A Fluorescence Inner-Filter Effect Based Sensing Platform for Turn-On Detection of Glutathione in Human Serum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of Bulk δ-MnO2 and MnO2 NSs
2.3. Preparation of MoS2 QDs
2.4. Detection of GSH
2.5. Analysis of GSH in Human Serum Samples
3. Results and Discussion
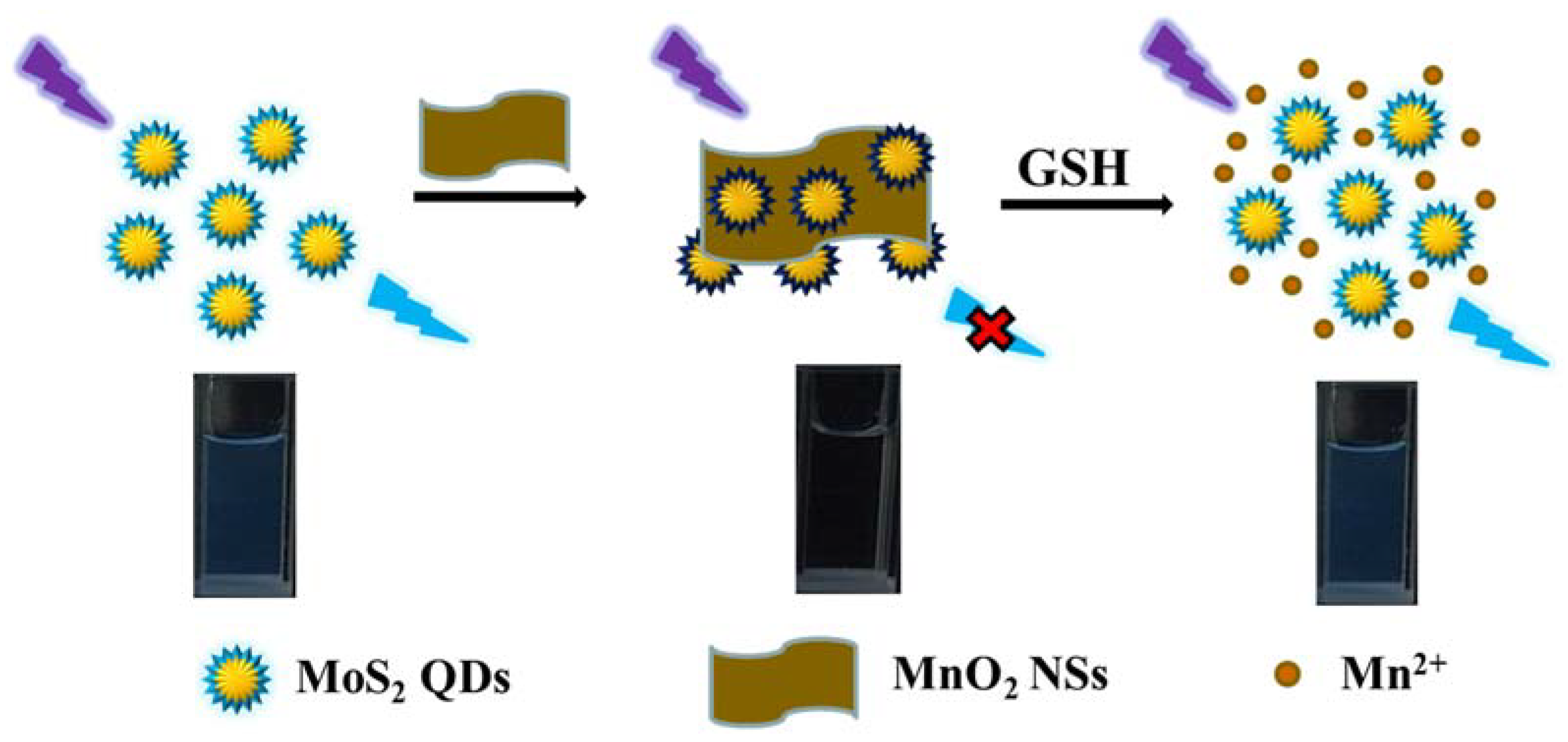
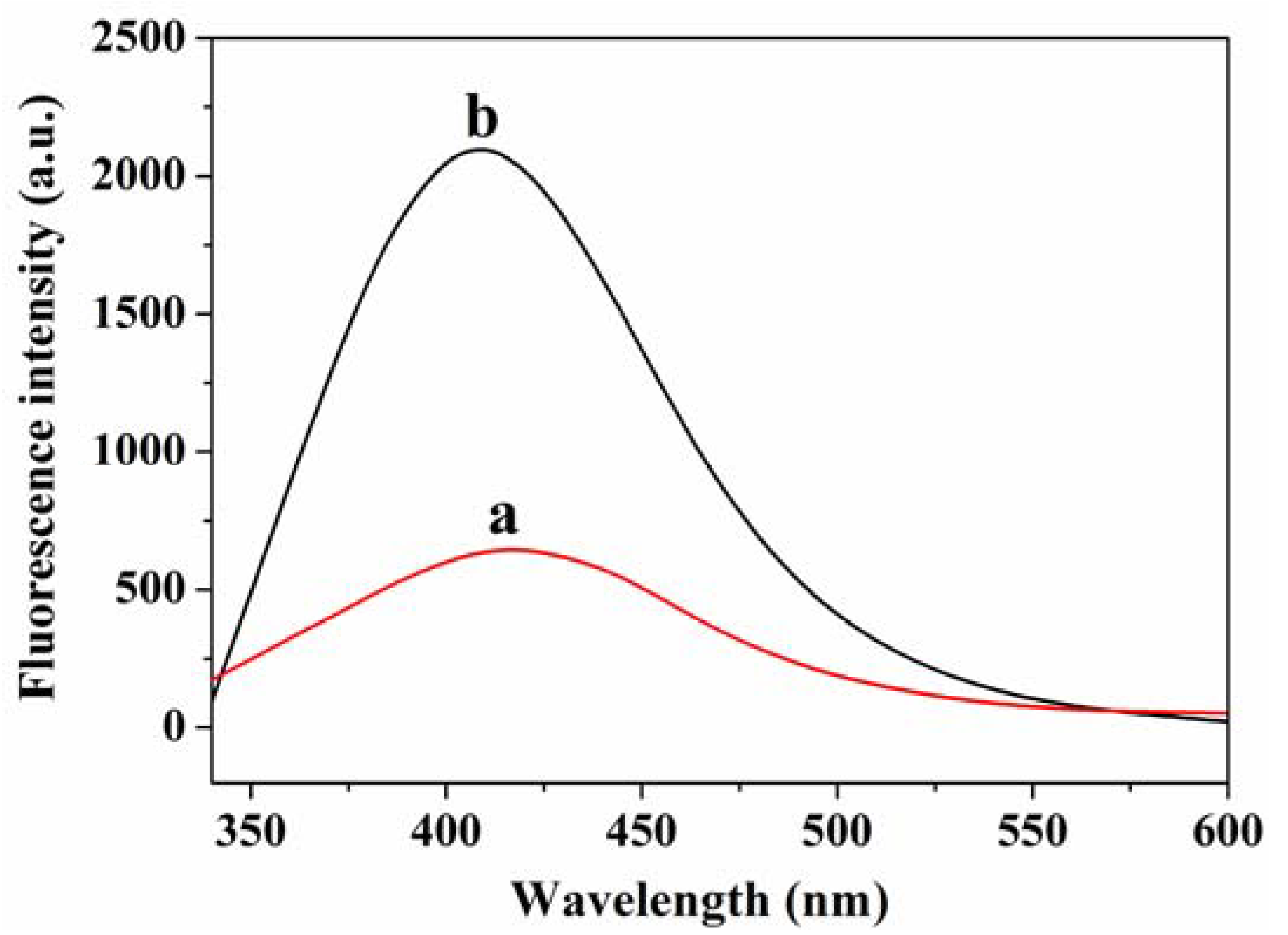
3.1. Fluorescence Sensing Principle of GSH
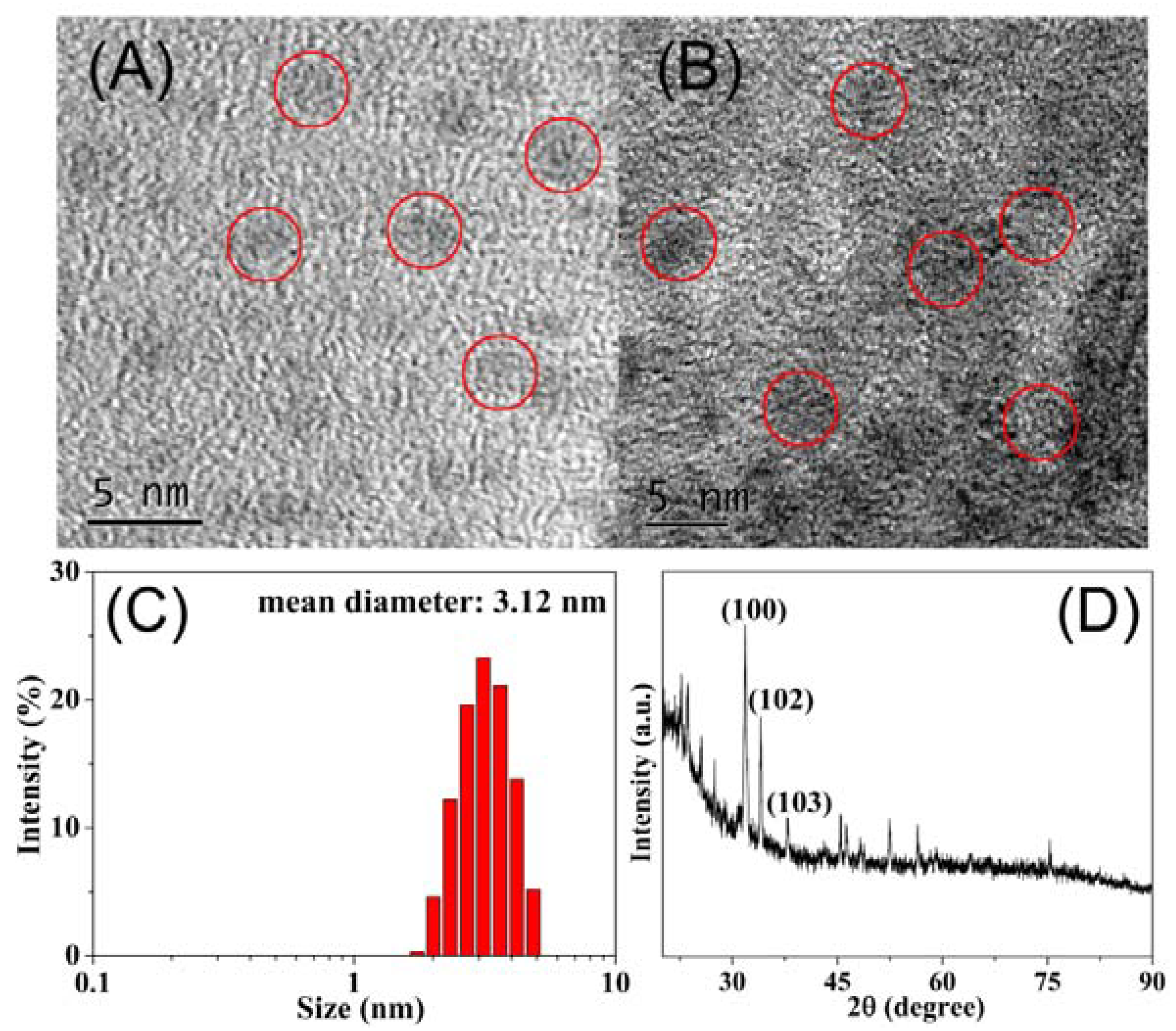
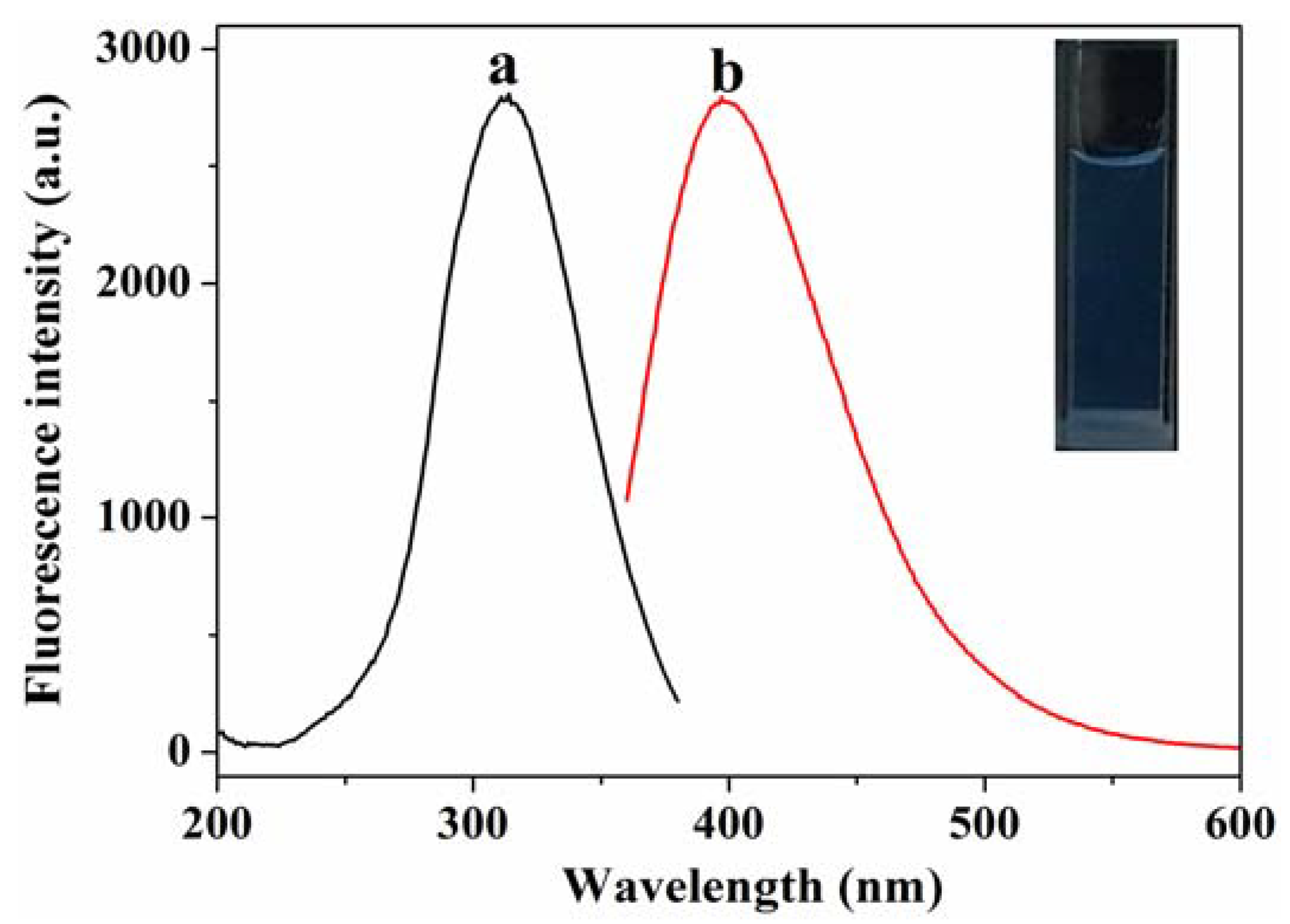
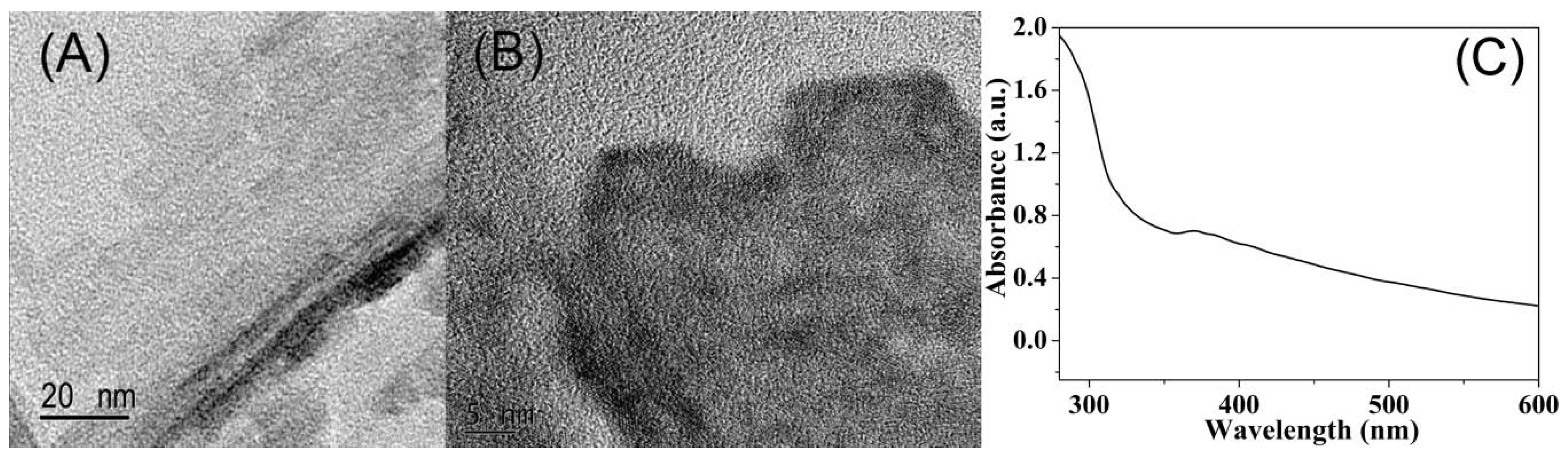
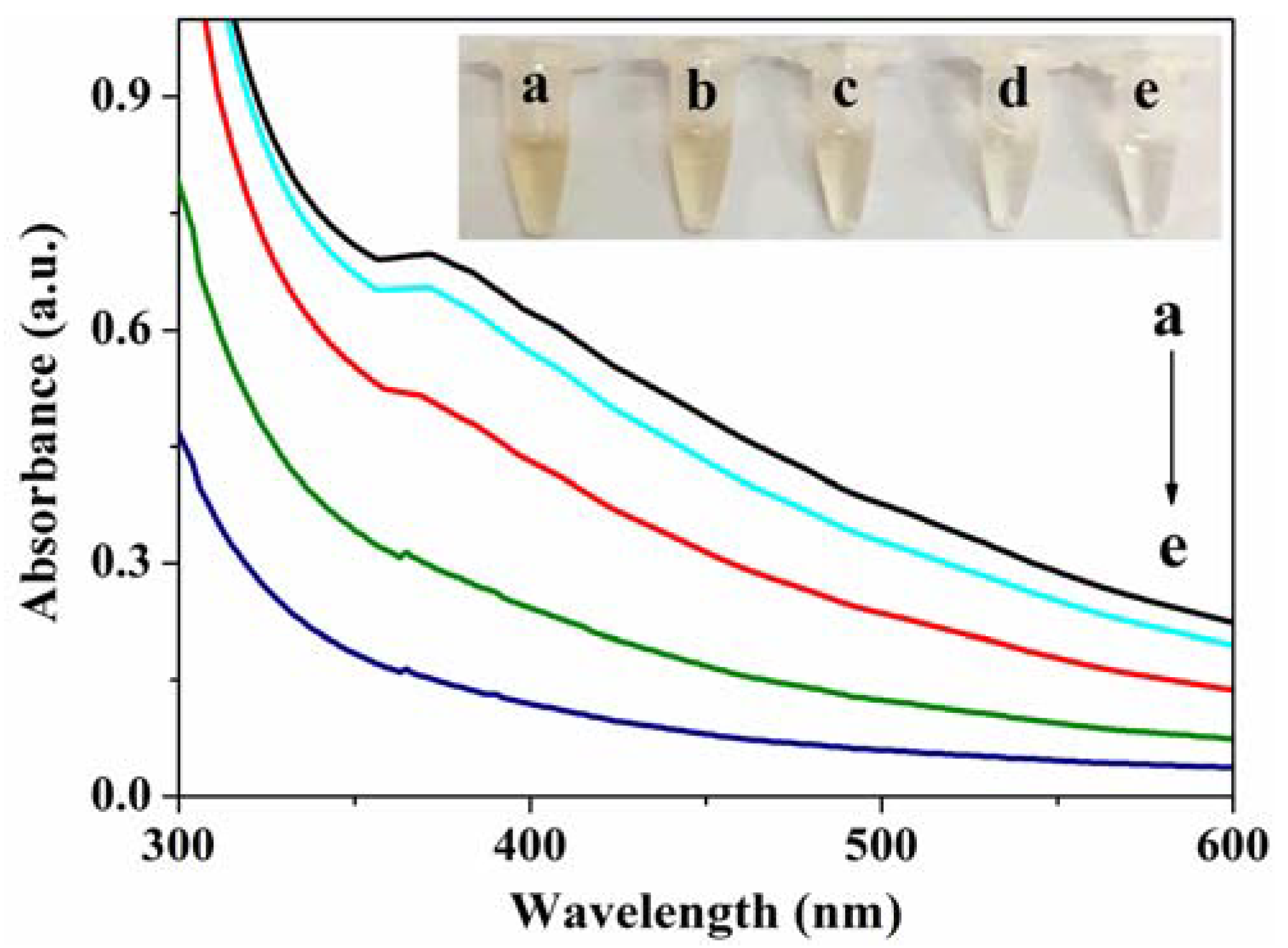
3.2. Characterization of MoS2 QDs and MnO2 NSs
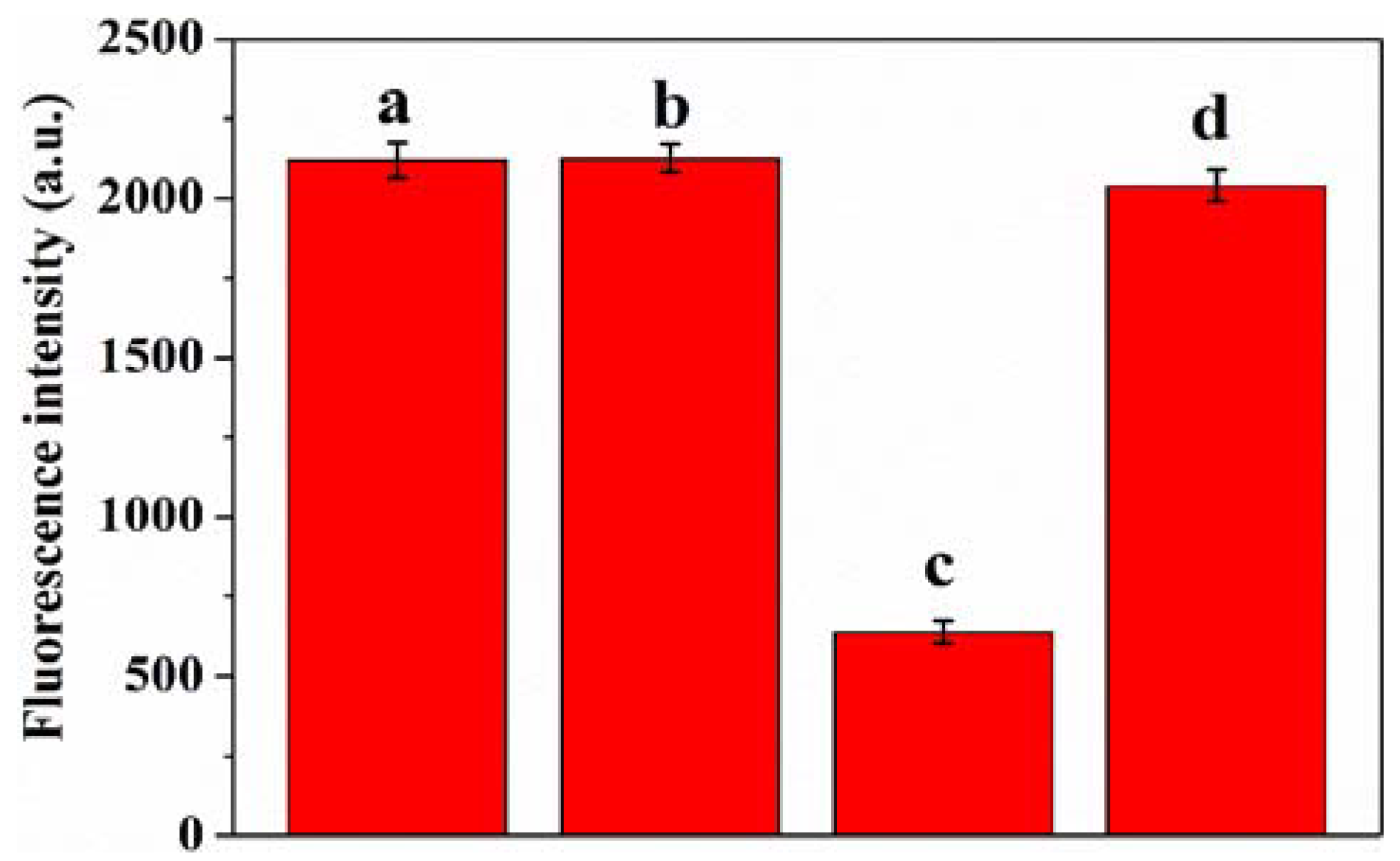
3.3. Feasibility Analysis of GSH Detection
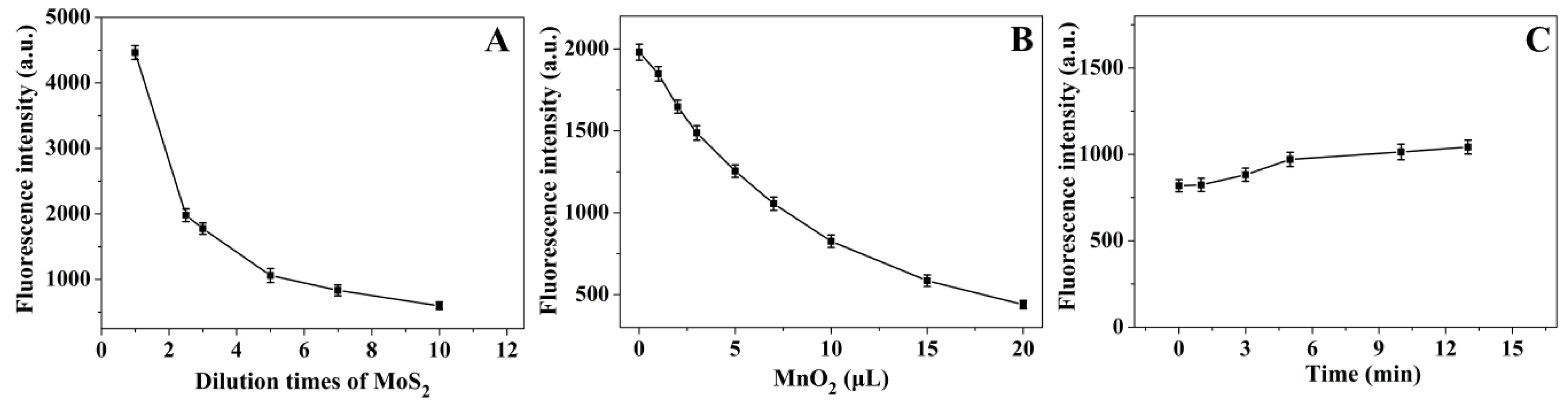
3.4. Optimization of Experimental Conditions
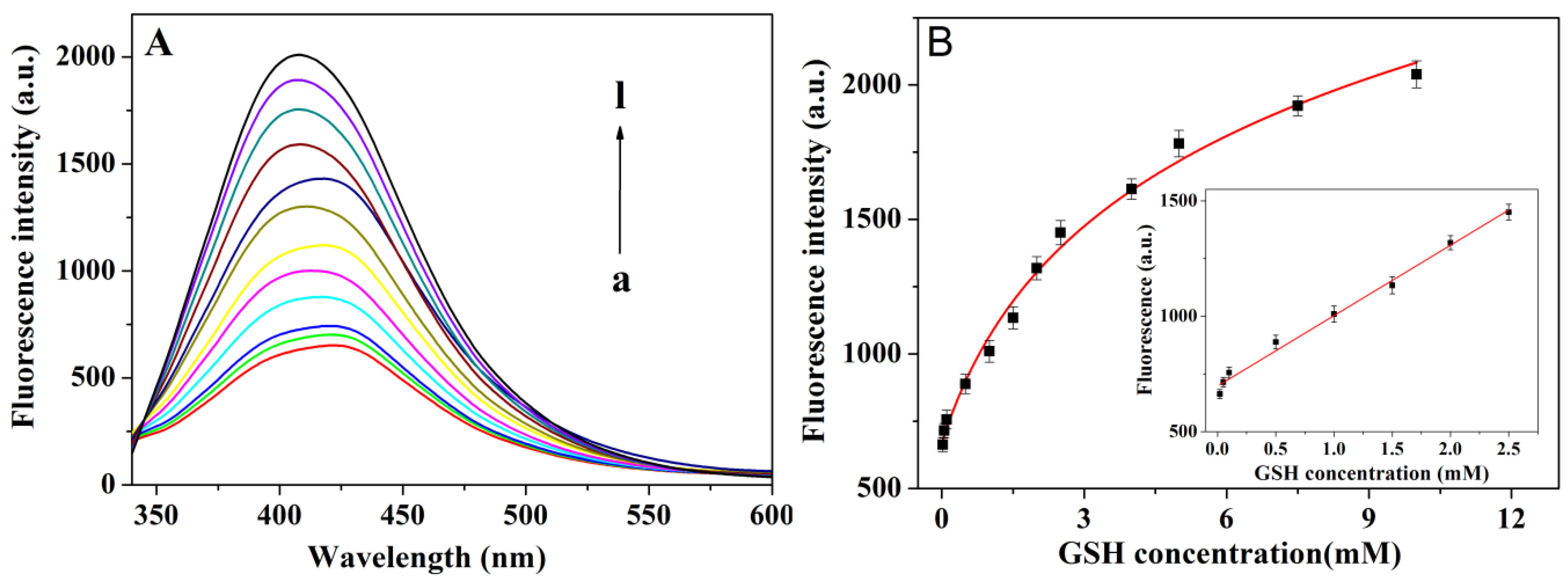
3.5. GSH Detection Sensitivity
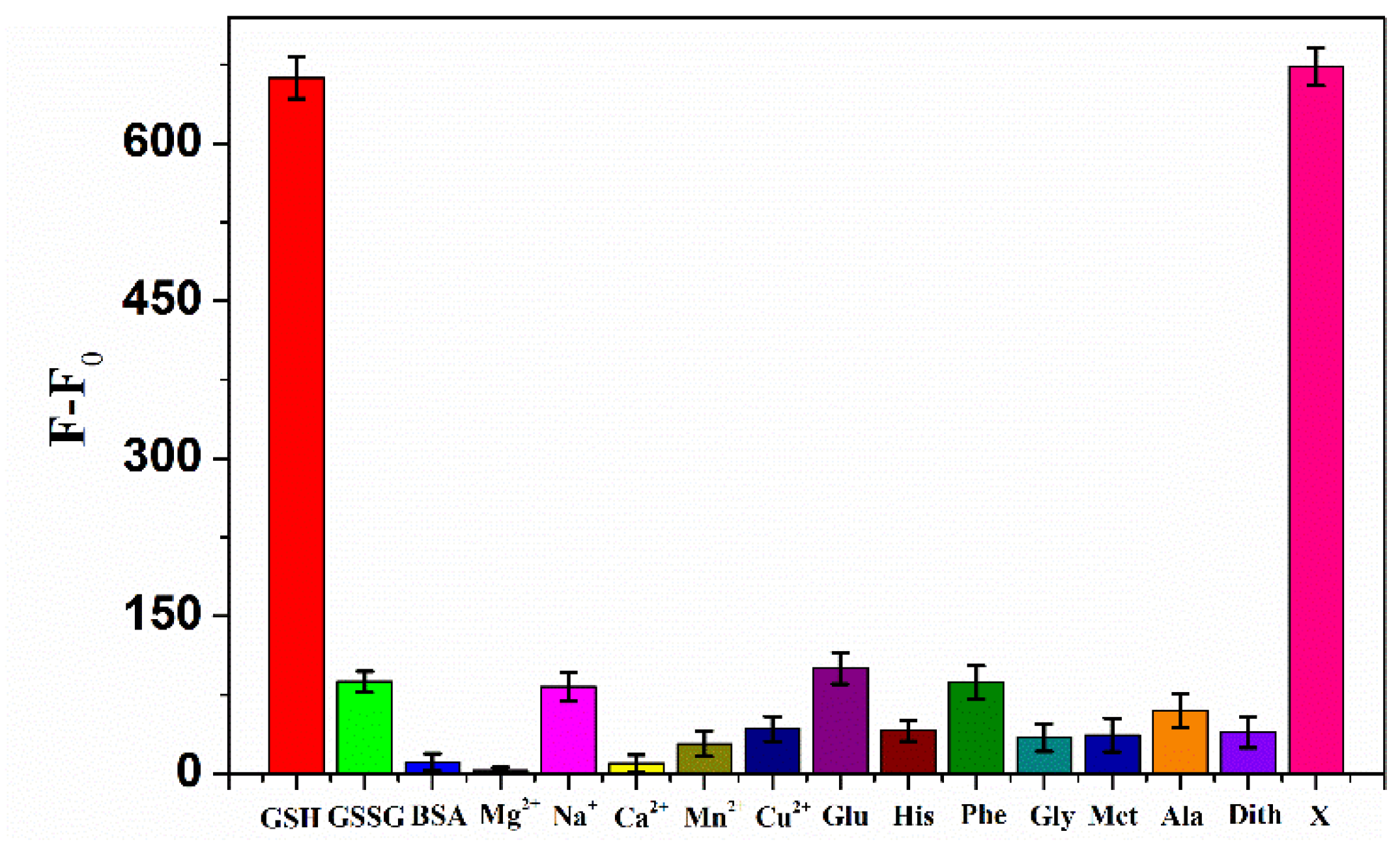
3.6. Interference Detection Results
3.7. GSH Detection in Human Serum
3.8. Reaction Mechanism Discussion
3.9. Stability Investigation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Pocernich, C.B.; Butterfield, D.A. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim. Biophys. Acta 2012, 1822, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, H.; Sun, Y.S.; Qu, B. A disulfide bound-molecular beacon as a fluorescent probe for the detection of reduced glutathione and its application in cells. Chem. Commun. 2012, 48, 3221–3223. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.; Haroune, N.; Williams, L.; Gronow, M. Determination of cellular glutathione: Glutathione disulfide ratio in prostate cancer cells by high performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2016, 1437, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chang, H.-T. Analysis of adenosine triphosphate and glutathione through gold nanoparticles assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2007, 79, 4852–4859. [Google Scholar] [CrossRef]
- Saha, A.; Jana, N.R. Detection of cellular glutathione and oxidized glutathione using magnetic–plasmonic nanocomposite-based “Turn-Off” surface enhanced raman scattering. Anal. Chem. 2013, 85, 9221–9228. [Google Scholar] [CrossRef]
- Wawegama, N.K.; Browning, G.F.; Kanci, A.; Marenda, M.S.; Markham, P.F. Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle. Clin. Vaccine Immunol. 2014, 21, 196–202. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, G.; Xu, L.; Li, B.; Cai, Q.; Jiang, H.; Zhou, X. Facile and controllable one-step fabrication of molecularly imprinted polymer membrane by magnetic field directed self-assembly for electrochemical sensing of glutathione. Anal. Chim. Acta 2015, 886, 37–47. [Google Scholar] [CrossRef]
- Yoon, S.A.; Kim, W.; Sharma, A.; Verwilst, P.; Won, M.; Lee, M.H. A fluorescent Cy7-mercaptopyridine for the selective detection of glutathione over homocysteine and cysteine. Sensors 2018, 18, 2897. [Google Scholar] [CrossRef]
- Niu, L.Y.; Guan, Y.S.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Chen, Y.Z.; Zheng, H.R.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Design strategies of fluorescent probes for selective detection among biothiols. Chem. Soc. Rev. 2015, 44, 6143–6160. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hepel, M. “Molecular beacon”-based fluorescent assay for selective detection of glutathione and cysteine. Anal. Chem. 2011, 83, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zheng, Z.; Yang, J.; Wu, Y.; Lu, F.; Chen, Y.; Gao, W. A novel and sensitive fluorescence sensor for glutathione detection by controlling the surface passivation degree of carbon quantum dots. Talanta 2017, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Meng, H.; Zhang, L.; Ji, D.; Liu, J.; Yu, F.; Qu, L.; Li, Z. A facile fluorescence lateral flow biosensor for glutathione detection based on quantum dots-MnO2 nanocomposites. Sens. Actuators B Chem. 2018, 260, 770–777. [Google Scholar]
- Dai, C.; Yang, C.; Yan, X. Self-quenched gold nanoclusters for turn-on fluorescence imaging of intracellular glutathione. Nano Res. 2018, 11, 2488–2497. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Zhou, X.; Hu, J. A label-free turn-on-off fluorescent sensor for the sensitive detection of cysteine via blocking the Ag+-enhancing glutathione-capped gold nanoclusters. Talanta 2018, 179, 742–752. [Google Scholar] [CrossRef]
- Iqbal, A.; Iqbal, K.; Xu, L.; Li, B.; Gong, D.; Liu, X.; Guo, Y.; Liu, W.; Qin, W.; Guo, H. Heterogeneous synthesis of nitrogen-doped carbon dots prepared via anhydrous citric acid and melamine for selective and sensitive turn on-off-on detection of Hg (II), glutathione and its cellular imaging. Sens. Actuators B Chem. 2018, 255, 1130–1138. [Google Scholar]
- Wu, D.; Li, G.; Chen, X.; Qiu, N.; Shi, X.; Chen, G.; Sun, Z.; You, J.; Wu, Y. Fluorometric determination and imaging of glutathione based on a thiol-triggered inner filter effect on the fluorescence of carbon dots. Microchim. Acta 2017, 184, 1923–1931. [Google Scholar] [CrossRef]
- Gogoi, S.; Khan, R. NIR upconversion characteristics of carbon dots for selective detection of glutathione. New J. Chem. 2018, 42, 6399–6407. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem. Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Chen, L. Switch-on fluorescent strategy based on crystal violet-functionalized CdTe quantum dots for detecting L-cysteine and glutathione in water and urine. Anal. Bioanal. Chem. 2017, 409, 6081–6090. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Qian, Z.; Xia, Y.; Zhu, C. Gold nanocluster-based fluorescent probes for near-infrared and turn-on sensing of glutathione in living cells. Langmuir 2012, 28, 3945–3951. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yan, Y.; Cao, X.; Zhang, C.; Ding, C.; Xian, Y. A facile and one-step ethanol-thermal synthesis of MoS2 quantum dots for two-photon fluorescence imaging. J. Mater. Chem. B 2016, 4, 27–31. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, A.Y.; Huang, D.; Chai, Y.Q.; Zhuo, Y.; Yuan, R. MoS2 quantum dots as new electrochemiluminescence emitters for ultrasensitive bioanalysis of lipopolysaccharide. Anal. Chem. 2017, 89, 8335–8342. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Y.; Wang, F.; Wang, H.; An, Y.; Gao, Z.; Xu, F.; Jiang, K. Oxygen-incorporated few-layer MoS2 vertically aligned on three-dimensional graphene matrix for enhanced catalytic performances in quantum dot sensitized solar cells. Carbon 2017, 123, 756–766. [Google Scholar] [CrossRef]
- Yuan, P.; Walt, D.R. Calculation for fluorescence modulation by absorbing species and its application to measurements using optical fibers. Anal. Chem. 1987, 59, 2391–2394. [Google Scholar] [CrossRef]
- Shao, N.; Zhang, Y.; Cheung, S.M.; Yang, R.H.; Chan, W.H.; Mo, T.; Li, K.; Liu, F. Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative. Anal. Chem. 2005, 77, 7294–7303. [Google Scholar] [CrossRef]
- Zhai, W.Y.; Wang, C.X.; Yu, P.; Wang, Y.X.; Mao, L.Q. Single-layer MnO2 nanosheets suppressed fluorescence of 7-hydroxycoumarin: Mechanistic study and application for sensitive sensing of ascorbic acid in vivo. Anal. Chem. 2014, 86, 12206–12213. [Google Scholar] [CrossRef]
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Jing, X.; Liu, X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens. Bioelectron. 2017, 90, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Yoshida, Y.; Kageyama, H.; Saito, G.; Ishigaki, T.; Yu, F.; Kawamata, J. Room-temperature synthesis of manganese oxide monosheets. J. Am. Chem. Soc. 2008, 130, 15938–15943. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.J.; Zhang, S.; Liu, S.; Cai, Y.; Low, M.; Teng, C.P.; Phang, I.Y.; Cheng, Y.; Duei, K.L.; Srinivasan, B.M.; et al. Protein induces layer-by-layer exfoliation of transition metal dichalcogenides. J. Am. Chem. Soc. 2015, 137, 6152–6155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, Y. Molybdenum disulfide quantum dots as a photoluminescence sensing platform for 2,4,6-trinitrophenol detection. Anal. Chem. 2014, 86, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Zheng, C.; Guo, S.-S.; Li, J.; Yang, H.-H.; Chen, G. Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet–MnO2 sandwich nanocomposite. Anal. Chem. 2014, 86, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Xie, X.; Vendrell, M.; Chang, Y.T.; Liu, X. Intracellular glutathione detection using MnO2-nanosheet-modified upconversion nanoparticles. J. Am. Chem. Soc. 2011, 133, 20168–20171. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, X.; Chai, R.; Xing, C.; Li, H.; Yin, X. A magnetic/fluorometric bimodal sensor based on carbon dots MnO2 platform for glutathione detection. Nanoscale 2016, 8, 13414–13421. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, X.; Lin, X.; Zheng, Y.; Wu, Y.; Chen, P.; Weng, S.; Lin, L.; Lin, X. Signal-on fluorescent sensor based on GQDs–MnO2 composite for glutathione. Anal. Methods 2016, 8, 2366–2374. [Google Scholar] [CrossRef]
- Feng, J.; Huang, P.; Shi, S.; Deng, K.; Wu, F. Colorimetric detection of glutathione in cells based on peroxidase-like activity of gold nanoclusters: A promising powerful tool for identifying cancer cells. Anal. Chim. Acta 2017, 967, 64–69. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Tang, L.; Chang, H.; Li, J. Graphene oxide amplified electrogenerated chemiluminescence of quantum dots and its selective sensing for glutathione from thiol-containing compounds. Anal. Chem. 2009, 81, 9710–9715. [Google Scholar] [CrossRef]
- Michelet, F.; Gueguen, R.; Leroy, P.; Wellman, M.; Nicolas, A.; Siest, G. Blood and plasma glutathione measured in healthy subjects by HPLC: Relation to sex, aging, biological variables, and life habits. Clin. Chem. 1995, 41, 1509–1517. [Google Scholar] [PubMed]
- Yu, F.; Li, P.; Wang, B.; Han, K. Reversible near-infrared fluorescent probe introducing tellurium to mimetic glutathione peroxidase for monitoring the redox cycles between peroxynitrite and glutathione in vivo. J. Am. Chem. Soc. 2013, 135, 7674–7680. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, S.; Shu, F.; Liu, Z. An MnO2 nanosheet as a label-free nanoplatform for homogeneous biosensing. Chem. Commun. 2014, 50, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
| Methods | Probes | Linear Range | LOD | References |
|---|---|---|---|---|
| Fluorescence | MnO2-UCNPs | - | 0.9 μM | [36] |
| Fluorescence | CdSe@ZnS QDs-MnO2 | 0.05~1.5 mM | 16.3 μM | [16] |
| Fluorescence | CDs-MnO2 NPs | 1~200 μM | 0.6 μM | [37] |
| Fluorescence | GQDs–MnO2 composite | 1~1000 μM | 0.45 μM | [38] |
| Colorimetric | MnO2NSs-TMB | 1~25 μM | 0.3 μM | [31] |
| Colorimetric | GSH-AuNCs-TMB | 2~25 μM | 0.42 μM | [39] |
| Electrochemistry | CdTe QDs | 24~214 μM | 8.3 μM | [40] |
| Fluorescence | MoS2 QDs-MnO2 NSs | 20~2500 μM | 1.0 μM | This work |
| Sample | Add/mM | Found/mM | Recovery/% | RSD/% |
|---|---|---|---|---|
| 1 | 0 | 0.193 | - | 9.6 |
| 2 | 0.1 | 0.104 | 104 | 2.1 |
| 3 | 1.0 | 0.992 | 99.2 | 2.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; You, X.; Fang, Q.; Li, X.; Li, G.; Chen, J.; Chen, W. A Fluorescence Inner-Filter Effect Based Sensing Platform for Turn-On Detection of Glutathione in Human Serum. Sensors 2019, 19, 228. https://doi.org/10.3390/s19020228
Tang S, You X, Fang Q, Li X, Li G, Chen J, Chen W. A Fluorescence Inner-Filter Effect Based Sensing Platform for Turn-On Detection of Glutathione in Human Serum. Sensors. 2019; 19(2):228. https://doi.org/10.3390/s19020228
Chicago/Turabian StyleTang, Shurong, Xiuhua You, Quanhui Fang, Xin Li, Guangwen Li, Jinghua Chen, and Wei Chen. 2019. "A Fluorescence Inner-Filter Effect Based Sensing Platform for Turn-On Detection of Glutathione in Human Serum" Sensors 19, no. 2: 228. https://doi.org/10.3390/s19020228
APA StyleTang, S., You, X., Fang, Q., Li, X., Li, G., Chen, J., & Chen, W. (2019). A Fluorescence Inner-Filter Effect Based Sensing Platform for Turn-On Detection of Glutathione in Human Serum. Sensors, 19(2), 228. https://doi.org/10.3390/s19020228





