Surface Plasmon Enhanced Light Scattering Biosensing: Size Dependence on the Gold Nanoparticle Tag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Au Seeds and AuNPs
2.3. Growth of AuNPs with Gold Enhancement Reagent
2.4. AuNP Conjugation with IgG
2.5. Confirmation of Bioconjugation with Dot Blot Assay
2.6. SPR Sensor Preparation and Optical Setup
2.7. Simulations
3. Results and Discussion
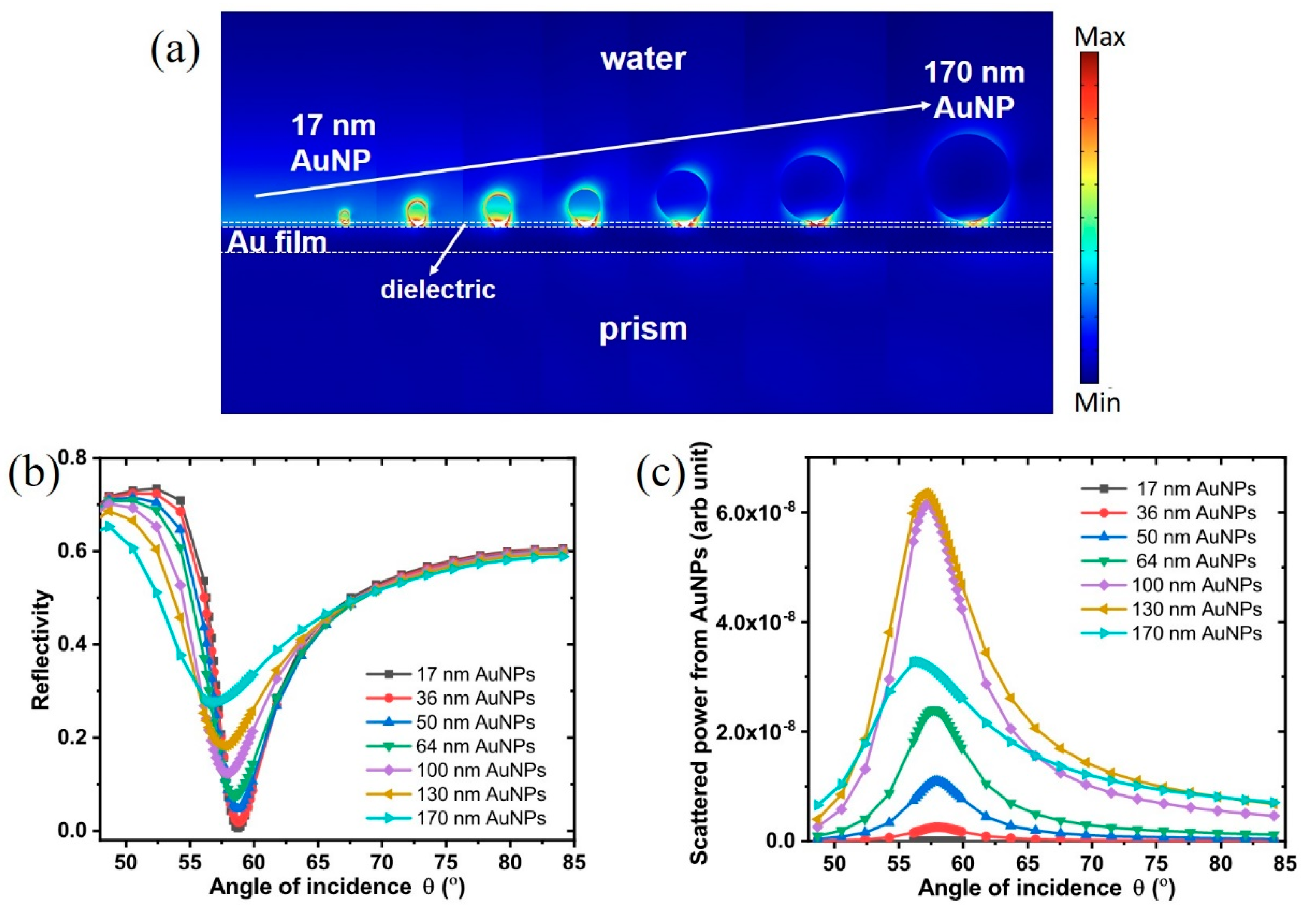
3.1. Theoretical Modeling
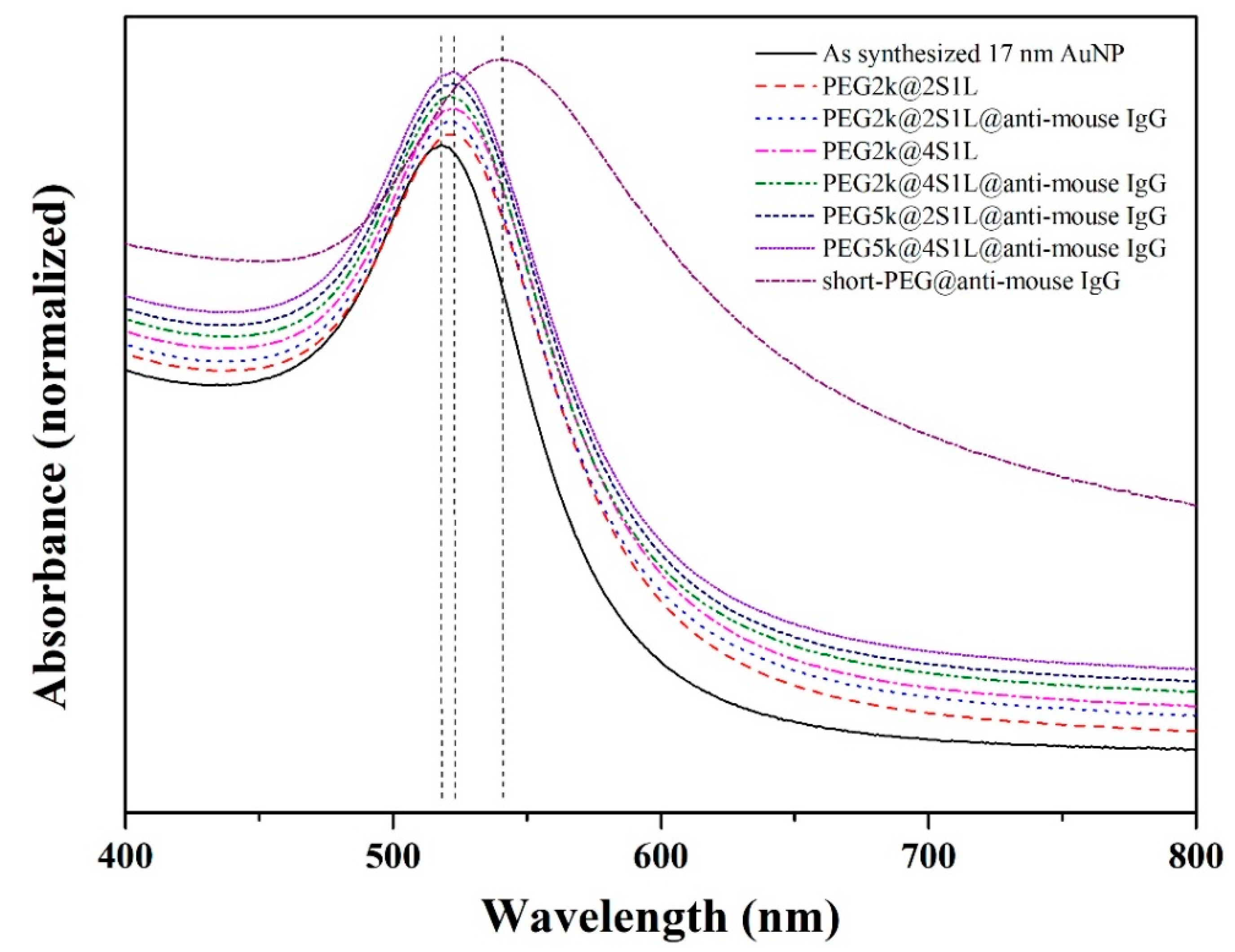
3.2. Optimization of Mixed PEG Coatings
3.3. Confirmation of the Immuno-Binding Efficiency Based on Dot Blot Assay
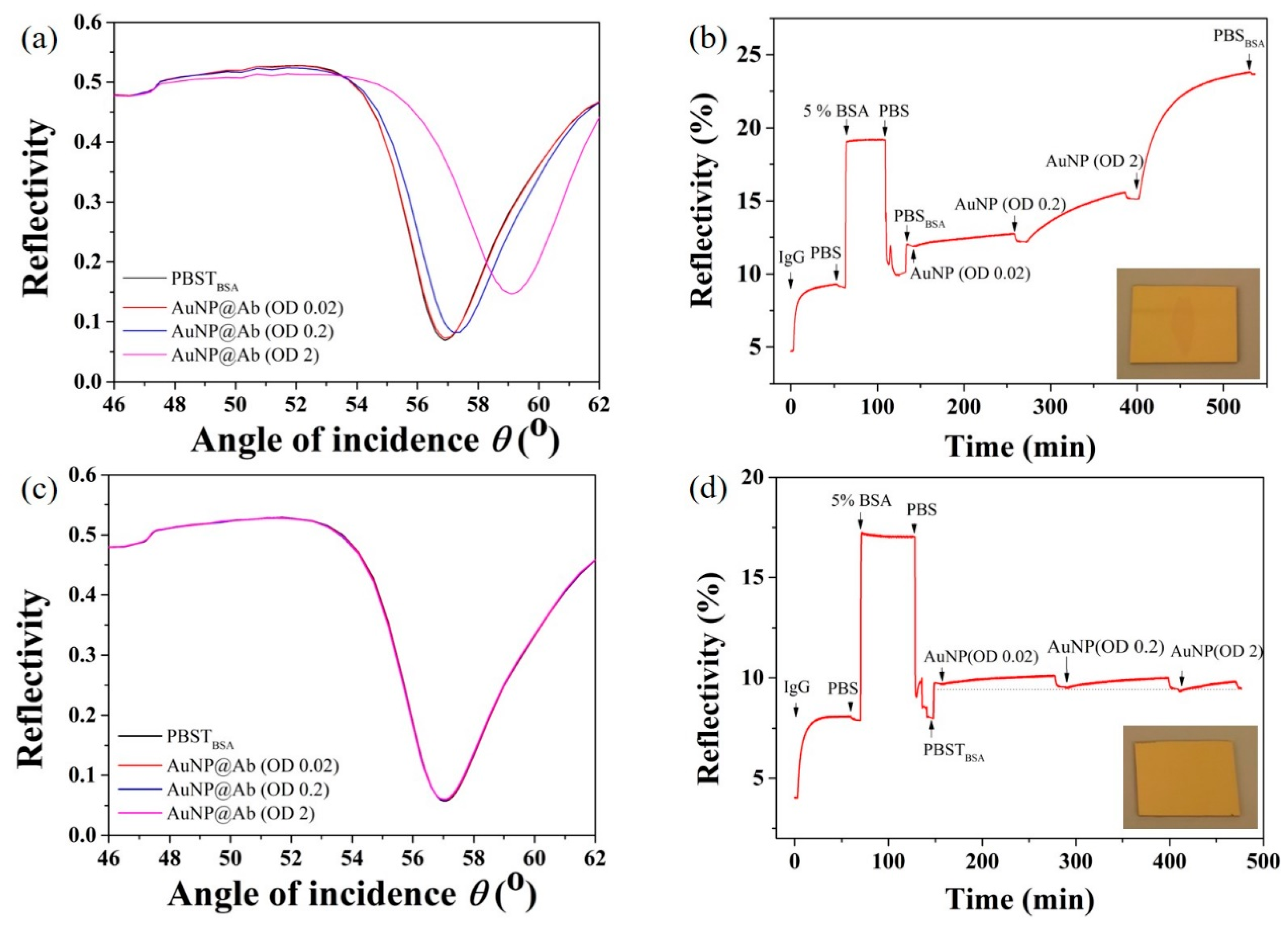
3.4. Confirmation of Immuno-Binding Efficiency Using SPR
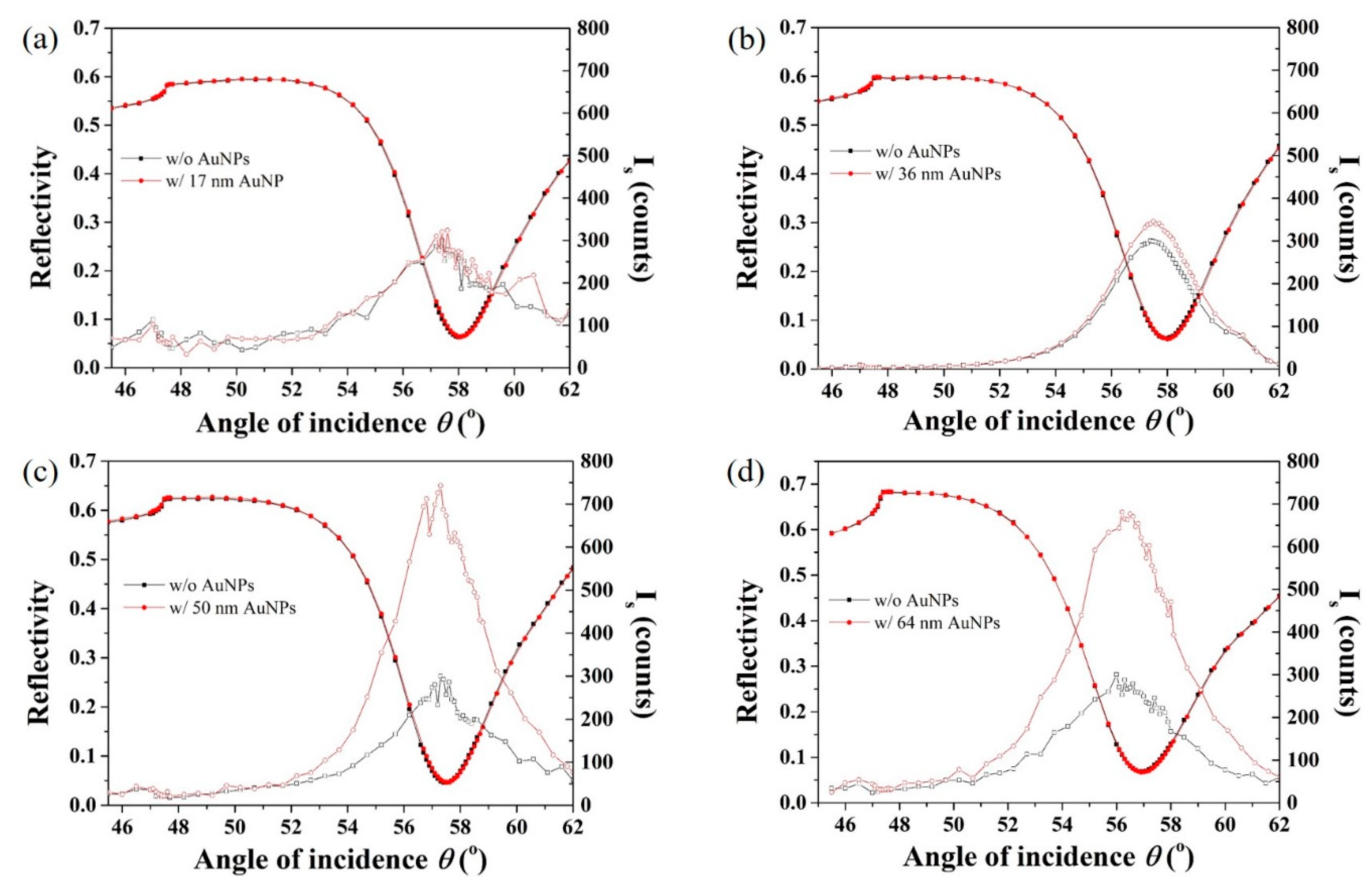
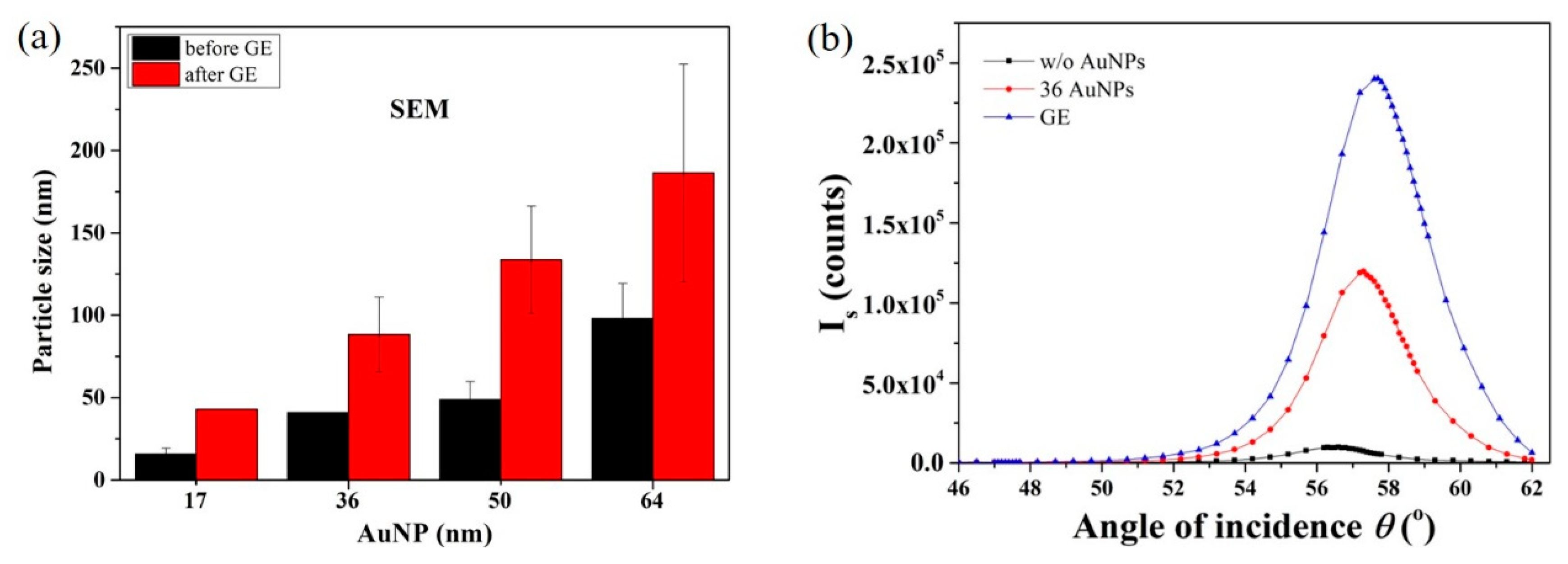
3.5. AuNP Size-Dependence in SP-LS Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abadian, P.N.; Kelley, C.P.; Goluch, E.D. Cellular analysis and detection using surface plasmon resonance techniques. Anal. Chem. 2014, 86, 2799–2812. [Google Scholar] [CrossRef]
- Mejard, R.; Griesser, H.J.; Thierry, B. Optical biosensing for label-free cellular studies. Trends Anal. Chem. 2014, 53, 178–186. [Google Scholar] [CrossRef]
- Yang, C.-T.; Méjard, R.G.; Griesser, H.J.; Bagnaninchi, P.O.; Thierry, B. Cellular micromotion monitored by long-range surface plasmon resonance with optical fluctuation analysis. Anal. Chem. 2015, 87, 1456–1461. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, R.; Wang, Q.; Huang, J.; Yang, X.; Wang, K.; Li, W.; Chen, N.; Li, Q. Detection of C-reactive protein using nanoparticle-enhanced surface plasmon resonance using an aptamer-antibody sandwich assay. Chem. Commun. 2016, 52, 3568–3571. [Google Scholar] [CrossRef]
- Yang, C.-T.; Wu, L.; Bai, P.; Thierry, B. Investigation of plasmonic signal enhancement based on long range surface plasmon resonance with gold nanoparticle tags. J. Mater. Chem. C 2016, 4, 9897–9904. [Google Scholar] [CrossRef]
- Šípová, H.; Homola, J. Surface plasmon resonance sensing of nucleic acids: A review. Anal. Chim. Acta 2013, 773, 9–23. [Google Scholar] [CrossRef]
- Masson, J.-F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef]
- Yu, F.; Persson, B.; Löfås, S.; Knoll, W. Attomolar sensitivity in bioassays based on surface plasmon fluorescence spectroscopy. J. Am. Chem. Soc. 2004, 126, 8902–8903. [Google Scholar] [CrossRef]
- Wang, Y.; Brunsen, A.; Jonas, U.; Dostalek, J.; Knoll, W. Prostate specific antigen biosensor based on long range surface plasmon-enhanced fluorescence spectroscopy and dextran hydrogel binding matrix. Anal. Chem. 2009, 81, 9625–9632. [Google Scholar] [CrossRef]
- Li, Y.; Lee, H.J.; Corn, R.M. Detection of protein biomarkers using RNA aptamer microarrays and enzymatically amplified surface plasmon resonance imaging. Anal. Chem. 2007, 79, 1082–1088. [Google Scholar] [CrossRef]
- Sendroiu, I.E.; Gifford, L.K.; Lupták, A.; Corn, R.M. Ultrasensitive DNA microarray biosensing via in situ RNA transcription-based amplification and nanoparticle-enhanced SPR imaging. J. Am. Chem. Soc. 2011, 133, 4271–4273. [Google Scholar] [CrossRef]
- He, L.; Musick, M.D.; Nicewarner, S.R.; Salinas, F.G.; Benkovic, S.J.; Natan, M.J.; Keating, C.D. Colloidal Au-enhanced surface plasmon resonance for ultrasensitive detection of DNA hybridization. J. Am. Chem. Soc. 2000, 122, 9071–9077. [Google Scholar] [CrossRef]
- Yang, C.-T.; Wu, L.; Liu, X.; Tran, N.T.; Bai, P.; Liedberg, B.; Wang, Y.; Thierry, B. Exploiting surface-plasmon-enhanced light scattering for the design of ultrasensitive biosensing modality. Anal. Chem. 2016, 88, 11924–11930. [Google Scholar] [CrossRef]
- Yang, C.-T.; Pourhassan-Moghaddam, M.; Wu, L.; Bai, P.; Thierry, B. Ultrasensitive detection of cancer prognostic miRNA biomarkers based on surface plasmon enhanced light scattering. ACS Sens. 2017, 2, 635–640. [Google Scholar] [CrossRef]
- Špringer, T.S.; Ermini, M.L.; Špačková, B.; Jabloňků, J.; Homola, J.Í. Enhancing sensitivity of surface plasmon resonance biosensors by functionalized gold nanoparticles: Size matters. Anal. Chem. 2014, 86, 10350–10356. [Google Scholar] [CrossRef]
- Zeng, S.; Yu, X.; Law, W.-C.; Zhang, Y.; Hu, R.; Dinh, X.-Q.; Ho, H.-P.; Yong, K.-T. Size dependence of Au NP-enhanced surface plasmon resonance based on differential phase measurement. Sens. Actuat. B Chem. 2013, 176, 1128–1133. [Google Scholar] [CrossRef]
- Zeng, S.; Sreekanth, K.V.; Shang, J.; Yu, T.; Chen, C.K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.P.; Kabashin, A.V. Graphene–gold metasurface architectures for ultrasensitive plasmonic biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef]
- Crut, A.; Maioli, P.; Del Fatti, N.; Vallée, F. Optical absorption and scattering spectroscopies of single nano-objects. Chem. Soc. Rev. 2014, 43, 3921–3956. [Google Scholar] [CrossRef]
- Agnarsson, B.R.; Lundgren, A.; Gunnarsson, A.; Rabe, M.; Kunze, A.; Mapar, M.; Simonsson, L.; Bally, M.; Zhdanov, V.P.; Höök, F. Evanescent light-scattering microscopy for label-free interfacial imaging: From single sub-100 nm vesicles to live cells. ACS Nano 2015, 9, 11849–11862. [Google Scholar] [CrossRef]
- Pai, J.-H.; Yang, C.-T.; Hsu, H.-Y.; Wedding, A.B.; Thierry, B. Development of a simplified approach for the fabrication of localised surface plasmon resonance sensors based on gold nanorods functionalized using mixed polyethylene glycol layers. Anal. Chim. Acta 2017, 974, 87–92. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Fan, A.; Cai, S.; Cao, Z.; Lau, C.; Lu, J. Hydroxylamine-amplified gold nanoparticles for the homogeneous detection of sequence-specific DNA. Analyst 2010, 135, 1400–1405. [Google Scholar] [CrossRef]
- Mejard, R.; Dostálek, J.; Huang, C.-J.; Griesser, H.; Thierry, B. Tuneable and robust long range surface plasmon resonance for biosensing applications. Opt. Mater. 2013, 35, 2507–2513. [Google Scholar] [CrossRef]
- Lyon, L.A.; Musick, M.D.; Smith, P.C.; Reiss, B.D.; Pena, D.J.; Natan, M.J. Surface plasmon resonance of colloidal Au-modified gold films. Sens. Actuat. B Chem. 1999, 54, 118–124. [Google Scholar] [CrossRef]
- Uchiho, Y.; Shimojo, M.; Furuya, K.; Kajikawa, K. Optical response of gold-nanoparticle-amplified surface plasmon resonance spectroscopy. J. Phys. Chem. C 2010, 114, 4816–4824. [Google Scholar] [CrossRef]
- Okamoto, T.; Yamaguchi, I. Optical absorption study of the surface plasmon resonance in gold nanoparticles immobilized onto a gold substrate by self-assembly technique. J. Phys. Chem. B 2003, 107, 10321–10324. [Google Scholar] [CrossRef]
- Driskell, J.D.; Lipert, R.J.; Porter, M.D. Labeled gold nanoparticles immobilized at smooth metallic substrates: Systematic investigation of surface plasmon resonance and surface-enhanced Raman scattering. J. Phys. Chem. B 2006, 110, 17444–17451. [Google Scholar] [CrossRef]
- Liu, T.; Thierry, B. A solution to the PEG dilemma: Efficient bioconjugation of large gold nanoparticles for biodiagnostic applications using mixed layers. Langmuir 2012, 28, 15634–15642. [Google Scholar] [CrossRef]
- Thierry, B.; Griesser, H.J. Dense PEG layers for efficient immunotargeting of nanoparticles to cancer cells. J. Mater. Chem. 2012, 22, 8810–8819. [Google Scholar] [CrossRef]
- Dagher, M.; Kleinman, M.; Ng, A.; Juncker, D. Ensemble multicolour FRET model enables barcoding at extreme FRET levels. Nat. Nanotechnol. 2018, 13, 925. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Tang, B.; Zhang, L.; Wang, C.; Ma, H.-T.; Pang, D.-W.; Zhang, Z.-L. On-demand one-step synthesis of small-sized fluorescent–magnetic bifunctional microparticles on a droplet-splitting chip. J. Mater. Chem. B 2018, 6, 961–965. [Google Scholar] [CrossRef]

| 17 nm AuNP | PEG2k_S2L1 | PEG2k_S2L1_IgG | PEG2k_S4L1 | PEG2k_S4L1_IgG | PEG5k_S2L1_IgG | PEG5k_S4L1_IgG | |
|---|---|---|---|---|---|---|---|
| Size (nm) | 18.1 ± 0.1 | 30.6 ± 0.1 | 34.9 ± 0.3 | 32.2 ± 0.1 | 51.1 ± 0.1 | 35.0 ± 0.4 | 34.6 ± 0.2 |
| PEG layer | - | 12.5 | 12.5 | 14.1 | 14.1 | - | - |
| IgG layer | - | - | 4.3 | - | 18.9 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-T.; Xu, Y.; Pourhassan-Moghaddam, M.; Tran, D.P.; Wu, L.; Zhou, X.; Thierry, B. Surface Plasmon Enhanced Light Scattering Biosensing: Size Dependence on the Gold Nanoparticle Tag. Sensors 2019, 19, 323. https://doi.org/10.3390/s19020323
Yang C-T, Xu Y, Pourhassan-Moghaddam M, Tran DP, Wu L, Zhou X, Thierry B. Surface Plasmon Enhanced Light Scattering Biosensing: Size Dependence on the Gold Nanoparticle Tag. Sensors. 2019; 19(2):323. https://doi.org/10.3390/s19020323
Chicago/Turabian StyleYang, Chih-Tsung, Yi Xu, Mohammad Pourhassan-Moghaddam, Duy Phu Tran, Lin Wu, Xin Zhou, and Benjamin Thierry. 2019. "Surface Plasmon Enhanced Light Scattering Biosensing: Size Dependence on the Gold Nanoparticle Tag" Sensors 19, no. 2: 323. https://doi.org/10.3390/s19020323
APA StyleYang, C.-T., Xu, Y., Pourhassan-Moghaddam, M., Tran, D. P., Wu, L., Zhou, X., & Thierry, B. (2019). Surface Plasmon Enhanced Light Scattering Biosensing: Size Dependence on the Gold Nanoparticle Tag. Sensors, 19(2), 323. https://doi.org/10.3390/s19020323







