Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds
Abstract
1. Introduction
2. Volatile Organic Compounds in the Environment
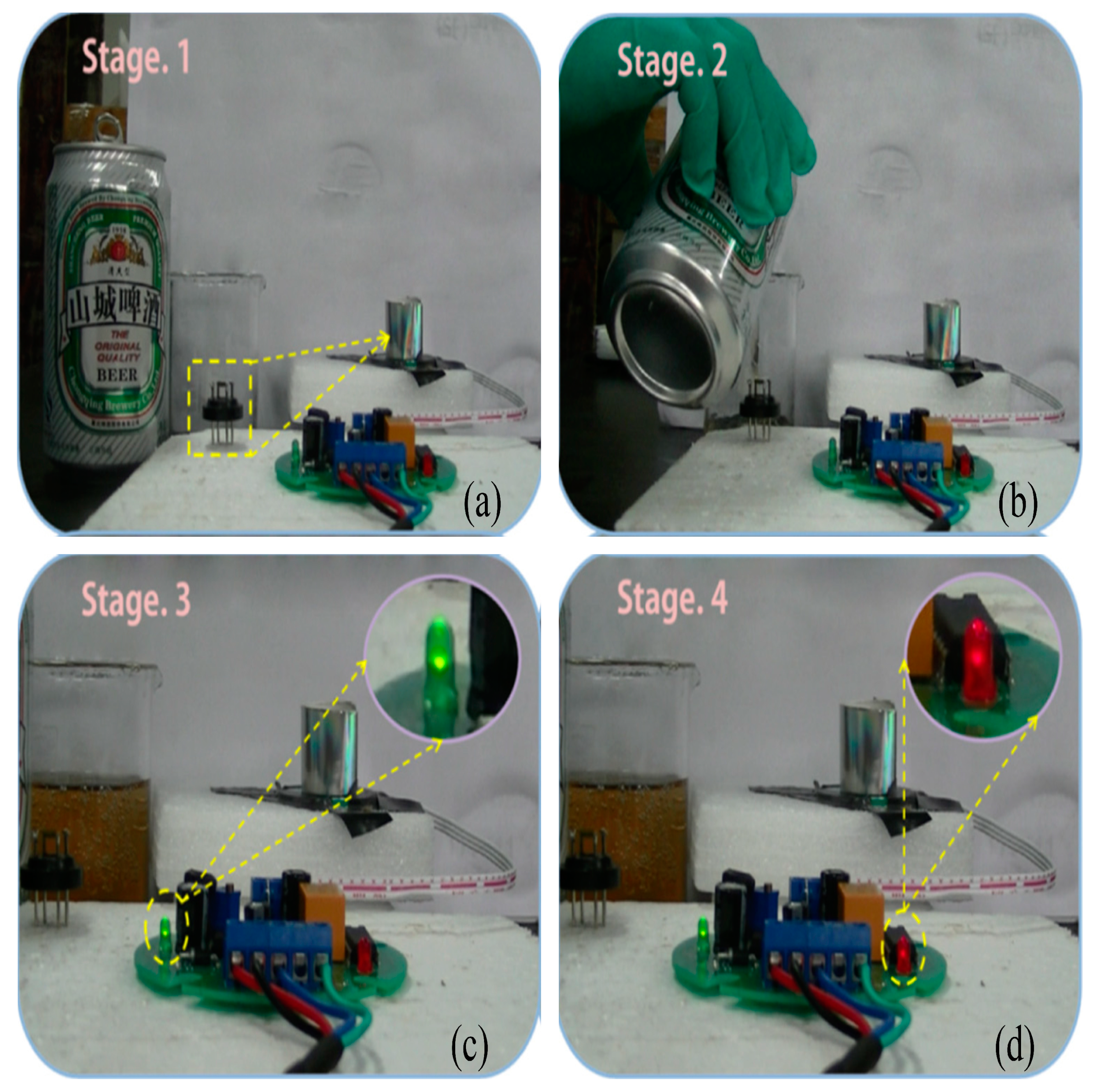
2.1. Ethanol
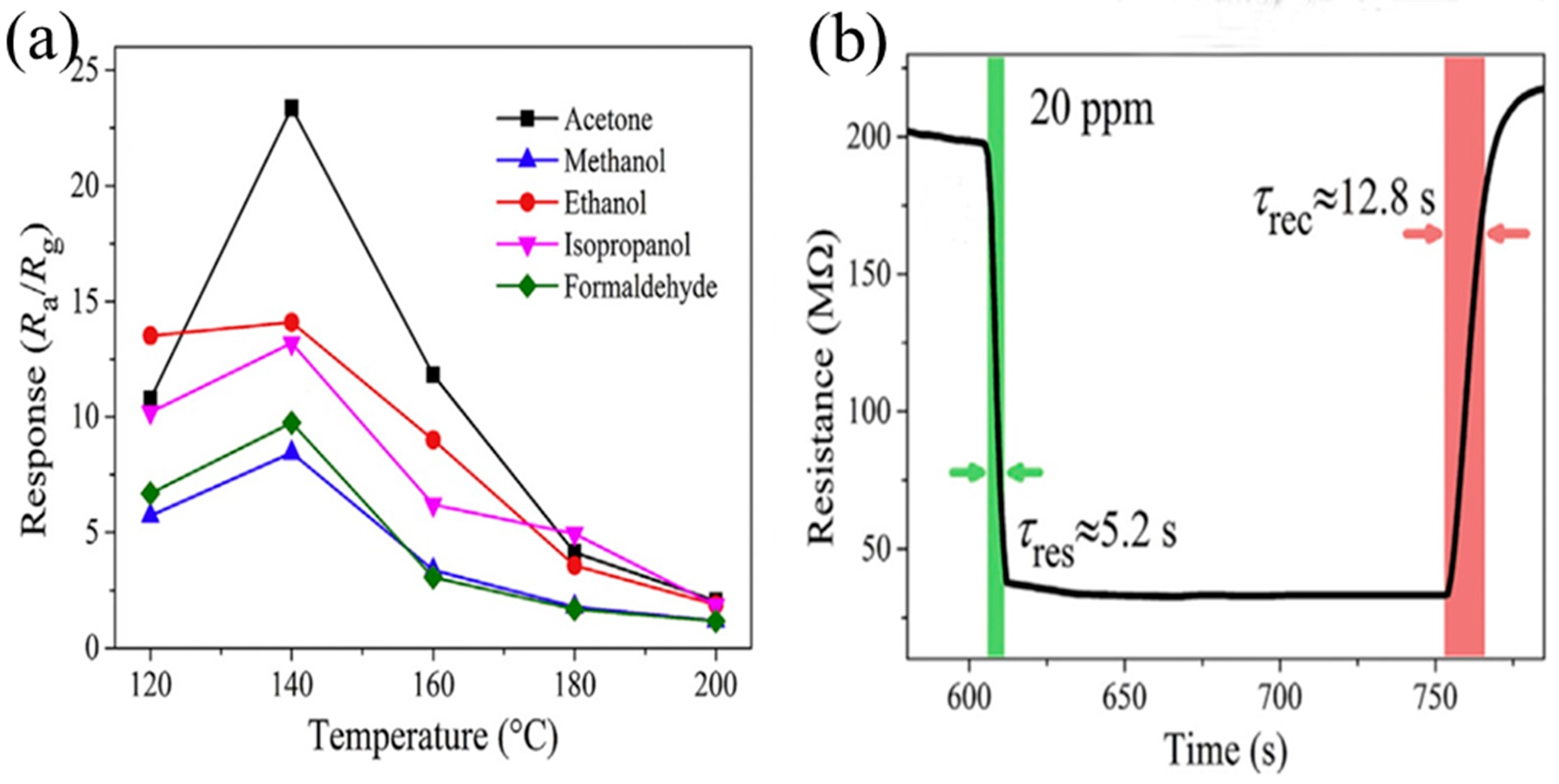
2.2. Acetone
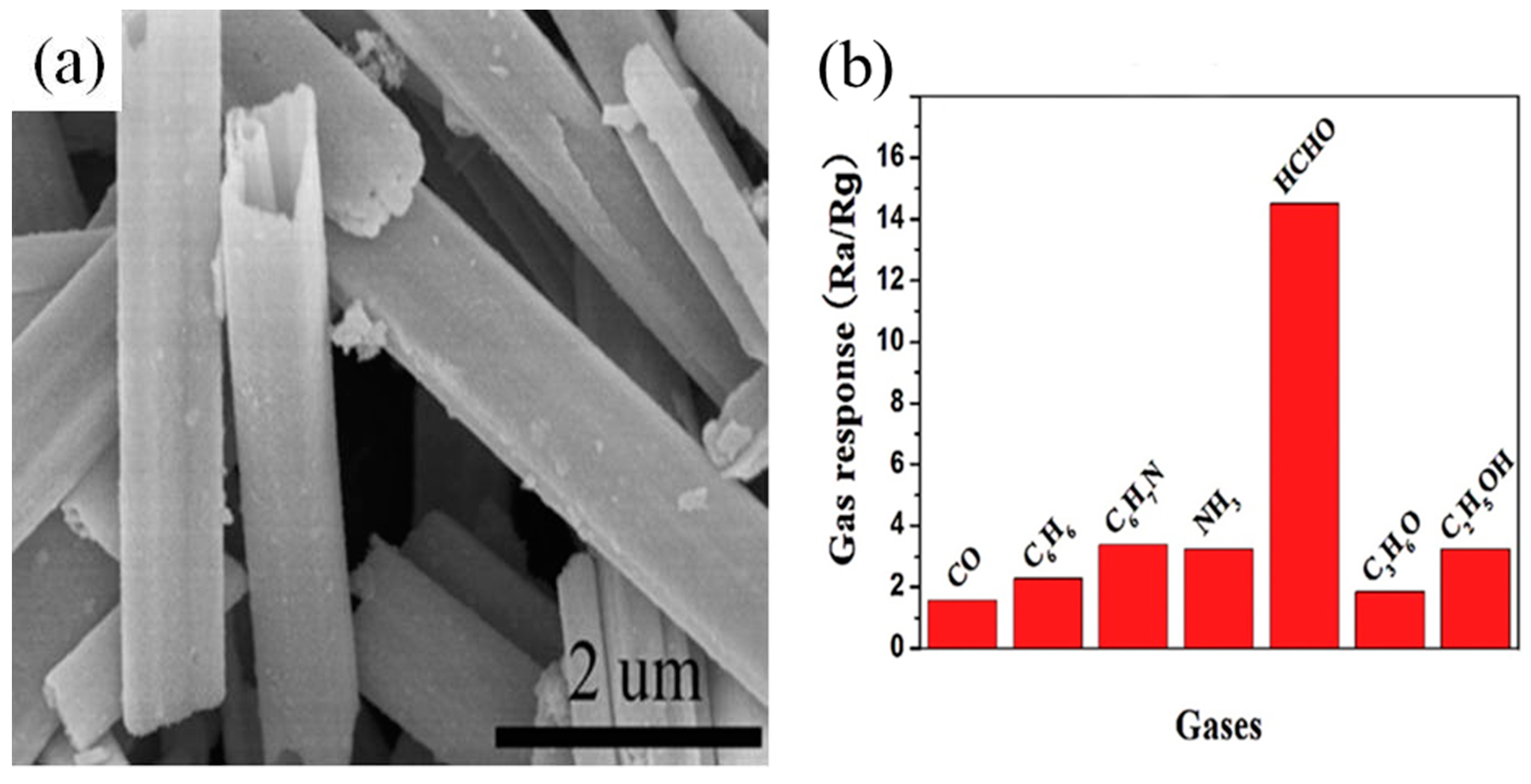
2.3. Formaldehyde
2.4. Toluene
2.5. Acetylene
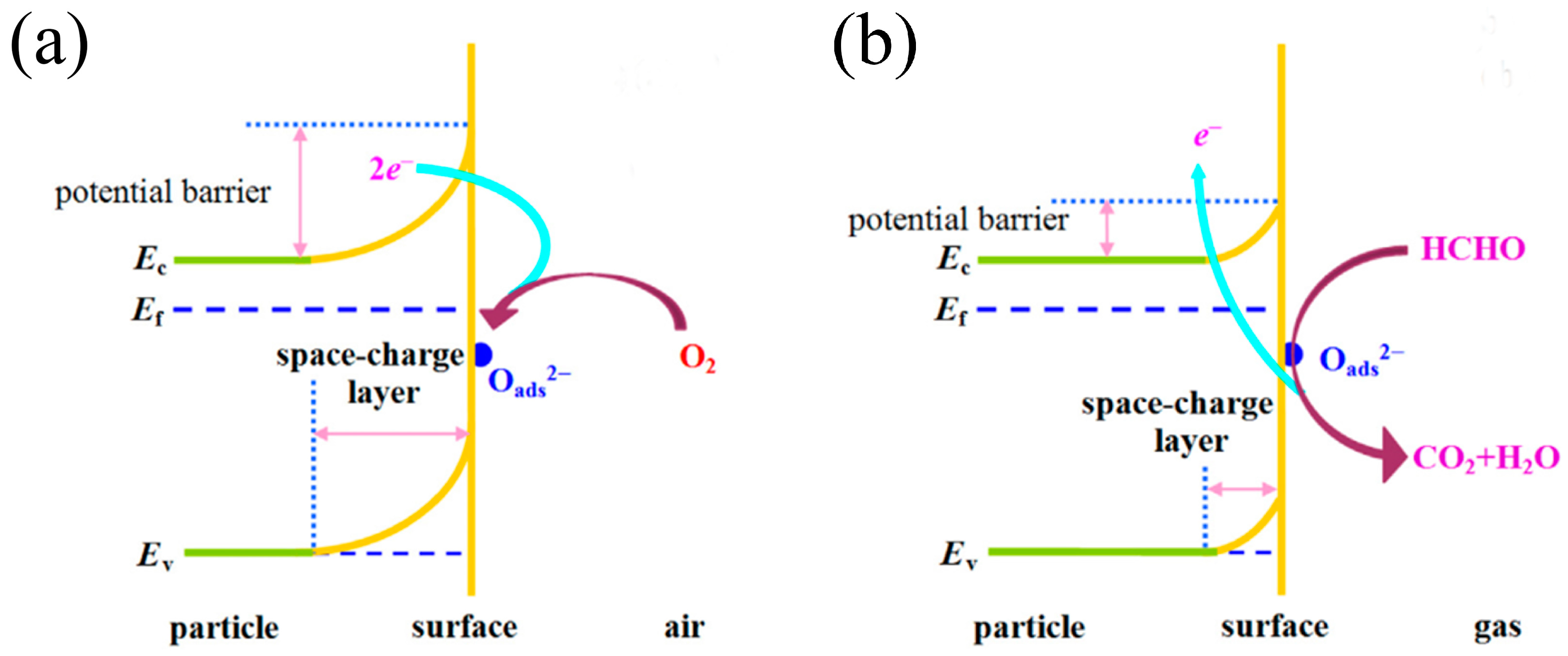
3. Gas-Sensing Mechanism of Semiconductor Metal Oxide Sensors towards Volatile Organic Compounds
4. Factors Affecting the Sensitivity of Semiconductor Metal Oxide Gas Sensors
4.1. Effect of the Microstructure
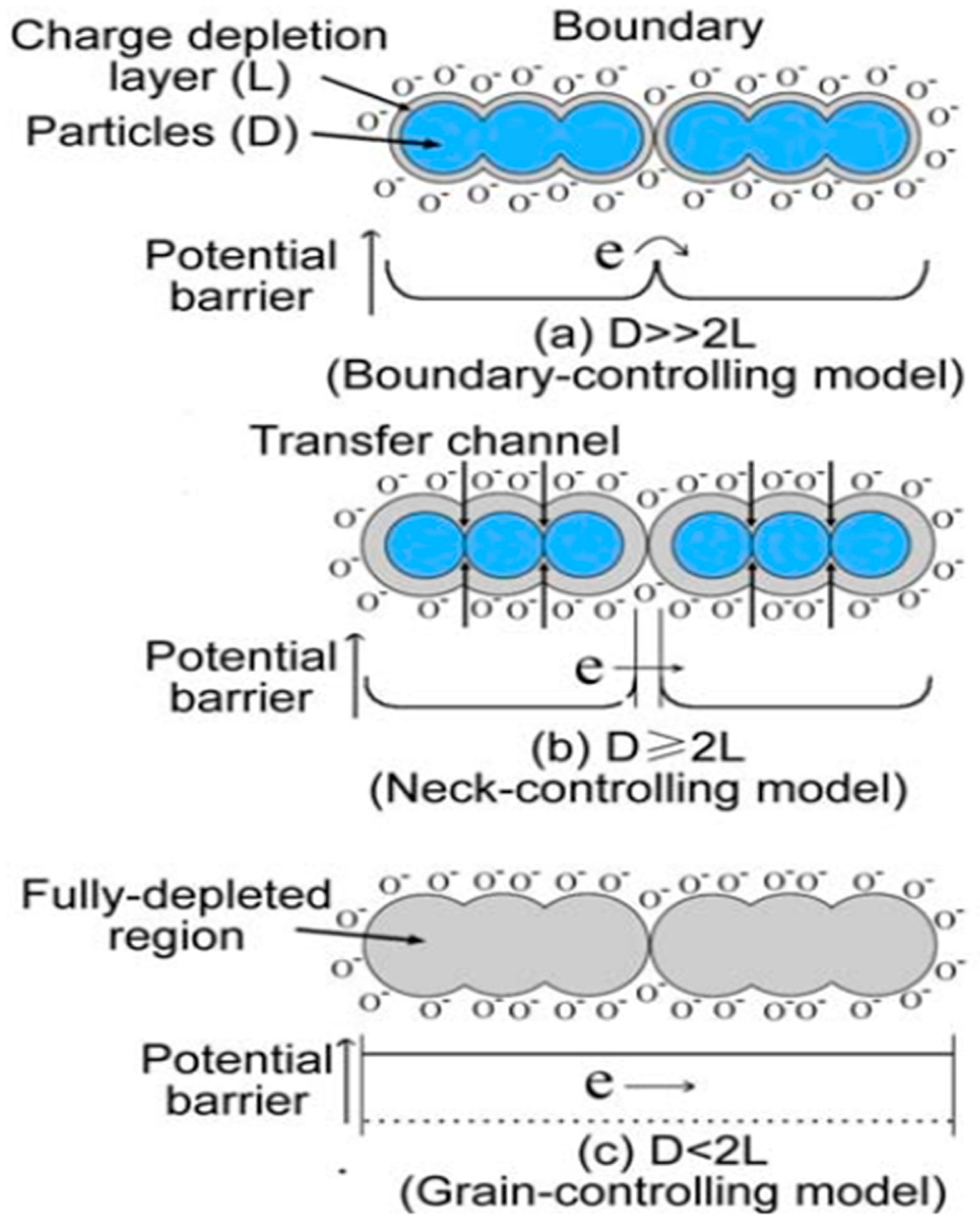
4.1.1. Grain Size
4.1.2. Number of Activated Adsorption Sites
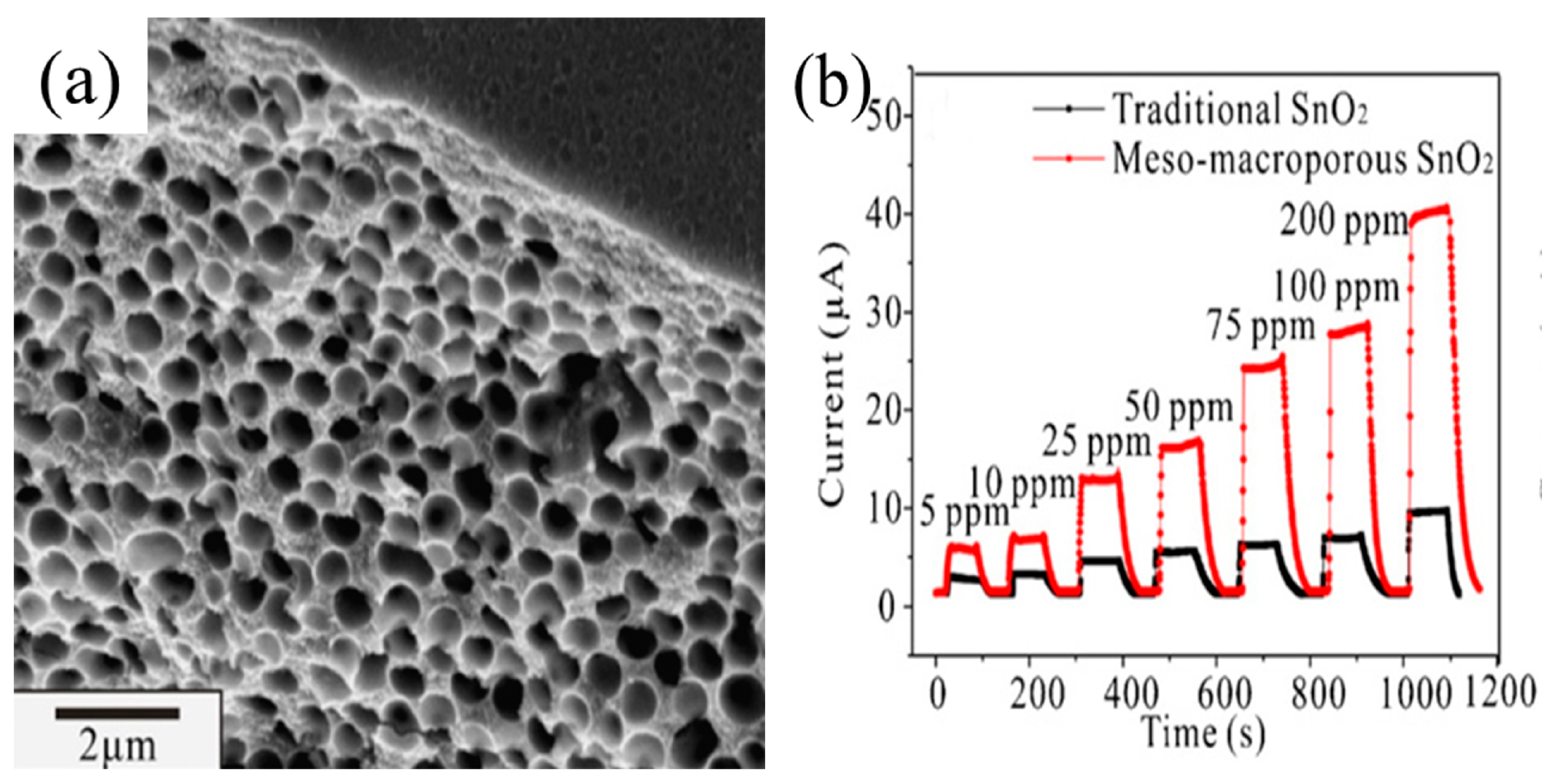
4.1.3. Gas Diffusion
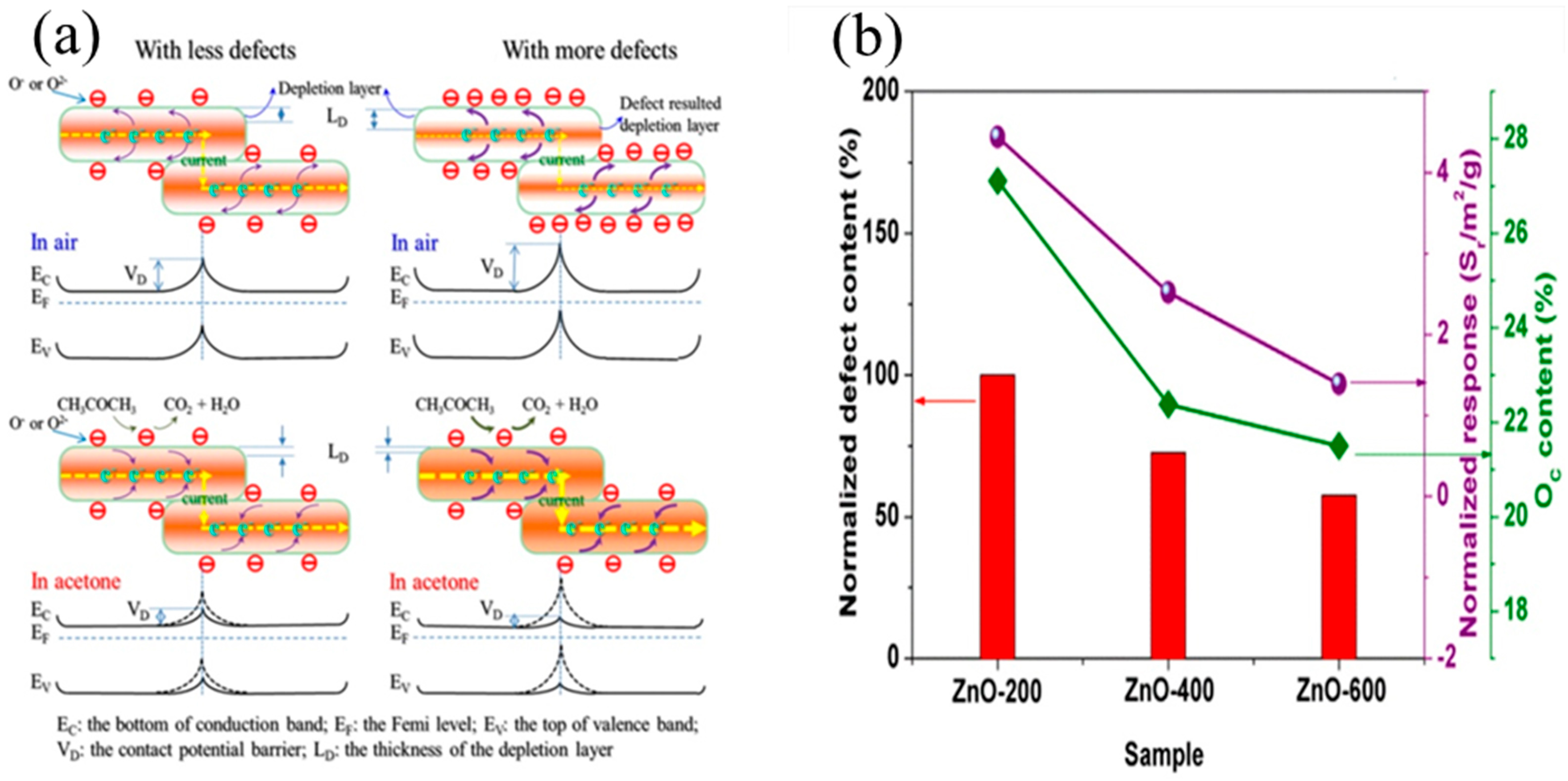
4.2. Effect of the Defects
4.3. Effect of the Catalyst
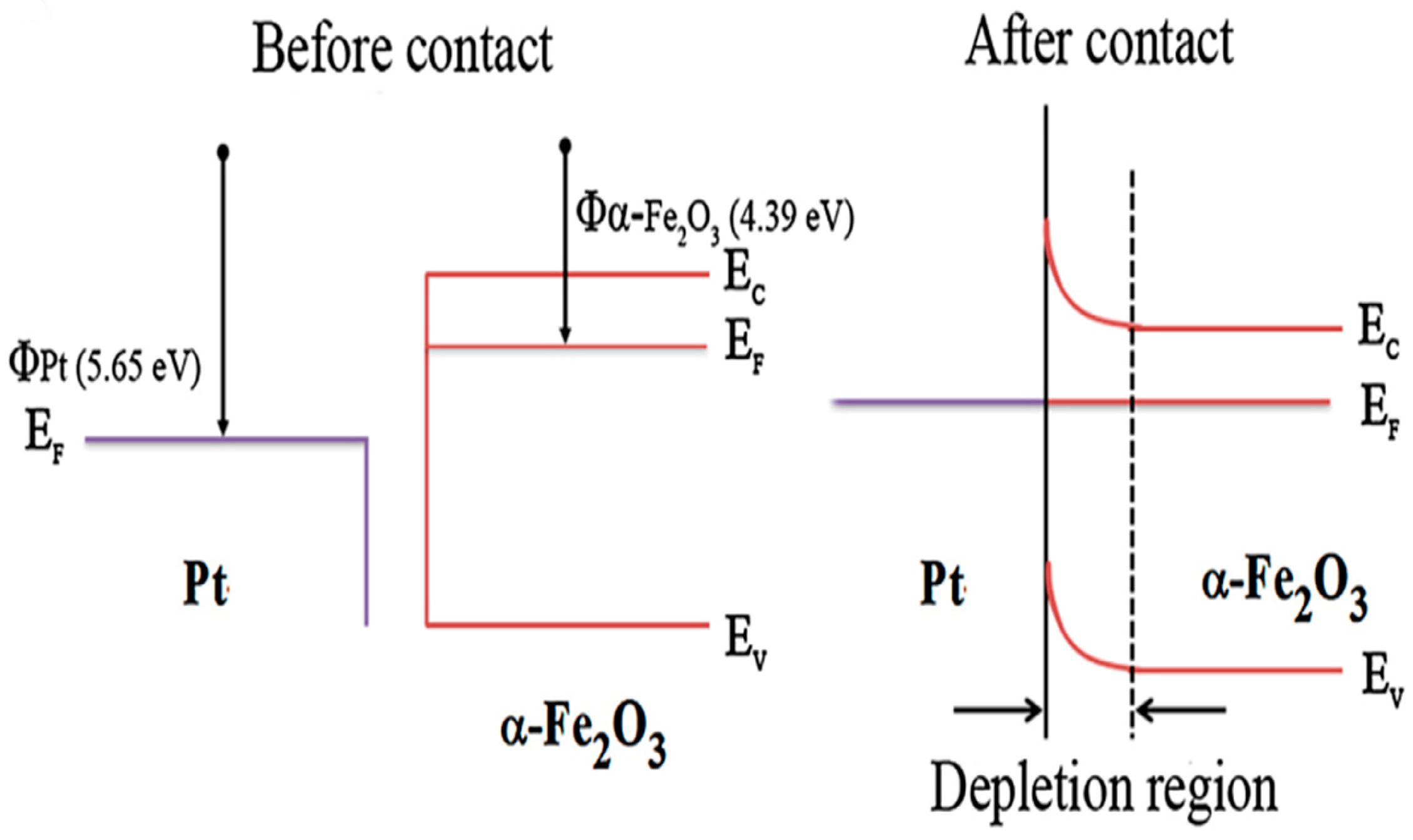
4.4. Effect of the Heterojunction
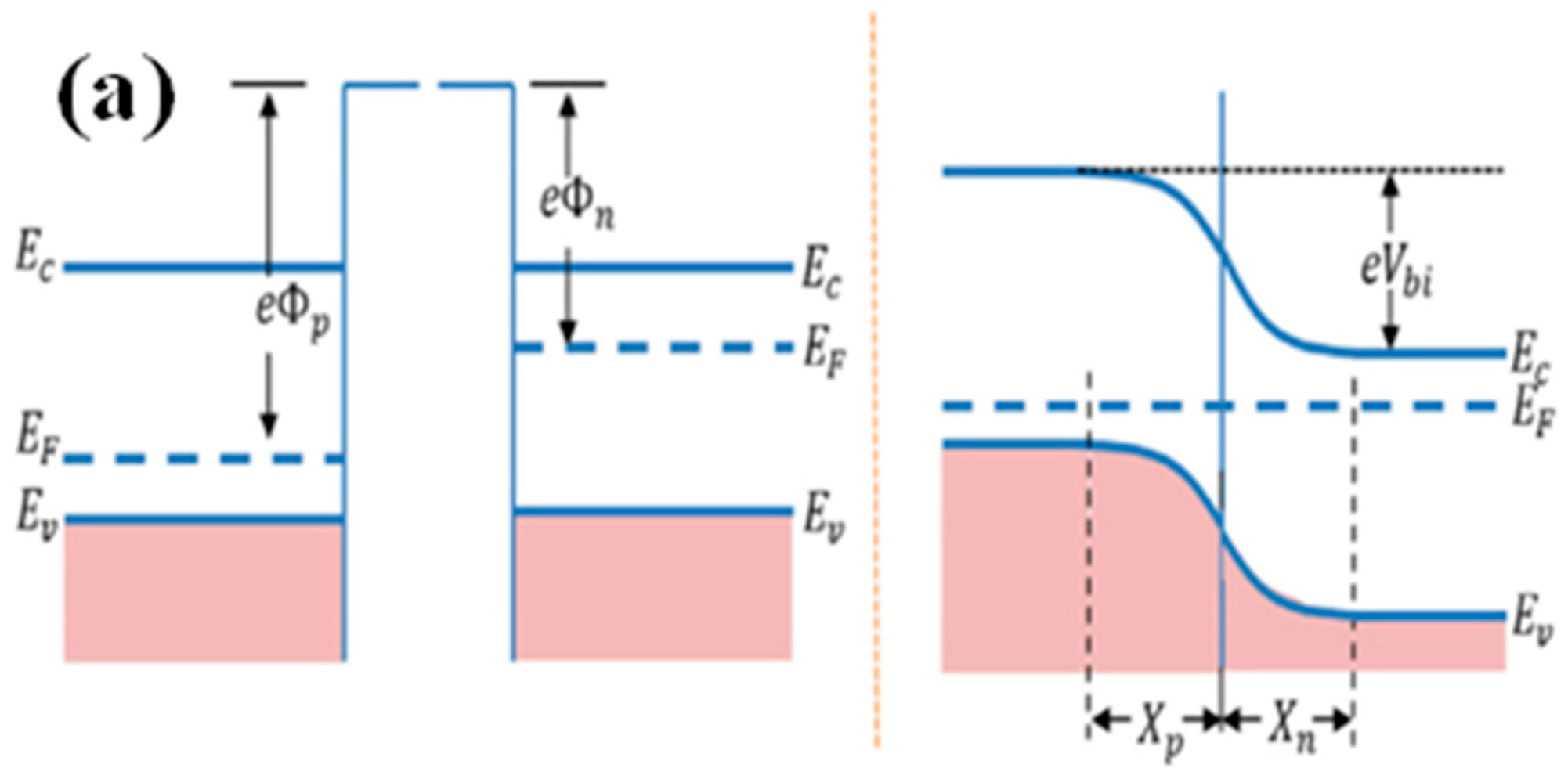
4.4.1. p–n or n–p Junction
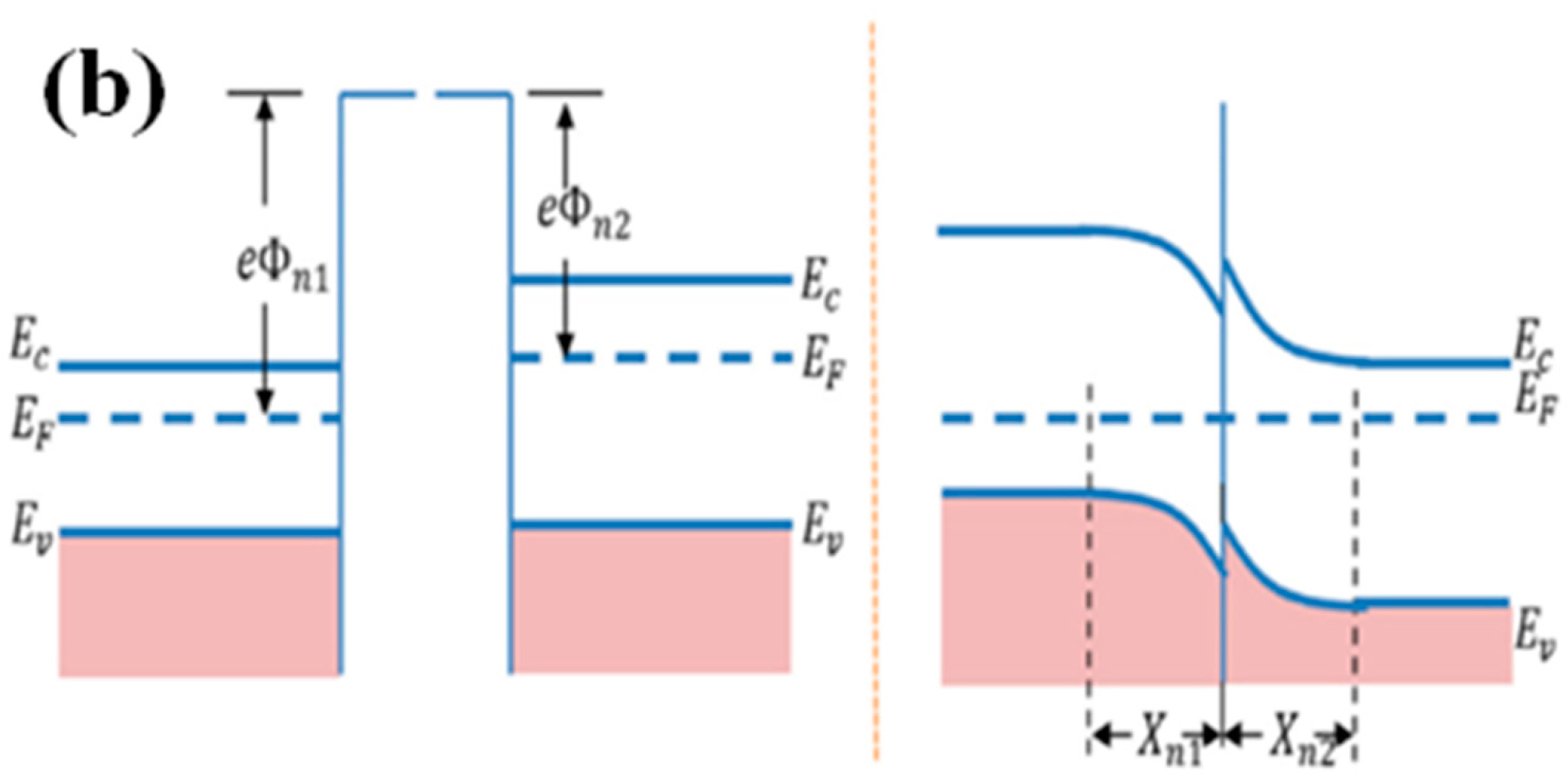
4.4.2. n–n or p–p Junction
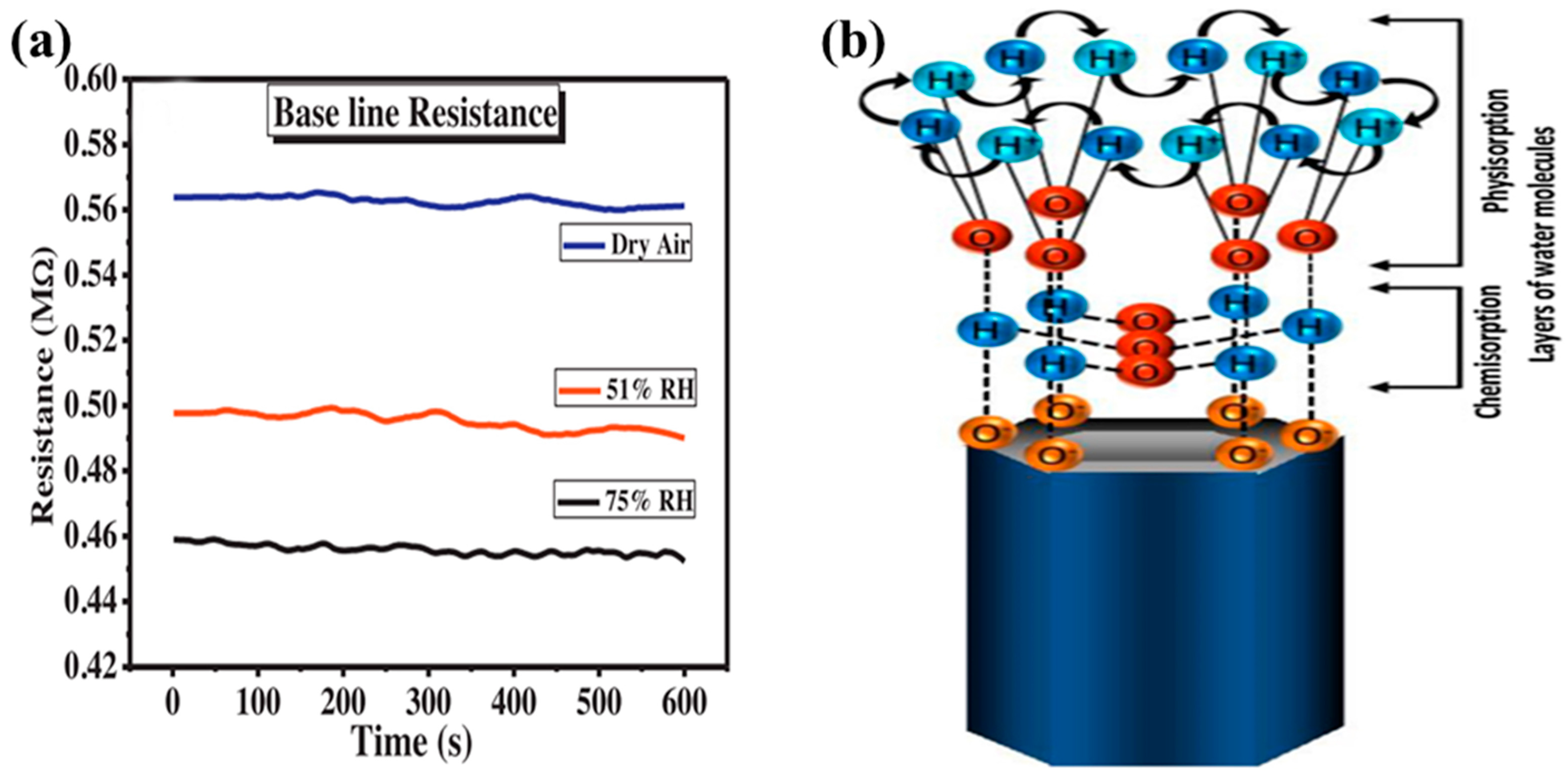
4.5. Effect of the Humidity
5. Semiconductor Metal Oxide Gas Sensors for the Detection of Volatile Organic Compounds
5.1. Ethanol Sensors
5.2. Acetone Sensors
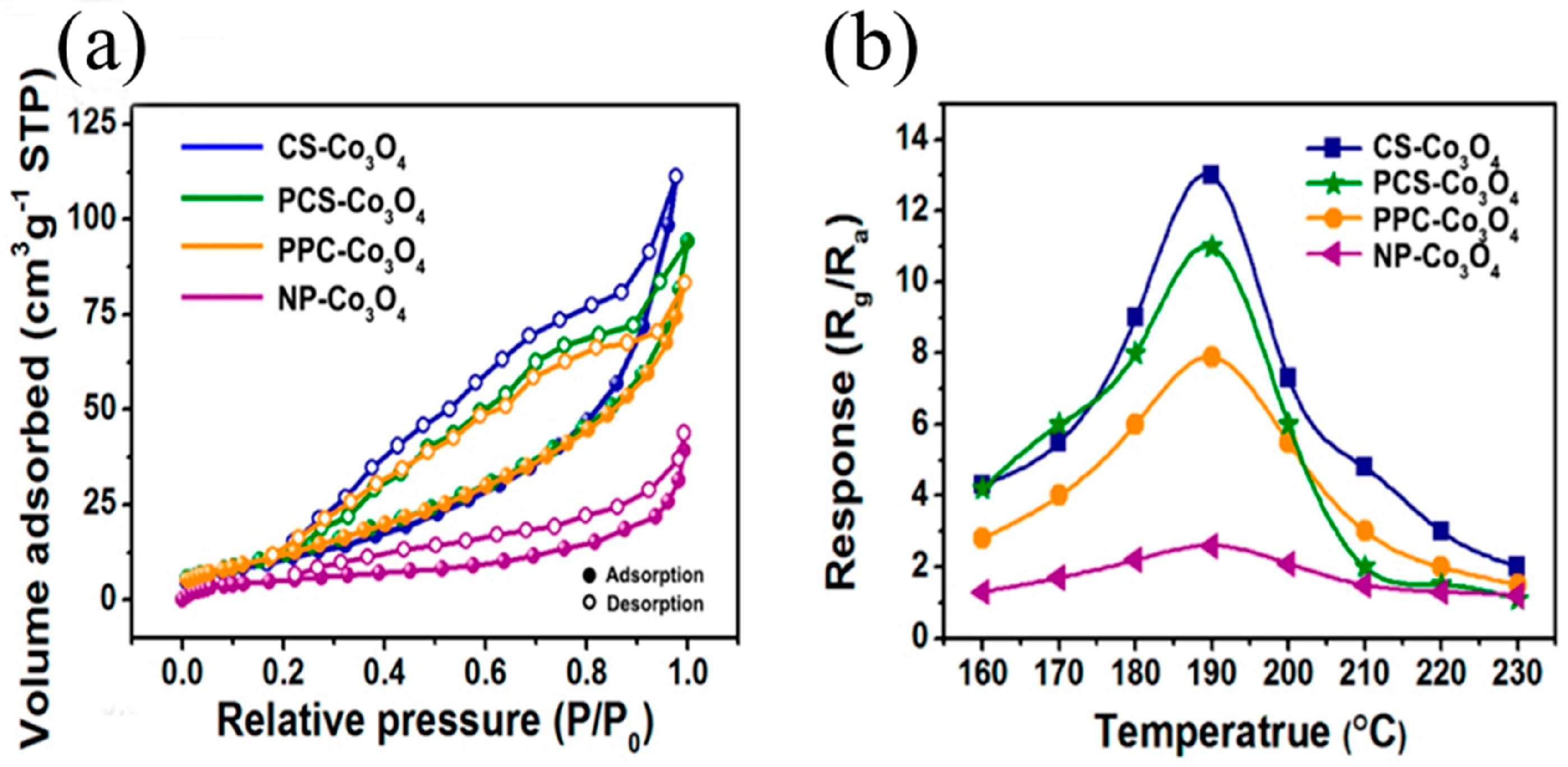
5.3. Formaldehyde Sensors
5.4. Toluene Sensors
5.5. Acetylene Sensors
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.L.; Nie, L.; Li, J.; Wang, Y.F.; Wang, G.; Wang, J.H.; Hao, Z.P. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar]
- Ralf, K. Volatile Organic Compounds in the Atmosphere; Blackwell: Oxford, UK, 2007; pp. 1–500. [Google Scholar]
- Ren, F.; Gao, L.; Yuan, Y.; Zhang, Y.; Alqrni, A.; Al-Dossary, O.M.; Xu, J. Enhanced BTEX gas-sensing performance of CuO/SnO2 composite. Sens. Actuators B Chem. 2016, 223, 914–920. [Google Scholar]
- Yan, H.; Song, P.; Zhang, S.; Zhang, J.; Yang, Z.; Wang, Q. Au nanoparticles modified MoO3 nanosheets with their enhanced properties for gas sensing. Sens. Actuators B Chem. 2016, 236, 201–207. [Google Scholar]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar]
- Arafat, M.M.; Haseeb, A.S.M.A.; Akbar, S.A.; Quadir, M.Z. In-situ fabricated gas sensors based on one dimensional core-shell TiO2-Al2O3 nanostructures. Sens. Actuators B Chem. 2017, 238, 972–984. [Google Scholar]
- Majhi, S.M.; Rai, P.; Yu, Y.T. Facile Approach to Synthesize Au@ZnO Core-Shell Nanoparticles and Their Application for Highly Sensitive and Selective Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar]
- Moran-Lazaro, J.P.; Lopez-Urias, F.; Munoz-Sandoval, E.; Blanco-Alonso, O.; Sanchez-Tizapa, M.; Carreon-Alvarez, A.; Guillen-Bonilla, H.; Olvera-Amador, M.L.; Guillen-Bonilla, A.; Rodriguez-Betancourtt, V.M. Synthesis, Characterization, and Sensor Applications of Spinel ZnCo2O4 Nanoparticles. Sensors 2016, 16, 2162. [Google Scholar]
- Teimoori, F.; Khojier, K.; Dehnavi, N.Z. Investigation of sensitivity and selectivity of ZnO thin film to volatile organic compounds. J. Theor. Appl. Phys. 2017, 11, 157–163. [Google Scholar]
- Feng, C.; Wang, C.; Zhang, H.; Li, X.; Wang, C.; Cheng, P.; Ma, J.; Sun, P.; Gao, Y.; Zhang, H.; Sun, Y.; Zheng, J.; Lu, G. Enhanced sensitive and selective xylene sensors using W-doped NiO nanotubes. Sens. Actuators B Chem. 2015, 221, 1475–1482. [Google Scholar]
- Wang, L.; Li, J.; Wang, Y.; Yu, K.; Tang, X.; Zhang, Y.; Wang, S.; Wei, C. Construction of 1D SnO2-coated ZnO nanowire heterojunction for their improved n-butylamine sensing performances. Sci. Rep. 2016, 6, 1–12. [Google Scholar]
- Wang, L.; Zhang, R.; Zhou, T.; Lou, Z.; Deng, J.; Zhang, T. Concave Cu2O octahedral nanoparticles as an advanced sensing material for benzene (C6H6) and nitrogen dioxide (NO2) detection. Sens. Actuators B Chem. 2016, 223, 311–317. [Google Scholar]
- Zeng, Q.Z.; Ma, S.Y.; Jin, W.X.; Yang, H.M.; Chen, H.; Ge, Q.; Ma, L. Hydrothermal synthesis of monodisperse α-Fe2O3 hollow microspheroids and their high gas-sensing properties. J. Alloys Compd. 2017, 705, 427–437. [Google Scholar]
- Bonyani, M.; Lee, J.K.; Sun, G.-J.; Lee, S.; Ko, T.; Lee, C. Benzene sensing properties and sensing mechanism of Pd-decorated Bi2O3-core/ZnO-shell nanorods. Thin Solid Films 2017, 636, 257–266. [Google Scholar]
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of aldehydes in beer using solid-phase microextraction with on-fiber derivatization and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944. [Google Scholar]
- Teixeira, L.S.; Leao, E.S.; Dantas, A.F.; Pinheiro, H.L.; Costa, A.C.; de Andrade, J.B. Determination of formaldehyde in Brazilian alcohol fuels by flow-injection solid phase spectrophotometry. Talanta 2004, 64, 711–715. [Google Scholar]
- Kumar, P.; Deep, A.; Kim, K.H.; Brown, R.J.C. Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar]
- Wei, Y.; Wang, X.; Yi, G.; Zhou, L.; Cao, J.; Sun, G.; Chen, Z.; Bala, H.; Zhang, Z. Hydrothermal synthesis of Ag modified ZnO nanorods and their enhanced ethanol-sensing properties. Mater. Sci. Semicond. Proc. 2018, 75, 327–333. [Google Scholar]
- Wang, X.; Gao, M. Porous Co3O4/SnO2 quantum dot (QD) heterostructures with abundant oxygen vacancies and Co2+ ions for highly efficient gas sensing and oxygen evolution reaction. Nanoscale 2018, 10, 12045–12053. [Google Scholar]
- Liu, X.; Jiang, L.; Jiang, X.; Tian, X.; Sun, X.; Wang, Y.; He, W.; Hou, P.; Deng, X.; Xu, X. Synthesis of Ce-doped In2O3 nanostructure for gas sensor applications. Appl. Surf. Sci. 2018, 428, 478–484. [Google Scholar]
- Li, F.; Ruan, S.; Zhang, N.; Yin, Y.; Guo, S.; Chen, Y.; Zhang, H.; Li, C. Synthesis and characterization of Cr-doped WO3 nanofibers for conductometric sensors with high xylene sensitivity. Sens. Actuators B Chem. 2018, 265, 355–364. [Google Scholar]
- Feng, C.; Kou, X.; Chen, B.; Qian, G.; Sun, Y.; Lu, G. One-pot synthesis of in doped NiO nanofibers and their gas sensing properties. Sens. Actuators B Chem. 2017, 253, 584–591. [Google Scholar]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2 (n)-NiO (p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B Chem. 2018, 258, 204–214. [Google Scholar]
- Han, X.; Sun, Y.; Feng, Z.; Zhang, G.; Chen, Z.; Zhan, J. Au-deposited porous single-crystalline ZnO nanoplates for gas sensing detection of total volatile organic compounds. RSC Adv. 2016, 6, 37750–37756. [Google Scholar]
- Li, T.; Zeng, W.; Wang, Z. Quasi-one-dimensional metal-oxide-based heterostructural gas-sensing materials: A review. Sens. Actuators B Chem. 2015, 221, 1570–1585. [Google Scholar]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar]
- Lin, T.; Lv, X.; Li, S.; Wang, Q. The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors 2017, 17, 2779. [Google Scholar]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar]
- Khalil, A.; Kim, J.J.; Tuller, H.L.; Rutledge, G.C.; Hashaikeh, R. Gas sensing behavior of electrospun nickel oxide nanofibers: Effect of morphology and microstructure. Sens. Actuators B Chem. 2016, 227, 54–64. [Google Scholar]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar]
- Kabcum, S.; Tammanoon, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Role of molybdenum substitutional dopants on H2S-sensing enhancement of flame-spray-made SnO2 nanoparticulate thick films. Sens. Actuators B Chem. 2016, 235, 678–690. [Google Scholar]
- Downie, N.A.; Maran & Co. Industrial Gases; Kluwer Academic: New York, NY, USA, 2002; pp. 1–559. [Google Scholar]
- Kuwahara, M.; Nishioka, M.; Yoshida, M.; Fujita, K.I. A Sustainable Method for the Synthesis of Acetic Acid Based on Dehydrogenation of an Ethanol-Water Solution Catalyzed by an Iridium Complex Bearing a Functional Bipyridonate Ligand. ChemCatChem 2018, 10, 3636–3640. [Google Scholar]
- Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol. 2018, 76, 533–542. [Google Scholar]
- Van Opstaele, F.; Goiris, K.; De Rouck, G.; Aerts, G.; De Cooman, L. Production of novel varietal hop aromas by supercritical fluid extraction of hop pellets-Part 2: Preparation of single variety floral, citrus, and spicy hop oil essences by density programmed supercritical fluid extraction. J. Supercrit. Fluid 2012, 71, 147–161. [Google Scholar]
- Leonardi, S.G.; Mirzaei, A.; Bonavita, A.; Santangelo, S.; Frontera, P.; Panto, F.; Antonucci, P.L.; Neri, G. A comparison of the ethanol sensing properties of alpha-iron oxide nanostructures prepared via the sol-gel and electrospinning techniques. Nanotechnology 2016, 27, 075502. [Google Scholar]
- Lian, X.; Li, Y.; Tong, X.; Zou, Y.; Liu, X.; An, D.; Wang, Q. Synthesis of Ce-doped SnO2 nanoparticles and their acetone gas sensing properties. Appl. Surf. Sci. 2017, 407, 447–455. [Google Scholar]
- Cheng, L.; Ma, S.Y.; Wang, T.T.; Luo, J. Synthesis and enhanced acetone sensing properties of 3D porous flower-like SnO2 nanostructures. Mater. Lett. 2015, 143, 84–87. [Google Scholar]
- Koo, W.T.; Yu, S.; Choi, S.J.; Jang, J.S.; Cheong, J.Y.; Kim, I.D. Nanoscale PdO Catalyst Functionalized Co3O4 Hollow Nanocages Using MOF Templates for Selective Detection of Acetone Molecules in Exhaled Breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar]
- Ge, W.; Chang, Y.; Natarajan, V.; Feng, Z.; Zhan, J.; Ma, X. In2O3-SnO2 hybrid porous nanostructures delivering enhanced formaldehyde sensing performance. J. Alloys Compd. 2018, 746, 36–44. [Google Scholar]
- Gu, C.; Cui, Y.; Wang, L.; Sheng, E.; Shim, J.J.; Huang, J. Synthesis of the porous NiO/SnO2 microspheres and microcubes and their enhanced formaldehyde gas sensing performance. Sens. Actuators B Chem. 2017, 241, 298–307. [Google Scholar]
- Wei, W.; Guo, S.; Chen, C.; Sun, L.; Chen, Y.; Guo, W.; Ruan, S. High sensitive and fast formaldehyde gas sensor based on Ag-doped LaFeO3 nanofibers. J. Alloys Compd. 2017, 695, 1122–1127. [Google Scholar]
- Shen, Z.; Zhang, X.; Ma, X.; Mi, R.; Chen, Y.; Ruan, S. The significant improvement for BTX (benzene, toluene and xylene) sensing performance based on Au-decorated hierarchical ZnO porous rose-like architectures. Sens. Actuators B Chem. 2018, 262, 86–94. [Google Scholar]
- Kawamura, K.; Vestergaard, M.D.; Ishiyama, M.; Nagatani, N.; Hashiba, T.; Tamiya, E. Development of a novel hand-held toluene gas sensor: Possible use in the prevention and control of sick building syndrome. Measurement 2006, 39, 490–496. [Google Scholar]
- Sui, L.; Zhang, X.; Cheng, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Au-Loaded Hierarchical MoO3 Hollow Spheres with Enhanced Gas-Sensing Performance for the Detection of BTX (Benzene, Toluene, And Xylene) And the Sensing Mechanism. ACS Appl. Mater. Interfaces 2017, 9, 1661–1670. [Google Scholar]
- Chen, W.D.; Burie, J.; Boucher, D. Investigation on infrared laser absorption spectroscopy measurement of acetylene trace quantities. Infrared Phys. Technol. 2000, 41, 339–348. [Google Scholar]
- Zhang, L.; Zhao, J.; Zheng, J.; Li, L.; Zhu, Z. Hydrothermal synthesis of hierarchical nanoparticle-decorated ZnO microdisks and the structure-enhanced acetylene sensing properties at high temperatures. Sens. Actuators B Chem. 2011, 158, 144–150. [Google Scholar]
- Li, C.; Su, Y.; Lv, X.; Xia, H.; Wang, Y. Electrochemical acetylene sensor based on Au/MWCNTs. Sens. Actuators B Chem. 2010, 149, 427–431. [Google Scholar]
- Wang, J.; Zeng, W.; Wang, Z. Assembly of 2D nanosheets into 3D flower-like NiO: Synthesis and the influence of petal thickness on gas-sensing properties. Ceram. Int. 2016, 42, 4567–4573. [Google Scholar]
- Yuliarto, B.; Gumilar, G.; Septiani, N.L.W. SnO2 Nanostructure as Pollutant Gas Sensors: Synthesis, Sensing Performances, and Mechanism. Adv. Mater. Sci. Eng. 2015, 2015, 1–14. [Google Scholar]
- Wang, Z.; Wang, D.; Sun, J. Controlled synthesis of defect-rich ultrathin two-dimensional WO3 nanosheets for NO2 gas detection. Sens. Actuators B Chem. 2017, 245, 828–834. [Google Scholar]
- Shankar, P.; Rayappan, J.B.B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases-A review. Sci. Lett. J. 2015, 4, 1–18. [Google Scholar]
- Wang, C.; Li, X.; Feng, C.; Sun, Y.; Lu, G. Nanosheets assembled hierarchical flower-like WO3 nanostructures: Synthesis, characterization, and their gas sensing properties. Sens. Actuators B Chem. 2015, 210, 75–81. [Google Scholar]
- Wang, S.; Yang, J.; Zhang, H.; Wang, Y.; Gao, X.; Wang, L.; Zhu, Z. One-pot synthesis of 3D hierarchical SnO2 nanostructures and their application for gas sensor. Sens. Actuators B Chem. 2015, 207, 83–89. [Google Scholar]
- Wang, Y.; Zhang, B.; Liu, J.; Yang, Q.; Cui, X.; Gao, Y.; Chuai, X.; Liu, F.; Sun, P.; Liang, X.; Sun, Y.; Lu, G. Au-loaded mesoporous WO3: Preparation and n -butanol sensing performances. Sens. Actuators B Chem. 2016, 236, 67–76. [Google Scholar]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B Chem. 2017, 238, 264–273. [Google Scholar]
- Wei, F.; Zhang, H.; Nguyen, M.; Ying, M.; Gao, R.; Jiao, Z. Template-free synthesis of flower-like SnO2 hierarchical nanostructures with improved gas sensing performance. Sens. Actuators B Chem. 2015, 215, 15–23. [Google Scholar]
- Chen, M.; Zhang, Y.; Zhang, J.; Li, K.; Lv, T.; Shen, K.; Zhu, Z.; Liu, Q. Facile lotus-leaf-templated synthesis and enhanced xylene gas sensing properties of Ag-LaFeO3 nanoparticles. J. Mater. Chem. C 2018, 6, 6138–6145. [Google Scholar]
- Song, X.; Xu, Q.; Zhang, T.; Song, B.; Li, C.; Cao, B. Room-temperature, high selectivity and low-ppm-level triethylamine sensor assembled with Au decahedrons-decorated porous α-Fe2O3 nanorods directly grown on flat substrate. Sens. Actuators B Chem. 2018, 268, 170–181. [Google Scholar]
- Subbiah, D.K.; Kulandaisamy, A.J.; George, R.B.; Shankar, P.; Mani, G.K.; Jayanth Babu, K.; Rayappan, J.B.B. Nano ceria as xylene sensor-Role of cerium precursor. J. Alloys Compd. 2018, 753, 771–780. [Google Scholar]
- Shi, S.; Zhang, F.; Lin, H.; Wang, Q.; Shi, E.; Qu, F. Enhanced triethylamine-sensing properties of P-N heterojunction Co3O4/In2O3 hollow microtubes derived from metal-organic frameworks. Sens. Actuators B Chem. 2018, 262, 739–749. [Google Scholar]
- Vijayakumar, Y.; Mani, G.K.; Ponnusamy, D.; Shankar, P.; Kulandaisamy, A.J.; Tsuchiya, K.; Rayappan, J.B.B.; Ramana Reddy, M.V. V2O5 nanofibers: Potential contestant for high performance xylene sensor. J. Alloys Compd. 2018, 731, 805–812. [Google Scholar]
- Liu, C.; Lu, H.; Zhang, J.; Gao, J.; Zhu, G.; Yang, Z.; Yin, F.; Wang, C. Crystal facet-dependent p-type and n-type sensing responses of TiO2 nanocrystals. Sens. Actuators B Chem. 2018, 263, 557–567. [Google Scholar]
- Xu, T.T.; Xu, Y.M.; Zhang, X.F.; Deng, Z.P.; Huo, L.H.; Gao, S. Enhanced H2S Gas-Sensing Performance of Zn2SnO4 Lamellar Micro-Spheres. Front. Chem. 2018, 6, 1–5. [Google Scholar]
- Han, B.; Liu, X.; Xing, X.; Chen, N.; Xiao, X.; Liu, S.; Wang, Y. A high response butanol gas sensor based on ZnO hollow spheres. Sens. Actuators B Chem. 2016, 237, 423–430. [Google Scholar]
- Gaidan, I.; Brabazon, D.; Ahad, I.U. Response of a Zn2TiO4 Gas Sensor to Propanol at Room Temperature. Sensors 2017, 17, 1995. [Google Scholar]
- Rothschild, A.; Komem, Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar]
- Xu, C.N.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain-size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar]
- Liang, S.; Li, J.; Wang, F.; Qin, J.; Lai, X.; Jiang, X. Highly sensitive acetone gas sensor based on ultrafine α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 923–927. [Google Scholar]
- Zhang, R.; Zhou, T.; Wang, L.; Zhang, T. Metal-organic frameworks-derived hierarchical Co3O4 structures as efficient sensing materials for acetone detection. ACS Appl. Mater. Interfaces 2018, 10, 9765–9773. [Google Scholar]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film senmiconductor gas sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar]
- Li, H.; Meng, F.; Liu, J.; Sun, Y.; Jin, Z.; Kong, L.; Hu, Y.; Liu, J. Synthesis and gas sensing properties of hierarchical meso-macroporous SnO2 for detection of indoor air pollutants. Sens. Actuators B Chem. 2012, 166–167, 519–525. [Google Scholar]
- Li, S.M.; Zhang, L.X.; Zhu, M.Y.; Ji, G.J.; Zhao, L.X.; Yin, J.; Bie, L.J. Acetone sensing of ZnO nanosheets synthesized using room-temperature precipitation. Sens. Actuators B Chem. 2017, 249, 611–623. [Google Scholar]
- Rai, P.; Majhi, S.M.; Yu, Y.T.; Lee, J.H. Noble metal@metal oxide semiconductor core@shell nano-architectures as a new platform for gas sensor applications. RSC Adv. 2015, 5, 76229–76248. [Google Scholar]
- Liu, C.; Gao, H.; Wang, L.; Wang, T.; Yang, X.; Sun, P.; Gao, Y.; Liang, X.; Liu, F.; Song, H.; Lu, G. Facile synthesis and the enhanced sensing properties of Pt-loaded α-Fe2O3 porous nanospheres. Sens. Actuators B Chem. 2017, 252, 1153–1162. [Google Scholar]
- Xiong, Y.; Xu, W.; Zhu, Z.; Xue, Q.; Lu, W.; Ding, D.; Zhu, L. ZIF-derived porous ZnO-Co3O4 hollow polyhedrons heterostructure with highly enhanced ethanol detection performance. Sens. Actuators B Chem. 2017, 253, 523–532. [Google Scholar]
- Zhang, B.; Fu, W.; Meng, X.; Ruan, A.; Su, P.; Yang, H. Enhanced ethanol sensing properties based on spherical-coral-like SnO2 nanorods decorated with α-Fe2O3 nanocrystallites. Sens. Actuators B Chem. 2018, 261, 505–514. [Google Scholar]
- Acharyya, D.; Bhattacharyya, P. Alcohol sensing performance of ZnO hexagonal nanotubes at low temperatures: A qualitative understanding. Sens. Actuators B Chem. 2016, 228, 373–386. [Google Scholar]
- Li, L.; Zhang, C.; Zhang, R.; Gao, X.; He, S.; Liu, M.; Li, X.; Chen, W. 2D ultrathin Co3O4 nanosheet array deposited on 3D carbon foam for enhanced ethanol gas sensing application. Sens. Actuators B Chem. 2017, 244, 664–672. [Google Scholar]
- Choi, Y.H.; Kim, D.H.; Hong, S.H. CuBi2O4 prepared by the polymerized complex method for gas-sensing applications. ACS Appl. Mater. Interfaces 2018, 10, 14901–14913. [Google Scholar]
- Rajesh, N.; Kannan, J.C.; Krishnakumar, T.; Leonardi, S.G.; Neri, G. Sensing behavior to ethanol of tin oxide nanoparticles prepared by microwave synthesis with different irradiation time. Sens. Actuators B Chem. 2014, 194, 96–104. [Google Scholar]
- Tan, W.; Tan, J.; Li, L.; Dun, M.; Huang, X. Nanosheets-assembled hollowed-out hierarchical Co3O4 microrods for fast response/recovery gas sensor. Sens. Actuators B Chem. 2017, 249, 66–75. [Google Scholar]
- Park, S.; Ko, H.; An, S.; Lee, W.I.; Lee, S.; Lee, C. Synthesis and ethanol sensing properties of CuO nanorods coated with In2O3. Ceram. Int. 2013, 39, 5255–5262. [Google Scholar]
- Huang, F.; Yang, W.; He, F.; Liu, S. Controlled synthesis of flower-like In2O3 microrods and their highly improved selectivity toward ethanol. Sens. Actuators B Chem. 2016, 235, 86–93. [Google Scholar]
- Zhang, Y.; Duan, Z.; Zou, H.; Ma, M. Fabrication of electrospun LaFeO3 nanotubes via annealing technique for fast ethanol detection. Mater. Lett. 2018, 215, 58–61. [Google Scholar]
- Cheng, Y.; Guo, H.; Wang, Y.; Zhao, Y.; Li, Y.; Liu, L.; Li, H.; Duan, H. Low cost fabrication of highly sensitive ethanol sensor based on Pd-doped α-Fe2O3 porous nanotubes. Mater. Res. Bull. 2018, 105, 21–27. [Google Scholar]
- Chen, D.; Yi, J. One-pot electrospinning and gas-sensing properties of LaMnO3 perovskite/SnO2 heterojunction nanofibers. J. Nanopart. Res. 2018, 20, 1–10. [Google Scholar]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar]
- Yuan, Z.; Yin, L.; Ding, H.; Huang, W.; Shuai, C.; Deng, J. One-step synthesis of single-crystalline ZnO nanowires for the application of gas sensor. J. Mater. Sci. Mater. Electron. 2018, 29, 11559–11565. [Google Scholar]
- Choi, K.S.; Park, S.; Chang, S.P. Enhanced ethanol sensing properties based on SnO2 nanowires coated with Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 871–879. [Google Scholar]
- Tharsika, T.; Haseeb, A.S.; Akbar, S.A.; Sabri, M.F.; Hoong, W.Y. Enhanced ethanol gas sensing properties of SnO2-core/ZnO-shell nanostructures. Sensors 2014, 14, 14586–14600. [Google Scholar]
- Le, D.T.T.; Trung, D.D.; Chinh, N.D.; Binh, B.T.T.; Hong, H.S.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. Facile synthesis of SnO2-ZnO core-shell nanowires for enhanced ethanol-sensing performance. Curr. Appl. Phys. 2013, 13, 1637–1642. [Google Scholar]
- Zoolfakar, A.S.; Ahmad, M.Z.; Rani, R.A.; Ou, J.Z.; Balendhran, S.; Zhuiykov, S.; Latham, K.; Wlodarski, W. Nanostructured copper oxides as ethanol vapour sensors. Sens. Actuators B Chem. 2013, 185, 620–627. [Google Scholar]
- Bagal, L.K.; Patil, J.Y.; Vaishampayan, M.V.; Mulla, I.S.; Suryavanshi, S.S. Effect of Pd and Ce on the enhancement of ethanol vapor response of SnO2 thick films. Sens. Actuators B Chem. 2015, 207, 383–390. [Google Scholar]
- Lu, Y.; Ma, Y.H.; Ma, S.Y.; Jin, W.X.; Yan, S.H.; Xu, X.L.; Chen, Q. Curly porous NiO nanosheets with enhanced gas-sensing properties. Mater. Lett. 2017, 190, 252–255. [Google Scholar]
- Xu, J.; Xue, Z.; Qin, N.; Cheng, Z.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuators B Chem. 2017, 242, 148–157. [Google Scholar]
- Li, T.; Zeng, W.; Long, H.; Wang, Z. Nanosheet-assembled hierarchical SnO2 nanostructures for efficient gas-sensing applications. Sens. Actuators B Chem. 2016, 231, 120–128. [Google Scholar]
- Yu, X.; Zeng, W. Fabrication and gas-sensing performance of nanorod-assembled SnO2 nanostructures. J. Mater. Sci. Mater. Electron. 2016, 27, 7448–7453. [Google Scholar]
- Guo, J.; Zhang, J.; Gong, H.; Ju, D.; Cao, B. Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor. Sens. Actuators B Chem. 2016, 226, 266–272. [Google Scholar]
- Miao, R.; Zeng, W. Hydrothermal synthesis of flake-flower NiO architectures: Structure, growth and gas-sensing properties. Mater. Lett. 2016, 171, 200–203. [Google Scholar]
- Zhang, B.; Fu, W.; Li, H.; Fu, X.; Wang, Y.; Bala, H.; Wang, X.; Sun, G.; Cao, J.; Zhang, Z. Synthesis and characterization of hierarchical porous SnO2 for enhancing ethanol sensing properties. Appl. Surf. Sci. 2016, 363, 560–565. [Google Scholar]
- Zhang, J.; Song, P.; Li, J.; Yang, Z.; Wang, Q. Template-assisted synthesis of hierarchical MoO3 microboxes and their high gas-sensing performance. Sens. Actuators B Chem. 2017, 249, 458–466. [Google Scholar]
- Wang, S.; Yu, W.; Cheng, C.; Zhang, T.; Ge, M.; Sun, Y.; Dai, N. Fabrication of mesoporous SnO2 nanocubes with superior ethanol gas sensing property. Mater. Res. Bull. 2017, 89, 267–272. [Google Scholar]
- Zhou, T.; Zhang, T.; Zhang, R.; Deng, J.; Lou, Z.; Lu, G.; Wang, L. Highly sensitive sensing platform based on ZnSnO3 hollow cubes for detection of ethanol. Appl. Surf. Sci. 2017, 400, 262–268. [Google Scholar]
- Yang, X.; Gao, H.; Zhao, L.; Wang, T.; Sun, P.; Liu, F.; Lu, G. Enhanced gas sensing properties of monodisperse Zn2SnO4 octahedron functionalized by PdO nanoparticals. Sens. Actuators B Chem. 2018, 266, 302–310. [Google Scholar]
- Cao, S.; Chen, H. Nanorods assembled hierarchical urchin-like WO3 nanostructures: Hydrothermal synthesis, characterization, and their gas sensing properties. J. Alloys Compd. 2017, 702, 644–648. [Google Scholar]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Tan, W.; Lin, Z.; Huang, X. Synthesis of hollow and hollowed-out Co3O4 microspheres assembled by porous ultrathin nanosheets for ethanol gas sensors: Responding and recovering in one second. Sens. Actuators B Chem. 2017, 249, 44–52. [Google Scholar]
- Huang, X.; Ren, Z.B.; Zheng, X.H.; Tang, D.P.; Wu, X.; Lin, C. A facile route to batch synthesis CuO hollow microspheres with excellent gas sensing properties. J. Mater. Sci. Mater. Electron. 2018, 29, 5969–5974. [Google Scholar]
- Zhao, Q.; Ma, G.; Zhai, C.; Yang, X.; Zhang, M. Facile synthesis of nanosheets-assembled hierarchical copper oxide microspheres and their ethanol gas sensing properties. New J. Chem. 2017, 41, 15042–15048. [Google Scholar]
- Yang, B.; Liu, J.; Qin, H.; Liu, Q.; Jing, X.; Zhang, H.; Li, R.; Huang, G.; Wang, J. Co3O4 nanoparticle-decorated hierarchical flower-like α-Fe2O3 microspheres: Synthesis and ethanol sensing properties. J. Alloys Compd. 2017, 727, 52–62. [Google Scholar]
- Wei, Y.; Wang, X.; Yi, G.; Zhou, L.; Cao, J.; Sun, G.; Hari, B. Synthesis and characterization of monodisperse hollow SnO2 microspheres and their enhanced sensing properties to ethanol. J. Porous Mater. 2017, 25, 1099–1104. [Google Scholar]
- Wang, H.; Wei, S.; Zhang, F.; Li, Y.; Liu, L.; Guo, X.; Song, L. Sea urchin-like SnO2/Fe2O3 microspheres for an ethanol gas sensor with high sensitivity and fast response/recovery. J. Mater. Sci. Mater. Electron. 2017, 28, 9969–9973. [Google Scholar]
- Liu, T.; Liu, J.; Liu, Q.; Rumin, L.; Zhang, H.; Jing, X.; Wang, J. Shape-controlled fabrication and enhanced gas sensing properties of uniform sphere-like ZnFe2O4 hierarchical architectures. Sens. Actuators B Chem. 2017, 250, 111–120. [Google Scholar]
- Kim, B.Y.; Cho, J.S.; Yoon, J.W.; Na, C.W.; Lee, C.S.; Ahn, J.H.; Kang, Y.C.; Lee, J.H. Extremely sensitive ethanol sensor using Pt-doped SnO2 hollow nanospheres prepared by Kirkendall diffusion. Sens. Actuators B Chem. 2016, 234, 353–360. [Google Scholar]
- Qiang, Z.; Ma, S.Y.; Jiao, H.Y.; Wang, T.T.; Jiang, X.H.; Jin, W.X.; Yang, H.M.; Chen, H. Highly sensitive and selective ethanol sensors using porous SnO2 hollow spheres. Ceram. Int. 2016, 42, 18983–18990. [Google Scholar]
- Wang, B.; Sun, L.; Wang, Y. Template-free synthesis of nanosheets-assembled SnO2 hollow spheres for enhanced ethanol gas sensing. Mater. Lett. 2018, 218, 290–294. [Google Scholar]
- Zhang, R.; Zhou, T.; Wang, L.; Lou, Z.; Deng, J.; Zhang, T. The synthesis and fast ethanol sensing properties of core-shell SnO2@ZnO composite nanospheres using carbon spheres as templates. New J. Chem. 2016, 40, 6796–6802. [Google Scholar]
- Wang, Q.; Yao, N.; Liu, C.; An, D.; Li, Y.; Zou, Y.; Tong, X. Synthesis of hollow ZnSnO3 nanospheres with high ethanol sensing properties. J. Nanomater. 2016, 2016, 1–5. [Google Scholar]
- An, D.; Mao, N.; Deng, G.; Zou, Y.; Li, Y.; Wei, T.; Lian, X. Ethanol gas-sensing characteristic of the Zn2SnO4 nanospheres. Ceram. Int. 2016, 42, 3535–3541. [Google Scholar]
- Jia, X.; Tian, M.; Dai, R.; Lian, D.; Han, S.; Wu, X.; Song, H. One-pot template-free synthesis and highly ethanol sensing properties of ZnSnO3 hollow microspheres. Sens. Actuators B Chem. 2017, 240, 376–385. [Google Scholar]
- Wang, X.; Ding, B.; Liu, Y.; Zhu, X.; Li, H.; Xia, M.; Fu, H.; Li, M. Synthesis of 3D flower-like ZnSnO3 and improvement of ethanol-sensing properties at room temperature based on nano-TiO2 decoration and UV radiation. Sens. Actuators B Chem. 2018, 264, 119–127. [Google Scholar]
- Staerz, A.; Weimar, U.; Barsan, N. Understanding the Potential of WO3 Based Sensors for Breath Analysis. Sensors 2016, 16, 1815. [Google Scholar]
- Gunawan, P.; Mei, L.; Teo, J.; Ma, J.; Highfield, J.; Li, Q.; Zhong, Z. Ultrahigh sensitivity of Au/1D alpha-Fe2O3 to acetone and the sensing mechanism. Langmuir 2012, 28, 14090–14099. [Google Scholar]
- Gu, F.; Chen, H.; Han, D.; Wang, Z. Metal–organic framework derived Au@ZnO yolk-shell nanostructures and their highly sensitive detection of acetone. RSC Adv. 2016, 6, 29727–29733. [Google Scholar]
- Khandekara, M.S.; Tarwal, N.L.; Mulla, I.S.; Suryavanshi, S.S. Nanocrystalline Ce doped CoFe2O4 as an acetone gas sensor. Ceram. Int. 2014, 40, 447–452. [Google Scholar]
- Geng, W.; Ge, S.; He, X.; Zhang, S.; Gu, J.; Lai, X.; Wang, H.; Zhang, Q. Volatile organic compound gas-sensing properties of bimodal porous alpha-Fe2O3 with ultrahigh sensitivity and fast response. ACS Appl. Mater. Interfaces 2018, 10, 13702–13711. [Google Scholar]
- Karmaoui, M.; Leonardi, S.G.; Latino, M.; Tobaldi, D.M.; Donato, N.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A.; Neri, G. Pt-decorated In2O3 nanoparticles and their ability as a highly sensitive (<10 ppb) acetone sensor for biomedical applications. Sens. Actuators B Chem. 2016, 230, 697–705. [Google Scholar]
- Zhang, J.; Song, J.M.; Niu, H.L.; Mao, C.J.; Zhang, S.Y.; Shen, Y.H. ZnFe2O4 nanoparticles: Synthesis, characterization, and enhanced gas sensing property for acetone. Sens. Actuators B Chem. 2015, 221, 55–62. [Google Scholar]
- Zhang, H.; Qin, H.; Gao, C.; Zhou, G.; Chen, Y.; Hu, J. UV light illumination can improve the sensing properties of LaFeO3 to acetone vapor. Sensors 2018, 18, 1990. [Google Scholar]
- Wang, S.; Cao, J.; Cui, W.; Fan, L.; Li, X.; Li, D. Facile synthesis of bamboo raft-like Co3O4 with enhanced acetone gas sensing performances. J. Alloys Compd. 2018, 758, 45–53. [Google Scholar]
- Tan, J.; Huang, X. Ultra-thin nanosheets-assembled hollowed-out hierarchical α-Fe2O3 nanorods: Synthesis via an interface reaction route and its superior gas sensing properties. Sens. Actuators B Chem. 2016, 237, 159–166. [Google Scholar]
- Li, L.; Tan, J.; Dun, M.; Huang, X. Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens. Actuators B Chem. 2017, 248, 85–91. [Google Scholar]
- Guo, X.; Zhang, J.; Ni, M.; Liu, L.; Lian, H.; Wang, H. Comparison of gas sensing properties based on hollow and porous α-Fe2O3 nanotubes. J. Mater. Sci. Mater. Electron. 2016, 27, 11262–11267. [Google Scholar]
- Zhang, Z.; Zhu, L.; Wen, Z.; Ye, Z. Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens. Actuators B Chem. 2017, 238, 1052–1059. [Google Scholar]
- Inyawilert, K.; Wisitsora-at, A.; Tuantranont, A.; Singjai, P.; Phanichphant, S.; Liewhiran, C. Ultra-rapid VOCs sensors based on sparked-In2O3 sensing films. Sens. Actuators B Chem. 2014, 192, 745–754. [Google Scholar]
- Muthukrishnan, K.; Vanaraja, M.; Boomadevi, S.; Karn, R.K.; Singh, V.; Singh, P.K.; Pandiyan, K. Studies on acetone sensing characteristics of ZnO thin film prepared by sol-gel dip coating. J. Alloys Compd. 2016, 673, 138–143. [Google Scholar]
- Wang, H.; Yan, L.; Li, S.; Li, Y.; Liu, L.; Du, L.; Duan, H.; Cheng, Y. Acetone sensors based on microsheet-assembled hierarchical Fe2O3 with different Fe3+ concentrations. Appl. Phys. A 2018, 124, 1–9. [Google Scholar]
- Wang, Q.; Yao, N.; An, D.; Li, Y.; Zou, Y.; Lian, X.; Tong, X. Enhanced gas sensing properties of hierarchical SnO2 nanoflower assembled from nanorods via a one-pot template-free hydrothermal method. Ceram. Int. 2016, 42, 15889–15896. [Google Scholar]
- Peng, C.; Guo, J.; Yang, W.; Shi, C.; Liu, M.; Zheng, Y.; Xu, J.; Chen, P.; Huang, T.; Yang, Y. Synthesis of three-dimensional flower-like hierarchical ZnO nanostructure and its enhanced acetone gas sensing properties. J. Alloys Compd. 2016, 654, 371–378. [Google Scholar]
- Liu, C.; Wang, B.; Wang, T.; Liu, J.; Sun, P.; Chuai, X.; Lu, G. Enhanced gas sensing characteristics of the flower-like ZnFe2O4/ZnO microstructures. Sens. Actuators B Chem. 2017, 248, 902–909. [Google Scholar]
- Wang, X.; Li, Y.; Liu, L.; Wang, L.; Wang, H.; Guo, X. Synthesis of flower-like porous ZnO and their ultrahigh acetone sensing properties. J. Porous Mater. 2016, 24, 463–468. [Google Scholar]
- Wang, B.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Chuai, X.; Lu, G. Gas sensing with yolk-shell LaFeO3 microspheres prepared by facile hydrothermal synthesis. Sens. Actuators B Chem. 2018, 258, 1215–1222. [Google Scholar]
- Zhang, S.; Song, P.; Zhang, J.; Yan, H.; Li, J.; Yang, Z.; Wang, Q. Highly sensitive detection of acetone using mesoporous In2O3 nanospheres decorated with Au nanoparticles. Sens. Actuators B Chem. 2017, 242, 983–993. [Google Scholar]
- Xiao, H.; Xue, C.; Song, P.; Li, J.; Wang, Q. Preparation of porous LaFeO3 microspheres and their gas-sensing property. Appl. Surf. Sci. 2015, 337, 65–71. [Google Scholar]
- Li, H.; Xie, W.; Liu, B.; Wang, Y.; Xiao, S.; Duan, X.; Li, Q.; Wang, T. Ultra-fast and highly-sensitive gas sensing arising from thin SnO2 inner wall supported hierarchical bilayer oxide hollow spheres. Sens. Actuators B Chem. 2017, 240, 349–357. [Google Scholar]
- Zhou, X.; Liu, J.; Wang, C.; Sun, P.; Hu, X.; Li, X.; Shimanoe, K.; Yamazoe, N.; Lu, G. Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens. Actuators B Chem. 2015, 206, 577–583. [Google Scholar]
- Zhou, X.; Wang, B.; Sun, H.; Wang, C.; Sun, P.; Li, X.; Hu, X.; Lu, G. Template-free synthesis of hierarchical ZnFe2O4 yolk-shell microspheres for high-sensitivity acetone sensors. Nanoscale 2016, 8, 5446–5453. [Google Scholar]
- Qu, F.; Shang, W.; Thomas, T.; Ruan, S.; Yang, M. Self-template derived ZnFe2O4 double-shell microspheres for chemresistive gas sensing. Sens. Actuators B Chem. 2018, 265, 625–631. [Google Scholar]
- Song, X.Z.; Qiao, L.; Sun, K.M.; Tan, Z.; Ma, W.; Kang, X.L.; Sun, F.F.; Huang, T.; Wang, X.F. Triple-shelled ZnO/ZnFe2O4 heterojunctional hollow microspheres derived from Prussian Blue analogue as high-performance acetone sensors. Sens. Actuators B Chem. 2018, 256, 374–382. [Google Scholar]
- Li, Y.X.; Guo, Z.; Su, Y.; Jin, X.B.; Tang, X.H.; Huang, J.R.; Huang, X.J.; Li, M.Q.; Liu, J.H. Hierarchical Morphology-Dependent Gas-Sensing Performances of Three-Dimensional SnO2 Nanostructures. ACS Sens. 2016, 2, 102–110. [Google Scholar]
- Liu, J.; Huang, H.; Zhao, H.; Yan, X.; Wu, S.; Li, Y.; Wu, M.; Chen, L.; Yang, X.; Su, B.L. Enhanced gas sensitivity and selectivity on aperture-controllable 3D interconnected macro-mesoporous ZnO nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 8583–8590. [Google Scholar]
- Guo, W. One-pot synthesis of urchin-like ZnO nanostructure and its enhanced acetone gas sensing properties. J. Mater. Sci. Mater. Electron. 2016, 28, 963–972. [Google Scholar]
- Ma, X.; Zhou, X.; Gong, Y.; Han, N.; Liu, H.; Chen, Y. MOF-derived hierarchical ZnO/ZnFe2O4 hollow cubes for enhanced acetone gas-sensing performance. RSC Adv. 2017, 7, 34609–34617. [Google Scholar]
- Song, X.Z.; Meng, Y.L.; Tan, Z.; Qiao, L.; Huang, T.; Wang, X.F. Concave ZnFe2O4 hollow octahedral nanocages derived from Fe-doped MOF-5 for high-performance acetone sensing at low-energy consumption. Inorg. Chem. 2017, 56, 13646–13650. [Google Scholar]
- Wang, X.; Zhang, S.; Shao, M.; Huang, J.; Deng, X.; Hou, P.; Xu, X. Fabrication of ZnO/ZnFe2O4 hollow nanocages through metal organic frameworks route with enhanced gas sensing properties. Sens. Actuators B Chem. 2017, 251, 27–33. [Google Scholar]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar]
- Upadhyay, S.B.; Mishra, R.K.; Sahay, P.P. Cr-doped WO3 nanosheets: Structural, optical and formaldehyde sensing properties. Ceram. Int. 2016, 42, 15301–15310. [Google Scholar]
- Wang, J.; Gan, X.; Li, Z.; Zhou, K. Microstructure and gas sensing property of porous spherical In2O3 particles prepared by hydrothermal method. Powder Technol. 2016, 303, 138–146. [Google Scholar]
- Zhang, S.; Song, P.; Yang, Z.; Wang, Q. Facile hydrothermal synthesis of mesoporous In2O3 nanoparticles with superior formaldehyde-sensing properties. Phys. E 2018, 97, 38–44. [Google Scholar]
- Gu, F.; Li, C.; Han, D.; Wang, Z. Manipulating the defect structure (VO) of In2O3 nanoparticles for enhancement of formaldehyde detection. ACS Appl. Mater. Interfaces 2018, 10, 933–942. [Google Scholar]
- Zhang, Y.; Xie, L.Z.; Yuan, C.X.; Zhang, C.L.; Liu, S.; Peng, Y.Q.; Li, H.R.; Zhang, M. A ppb-level formaldehyde gas sensor based on rose-like nickel oxide nanoparticles prepared using electrodeposition process. Nano 2016, 11, 1–9. [Google Scholar]
- Cao, J.; Zhang, H.; Yan, X. Facile fabrication and enhanced formaldehyde gas sensing properties of nanoparticles-assembled chain-like NiO architectures. Mater. Lett. 2016, 185, 40–42. [Google Scholar]
- Zhang, W.; Cheng, X.; Zhang, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. High selectivity to ppb-level HCHO sensor based on mesoporous tubular SnO2 at low temperature. Sens. Actuators B Chem. 2017, 247, 664–672. [Google Scholar]
- Guo, W.; Fu, M.; Zhai, C.; Wang, Z. Hydrothermal synthesis and gas-sensing properties of ultrathin hexagonal ZnO nanosheets. Ceram. Int. 2014, 40, 2295–2298. [Google Scholar]
- Hussaina, S.; Aslam, N.; Yang, X.Y.; Javed, M.S.; Xu, Z.W.; Wang, M.S.; Liu, G.W.; Qiao, G.J. Unique polyhedron CeO2 nanostructures for superior formaldehyde gas sensing performances. Ceram. Int. 2018, 44, 19624–19630. [Google Scholar]
- Wang, S.; Cao, J.; Cui, W.; Li, X.; Li, D. Facile synthesis and excellent formaldehyde gas sensing properties of novel spindle-like In2O3 porous polyhedra. Sens. Actuators B Chem. 2016, 237, 944–952. [Google Scholar]
- Yu, H.; Yang, T.; Wang, Z.; Li, Z.; Xiao, B.; Zhao, Q.; Zhang, M. Facile synthesis cedar-like SnO2 hierarchical micro-nanostructures with improved formaldehyde gas sensing characteristics. J. Alloys Compd. 2017, 724, 121–129. [Google Scholar]
- Zhang, S.; Song, P.; Li, J.; Zhang, J.; Yang, Z.; Wang, Q. Facile approach to prepare hierarchical Au-loaded In2O3 porous nanocubes and their enhanced sensing performance towards formaldehyde. Sens. Actuators B Chem. 2017, 241, 1130–1138. [Google Scholar]
- Zhou, T.; Zhang, T.; Zhang, R.; Lou, Z.; Deng, J.; Wang, L. Hollow ZnSnO3 cubes with controllable shells enabling highly efficient chemical sensing detection of formaldehyde vapors. ACS Appl. Mater. Interfaces 2017, 9, 14525–14533. [Google Scholar]
- Fu, Q.; Ai, M.; Duan, Y.; Lu, L.; Tian, X.; Sun, D.; Xu, Y.; Sun, Y. Synthesis of uniform porous NiO nanotetrahedra and their excellent gas-sensing performance toward formaldehyde. RSC Adv. 2017, 7, 52312–52320. [Google Scholar]
- Wang, S.; Cao, J.; Cui, W.; Fan, L.; Li, X.; Li, D. Oxygen vacancies and grain boundaries potential barriers modulation facilitated formaldehyde gas sensing performances for In2O3 hierarchical architectures. Sens. Actuators B Chem. 2018, 255, 159–165. [Google Scholar]
- Zhang, W.; Zhang, W.; Chen, B.; Shao, R.; Guan, R.; Zhang, W.; Zhang, Q.; Hou, G.; Yue, L. Controllable biomolecule-assisted synthesis and gas sensing properties of In2O3 micro/nanostructures with double phases. Sens. Actuators B Chem. 2017, 239, 270–278. [Google Scholar]
- San, X.; Zhao, G.; Wang, G.; Shen, Y.; Meng, D.; Zhang, Y.; Meng, F. Assembly of 3D flower-like NiO hierarchical architectures by 2D nanosheets: Synthesis and their sensing properties to formaldehyde. RSC Adv. 2017, 7, 3540–3549. [Google Scholar]
- Meng, D.; Liu, D.; Wang, G.; San, X.; Shen, Y.; Jin, Q.; Meng, F. CuO hollow microspheres self-assembled with nanobars: Synthesis and their sensing properties to formaldehyde. Vacuum 2017, 144, 272–280. [Google Scholar]
- Zhou, W.; Wu, Y.P.; Wang, X.; Qiao, X.Q.; Hou, D.F.; Zhao, J.; Li, D.S. In-MOFs-derived microsphere In2O3 for highly sensitive detecting formaldehyde vapor. Inorg. Chem. Commun. 2017, 85, 100–104. [Google Scholar]
- Zou, Y.; Wang, H.; Lai, X.; Li, X.; Zhou, X.; Lin, G.; Liu, D.; Chen, J.; Xin, H. Synthesis and enhanced formaldehyde-sensing properties of In2O3 hollow spheres with thin shells. J. Electron. Mater. 2017, 47, 2165–2170. [Google Scholar]
- Yang, J.; Wang, S.; Dong, R.; Zhang, L.; Zhu, Z.; Gao, X. One-pot synthesis of SnO2 hollow microspheres and their formaldehyde sensor application. Mater. Lett. 2016, 184, 9–12. [Google Scholar]
- Xing, R.; Xu, L.; Song, J.; Zhou, C.; Li, Q.; Liu, D.; Hong, W. Preparation and gas sensing properties of In2O3/Au nanorods for detection of volatile organic compounds in exhaled breath. Sci. Rep. 2015, 5, 1–14. [Google Scholar]
- Suematsu, K.; Watanabe, K.; Tou, A.; Sun, Y.; Shimanoe, K. Ultraselective toluene-gas sensor: Nanosized gold loaded on zinc oxide nanoparticles. Anal. Chem. 2018, 90, 1959–1966. [Google Scholar]
- Xie, N.; Guo, L.; Chen, F.; Kou, X.; Wang, C.; Ma, J.; Sun, Y.; Liu, F.; Liang, X.; Gao, Y.; Yan, X.; Lu, G. Enhanced sensing properties of SnO2 nanofibers with a novel structure by carbonization. Sens. Actuators B Chem. 2018, 271, 44–53. [Google Scholar]
- Kim, N.H.; Choi, S.J.; Yang, D.J.; Bae, J.; Park, J.; Kim, I.D. Highly sensitive and selective hydrogen sulfide and toluene sensors using Pd functionalized WO3 nanofibers for potential diagnosis of halitosis and lung cancer. Sens. Actuators B Chem. 2014, 193, 574–581. [Google Scholar]
- Kim, J.H.; Kim, S.S. Realization of ppb-scale toluene-sensing abilities with Pt-functionalized SnO2-ZnO core-shell nanowires. ACS Appl. Mater. Interfaces 2015, 7, 17199–17208. [Google Scholar]
- Seo, M.H.; Yuasa, M.; Kida, T.; Huh, J.S.; Yamazoe, N.; Shimanoe, K. Microstructure control of TiO2 nanotubular films for improved VOC sensing. Sens. Actuators B Chem. 2011, 154, 251–256. [Google Scholar]
- Sun, Y.; Wei, Z.; Zhang, W.; Li, P.; Lian, K.; Hu, J. Synthesis of brush-like ZnO nanowires and their enhanced gas-sensing properties. J. Mater. Sci. 2015, 51, 1428–1436. [Google Scholar]
- Wang, C.; Wang, T.; Wang, B.; Zhou, X.; Cheng, X.; Sun, P.; Zheng, J.; Lu, G. Design of alpha-Fe2O3 nanorods functionalized tubular NiO nanostructure for discriminating toluene molecules. Sci. Rep. 2016, 6, 1–9. [Google Scholar]
- Shan, H.; Liu, C.; Liu, L.; Zhang, J.; Li, H.; Liu, Z.; Zhang, X.; Bo, X.; Chi, X. Excellent toluene sensing properties of SnO2-Fe2O3 interconnected nanotubes. ACS Appl. Mater. Interfaces 2013, 5, 6376–6380. [Google Scholar]
- Wang, T.; Huang, Z.; Yu, Z.; Wang, B.; Wang, H.; Sun, P.; Suo, H.; Gao, Y.; Sun, Y.; Li, T.; Lu, G. Low operating temperature toluene sensor based on novel α-Fe2O3/SnO2 heterostructure nanowire arrays. RSC Adv. 2016, 6, 52604–52610. [Google Scholar]
- Wang, L.; Deng, J.; Lou, Z.; Zhang, T. Nanoparticles-assembled Co3O4 nanorods p-type nanomaterials: One-pot synthesis and toluene-sensing properties. Sens. Actuators B Chem. 2014, 201, 1–6. [Google Scholar]
- Li, J.; Wang, L.; Liu, H.; Zhao, J.; Li, X.; Wei, H.; Han, Y. Synthesis and enhanced toluene gas sensing properties of 1-D α-MoO3/Fe2(MoO4)3 heterostructure. J. Alloys Compd. 2017, 694, 939–945. [Google Scholar]
- Zhang, K.; Yang, X.; Wang, Y.; Bing, Y.; Qiao, L.; Liang, Z.; Yu, S.; Zeng, Y.; Zheng, W. Pd-loaded SnO2 ultrathin nanorod-assembled hollow microspheres with the significant improvement for toluene detection. Sens. Actuators B Chem. 2017, 243, 465–474. [Google Scholar]
- Dong, H.; Liu, Y.; Li, G.; Wang, X.; Xu, D.; Chen, Z.; Zhang, T.; Wang, J.; Zhang, L. Hierarchically rosette-like In2O3 microspheres for volatile organic compounds gas sensors. Sens. Actuators B Chem. 2013, 178, 302–309. [Google Scholar]
- Zhang, Y.; Li, D.; Qin, L.; Liu, D.; Liu, Y.; Liu, F.; Song, H.; Wang, Y.; Lu, G. Preparation of Au-loaded TiO2 pecan-kernel-like and its enhanced toluene sensing performance. Sens. Actuators B Chem. 2018, 255, 2240–2247. [Google Scholar]
- Qiao, L.; Bing, Y.; Wang, Y.; Yu, S.; Liang, Z.; Zeng, Y. Enhanced toluene sensing performances of Pd-loaded SnO2 cubic nanocages with porous nanoparticle-assembled shells. Sens. Actuators B Chem. 2017, 241, 1121–1129. [Google Scholar]
- Bing, Y.; Zeng, Y.; Liu, C.; Qiao, L.; Zheng, W. Synthesis of double-shelled SnO2 nano-polyhedra and their improved gas sensing properties. Nanoscale 2015, 7, 3276–3284. [Google Scholar]
- Lou, Z.; Deng, J.; Wang, L.; Wang, L.; Fei, T.; Zhang, T. Toluene and ethanol sensing performances of pristine and PdO-decorated flower-like ZnO structures. Sens. Actuators B Chem. 2013, 176, 323–329. [Google Scholar]
- Bing, Y.; Liu, C.; Qiao, L.; Zeng, Y.; Yu, S.; Liang, Z.; Liu, J.; Luo, J.; Zheng, W. Multistep synthesis of non-spherical SnO2@SnO2 yolk-shell cuboctahedra with nanoparticle-assembled porous structure for toluene detection. Sens. Actuators B Chem. 2016, 231, 365–375. [Google Scholar]
- Dong, C.; Liu, X.; Xiao, X.; Du, S.; Wang, Y. Monodisperse ZnFe2O4 nanospheres synthesized by a nonaqueous route for a highly slective low-ppm-level toluene gas sensor. Sens. Actuators B Chem. 2017, 239, 1231–1236. [Google Scholar]
- Iftekhar Uddin, A.S.M.; Chung, G.S. Effects of Ag nanoparticles decorated on ZnO nanorods under visible light illumination on flexible acetylene gas sensing properties. J. Electroceram. 2017, 40, 42–49. [Google Scholar]
- Uddin, A.S.M.I.; Yaqoob, U.; Phan, D.T.; Chung, G.S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2016, 222, 536–543. [Google Scholar]
- Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Phanichphant, S. Acetylene sensor based on Pt/ZnO thick films as prepared by flame spray pyrolysis. Sens. Actuators B Chem. 2011, 152, 155–161. [Google Scholar]
- Iftekhar Uddin, A.S.M.; Phan, D.T.; Chung, G.S. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 207, 362–369. [Google Scholar]
- Zhou, Q.; Tang, C.; Zhu, S.P.; Chen, W.G.; Li, J. Synthesis, characterisation and sensing properties of Sm2O3 doped SnO2 nanorods to C2H2 gas extracted from power transformer oil. Mater. Technol. 2016, 31, 364–370. [Google Scholar]
- Zhang, H.; Chen, W.; Jin, L.; Cui, F. Hierarchically porous WO3 microstructures with networks for acetylene sensing application. Mater. Lett. 2018, 214, 198–201. [Google Scholar]
- Wang, B.; Jin, H.T.; Zheng, Z.Q.; Zhou, Y.H.; Gao, C. Low-temperature and highly sensitive C2H2 sensor based on Au decorated ZnO/In2O3 belt-tooth shape nano-heterostructures. Sens. Actuators B Chem. 2017, 244, 344–356. [Google Scholar]
- Qiao, P.Y.; Zhang, L.X.; Zhu, M.Y.; Yin, Y.Y.; Zhao, Z.W.; Sun, H.N.; Dong, J.Y.; Bie, L.J. Acetylene sensing enhancement of mesoporous ZnO nanosheets with morphology and defect induced structural sensitization. Sens. Actuators B Chem. 2017, 250, 189–197. [Google Scholar]
- Li, C.; Lin, Y.; Li, F.; Zhu, L.; Meng, F.; Sun, D.; Zhou, J.; Ruan, S. Synthesis and highly enhanced acetylene sensing properties of Au nanoparticle-decorated hexagonal ZnO nanorings. RSC Adv. 2015, 5, 87132–87138. [Google Scholar]
- Chen, W.G.; Gao, T.Y.; Li, Q.Z.; Gan, H.I. Enhanced gas sensing properties of flower-like ZnO nanostructure to acetylene. Mater. Technol. 2014, 30, 96–100. [Google Scholar]
- Shen, Z.; Zhang, X.; Ma, X.; Chen, Y.; Liu, M.; Chen, C.; Ruan, S. Synthesis of hierarchical 3D porous ZnO microspheres decorated by ultra-small Au nanoparticles and its highly enhanced acetylene gas sensing ability. J. Alloys Compd. 2018, 731, 1029–1036. [Google Scholar]
- Lin, Y.; Li, C.; Wei, W.; Li, Y.; Wen, S.; Sun, D.; Chen, Y.; Ruan, S. A new type of acetylene gas sensor based on a hollow heterostructure. RSC Adv. 2015, 5, 61521–61527. [Google Scholar]
- Iftekhar Uddin, A.S.M.; Lee, K.W.; Chung, G.S. Acetylene gas sensing properties of an Ag-loaded hierarchical ZnO nanostructure-decorated reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 216, 33–40. [Google Scholar]
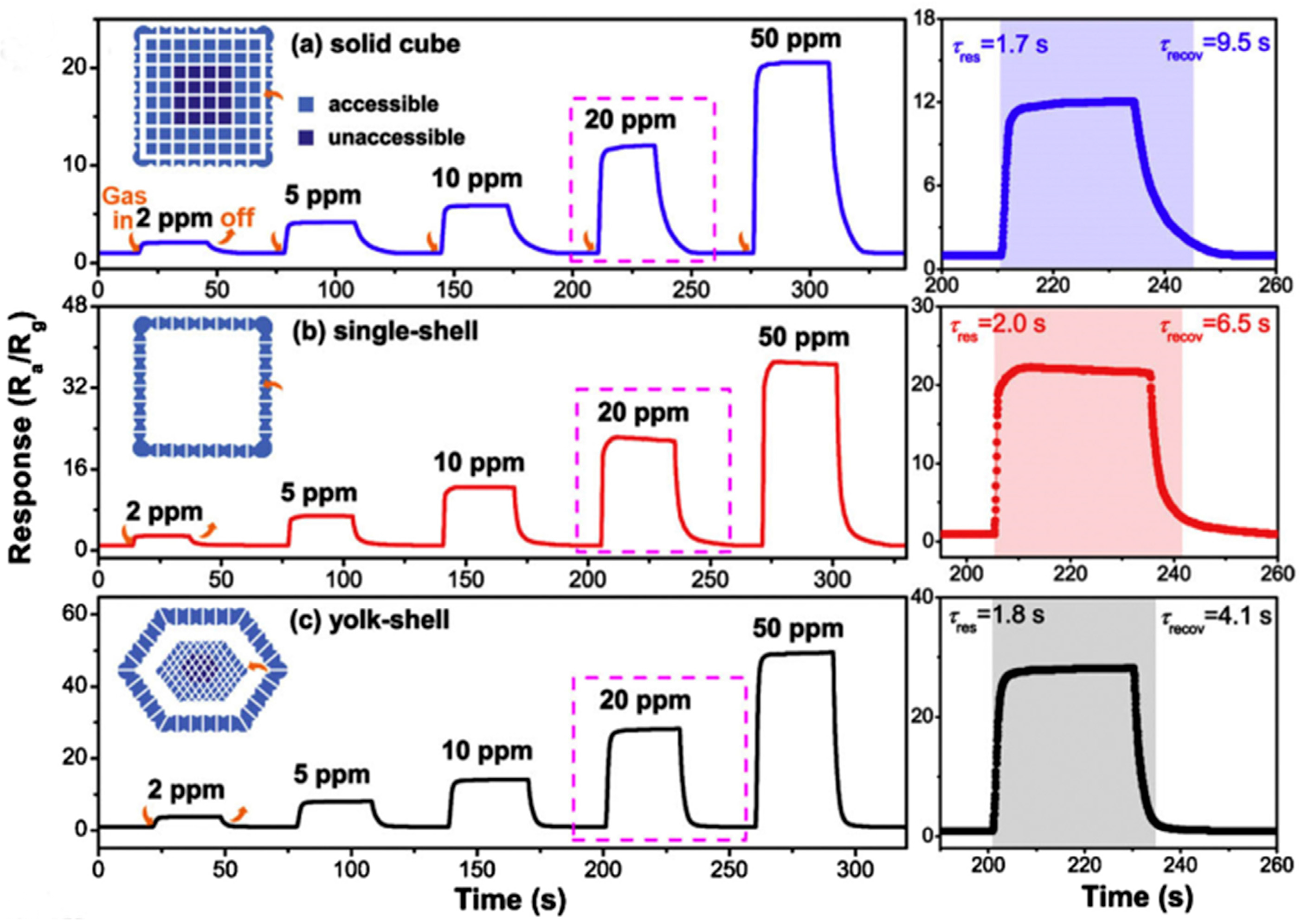
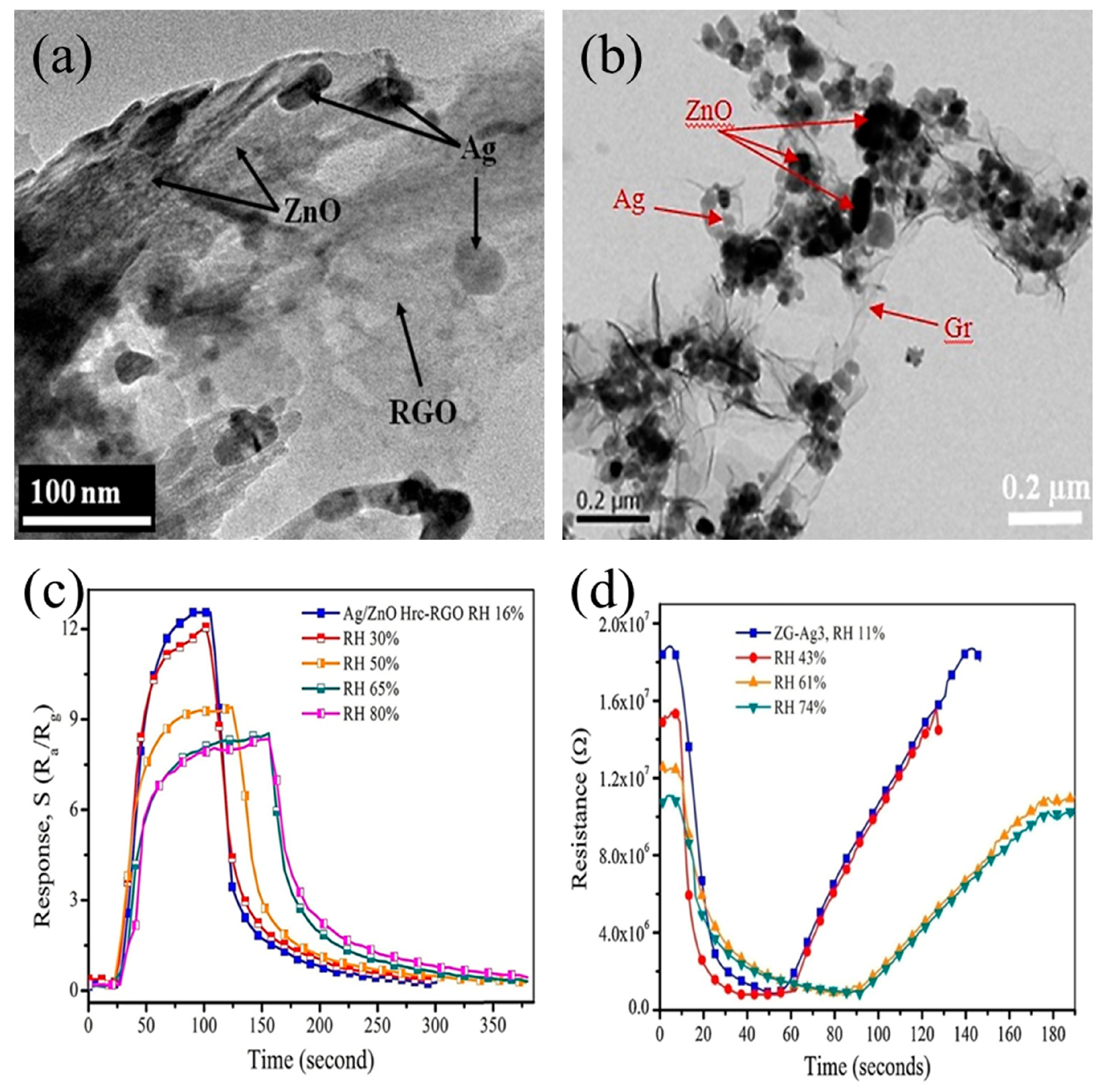
| Gases | Chemical Formula | Formula Weight (g/mol) | IDLH (ppm) | TLV (ppm) |
|---|---|---|---|---|
| Ethanol | C2H5OH | 46.07 | 3300 | 1000 |
| Acetone | CH3COCH3 | 58.08 | 20,000 | 750 |
| Formaldehyde | HCHO | 30.03 | 20 | 0.75 |
| Toluene | C7H8 | 92.14 | 500 | 100 |
| Acetylene | C2H2 | 26.04 | NA | NA |
| Order | Dimensions | Materials | Synthesis Method | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0D | CuBi2O4 powders | polymerized complex method | 1000 | 5 | 400 | 57 | 294 | 10.4 b | [85] |
| 2 | SnO2 nanoparticles | microwave treatment | 250 | NA | 100 | 16 | 25 | 30 c | [86] | |
| 3 | 1D | Co3O4 microrods | interfacial-reaction | 100 | NA | 220 | 0.8 | 10.8 | 9.8 b | [87] |
| 4 | CuO/In2O3 nanorods | thermal evaporation and sputtering | 50 | NA | 300 | 53 | 149 | 3.82 a | [88] | |
| 5 | In2O3 microrods | hydrothermal | 100 | 1 | 300 | 15 | 20 | 18.33 a | [89] | |
| 6 | LaFeO3 nanotubes | electrospinning | 100 | NA | 160 | 2 | 4 | 9.4 b | [90] | |
| 7 | Pd/Fe2O3 nanotubes | electrospinning | 50 | 0.1 | 240 | 8 | 30 | 65.4 a | [91] | |
| 8 | ZnO nanotubes | electrodeposition and electrochemical etching | 700 | 1 | RT | 4.56 min | 1.53 min | 64.17% c | [83] | |
| 9 | LaMnO3/SnO2 nanofibers | electrospinning | 100 | NA | 260 | 6 | 34 | 20 a | [92] | |
| 10 | WO3/SnO2 nanofibers | coaxial electrospinning | 10 | NA | 280 | 18.5 | 282 | 5.09 a | [93] | |
| 11 | ZnO nanowires | solvothermal | 500 | NA | 340 | 6 | 26 | 10.68 a | [94] | |
| 12 | SnO2/Fe2O3 nanowires | VLS, hydrothermal and spin coating | 5 | NA | 300 | 100 | 300 | 3.07 a | [95] | |
| 13 | SnO2/ZnO nanowires | carbon-assisted thermal evaporation | 400 | NA | 400 | NA | NA | 128 c | [96] | |
| 14 | SnO2/ZnO nanowires | thermal evaporation and spray-coating | 100 | NA | 400 | NA | NA | 14.1a | [97] | |
| 15 | 2D | CuO films | RF sputtering | 12.5 | NA | 180 | 31 | 52 | 2.2 b | [98] |
| 16 | Pd/Ce/SnO2 films | co-precipitation | 100 | NA | 250 | 6 | 20 | 88 c | [99] | |
| 17 | NiO nanosheets | hydrothermal | 50 | 1 | 240 | 4 | 7 | 11.15 b | [100] | |
| 18 | ZnO nanosheets | hydrothermal | 50 | 1 | 330 | 15 | 12 | 83.6 a | [101] | |
| 19 | 3D | SnO2 nanoflowers | hydrothermal | 200 | 4.52 | 240 | 10 | 16 | 62.2 a | [102] |
| 20 | SnO2 nanoflowers | hydrothermal | 100 | NA | 300 | 10 | 16 | 47.29 a | [103] | |
| 21 | Au/SnO2 hierarchical structures | hydrothermal | 100 | NA | 340 | 5 | 10 | 18 a | [104] | |
| 22 | NiO hierarchical structures | hydrothermal | 400 | NA | 300 | 4 | 8 | 32 b | [105] | |
| 23 | SnO2 macropores structures | sol-gel method | 500 | NA | 240 | NA | NA | 70.94 a | [106] | |
| 24 | MoO3 microboxes | hydrothermal | 100 | 1 | 260 | 15 | 5 | 78 a | [107] | |
| 25 | SnO2 nanocubes | hydrothermal | 100 | 1 | 200 | 23 | 21 | 1670.5 a | [108] | |
| 26 | ZnSnO3 nanocubes | co-precipitation | 100 | NA | 260 | 4 | 276 | 34.1 a | [109] | |
| 27 | PdO/Zn2SnO4 octahedrons | hydrothermal and wet impregnation treatment | 100 | 0.5 | 250 | 1 | 206 | 82.3 a | [110] | |
| 28 | WO3 urchin-like structures | hydrothermal | 100 | NA | 350 | 28 | 12 | 68.56 a | [111] | |
| 29 | Co3O4 microspheres | interfacial-reaction | 100 | 1 | 220 | 0.1 | 0.7 | 38.2 b | [112] | |
| 30 | CuO microspheres | precipitation | 400 | NA | 250 | 17 | 11.9 | 5.6 b | [113] | |
| 31 | CuO microspheres | hydrothermal | 100 | NA | 200 | 2 | 8 | 390% c | [114] | |
| 32 | Fe2O3/Co3O4 microspheres | solution route | 100 | NA | 170 | 3.3 | 5.4 | 16.1 b | [115] | |
| 33 | SnO2 microspheres | ion exchange method | 200 | NA | 260 | NA | NA | 103.1 a | [116] | |
| 34 | SnO2/Fe2O3 microspheres | hydrothermal | 100 | 0.1 | 260 | 3 | 4 | 41.7 a | [117] | |
| 35 | ZnFe2O4 microspheres | solvothermal | 10 | 0.5 | 180 | 5.1 | 6.5 | 6.85 a | [118] | |
| 36 | Pt/SnO2 nanospheres | spray drying and Kirkendall diffusion | 5 | 0.25 | 325 | 1 | 1577 | 1399.9 a | [119] | |
| 37 | SnO2 nanospheres | precipitation | 200 | NA | 260 | 10 | 8 | 274.5 a | [120] | |
| 38 | SnO2 nanospheres | hydrothermal | 100 | 0.5 | 350 | 5 | NA | 10.5 a | [121] | |
| 39 | SnO2/ZnO nanospheres | self-sacrificial template | 50 | NA | 270 | 0.4 | 235 | 7.5 a | [122] | |
| 40 | ZnSnO3 nanospheres | hydrothermal | 100 | NA | 200 | 4 | 30 | 32 a | [123] | |
| 41 | Zn2SnO4 nanospheres | hydrothermal | 50 | 5 | 180 | NA | NA | 23.4 a | [124] |
| Order | Dimensions | Materials | Synthesis Method | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0D | Au/ZnO nanoparticles | MOF template | 10 | 0.05 | 280 | 15 | 12 | 43 a | [129] |
| 2 | Ce/CoFe2O4 nanocrystallites | molten-salt | 2000 | NA | 200 | 38 | 61 | 157 b | [130] | |
| 3 | Fe2O3 nanoparticles | hard template | 100 | NA | 300 | 6 | NA | 26.3 a | [131] | |
| 4 | Pt/In2O3 nanoparticles | sol-gel | 1.56 | 0.01 | 200 | 25 | 120 | 12 a | [132] | |
| 5 | ZnFe2O4 nanoparticles | hydrothermal | 200 | NA | 200 | NA | NA | 39.5 a | [133] | |
| 6 | LaFeO3 powders | sol-gel method | 10 | NA | 200 | 21 | 6 | 7.83 b | [134] | |
| 7 | 1D | Co3O4 nanochains | solution route | 200 | 5 | 180 | 32 | 35 | 10.5 b | [135] |
| 8 | Fe2O3 nanorods | interfacial-reaction | 100 | NA | 280 | 0.4 | 2.4 | 32.5 a | [136] | |
| 9 | ZnFe2O4 nanorods | hydrothermal | 100 | NA | 260 | 1 | 11 | 52.8 a | [137] | |
| 10 | Fe2O3 nanotubes | electrospinning | 100 | NA | 240 | 9 | 3 | 11 a | [138] | |
| 11 | 2D | Co3O4 nanosheets | fluorine-assisted hydrothermal | 1000 | NA | 111 | NA | NA | 36.5 b | [139] |
| 12 | ZnO nanosheets | precipitation | 200 | 0.81 | 300 | 18.71 | 13.75 | 106.1 a | [78] | |
| 13 | In2O3 films | sparking process | 2000 | NA | 350 | 3 | NA | 117 a | [140] | |
| 14 | ZnO films | sol-gel dip coating | 50 | 2 | RT | 60 | 28 | 490 a | [141] | |
| 15 | ZnO films | e-beam and post-annealing | 100 | 10 | 280 | 6 | 18 | 30 a | [9] | |
| 16 | 3D | Fe2O3 microflowers | hydrothermal | 100 | NA | 220 | 8 | 19 | 52 a | [142] |
| 17 | SnO2 nanoflowers | hydrothermal | 50 | NA | 170 | 3 | 28 | 29.2 a | [143] | |
| 18 | ZnO nanoflowers | hydrothermal | 100 | NA | 300 | 7 | NA | 18.6 a | [144] | |
| 19 | ZnFe2O4/ZnO microflowers | solution route | 50 | 1 | 250 | 2 | 25 | 8.3 a | [145] | |
| 20 | ZnO porous flowers | hydrothermal | 50 | 0.25 | 280 | 2 | 23 | 97.8 a | [146] | |
| 21 | LaFeO3 microspheres | hydrothermal | 100 | 1 | 225 | 5 | 25 | 25.5 b | [147] | |
| 22 | Au/In2O3 nanospheres | hydrothermal | 10 | NA | 320 | 4 | 9 | 53.08 b | [148] | |
| 23 | LaFeO3 microspheres | hydrothermal | 100 | NA | 260 | 9 | 17 | 29 b | [149] | |
| 24 | SnO2/Fe2O3 nanospheres | self-sacrificial template | 100 | NA | 260 | 3 | 6 | 47.1 a | [150] | |
| 25 | ZnFe2O4 nanospheres | solvothermal | 30 | 0.8 | 200 | 9 | 272 | 11.8 a | [151] | |
| 26 | ZnFe2O4 microspheres | solvothermal | 50 | 0. 5 | 200 | NA | NA | 28.3 a | [152] | |
| 27 | ZnFe2O4 microspheres | hydrothermal | 20 | 0.13 | 206 | NA | NA | 13.6 a | [153] | |
| 28 | ZnO/ZnFe2O4 microspheres | self-sacrificial template | 20 | NA | 140 | 5.2 | 12.8 | 5.9 a | [154] | |
| 29 | SnO2 hierarchical structures | hydrothermal | 100 | 0.05 | 325 | NA | NA | 175 a | [155] | |
| 30 | ZnO macro-mesoporous structures | layer-by-layer filtration deposition | 100 | NA | 260 | 13 | 50 | 137 a | [156] | |
| 31 | ZnO urchin-like structures | hydrothermal | 10 | NA | 300 | 42 | 10 | 58.1 a | [157] | |
| 32 | ZnO/ZnFe2O4 microcubes | MOF template | 5 | 0.5 | 250 | 5.6 min | 6.0 min | 9.4 a | [158] | |
| 33 | ZnFe2O4 octahedral nanocages | thermal decomposition | 200 | NA | 120 | NA | NA | 64.4 a | [159] | |
| 34 | ZnO/ZnFe2O4 nanocages | self-sacrificial template | 100 | 1 | 290 | 8 | 32 | 25.8 a | [160] |
| Order | Dimensions | Materials | Synthesis Method | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0D | In2O3 particles | hydrothermal | 50 | NA | 130 | 37 | 48 | 86 a | [163] |
| 2 | In2O3 nanoparticles | hydrothermal | 10 | NA | 280 | 4 | 8 | 20 a | [164] | |
| 3 | In2O3 nanoparticles | hydrothermal | 100 | NA | 230 | 100 | 70 | 80 a | [165] | |
| 4 | NiO nanoparticles | electrodeposition | 1 | NA | 230 | 53.7 | 13.3 | 3.43 b | [166] | |
| 5 | 1D | NiO nanochains | electrospinning | 50 | 1 | 210 | 1 | 10 | NA | [167] |
| 6 | SnO2 microtubes | solvothermal | 100 | 0.01 | 92 | NA | NA | 26.2 a | [168] | |
| 7 | 2D | ZnO nanosheets | hydrothermal | 50 | NA | 350 | 9 | 11 | 37.8 a | [169] |
| 8 | 3D | CeO2 polyhedron | hydrothermal | 150 | 1 | 220 | 21 | 16 | 172 a | [170] |
| 9 | In2O3 polyhedrons | solution route | 20 | NA | 240 | 1 | 2 | 8.2 a | [171] | |
| 10 | SnO2 cedar-like structures | hydrothermal | 100 | 1 | 200 | 1 | 13 | 13.3 a | [172] | |
| 11 | Au/In2O3 nanocubes | hydrothermal | 100 | NA | 240 | 3 | 8 | 37 b | [173] | |
| 12 | ZnSnO3 multishelled cubes | coprecipitation and alkaline etching route | 100 | NA | 220 | 1 | 59 | 37.2 a | [174] | |
| 13 | NiO nanotetrahedra | solvothermal | 50 | NA | 250 | NA | NA | 11.6 b | [175] | |
| 14 | In2O3 hierarchical structures | solution route | 100 | 1 | 260 | 1 | 8 | 8.6 a | [176] | |
| 15 | In2O3 microflowers | hydrothermal/solvothermal | 50 | 0.5 | 190 | 25 | 65 | 90 a | [177] | |
| 16 | NiO microflowers | solvothermal | 100 | NA | 200 | 30 | 56 | 3.5 b | [178] | |
| 17 | CuO microspheres | hydrothermal | 100 | NA | 300 | 26 | 28 | 3.2 b | [179] | |
| 18 | In2O3 microspheres | thermal decomposition | 50 | 10 | 175 | 25 | 104 | 30 a | [180] | |
| 19 | In2O3 microspheres | self-sacrificial template | 100 | NA | 300 | 21 | 7 | 40.9 a | [181] | |
| 20 | SnO2 microspheres | hydrothermal | 200 | 1 | 300 | NA | NA | 9 a | [182] |
| Order | Dimensions | Materials | Synthesis Method | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0D | Au/ZnO nanoparticles | coprecipitation | 100 | NA | 377 | NA | 6 min | 92 a | [184] |
| 2 | 1D | Pd/SnO2 nanofibers | electrospinning and carbonization | 100 | 0.5 | 250 | 3 | 35 | 24.6 a | [185] |
| 3 | Pd/WO3 nanofibers | electrospinning | 1 | 0.02 | 350 | 10.9 | 16.1 | 5.5 b | [186] | |
| 4 | Pt/SnO2/ZnO nanowires | vapor-liquid-solid, atomic layer deposition and γ-ray radiolysis | 0.1 | NA | 300 | NA | NA | 279 a | [187] | |
| 5 | TiO2 nanotubes | hydrothermal | 50 | NA | 500 | 110–130 | 800–1150 | 3 a | [188] | |
| 6 | ZnO nanowires | electrospinning and hydrothermal | 100 | 1 | 240 | 9 | 4 | 12.7 a | [189] | |
| 7 | Fe2O3/NiO nanotubes | hydrothermal | 5 | 0.5 | 275 | 10 | 24 | 8.8 b | [190] | |
| 8 | SnO2/Fe2O3 nanotubes | electrospinning | 50 | 0.05 | 260 | 5 | 11 | 25.3 a | [191] | |
| 9 | Fe2O3/SnO2 nanowires | ultrasonic spray pyrolysis and hydrothermal | 100 | NA | 90 | 20 | 15 | 49.7% c | [192] | |
| 10 | Co3O4 nanorods | solvothermal | 200 | NA | 200 | 90 | 55 | 35 b | [193] | |
| 11 | MoO3/Fe2(MoO4)3 nanobelts | hydrothermal | 50 | NA | 250 | NA | NA | 5.3 a | [194] | |
| 12 | 3D | Pd/SnO2 microspheres | hydrothermal | 20 | 0.1 | 230 | 0.48 | 5.5 | 52.9 a | [195] |
| 13 | In2O3 microspheres | hydrothermal | 50 | 0.5 | 350 | 12 | 25 | 85% c | [196] | |
| 14 | Au/TiO2 pecan-kernel-like structures | hydrothermal and precipitation | 100 | NA | 375 | 4 | 5 | 7.3 a | [197] | |
| 15 | Pd/SnO2 cubic nanocages | self-sacrificial template and precipitation | 20 | 0.1 | 230 | 0.4 | 16.5 | 41.4 a | [198] | |
| 16 | SnO2 nanocages | self-sacrificial template | 20 | NA | 250 | 2.3 | 5.8 | 33.4 a | [199] | |
| 17 | PdO/ZnO nanoflowers | hydrothermal | 100 | NA | 240 | 1 | 9 | 10.9 a | [200] | |
| 18 | SnO2/SnO2 cuboctahedra | anneal-etching | 20 | NA | 250 | 1.8 | 4.1 | 28.6 a | [201] | |
| 19 | ZnFe2O4 nanospheres | nonaqueous route | 100 | 1 | 300 | 18.14 | 29.2 | 9.98 a | [202] |
| Order | Dimensions | Materials | Synthesis Method | Conc. (ppm) | LOD (ppm) | Temp. (°C) | τres (s) | τrec (s) | Resp. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0D | Pt/ZnO nanoparticles | flame spray pyrolysis | 10000 | NA | 300 | 6 | 60 | 836 a | [206] |
| 2 | Ag/ZnO/RGO hybrid nanostructures | chemical route | 100 | 1 | 150 | 25 | 80 | 21.2 a | [207] | |
| 3 | 1D | Sm2O3/SnO2 nanorods | hydrothermal | 100 | NA | 260 | 8 | 9 | 50.5 a | [208] |
| 4 | WO3 nanowires | hydrothermal | 200 | NA | 300 | 6 | 7 | 58 a | [209] | |
| 5 | Au/ZnO/In2O3 belt-tooth | chemical vapor deposition | 100 | NA | 90 | 8.5 | NA | 5 a | [210] | |
| 6 | 2D | ZnO nanosheets | hydrothermal | 100 | 1 | 400 | 11 | 5 | 101.1 a | [205] |
| 7 | Au/ZnO nanorings | hydrothermal and deposition | 100 | 0.5 | 255 | 4 | 3 | 28 a | [211] | |
| 8 | 3D | ZnO nanoflowers | hydrothermal | 200μL/L | NA | 375 | 8 | 11 | 48.2 a | [212] |
| 9 | Au/ZnO microspheres | hydrothermal and precipitation | 100 | 1 | 183.5 | 8 | 2 | 311.3 a | [213] | |
| 10 | NiO/SnO2 hierarchical structures | hydrothermal | 100 | 1 | 206 | 2 | 5 | 13.8 a | [214] | |
| 11 | Ag/ZnO/RGO hierarchical structures | photochemical | 100 | 3 | 200 | 57 | 90 | 12.3 a | [215] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. https://doi.org/10.3390/s19020233
Lin T, Lv X, Hu Z, Xu A, Feng C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors. 2019; 19(2):233. https://doi.org/10.3390/s19020233
Chicago/Turabian StyleLin, Tingting, Xin Lv, Zhineng Hu, Aoshu Xu, and Caihui Feng. 2019. "Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds" Sensors 19, no. 2: 233. https://doi.org/10.3390/s19020233
APA StyleLin, T., Lv, X., Hu, Z., Xu, A., & Feng, C. (2019). Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors, 19(2), 233. https://doi.org/10.3390/s19020233





