Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
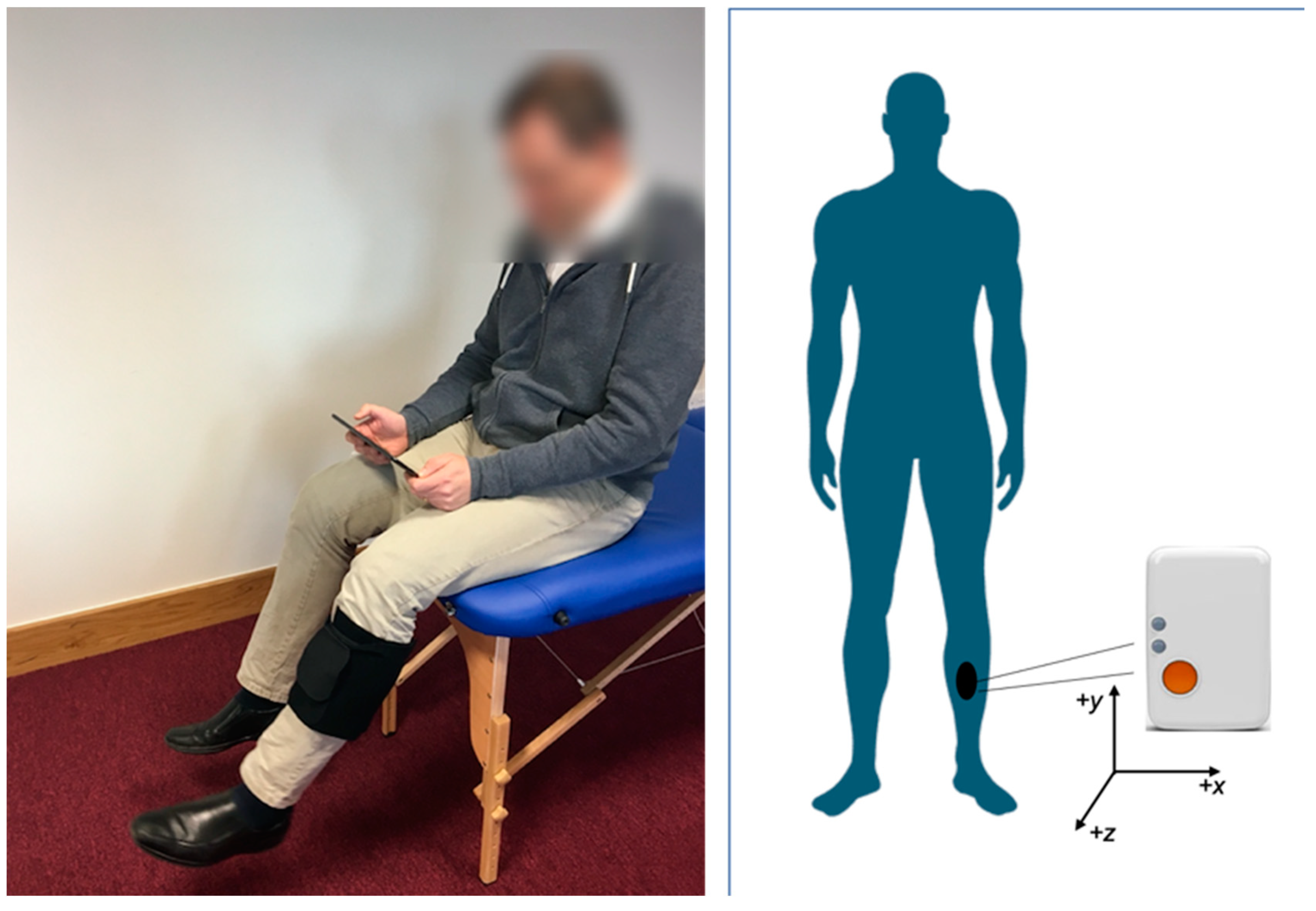
2.2. Prototype Exercise Biofeedback System
2.3. Experimental Procedure
2.4. Data Analysis
3. Results
3.1. Usability, Functionality and User Experience
It’s very simple to use, very simple. And it flows through on a good progression.[Acute 2]
Initially I said to you I wasn’t very computer literate but it’s very simple to use. Once you do it once or twice you can do it with your eyes closed essentially.[Acute 9]
I think it set me on a routine very, very quickly and a routine that I actually got to enjoy in a certain way. It was not like holding a sheet of paper… you became involved in it and so user friendly that I really think it was a great aid to me.[Acute 5]
It’s very helpful, it’s much better than leafing through static illustrations. It’s 3D real time. It made what are otherwise boring exercises more interesting.[Acute 9]
There is a bit of problem with the counting in it… Yeah sometimes it misses a few you do… it just runs away with itself.[Post-Acute 1]
Some of the technique feedback seems to be quite inconsistent.[Acute 9]
On the graph I don’t know what the interpretation is supposed to be.[Acute 6]
We had just the one where it says unusual behaviour, unexpected behaviour, please repeat the exercise. I think I was saying to you that on two occasions I actually repeated the exercise… I just said there was a glitch and it didn’t really bother me.[Acute 5]
It’s just very frustrating as I say it’s on the three that are really painful to do, and you struggle through them and you think well I think I have done them fairly well and then it says unexplained behaviour do them again and you just can’t.[Post-Acute 4]
3.2. Perceived Impact
It kept me doing physio when I might not have done it at home, especially with various things that have been happening at home. So it kept me doing physio and made sure I did it every day.[Post-Acute 3]
Well I can 100% tell you that I had a previous knee operation and I didn’t have an app and I did the exercises as diligently and frequently as I could, but I certainly didn’t do them with the thoroughness and regularity that I’ve done them this time[Acute 3]
I found it, I have to say, it made me do the exercises when I didn’t really want to do them, knowing I was being monitored, I do think it helped me a lot.[Acute 2]
I kind of felt that that app now made me do my exercises, the three times a day, and secondly at least it was recording it and you could see, that’s what I liked, you could see the progress from, I’m sure you have the record there of the beginning ones weren’t great.[Acute 4]
Oh yes it made a huge difference, that was a great motivation… It just meant that I was in control of my situation and I didn’t feel the days were endless. I was looking forward to doing it to see how well I was doing.[Acute 7]
A huge incentive and even if you try it and say, I’m too tired tonight and then you say no, no I have to do it and I have to try and improve on it.[Acute 6]
That’s the important bit about it that it tells you straight away, you need feedback, there is no point in having the app if it doesn’t give you feedback… Because if you are waiting for someone to come in and check it out for you, that’s two to three weeks, but it’s two to three weeks of doing it wrong.[Post-Acute 2]
It’s ideal for somebody that’s coming straight out of hospital and they have to do the exercises on their own, because if you are not doing it correctly why are you bothering doing them in the first place. So that as a tool in itself is worth a hell of a lot.[Post-Acute 3]
Yes first couple of days that was very interesting and I liked the idea of looking at the little cartoon. But after 2 or 3 days it was for me, I thought it was unnecessary… I have no interest because I know very well if I’m doing better or not. So I find it unnecessary.[Acute 1]
I think the fact that I was almost keen for the next session, it led to a regularity and I think that has paid huge dividends in the exercises and in the result of the exercises on the leg. I really think it was extremely beneficial.[Acute 5]
It was so positive. It was just brilliant. It just meant that I was in control whereas normally I would be coming home and in the hospital it would be altogether different because there’s so much support there, but when you come home its gone except that I had that.[Acute 7]
3.3. Refinements
I think if you gave me progression on the exercises, well done, try this one now, I’d like that.[Post-Acute 5]
This is probably not possible, but to get the angle of that knee bend, if you knew that…for me that is where I’m really stuck so just to know that…I know it counts it and it said you did it right, but I’m not sure what the angle of the bend is, and I’m rather obsessed with that.[Post-Acute 4]
The other one thing that I’d love to be able to see is you know when you do the knee bends, I’d love to be able to see what angle you got… Now it just you know then the way at least you could say well I’ve gone from a 50 to a 75 rather than just ok it looks like I am doing it ok.[Acute 4]
And you know the way your Fitbit would have the circle that you have to fill the circle and obviously this system is basic bar charts… Yes that (the Fitbit) does make more sense.[Post-Acute 3]
It would be very difficult to rate it from the previous time, there is no linkage from the previous repetitions… So in a way you don’t know if you are doing better today than you did yesterday… The quality of how I’m doing them.[Acute 2]
I suppose it’s nice to see it really, but she needs to undergo a great makeover… Just a bit more human the graphics… I mean even if it was more a cartoon figure or something it doesn’t really matter.[Post-Acute 4]
If there was a games element to it you know you have unlocked the next level… or a medal or something.[Acute 4]
I think that’d be a mistake because then you are going to end up turning it into playtime rather than exercise time. I think it’ll lose the point of it. I don’t think you should be able to manipulate too much unless it is information gathering or correcting the programme. For me this is a medical instruction for exercise to improve your health... you’d end up wasting time and not doing the exercise.[Acute 9]
4. Discussions
5. Conclusions
Ethical Approval
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IMU | inertial measurement unit |
| SUS | system usability |
| SD | standard deviation |
| TKR | total knee replacement |
| UKR | unicompartmental knee replacement |
| uMARS | user version of the Mobile Application Rating Scale |
References
- Caulfield, B.M.; Donnelly, S. What is Connected Health and why will it change your practice? QJM 2013, 106, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.M.; Mawson, S.; Brownsell, S. Telerehabilitation: Enabling the remote delivery of healthcare, rehabilitation, and self management. Stud. Health Technol. Inform. 2009, 145, 231–248. [Google Scholar] [PubMed]
- Kairy, D.; Lehoux, P.; Vincent, C.; Visintin, M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil. Rehabil. 2009, 31, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Persson, U.; Caulfield, B. Biofeedback in rehabilitation. J. NeuroEng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, M.; Kelly, J.J.; Newman, J.M.; Sultan, A.A.; Khlopas, A.; Sodhi, N.; Bhave, A.; Kolczun, M.; Mont, M.A. The Role of Virtual Rehabilitation in Total and Unicompartmental Knee Arthroplasty. J. Knee Surg. 2019, 32, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Smittenaar, P.; Erhart-Hledik, J.C.; Kinsella, R.; Hunter, S.; Mecklenburg, G.; Perez, D. Translating Comprehensive Conservative Care for Chronic Knee Pain into a Digital Care Pathway: 12-Week and 6-Month Outcomes for the Hinge Health Program. JMIR Rehabil. Assist. Technol. 2017, 4, e4. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.D.; Nogueira, A.; Magalhães, I.; Guimarães, J.; Moreira, M.; Barradas, I.; Teixeira, L.; Tulha, J.; Seabra, R.; Lains, J.; et al. Home-based Rehabilitation with A Novel Digital Biofeedback System versus Conventional In-person Rehabilitation after Total Knee Replacement: A feasibility study. Sci. Rep. 2018, 8, 11299. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.H.M.; Anastasova-Ivanova, S.; Spulber, I.; Gulati, V.; Georgiou, P.; McGregor, A. An Attachable Clothing Sensor System for Measuring Knee Joint Angles. IEEE Sens. J. 2013, 13, 4090–4097. [Google Scholar] [CrossRef]
- Burns, A.; Greene, B.R.; McGrath, M.J.; O’Shea, T.J.; Kuris, B.; Ayer, S.M.; Stroiescu, F.; Cionca, V. SHIMMERTM—A Wireless Sensor Platform for Noninvasive Biomedical Research. IEEE Sens. J. 2010, 10, 1527–1534. [Google Scholar] [CrossRef]
- O’Reilly, M.; Caulfield, B.; Ward, T.; Johnston, W.; Doherty, C. Wearable Inertial Sensor Systems for Lower Limb Exercise Detection and Evaluation: A Systematic Review. Sports Med. 2018, 48, 1221–1246. [Google Scholar] [CrossRef]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. NeuroEng. Rehabil. 2014, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, M.A.; Whelan, D.F.; Ward, T.E.; Delahunt, E.; Caulfield, B.M. Technology in Strength and Conditioning: Assessing Bodyweight Squat Technique with Wearable Sensors. J. Strength Cond. Res. 2017, 31, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A.; Whelan, D.F.; Ward, T.E.; Delahunt, E.; Caulfield, B. Classification of lunge biomechanics with multiple and individual inertial measurement units. Sports Biomech. 2017, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Huang, B.; Argent, R.; Caulfield, B.; Kechadi, T. Automatic classification of knee rehabilitation exercises using a single inertial sensor: A case study. In Proceedings of the 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Las Vegas, NV, USA, 4–7 March 2018; pp. 21–24. [Google Scholar]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. Vol. 2007, 89, 780–785. [Google Scholar]
- Perry, M.A.; Hudson, H.S.; Meys, S.; Norrie, O.; Ralph, T.; Warner, S. Older adults’ experiences regarding discharge from hospital following orthopaedic intervention: A metasynthesis. Disabil. Rehabil. 2011, 34, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Zapata, B.C.; Fernández-Alemán, J.L.; Idri, A.; Toval, A. Empirical Studies on Usability of mHealth Apps: A Systematic Literature Review. J. Med. Syst. 2015, 39, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Yardley, L.; West, R.; Patrick, K.; Greaves, F. Developing and Evaluating Digital Interventions to Promote Behavior Change in Health and Health Care: Recommendations Resulting from an International Workshop. J. Med. Internet Res. 2017, 19, e232. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.H.M.; McGregor, A.H. Body-Worn Sensor Design: What Do Patients and Clinicians Want? Ann. Biomed. Eng. 2011, 39, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, P.J.; Hinman, R.S.; French, S.D.; Lonsdale, C.; Bennell, K.L. Improving adherence to exercise: Do people with knee osteoarthritis and physical therapists agree on the behavioural approaches likely to succeed? Arthr. Care Res. Hoboken 2018, 70, 388–397. [Google Scholar] [CrossRef]
- Argent, R.; Daly, A.; Caulfield, B. Patient Involvement with Home-Based Exercise Programs: Can Connected Health Interventions Influence Adherence? JMIR mHealth uHealth 2018, 6, e47. [Google Scholar] [CrossRef]
- Argent, R.; Slevin, P.; Bevilacqua, A.; Neligan, M.; Daly, A.; Caulfield, B. Clinician perceptions of a prototype wearable exercise biofeedback system for orthopaedic rehabilitation: A qualitative exploration. BMJ Open 2018, 8, e026326. [Google Scholar] [CrossRef] [PubMed]
- Dowsey, M.M.; Choong, P.F.M. The Utility of Outcome Measures in Total Knee Replacement Surgery. Int. J. Rheumatol. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A.; Slevin, P.; Ward, T.; Caulfield, B. A Wearable Sensor-Based Exercise Biofeedback System: Mixed Methods Evaluation of Formulift. JMIR mHealth uHealth 2018, 6, e33. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, J. Qualitative research practice: A guide for social science students and researchers (2nd edition). Int. J. Mark. Res. 2014, 56, 407. [Google Scholar] [CrossRef]
- Brooke, J. SUS: A ‘quick and dirty’ usability scale. In Usability Evaluation in Industry; Jordan, P., Thomas, B., McClelland, I., Weerdmeester, B., Eds.; Taylor and Francis: London, UK, 1996; pp. 189–194. [Google Scholar]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Wilson, H. Development and Validation of the User Version of the Mobile Application Rating Scale (uMARS). JMIR mHealth uHealth 2016, 4, e72. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An Empirical Evaluation of the System Usability Scale. Int. J. Hum.-Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. J. Usability Stud. 2009, 4, 114–123. [Google Scholar]
- Stoyanov, S.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR mHealth uHealth 2015, 3, 27. [Google Scholar] [CrossRef]
- Fereday, J.; Muir-Cochrane, E. Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development. Int. J. Qual. Methods 2006, 5, 80–92. [Google Scholar] [CrossRef]
- Boeije, H. A Purposeful Approach to the Constant Comparative Method in the Analysis of Qualitative Interviews. Qual. Quant. 2002, 36, 391–409. [Google Scholar] [CrossRef]
- Papi, E.; Belsi, A.; McGregor, A.H. A knee monitoring device and the preferences of patients living with osteoarthritis: A qualitative study. BMJ Open 2015, 5, 007980. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 9241-11 Ergonomics of Human-System Interaction-Part 11: Usability: Definitions and Concepts; Ergonomics of Human-System Interaction: Geneva, Switzerland, 2018. [Google Scholar]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to treatment adherence in physiotherapy outpatient clinics: A systematic review. Man. Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, N.M.C.; Nordin, M.; Hiebert, R.; Campello, M. Predictors of compliance with short-term treatment among patients with back pain. Rev. Panam. Salud Publica 2002, 12, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Peek, K.; Sanson-Fisher, R.; MacKenzie, L.; Carey, M.; Information, P.E.K.F.C. Interventions to aid patient adherence to physiotherapist prescribed self-management strategies: A systematic review. Physiotherapy 2016, 102, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bollen, J.; Dean, S.G.; Siegert, R.J.; Howe, T.E.; Goodwin, V.A.; Nepogodiev, D.; Chapman, S.J.; Glasbey, J.C.D.; Kelly, M.; Khatri, C.; et al. A systematic review of measures of self-reported adherence to unsupervised home-based rehabilitation exercise programmes, and their psychometric properties. BMJ Open 2014, 4, 005044. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, C.H.; Penninx, B.W.J.H.; Kempen, G.I.J.M.; Rejeski, W.J.; Miller, G.D.; Van Eijk, J.T.M.; Pahor, M.; Messier, S.P. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthr. Rheum. 2005, 53, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.R.; Laplante, P.A. User-Centered Design; Informa UK Limited: London, UK, 2010; pp. 1308–1317. [Google Scholar]
- Steele, R.; Lo, A.; Secombe, C.; Wong, Y.K. Elderly persons’ perception and acceptance of using wireless sensor networks to assist healthcare. Int. J. Med. Inform. 2009, 78, 788–801. [Google Scholar] [CrossRef]
- Blyth, F.M.; March, L.M.; Nicholas, M.K.; Cousins, M.J. Self-management of chronic pain: A population-based study. PAIN 2005, 113, 285–292. [Google Scholar] [CrossRef]
- Power, C.; Matthews, S. Origins of health inequalities in a national population sample. Lancet 1997, 350, 1584–1589. [Google Scholar] [CrossRef]

| Demographic Details | n = 15 | |
|---|---|---|
| Marital Status | Married | 86.6% |
| Single | 0% | |
| Widowed | 6.6% | |
| Other | 6.6% | |
| Lives with | Spouse | 46.6% |
| Family | 40% | |
| Alone | 13.3% | |
| Education | Degree Educated | 73.3% |
| Completed Secondary | 20% | |
| Completed Primary | 6.6% | |
| Technology Ownership | Mobile Phone | 100% |
| Smart Phone | 86.6% | |
| Tablet | 66.6% | |
| WiFi | 93.3% | |
| Health/Fitness App | 26.6% |
| uMARS Section (score out of five) | Mean (SD) |
|---|---|
| Engagement | 3.5 (0.69) |
| Functionality | 4.2 (0.34) |
| Aesthetics | 4.2 (0.45) |
| Information | 4.4 (0.34) |
| Overall Quality | 4.1 (0.39) |
| Perceived Impact | 4.4 (0.83) |
| Subjective App Quality | 4.2 (0.86) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argent, R.; Slevin, P.; Bevilacqua, A.; Neligan, M.; Daly, A.; Caulfield, B. Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System. Sensors 2019, 19, 432. https://doi.org/10.3390/s19020432
Argent R, Slevin P, Bevilacqua A, Neligan M, Daly A, Caulfield B. Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System. Sensors. 2019; 19(2):432. https://doi.org/10.3390/s19020432
Chicago/Turabian StyleArgent, Rob, Patrick Slevin, Antonio Bevilacqua, Maurice Neligan, Ailish Daly, and Brian Caulfield. 2019. "Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System" Sensors 19, no. 2: 432. https://doi.org/10.3390/s19020432
APA StyleArgent, R., Slevin, P., Bevilacqua, A., Neligan, M., Daly, A., & Caulfield, B. (2019). Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System. Sensors, 19(2), 432. https://doi.org/10.3390/s19020432





