Solvent Treatment of Wet-Spun PEDOT: PSS Fibers for Fiber-Based Wearable pH Sensing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
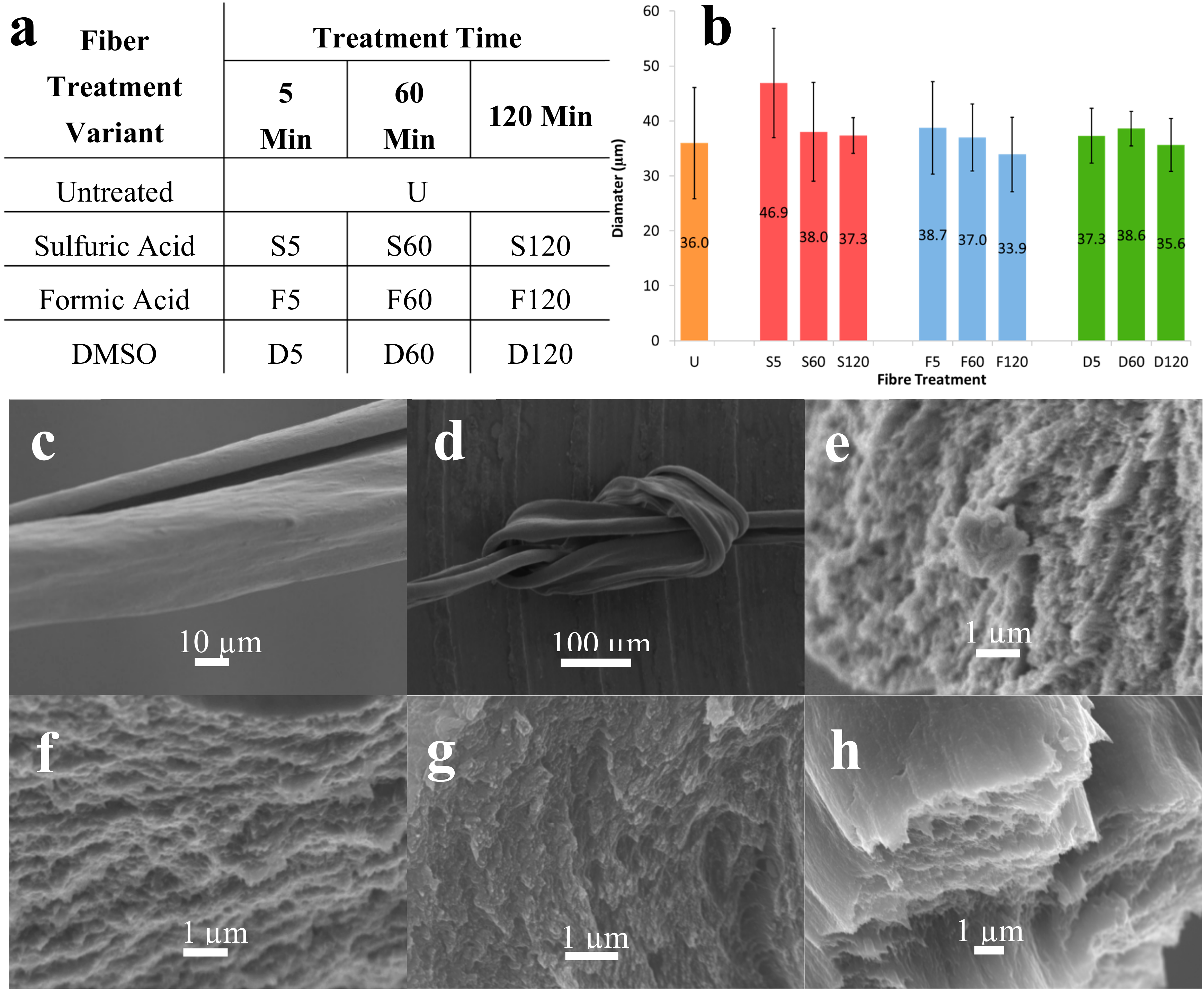
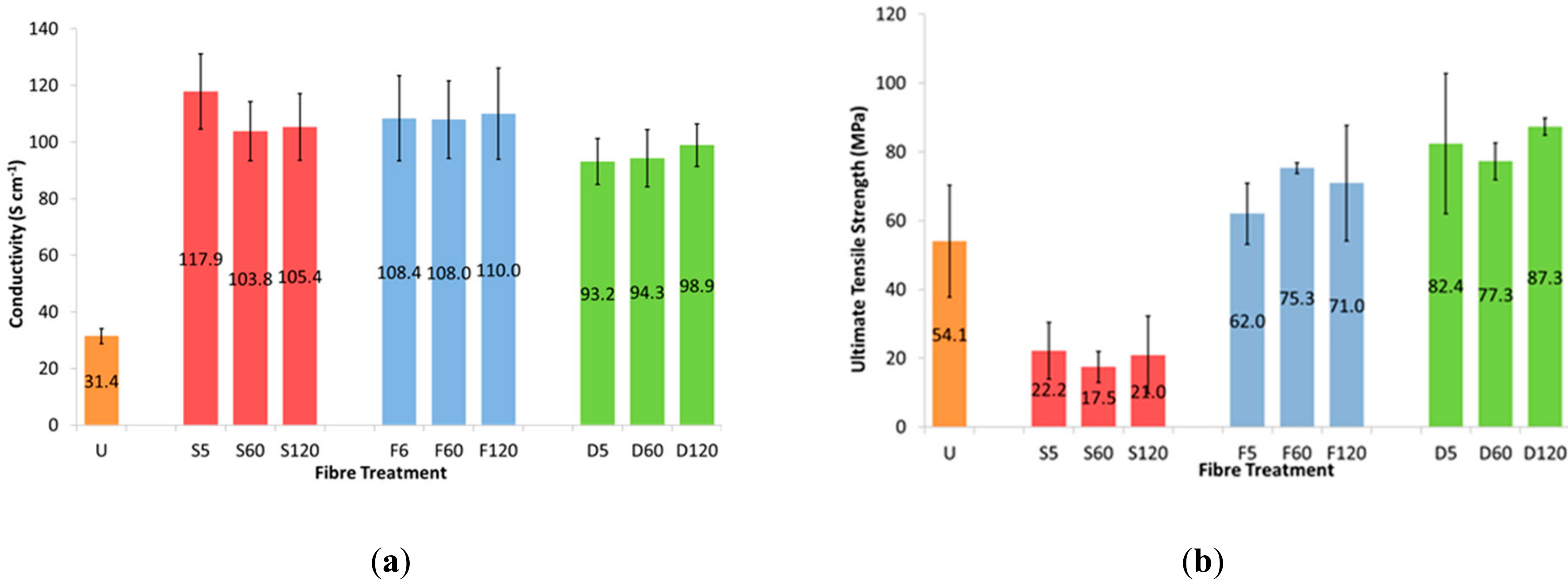
3.1. Effect of Solvent Treatment Time
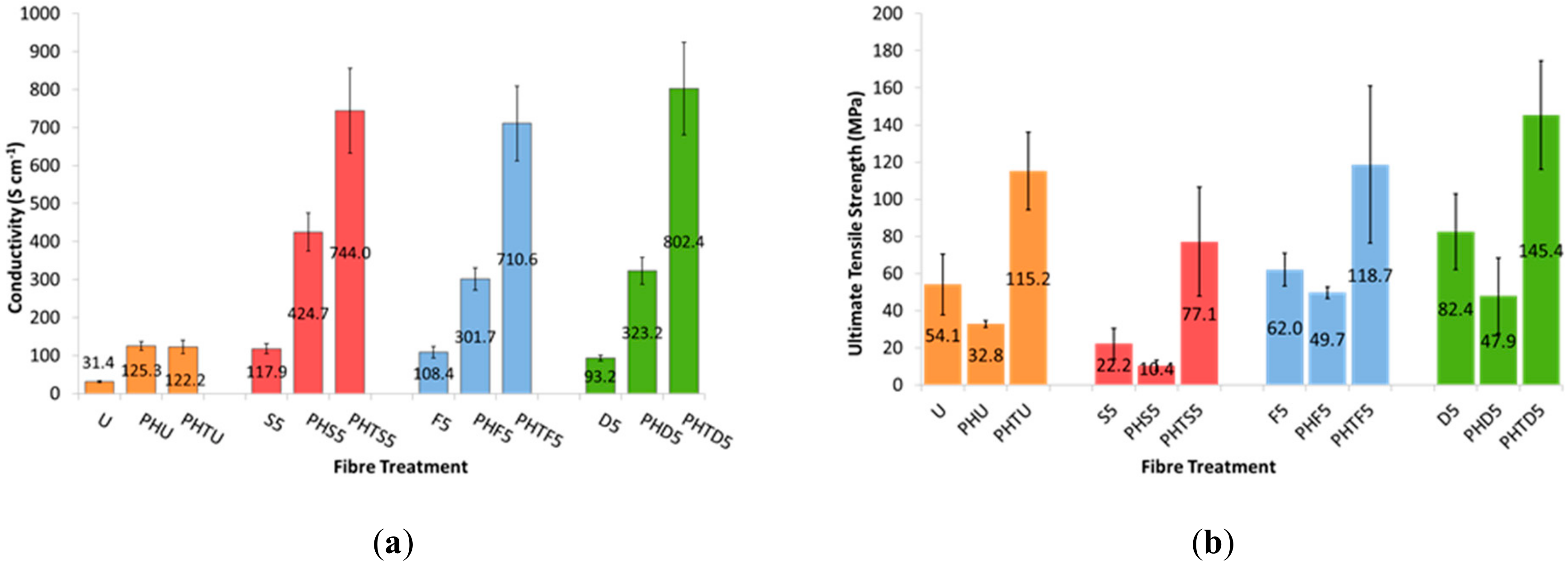
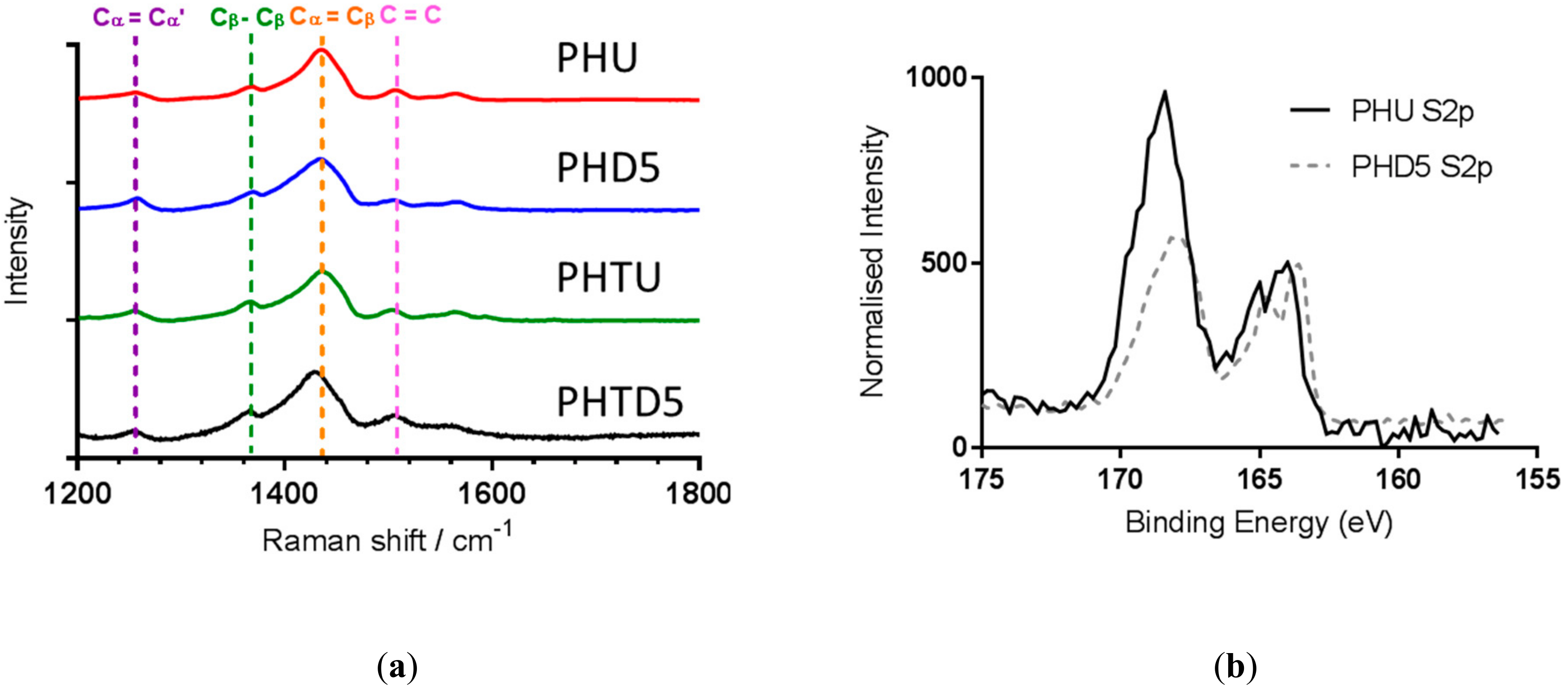
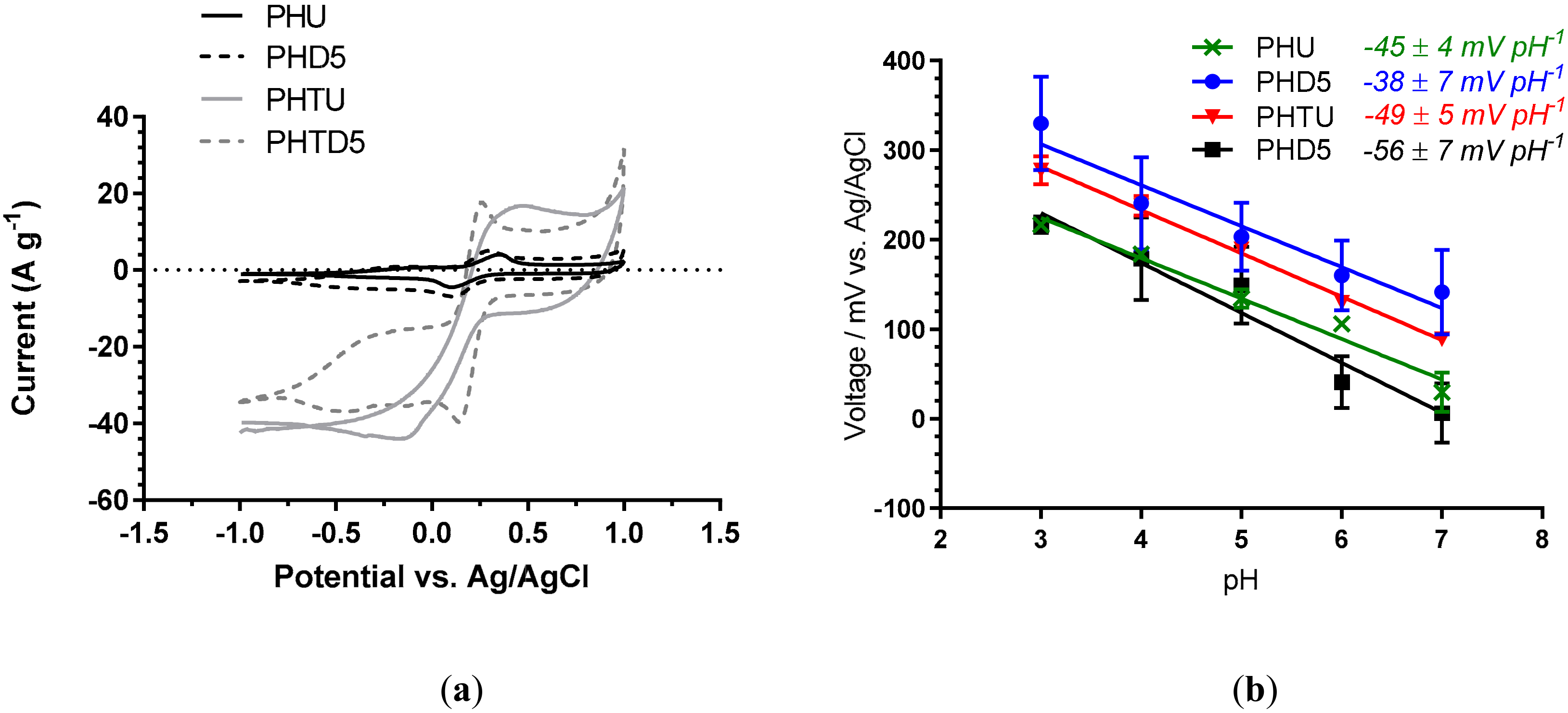
3.2. Effect of PEDOT: PSS Material Source
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdes-Ramirez, G.; Paixao, T.R.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with intergrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- Kuang, S.Y.; Chen, J.; Cheng, X.B.; Zhu, G.; Wang, Z.L. Two-dimensional rotary triboelectric nanogenerator as a portable and wearable power source for electronics. Nano Energy 2015, 17, 10–16. [Google Scholar] [CrossRef]
- Van den Brand, J.; de Kok, M.; Koetse, M.; Cauwe, M.; Verplancke, R.; Bossuyt, F.; Jablonski, M.; Vanfleteren, J. Flexible and stretchable electronics for wearable health devices. Solid State Electron. 2015, 113, 116–120. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, L.; Mak, C.L.; Huang, H.; Tang, W.M.; Chan, H.L. High-performance fiber-shaped supercapacitors using carbon fiber thread (CFT)@polyaniline and functionalized CFT electrodes for wearable/stretchable electronics. Nano Energy 2015, 11, 662–670. [Google Scholar] [CrossRef]
- Lynam, C.; Moulton, S.E.; Wallace, G.G. Carbon-Nanotube Biofibres. Adv. Mater. 2007, 19, 1244–1248. [Google Scholar] [CrossRef]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya-Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable one-step wet-spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: Towards multifunctional textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Spinks, G.M.; Mottaghitalab, V.; Bahrami-Samani, M.; Whitten, P.G.; Wallace, G.G. Carbon-nanotube-reinforced polyaniline fibers for high-strength artificial muscles. Adv. Mater. 2006, 18, 637–640. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G. A reactive wet spinning approach to polypyrrole fibres. J. Mat. Chem. 2011, 21, 6421–6426. [Google Scholar] [CrossRef] [Green Version]
- Jalili, R.; Razal, J.M.; Innis, P.C.; Wallace, G.G. One-step wet-spinning process of poly (3,4-ethylenedioxythiophene): Poly (styrenesulfonate) fibers and the origin of higher electrical conductivity. Adv. Funct. Mater. 2011, 21, 3363–3370. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Holze, R.; Wu, Y.P. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochim. Acta 2014, 122, 93–107. [Google Scholar] [CrossRef]
- Garcia-Torres, J.; Crean, C. Ternary composite solid-state flexible supercapacitor based on nanocarbons/manganese dioxide/PEDOT: PSS fibres. Mater. Des. 2018, 155, 194–202. [Google Scholar] [CrossRef]
- Garcia-Torres, J.; Roberts, A.J.; Slade, R.C.T.; Crean, C. One-step wet spinning process of CB/CNT/MnO2 nanotbues hybrid flexible fibres as electrodes for wearable supercapacitors. Electrochim. Acta 2019, 296, 481–490. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly (3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-Processed Metallic Conducting Polymer Films as Transparent Electrode of Optoelectronic Devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.; Chu, C. Highly Conductive PEDOT: PSS Treated with Formic Acid for ITO-Free Polymer Solar Cells. Adv. Mater. Interfaces 2014, 6, 2292–2299. [Google Scholar] [CrossRef]
- Cruz-Cruz, I.; Reyes-Reyes, M.; Anguilar-Fruitis, M.A.; Rodriguez, A.G.; Lopez-Sandoval, R. Study of the effect of DMSO concentration on the thickness of the PSS insulating barrier in PEDOT: PSS thin films. Synth. Met. 2010, 160, 1501–1506. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.Y.; Ho, K.C.; Chu, C.W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Zhou, J.; Qiang, E.; Li, R.; Xu, X.; Ventura, I.A.; Moussawi, A.; Anjum, D.H.; Hedhili, M.N.; Smilgies, D.M.; Lubineau, G.; et al. Semi-metallic, strong and stretchable wet-spun conjugated polymer microfibers. J. Mater. Chem. C 2015, 3, 2528–2538. [Google Scholar] [CrossRef] [Green Version]
- Okuzaki, H.; Harashina, Y.; Yan, H. Highly conductive PEDOT: PSS microfibres fabricated by wet-spinning and dip-treatmeant in ethylene glycol. Eur. Polym. J. 2009, 45, 256–261. [Google Scholar] [CrossRef]
- Seyedin, M.Z.; Razal, J.M.; Innis, P.C.; Wallace, G.G. Strain-responsive polyurethane/PEDOT: PSS elastomeric composite fibers with high electrical conductivity. Adv. Funct. Mater. 2014, 24, 2957–2966. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y. Cotton-based wearable PEDOT: PSS electronic sensor for detecting acetone vapour. Flex. Print. Electron. 2017, 2, 042001. [Google Scholar] [CrossRef]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In Situ Spectroelectrochemical Raman Studies of Poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807–6812. [Google Scholar] [CrossRef]
- Chiu, W.W.; Travas-Sejdic, J.; Cooney, R.P.; Bowmaker, G.A. Studies of dopant effects in poly(3,4-ethylenedioxythiophene) using Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1354–1361. [Google Scholar] [CrossRef]
- Luo, J.; Billep, D.; Waechtler, T.; Otto, T.; Toader, M.; Gordan, O.; Sheremet, E.; Martin, J.; Hietschold, M.; Zahn, D.R.T.; et al. Enhancement of the thermoelectric properties of PEDOT: PSS thin films by post-treatment. J. Mater. Chem. A 2013, 1, 7576–7583. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Li, M.; Chen, T.; Zhang, H.; Chen, Q.; Ding, B.; Liu, Y. A highly conductive poly (3,4-ethylenedioxythiophene): Poly (styrene sulfonate) film with the solvent bath treatment by dimethyl sulfoxide as cathode for polymer tantalum capacitor. Chem. Phys. Lett. 2016, 654, 86–91. [Google Scholar] [CrossRef]
- Vacca, A.; Mascia, M.; Rizzardini, S.; Corgiolu, S.; Palmas, D.; Bonfiglio, A.; Ricci, P. Preparation and characterisation of transparent and flexible PEDOT: PSS/PANi electrodes by ink-jet printing and electropolymerisation. RSC Adv. 2015, 79, 79600–79606. [Google Scholar] [CrossRef]
- Sarkar, B.; Jaisal, M.; Satapathy, D.K. Swelling kinetics and electrical charge transport in PEDOT: PSS thin films exposed to water vapor. J. Phys. Condes. Matter 2018, 30, 225101. [Google Scholar] [CrossRef]
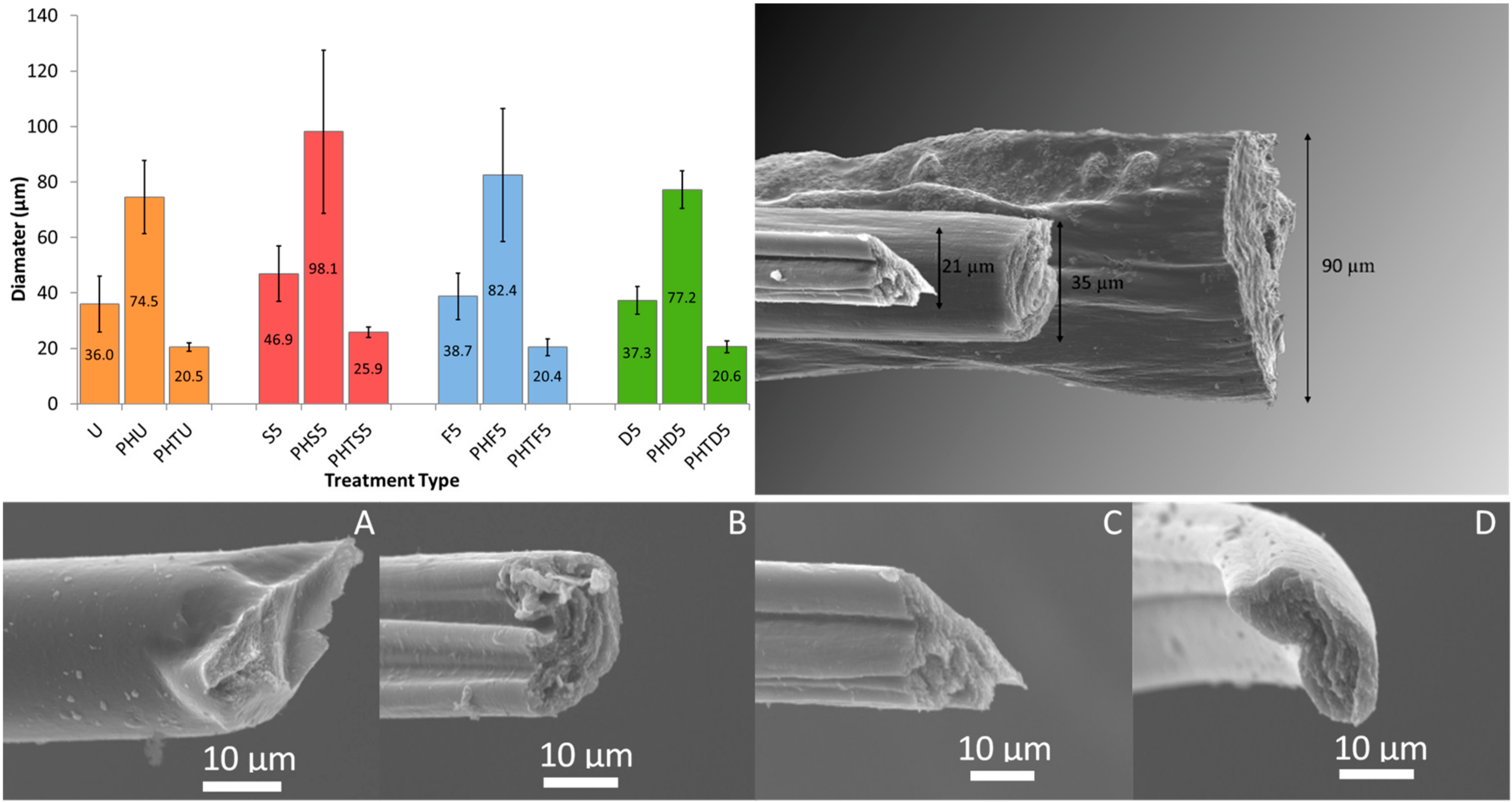
| 5 Min Solvent Treatment | |||||
|---|---|---|---|---|---|
| PEDOT: PSS Source | Fiber Diameter (µm) | Untreated | Sulfuric Acid | Formic Acid | DMSO |
| Orgacon | 35 | U | S5 | F5 | D5 |
| Clevios PH1000 | 90 | PHU | PHS5 | PHF5 | PHD5 |
| Clevios PH1000 | 20 * (* T = thin) | PHTU | PHTS5 | PHTF5 | PHTD5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid, D.O.; Smith, R.E.; Garcia-Torres, J.; Watts, J.F.; Crean, C. Solvent Treatment of Wet-Spun PEDOT: PSS Fibers for Fiber-Based Wearable pH Sensing. Sensors 2019, 19, 4213. https://doi.org/10.3390/s19194213
Reid DO, Smith RE, Garcia-Torres J, Watts JF, Crean C. Solvent Treatment of Wet-Spun PEDOT: PSS Fibers for Fiber-Based Wearable pH Sensing. Sensors. 2019; 19(19):4213. https://doi.org/10.3390/s19194213
Chicago/Turabian StyleReid, Daniel O., Rachel E. Smith, Jose Garcia-Torres, John F. Watts, and Carol Crean. 2019. "Solvent Treatment of Wet-Spun PEDOT: PSS Fibers for Fiber-Based Wearable pH Sensing" Sensors 19, no. 19: 4213. https://doi.org/10.3390/s19194213





