Non-Invasive Device for Blood Pressure Wave Acquisition by Means of Mechanical Transducer
Abstract
:1. Introduction
2. Materials and Methods
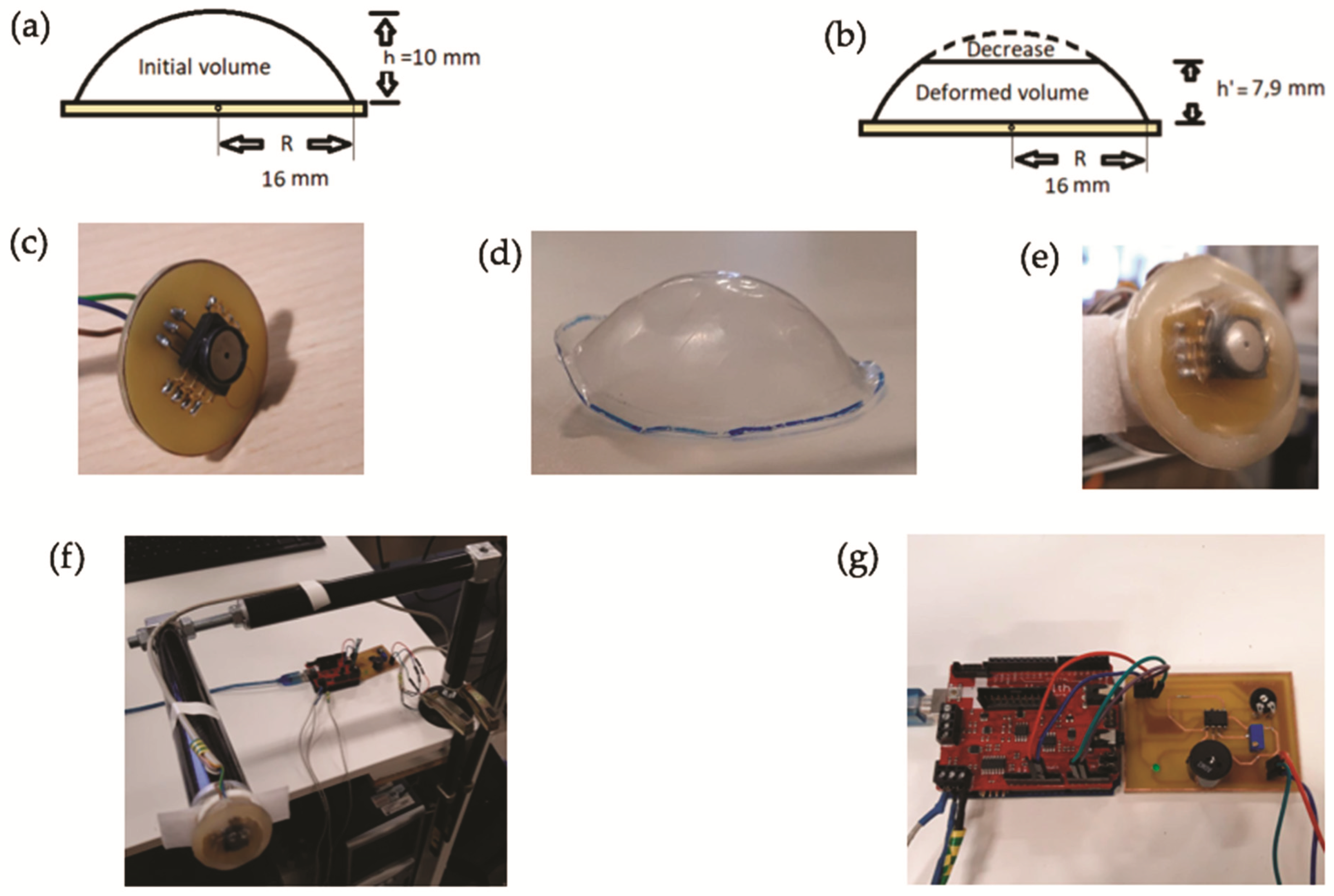
2.1. Device Description and Implementation
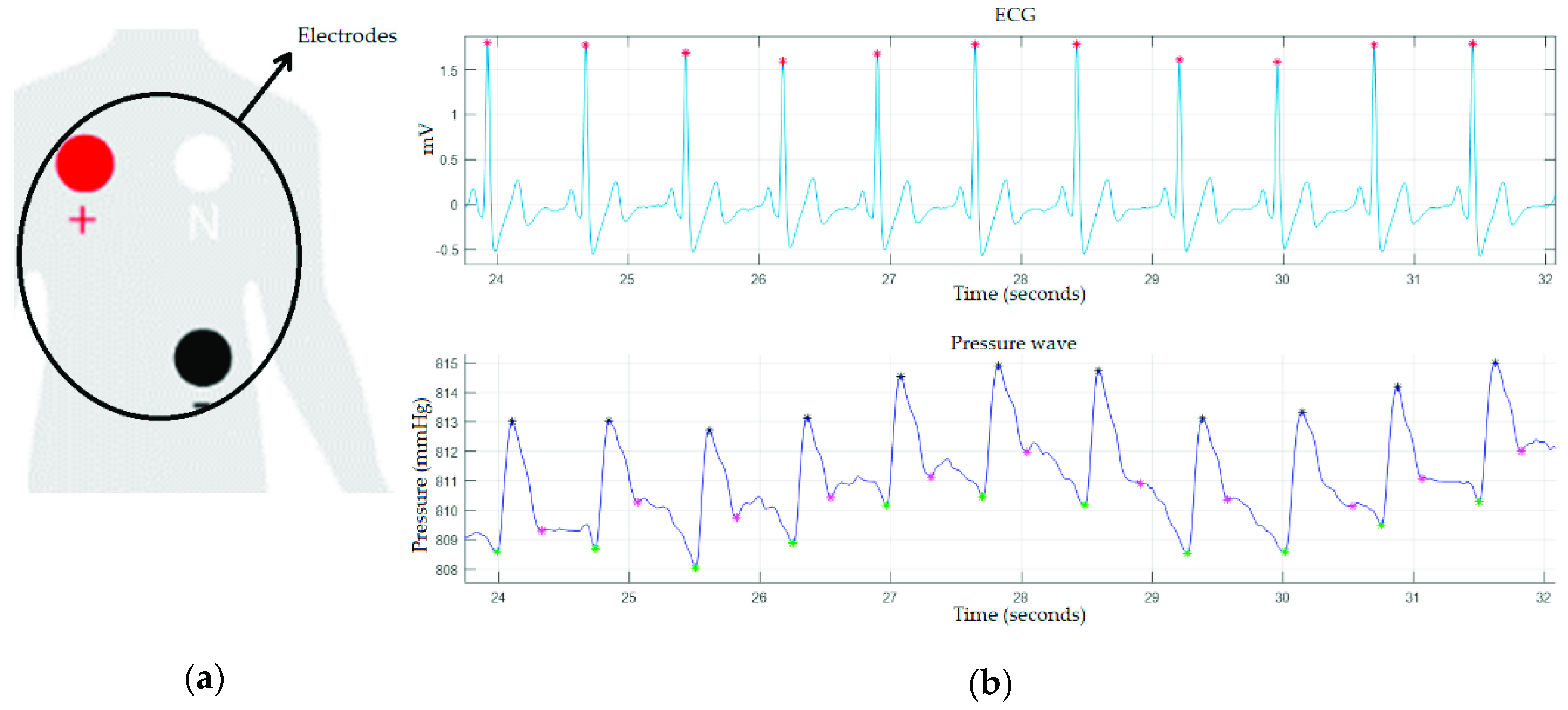
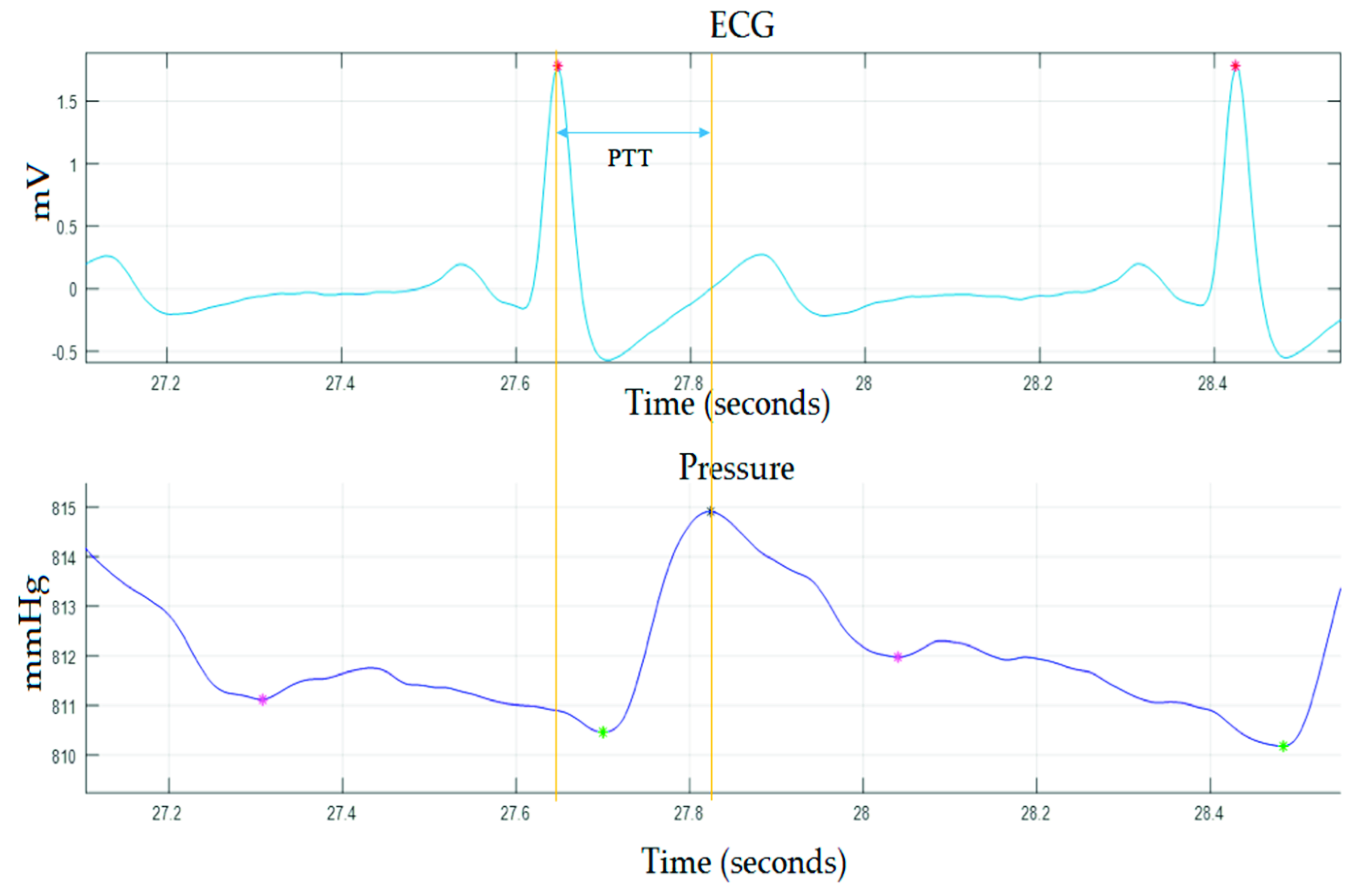
2.2. Validation of the Device
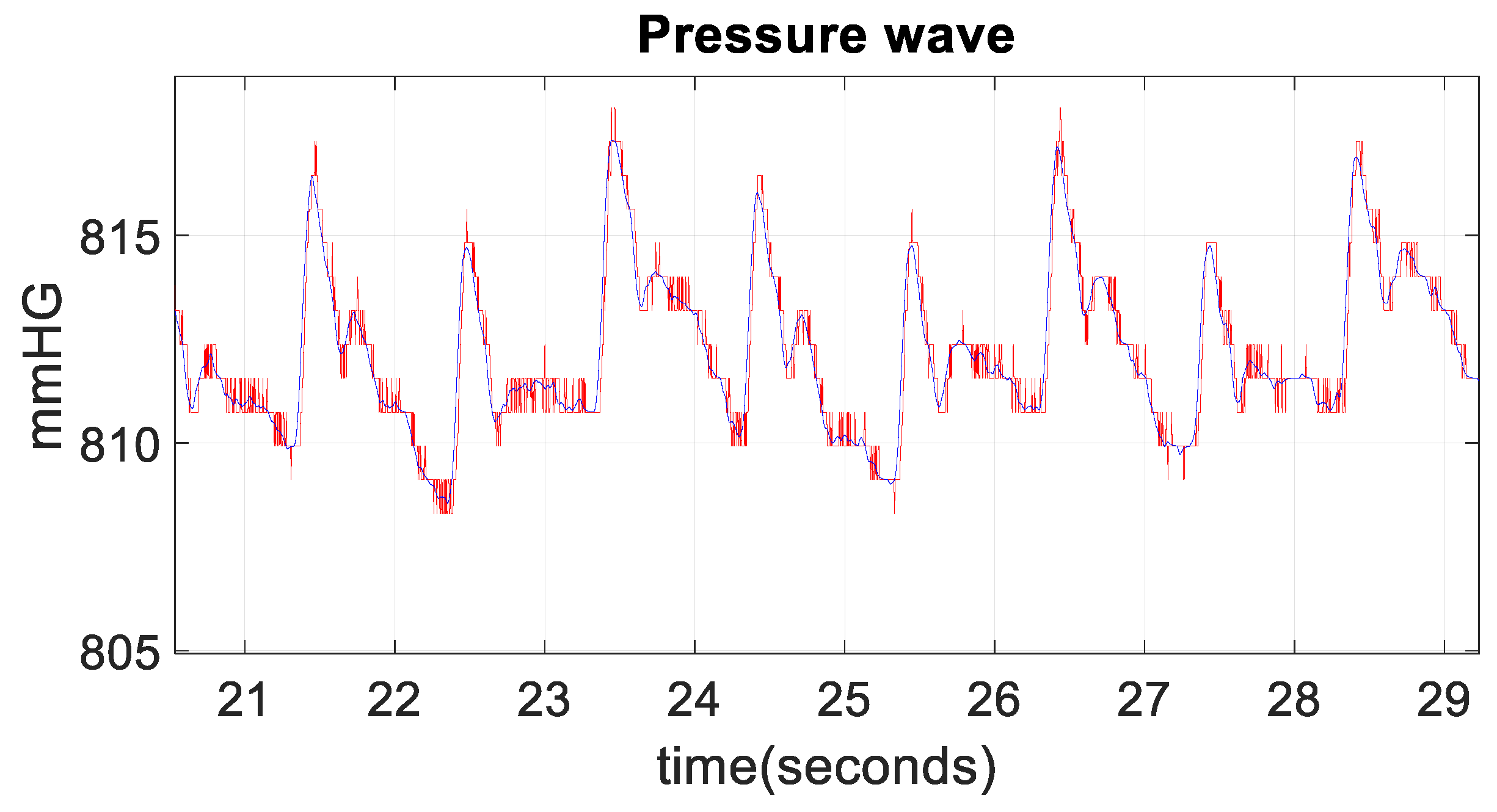
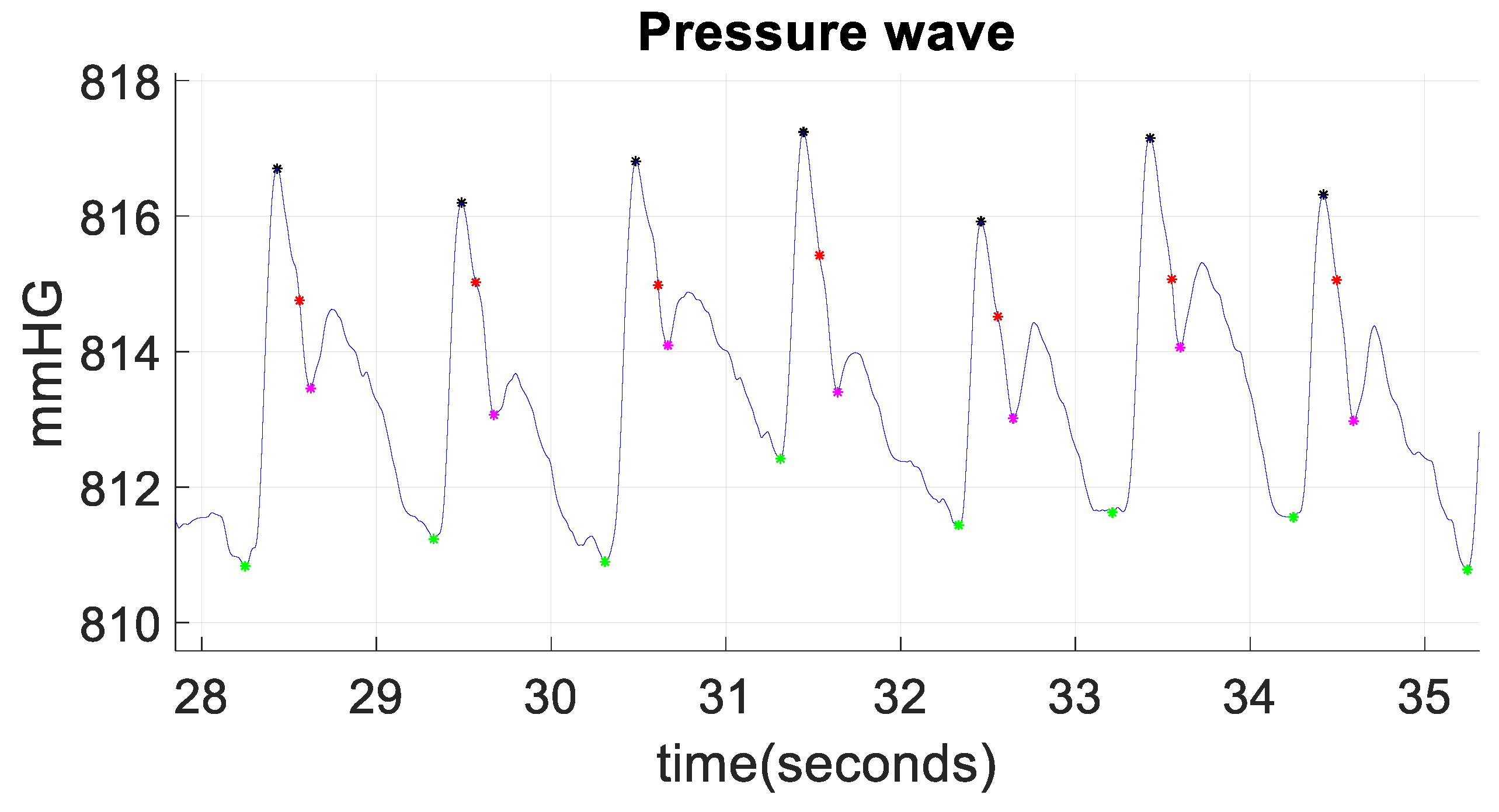
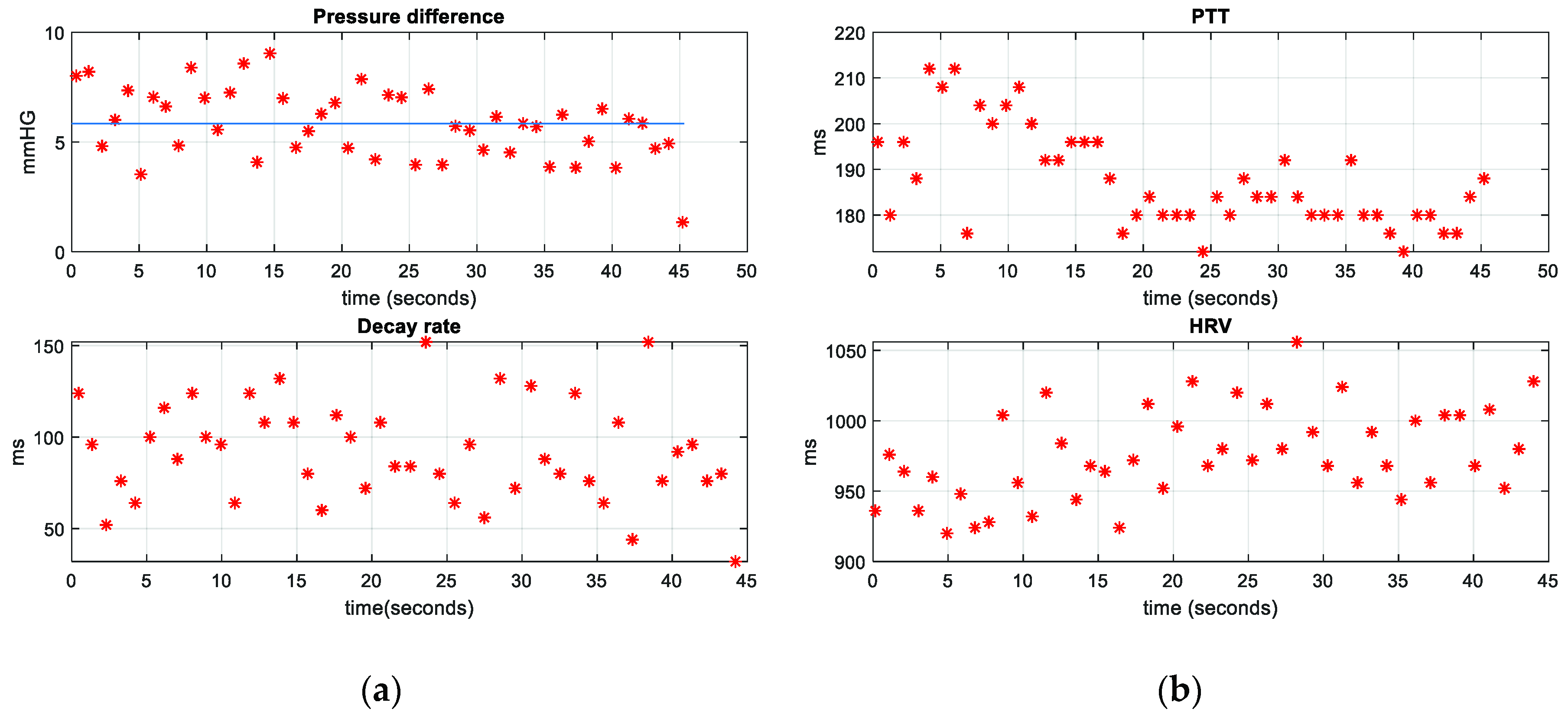
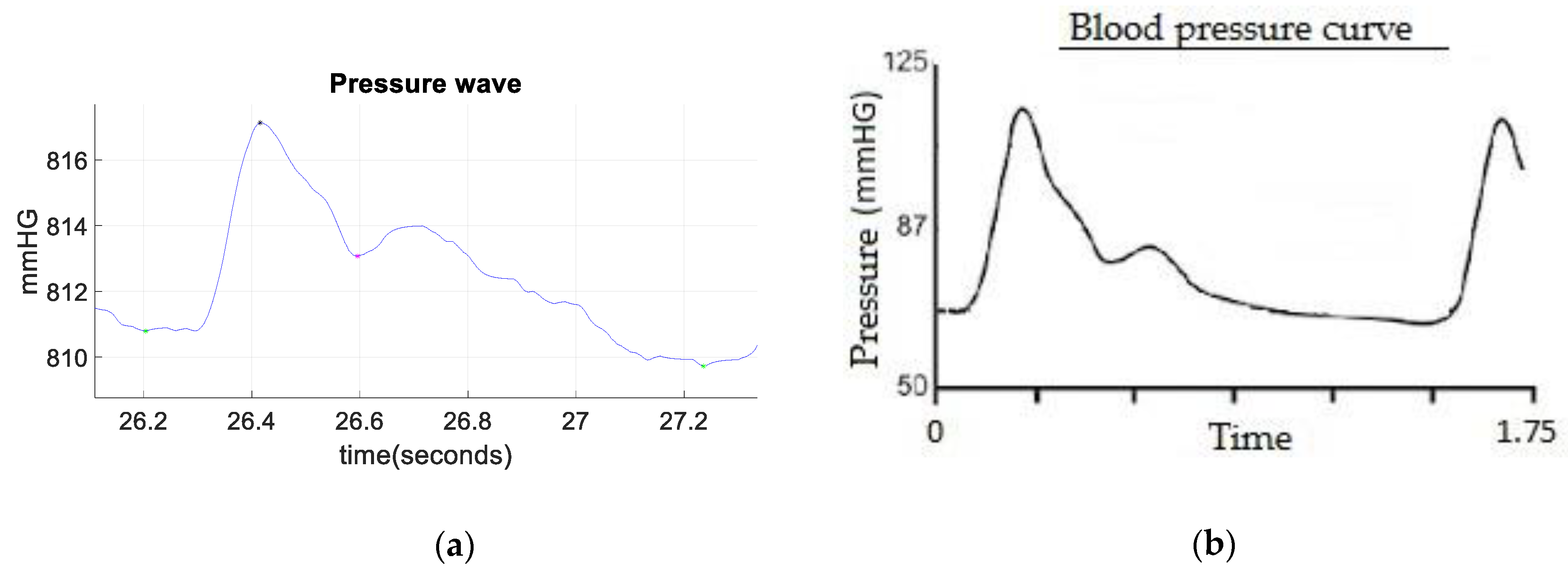
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ezzai, M.; López, A.D.; Rodgers, A.; Vander Hoorn, S.; Murray, C.J. Selected major risk factors and global and regional burden of disease. Lancet 2002, 360, 1347–1360. [Google Scholar] [CrossRef]
- A Global Brief on Hypertension. World Health Organization. Available online: https://apps.who.int/iris/bitstream/handle/10665/79059/WHO_DCO_WHD_2013.2_eng.pdf;jsessionid=DB8EACDFB98C92BB62F515584EC7F3FF?sequence=1 (accessed on 31 July 2019).
- Gonzalez, M. Concordance analysis between manual and automatic sphygmomanometers used in a military unit, in rest and after a physical exercise. Sanid. Mil. 2018, 74, 13–19. [Google Scholar]
- Mateu, A.; Ferrándiz, G.; Gruartmoner, J.; Mesquida, C.; Sabatier, Y. Techniques available for hemodynamic monitoring. Advantages and limitations. Med. Intensiv. 2012, 36, 434–444. [Google Scholar]
- Liton, E.; Morgan, M. The PiCCO monitor: A review. Anaesth. Intensive Care 2012, 40, 393–409. [Google Scholar]
- Hamilton, T.T.; Huber, L.M.; Jessen, M.E. PulseCO: A less-invasive method to monitor cardiac output from arterial pressure after cardiac surgery. Ann. Thorac. Surg. 2002, 74, 1408–1412. [Google Scholar] [CrossRef]
- Romagnoli, S.; Bevilacqua, S.; Lazzeri, C.; Ciappi, F.; Dini, D.; Pratesi, C.; Gensini, G.F.; Romano, S.M. Most care®: A minimally invasive system for hemodynamic monitoring powered by the pressure recording analytical method (PRAM). HSR Proc. Intensive Care Cardiovasc. Anesth. 2009, 1, 20–27. [Google Scholar]
- Ding, X. Continuous Blood Pressure Measurement From Invasive to Unobtrusive: Celebration of 200th Birth Anniversary of Carl Ludwig. IEEE J. Biomed. Health. Inform. 2016, 20, 1455–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazazzera, R.; Belhaj, Y.; Carrault, G. A New Wearable Device for Blood Pressure Estimation Using Photoplethysmogram. Sensors 2019, 11, 2557. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.H.; Cheng, D.C.; Su, C.H. A cuffless blood pressure measurement based on the Impedance plethysmography technique. Sensors 2017, 17, 1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Liu, J.K.; Wen, B.; He, Q.Y.; Li, Y.; Miao, F. Cuffless Blood Pressure Estimation Using Pressure Pulse Wave Signals. Sensors. 2018, 18, 4227. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, R.; Hahn, J.O.; Inan, O.T.; Mestha, L.K.; Kim, C.S.; Töreyin, H.; Kyal, S. Towards Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Rafiq, M.; Nasir, A.; Kashif, M. Continuous and Noninvasive Blood Pressure Estimation by Two-Sensor Measurement of Pulse Transit Time. In Proceedings of the 14th International Conference on Emerging Technologies (ICET), Islamabad, Pakistan, 21–22 November 2018; pp. 1–5. [Google Scholar]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.K.; Hsiai, T.K.; Chiao, J.-C.; Cao, H. Cuff-Less and Continuous Blood Pressure Monitoring: A Methodological Review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef]
- Huynh, T.H.; Jafari, R.; Chung, W. Noninvasive Cuffless Blood Pressure Estimation Using Pulse Transit Time and Impedance Plethysmography. IEEE Trans. Biomed. Eng. 2019, 66, 967–976. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, B.P.; Zhang, Y.; Yu, C.M.; Poon, C.C. Wearable cuff-less PTT-based system for overnight blood pressure monitoring. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 6103–6106. [Google Scholar]
- SphygmoCor. Available online: http://www.atcormedical.com.au/download/Active/Research_Manual_(CVMS).pdf (accessed on 26 August 2019).
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, L. Current assessment of pulse wave velocity: comprehensive review of validation studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Fabian, V.; Matera, L.; Bayerova, K.; Havlik, J.; Kremen, V.; Pudil, J.; Sajgalik, P.; Zemanek, D. Noninvasive Assessment of Aortic Pulse Wave Velocity by the Brachial Occlusion-Cuff Technique: Comparative Study. Sensors 2019, 19, 3467. [Google Scholar] [CrossRef]
- Xie, G.L.; Wnag, J.H.; Zhou, Y.; Xu, H.; Sun, J.H.; Yang, S.R. Association of high blood pressure with heart rate variability in children. Iran J. Pediatr. 2013, 23, 37–44. [Google Scholar]
- e-Health Sensor Platform V1.0 for Arduino and Raspberry Pi [Biometric/Medical Applications]. Available online: https://www.cooking-hacks.com/documentation/tutorials/ehealth-v1-biometric-sensor-platform-arduino-raspberry-pi-medical/ (accessed on 31 July 2019).
- Cymberknop, L.J.; Legnani, W.; Pessana, F.M.; Armentano, R.L. Procesamiento no lineal de señales cardiovasculares: marco conceptual para la detección de patologías. Proyecciones 2013, 11, 11–28. [Google Scholar]
- Lin, W.; Wang, H.; Samuel, O.W.; Li, G. Using a new PPG indicator to increase the accuracy of PTT-based continuous cuffless blood pressure estimation. In Conf. Proc. IEEE Eng. Med. Biol. Soc. 2017, 738–741. [Google Scholar]
- Park, E.K.; Cho, B.H.; Lee, J.Y.; Lee, J.S.; Kim, I.Y.; Kim, S.I. Continuous measurement of systolic blood pressure using the PTT and other parameters. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 3555–3558. [Google Scholar]
- Zocalo, Y.; Bai, D.; Farro, I.; Torrado, J.; Farro, F.; Luis Armentano, R.; Lluberas, R. Presión aórtica central y parámetros de reflexión de onda: Importancia clínica y análisis de consistencias y discrepancias con niveles de presión periférica. Rev. Urug. Cardiol. 2012, 27, 418–430, [Spanish]. [Google Scholar]
- Wang, K.L.; Cheng, H.M.; Chuang, S.Y.; Spurgeon, H.A.; Ting, C.T.; Lakatta, E.G.; Yin, F.C.; Chou, P.; Chen, C.H. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J. Hypertens. 2009, 27, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaisti, M.; Panula, T.; Leppänen, J.; Punkkinen, R.; Jafari Tadi, M.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, J.; Kostiainen, P. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. npj Digit. Med. 2019, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- AtCor Medical, Inc. (USA). SphygmoCor Vx. Brochure 2005. Available online: http://www.atcormedical.com.au/pdf/English/USA%20Letter/SphygmoCor%20Vx%20Datasheet%20DCN%20100516%20(English)%20USA.pdf (accessed on 29 September 2019).


| Gender | Age (Years) | Weight (kg) |
|---|---|---|
| Female | 28 | 65 |
| Male | 28 | 77 |
| Male | 27 | 82 |
| Male | 25 | 74 |
| Male | 24 | 76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrana-Vinaroz, D.; Vicente-Samper, J.M.; G. Juan, C.; Esteve-Sala, V.; Sabater-Navarro, J.M. Non-Invasive Device for Blood Pressure Wave Acquisition by Means of Mechanical Transducer. Sensors 2019, 19, 4311. https://doi.org/10.3390/s19194311
Zambrana-Vinaroz D, Vicente-Samper JM, G. Juan C, Esteve-Sala V, Sabater-Navarro JM. Non-Invasive Device for Blood Pressure Wave Acquisition by Means of Mechanical Transducer. Sensors. 2019; 19(19):4311. https://doi.org/10.3390/s19194311
Chicago/Turabian StyleZambrana-Vinaroz, David, Jose Maria Vicente-Samper, Carlos G. Juan, Vicente Esteve-Sala, and Jose Maria Sabater-Navarro. 2019. "Non-Invasive Device for Blood Pressure Wave Acquisition by Means of Mechanical Transducer" Sensors 19, no. 19: 4311. https://doi.org/10.3390/s19194311
APA StyleZambrana-Vinaroz, D., Vicente-Samper, J. M., G. Juan, C., Esteve-Sala, V., & Sabater-Navarro, J. M. (2019). Non-Invasive Device for Blood Pressure Wave Acquisition by Means of Mechanical Transducer. Sensors, 19(19), 4311. https://doi.org/10.3390/s19194311








