org/10.3390/s19020436





