Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects
Abstract
:1. Introduction:
2. Evolution of Nanotransducers for FET-Based Sensors
3. Antibody and its Fragments as Bio-Probes for FET Immunosensors
4. Nucleic Acid Probes for FET Biosensors
5. Conclusions and Future Prospects
Acknowledgments
Conflicts of Interest
References
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E. Biosensors for Cancer Markers Diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ligler, F.S.; Taitt, C.R.; Shriver-Lake, L.C.; Sapsford, K.E.; Shubin, Y.; Golden, J.P. Array Biosensor for Detection of Toxins. Anal. Bioanal. Chem. 2003, 377, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Bunney, J.; Williamson, S.; Atkin, D.; Jeanneret, M.; Cozzolino, D.; Chapman, J.; Power, A.; Chandra, S. The Use of Electrochemical Biosensors in Food Analysis. Curr. Res. Nutr. Food. Sci. 2017, 5, 83–95. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Man Cybern. Syst. 2010, 40, 1–12. [Google Scholar] [CrossRef]
- Chaplin, M.F.; Bucke, C. Biosensors. In Enzyme Technology, 1st ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed Electrical Detection of Cancer Markers with Nanowire Sensor Arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of Silicon Nanowire Devices for Ultrasensitive, Label-Free, Real-Time Detection of Biological and Chemical Species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-Free Immunodetection with CMOS-Compatible Semiconducting Nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Wu, C.-S.; Chuang, C.-K.; Pang, S.-T.; Pan, T.-M.; Yang, Y.-S.; Ko, F.-H. Real-Time and Label-Free Detection of the Prostate-Specific Antigen in Human Serum by a Polycrystalline Silicon Nanowire Field-Effect Transistor Biosensor. Anal. Chem. 2013, 85, 7912–7918. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hung, C.-H.; Hsiao, C.-Y.; Lin, H.-C.; Ko, F.-H.; Yang, Y.-S. Poly-Silicon Nanowire Field-Effect Transistor for Ultrasensitive and Label-Free Detection of Pathogenic Avian Influenza DNA. Biosens. Bioelectron. 2009, 24, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Duan, X.; Wei, Q.; Lieber, C.M. Directed Assembly of One-Dimensional Nanostructures into Functional Networks. Science 2001, 291, 630–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Cao, A.; Lieber, C.M. Large-Area Blown Bubble Films of Aligned Nanowires and Carbon Nanotubes. Nat. Nanotechnol. 2007, 2, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Ho, J.C.; Jacobson, Z.A.; Yerushalmi, R.; Alley, R.L.; Razavi, H.; Javey, A. Wafer-Scale Assembly of Highly Ordered Semiconductor Nanowire Arrays by Contact Printing. Nano Lett. 2008, 8, 20–25. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Cui, Y.; Wang, J.; Lieber, C.M. Indium Phosphide Nanowires as Building Blocks for Nanoscale Electronic and Optoelectronic Devices. Nature 2001, 409, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Panda, A.B.; Belman, N.; Efrima, S.; Golan, Y. A Semiconductor-Nanowire Assembly of Ultrahigh Junction Density by the Langmuir–Blodgett Technique. Adv. Mater. 2006, 18, 210–213. [Google Scholar] [CrossRef]
- Zheng, Z.; Gan, L.; Zhai, T. Electrospun Nanowire Arrays for Electronics and Optoelectronics. Sci. China Mater. 2016, 59, 200–216. [Google Scholar] [CrossRef]
- Weiss, N.O.; Duan, X. A Guide for Nanowire Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 15171–15172. [Google Scholar] [CrossRef]
- Heath, J.R. Superlattice Nanowire Pattern Transfer (SNAP). Acc. Chem. Res. 2008, 41, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Lin, Z.; Huang, Y.; Duan, X. Nanowire Electronics: From Nanoscale to Macroscale. Chem. Rev. 2019, 119, 9074–9135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hotani, K.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Hashimoto, M.; Mori, Y.; Osaka, T. Field Effect Transistor Biosensor Using Antigen Binding Fragment for Detecting Tumor Marker in Human Serum. Materials 2014, 7, 2490–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Chu, C.-J.; Teng, K.-N.; Su, Y.-J.; Chen, C.-D.; Tsai, L.-C.; Yang, Y.-S. Recovery Based Nanowire Field-Effect Transistor Detection of Pathogenic Avian Influenza DNA. Jpn. J. Appl. Phys. 2012, 51, 02BL02. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, H.-C.; Yang, Y.-S.; Huang, C.-J.; Chan, H.W.-H.; Hu, W.-P. Improved DNA Detection by Utilizing Electrically Neutral DNA Probe in Field-Effect Transistor Measurements as Evidenced by Surface Plasmon Resonance Imaging. Biosens. Bioelectron. 2013, 41, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-P.; Tsai, C.-C.; Yang, Y.-S.; Chan, H.W.-H.; Chen, W.-Y. Synergetic Improvements of Sensitivity and Specificity of Nanowire Field Effect Transistor Gene Chip by Designing Neutralized DNA as Probe. Sci. Rep. 2018, 8, 12598. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyaly, M.; Zhuang, X.; Lieber, C.M. Electrical Detection of Single Viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Zhang, L.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.-J.A.; Kang, T.G.; Chen, Y. Silicon Nanowire Biosensor for Highly Sensitive and Rapid Detection of Dengue Virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsiao, C.-Y.; Hung, C.-H.; Lo, Y.-R.; Lee, C.-C.; Su, C.-J.; Lin, H.-C.; Ko, F.-H.; Huang, T.-Y.; Yang, Y.-S. Ultrasensitive Detection of Dopamine Using a Polysilicon Nanowire Field-Effect Transistor. Chem. Commun. 2008, 44, 5749–5751. [Google Scholar] [CrossRef]
- Wu, C.-C.; Ko, F.-H.; Yang, Y.-S.; Hsia, D.-L.; Lee, B.-S.; Su, T.-S. Label-Free Biosensing of a Gene Mutation Using a Silicon Nanowire Field-Effect Transistor. Biosens. Bioelectron. 2009, 25, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-Y.; Hsu, W.-Y.; Yang, Y.-S.; Huang, J.-W.; Chung, Y.-L.; Chen, H. Immobilized Rolling Circle Amplification on Extended-Gate Field-Effect Transistors with Integrated Readout Circuits for Early Detection of Platelet-Derived Growth Factor. Anal. Bioanal. Chem. 2016, 408, 4785–4797. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-R.; Chen, H.-M.P.; Yang, Y.-S.; Lu, M.-P. Gas Sensing Ability on Polycrystalline-Silicon Nanowire. ECS J. Solid State Sci. Technol. 2018, 7, Q3104–Q3107. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold Regime Has the Optimal Sensitivity for Nanowire FET Biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Gao, X.P.A.; Lieber, C.M. Frequency Domain Detection of Biomolecules Using Silicon Nanowire Biosensors. Nano Lett. 2010, 10, 3179–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.-J.; Yeh, C.-S.; Liao, C.-K.; Tsai, L.-C.; Huang, C.-M.; Lin, H.-Y.; Shyue, J.-J.; Chen, Y.-T.; Chen, C.-D. Improving Nanowire Sensing Capability by Electrical Field Alignment of Surface Probing Molecules. Nano Lett. 2013, 13, 2564–2569. [Google Scholar] [CrossRef]
- Xie, P.; Xiong, Q.; Fang, Y.; Qing, Q.; Lieber, C.M. Local Electrical Potential Detection of DNA by Nanowire–Nanopore Sensors. Nat. Nanotechnol. 2012, 7, 119–125. [Google Scholar] [CrossRef]
- Gong, J.-R. Label-Free Attomolar Detection of Proteins Using Integrated Nanoelectronic and Electrokinetic Devices. Small 2010, 6, 967–973. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, B.; Xiang, J.; Qian, F.; Zheng, G.; Wang, H.; Mai, L.; Lieber, C.M. Rational Growth of Branched Nanowire Heterostructures with Synthetically Encoded Properties and Function. Proc. Natl. Acad. Sci. USA 2011, 108, 12212–12216. [Google Scholar] [CrossRef]
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-Free Biomarker Detection from Whole Blood. Nat. Nanotechnol. 2010, 5, 138–142. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Heller, I.; Kong, J.; Heering, H.A.; Williams, K.A.; Lemay, S.G.; Dekker, C. Individual Single-Walled Carbon Nanotubes as Nanoelectrodes for Electrochemistry. Nano Lett. 2005, 5, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Krapf, D.; Quinn, B.M.; Wu, M.-Y.; Zandbergen, H.W.; Dekker, C.; Lemay, S.G. Experimental Observation of Nonlinear Ionic Transport at the Nanometer Scale. Nano Lett. 2006, 6, 2531–2535. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J.; Chou, A.; Liu, J.; Losic, D.; Shapter, J.G.; Hibbert, D.B. The Effects of the Lengths and Orientations of Single-Walled Carbon Nanotubes on the Electrochemistry of Nanotube-Modified Electrodes. Electrochem. Commun. 2007, 9, 1677–1683. [Google Scholar] [CrossRef]
- Heller, I.; Janssens, A.M.; Mannik, J.; Minot, E.D.; Lemay, S.G.; Dekker, C. Identifying the Mechanism of Biosensing with Carbon Nanotube Transistors. Nano Lett. 2008, 8, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Coleman, K.S.; Azamian, B.R.; Bagshaw, C.B.; Green, M.L.H. Chemical and Biochemical Sensing with Modified Single Walled Carbon Nanotubes. Chem. Eur. J. 2003, 9, 3732–3739. [Google Scholar] [CrossRef]
- Gooding, J.J.; Wibowo, R.; Liu, J.; Yang, W.; Losic, D.; Orbons, S.; Mearns, F.J.; Shapter, J.G.; Hibbert, D.B. Protein Electrochemistry Using Aligned Carbon Nanotube Arrays. J. Am. Chem. Soc. 2003, 125, 9006–9007. [Google Scholar] [CrossRef]
- Li, J.; Ng, H.T.; Cassell, A.; Fan, W.; Chen, H.; Ye, Q.; Koehne, J.; Han, J.; Meyyappan, M. Carbon Nanotube Nanoelectrode Array for Ultrasensitive DNA Detection. Nano Lett. 2003, 3, 597–602. [Google Scholar] [CrossRef]
- Tsang, S.C.; Davis, J.J.; Green, M.L.H.; Hill, H.A.O.; Leung, Y.C.; Sadler, P.J. Immobilization of Small Proteins in Carbon Nanotubes: High-Resolution Transmission Electron Microscopy Study and Catalytic Activity. J. Chem. Soc. Chem. Commun. 1995, 17, 1803–1804. [Google Scholar] [CrossRef]
- Davis, J.J.; Green, M.L.H.; Hill, H.A.O.; Leung, Y.C.; Sadler, P.J.; Sloan, J.; Xavier, A.V.; Tsang, S.C. The Immobilisation of Proteins in Carbon Nanotubes. Inorg. Chim. Acta 1998, 272, 261–266. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. The Direct Electron Transfer of Glucose Oxidase and Glucose Biosensor Based on Carbon Nanotubes/Chitosan Matrix. Biosens. Bioelectron. 2005, 21, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Star, A.; Gabriel, J.-C.P.; Bradley, K.; Gruner, G. Electronic Detection of Specific Protein Binding Using Nanotube FET Devices. Nano Lett. 2003, 3, 459–463. [Google Scholar] [CrossRef]
- Star, A.; Tu, E.; Niemann, J.; Gabriel, J.-C.P.; Joiner, C.S.; Valcke, C. Label-Free Detection of DNA Hybridization Using Carbon Nanotube Network Field-Effect Transistors. Proc. Natl. Acad. Sci. USA 2006, 103, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L. Towards Chirality-Pure Carbon Nanotubes. Nanoscale 2010, 2, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Sun, H.; Gao, C. Superstructured Assembly of Nanocarbons: Fullerenes, Nanotubes, and Graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Chandran, G.T.; Li, X.; Ogata, A.; Penner, R.M. Electrically Transduced Sensors Based on Nanomaterials (2012–2016). Anal. Chem. 2017, 89, 249–275. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Chen, S.; Liu, L.; Zhou, J.; Jiang, S. Controlling Antibody Orientation on Charged Self-Assembled Monolayers. Langmuir 2003, 19, 2859–2864. [Google Scholar] [CrossRef]
- Chou, W.-C.; Hu, W.-P.; Yang, Y.-S.; Chan, H.W.-H.; Chen, W.Y. Neutralized Chimeric DNA Probe for the Improvement of GC-Rich RNA Detection Specificity on the Nanowire Field-Effect Transistor. Sci. Rep. 2019, 9, 11056. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, C.; Jiang, S.; Hu, G.; Li, X.; Zou, Y.; Liu, H.; Li, J.; Li, Z.; Wang, X.; et al. Graphene Foam Field-Effect Transistor for Ultra-Sensitive Label-Free Detection of ATP. Sens. Actuators B Chem. 2019, 284, 125–133. [Google Scholar] [CrossRef]
- Thakur, B.; Zhou, G.; Chang, J.; Pu, H.; Jin, B.; Sui, X.; Yuan, X.; Yang, C.-H.; Magruder, M.; Chen, J. Rapid Detection of Single E. coli Bacteria Using a Graphene-Based Field-Effect Transistor Device. Biosens. Bioelectron. 2018, 110, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhang, Y.; Nadappuram, B.P.; Akpinar, B.; Klenerman, D.; Ivanov, A.P.; Edel, J.B.; Korchev, Y. Nanopore Extended Field-Effect Transistor for Selective Single-Molecule Biosensing. Nat. Commun. 2017, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M. The Field Effect Transistor DNA Biosensor Based on ITO Nanowires in Label-Free Hepatitis B Virus Detecting Compatible with CMOS Technology. Biosens. Bioelectron. 2018, 105, 58–64. [Google Scholar] [CrossRef] [PubMed]
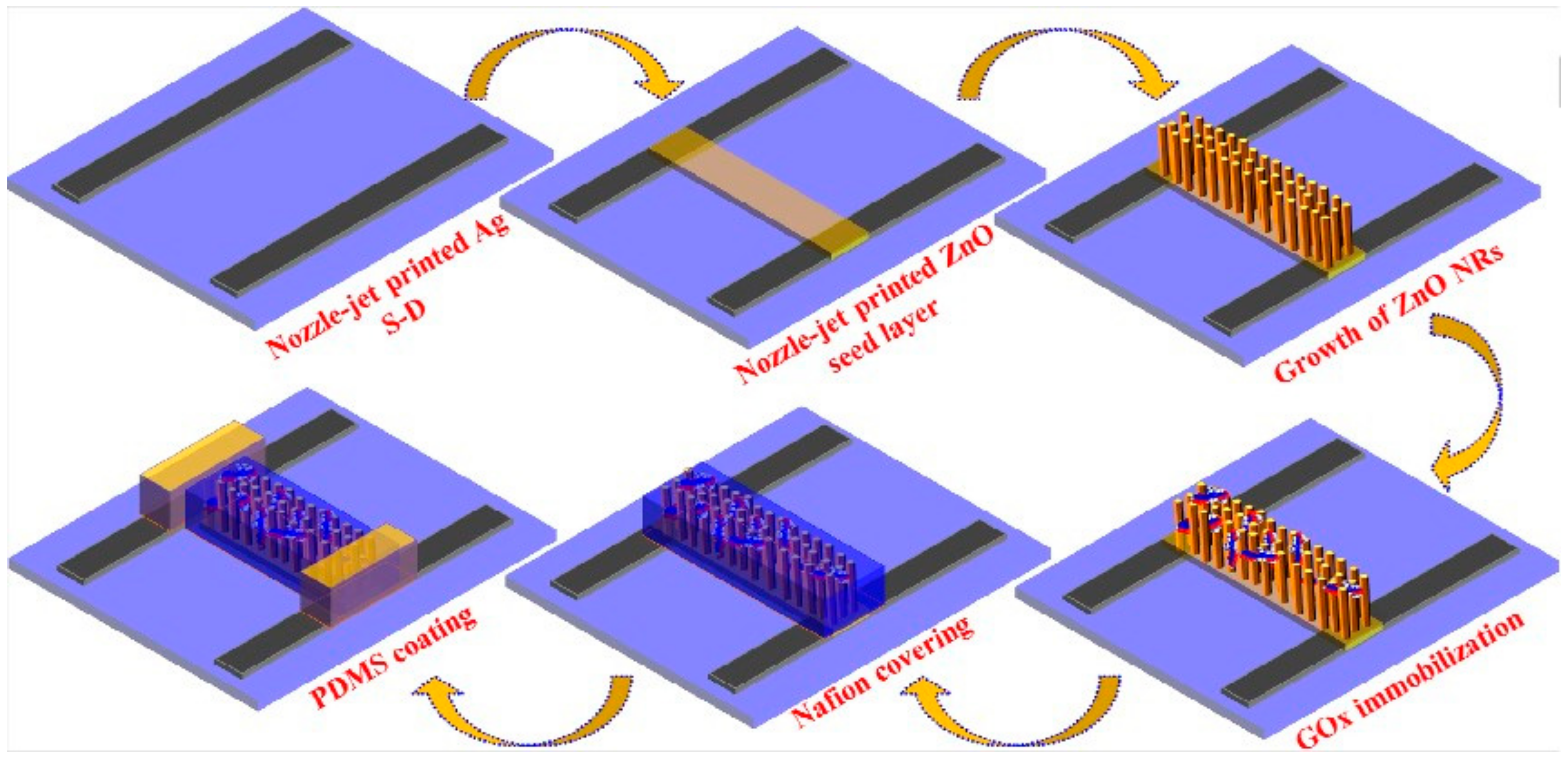
- Bhat, K.S.; Ahmad, R.; Yoo, J.-Y.; Hahn, Y.-B. Nozzle-Jet Printed Flexible Field-Effect Transistor Biosensor for High Performance Glucose Detection. J. Colloid Interface Sci. 2017, 506, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Fathollahzadeh, M.; Hosseini, M.; Norouzi, M.; Ebrahimi, A.; Fathipour, M.; Kolahdouz, M.; Haghighi, B. Immobilization of Glucose Oxidase on ZnO Nanorods Decorated Electrolyte-Gated Field Effect Transistor for Glucose Detection. J. Solid State Electrochem. 2018, 22, 61–67. [Google Scholar] [CrossRef]
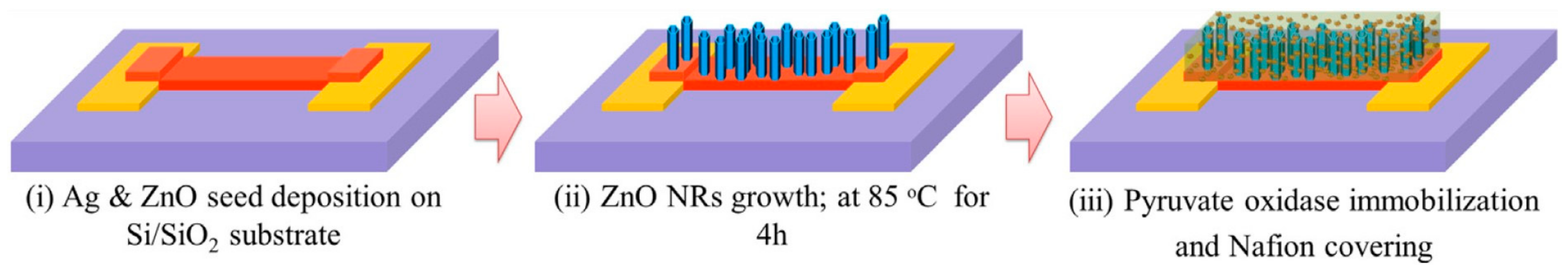
- Ahmad, R.; Ahn, M.-S.; Hahn, Y.-B. ZnO Nanorods Array Based Field-Effect Transistor Biosensor for Phosphate Detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef]
- Bao, Z.; Sun, J.; Zhao, X.; Li, Z.; Cui, S.; Meng, Q.; Zhang, Y.; Wang, T.; Jiang, Y. Top-Down Nanofabrication of Silicon Nanoribbon Field Effect Transistor (Si-NR FET) for Carcinoembryonic Antigen Detection. Int. J. Nanomed. 2017, 12, 4623–4631. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Lee, Y.-K.; Zhang, A. Direct Label-Free Protein Detection in High Ionic Strength Solution and Human Plasma Using Dual-Gate Nanoribbon-Based Ion-Sensitive Field-Effect Transistor Biosensor. Biosens. Bioelectron. 2018, 117, 276–282. [Google Scholar] [CrossRef]
- Ma, S.; Lee, Y.-K.; Zhang, A.; Li, X. Label-Free Detection of Cordyceps Sinensis Using Dual-Gate Nanoribbon-Based Ion-Sensitive Field-Effect Transistor Biosensor. Sens. Actuators B Chem. 2018, 264, 344–352. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Legallais, M.; Morisot, F.; Cazimajou, T.; Mouis, M.; Salem, B.; Stambouli, B.; Ternon, C. On the Development of Label-Free DNA Sensor Using Silicon Nanonet Field-Effect Transistors. Proceedings 2017, 1, 312. [Google Scholar] [CrossRef]
- Chu, C.-H.; Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chang, W.-H.; Lee, G.-Y.; Chyi, J.-I.; Chen, C.-C.; Shiesh, S.-C.; et al. Beyond the Debye Length in High Ionic Strength Solution: Direct Protein Detection with Field-Effect Transistors (FETs) in Human Serum. Sci. Rep. 2017, 7, 5256. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Chen, Y.-W.; Sarangadharan, I.; Hsu, C.-P.; Chen, C.-C.; Shiesh, S.-C.; Lee, G.-B.; Wang, Y.-L. Editors’ Choice—Field-Effect Transistor-Based Biosensors and a Portable Device for Personal Healthcare. ECS J. Solid State Sci. Technol. 2017, 6, Q71–Q76. [Google Scholar] [CrossRef]
- Kao, W.-C.; Chen, Y.-W.; Chu, C.-H.; Chang, W.-H.; Shiesh, S.-C.; Wang, Y.-L.; Lee, G.-B. Detection of C-reactive Protein on an Integrated Microfluidic System by Utilizing Field-Effect Transistors and Aptamers. Biomicrofluidics 2017, 11, 044105. [Google Scholar] [CrossRef]
- Sinha, A.; Tai, T.-Y.; Lee, G.-B.; Wang, Y.-L. Integrated Microfluidic System with Field Effect Transistor for Automatic Detection of Multiple Cardiovascular Biomarkers. In IEEE Micro Electro Mechanical Systems; IEEE: New York, NY, USA, 2018. [Google Scholar]
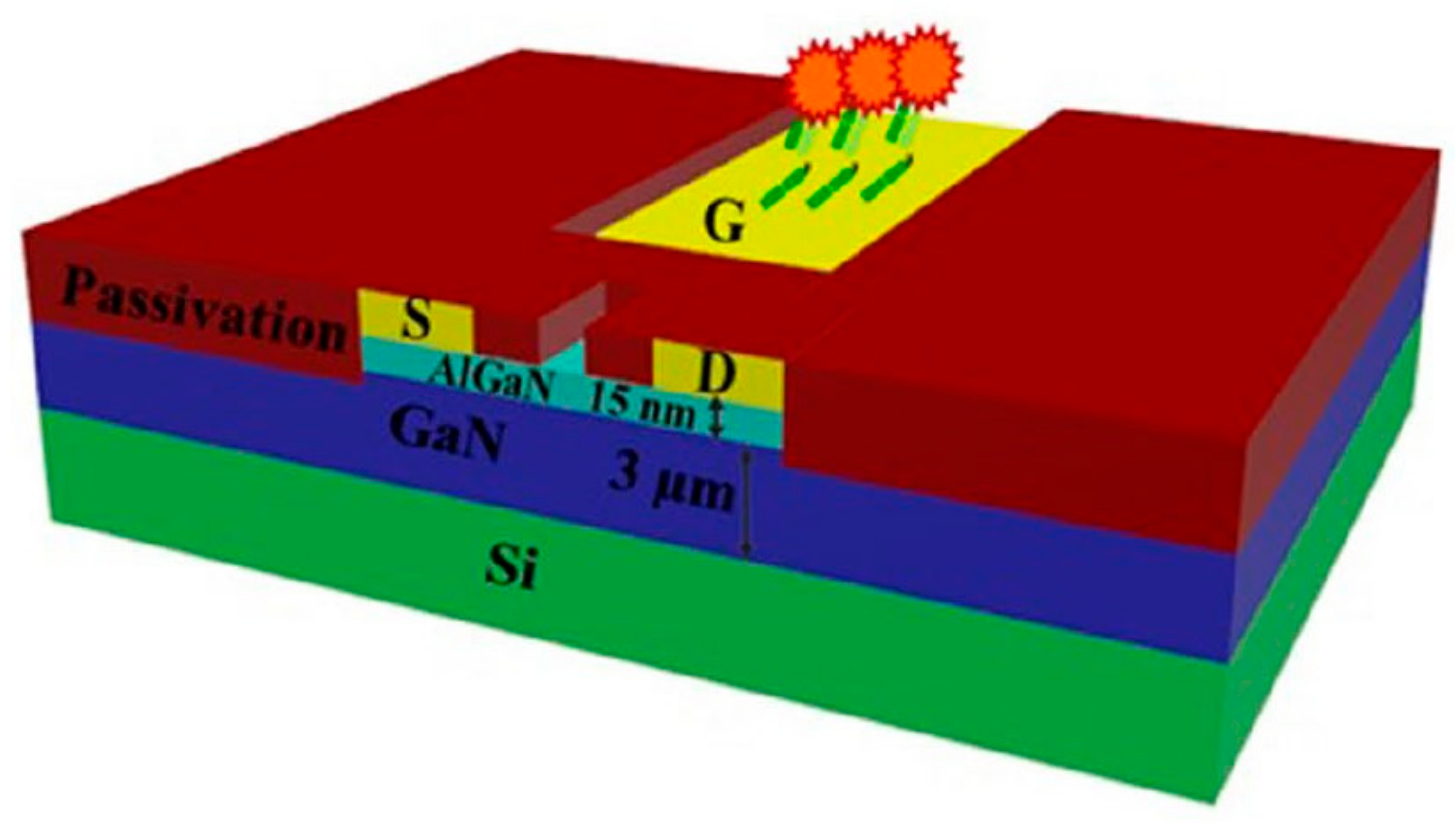
- Stock, D.; Muntze, G.M.; Figge, S.; Eickhoff, M. Ion Sensitive AlGaN/GaN Field-Effect Transistors with Monolithically Integrated Wheatstone Bridge for Temperature- and Drift Compensation in Enzymatic Biosensors. Sens. Actuators B Chem. 2018, 263, 20–26. [Google Scholar] [CrossRef]
- Jang, H.-J.; Lee, T.; Song, J.; Russell, L.; Li, H.; Dailey, J.; Searson, P.C.; Katz, H.E. Electronic Cortisol Detection Using an Antibody-Embedded Polymer Coupled to a Field-Effect Transistor. ACS Appl. Mater. Interfaces 2018, 10, 16233–16237. [Google Scholar] [CrossRef]
- Jeong, G.; Oh, J.; Jang, J. Fabrication of N-Doped Multidimensional Carbon Nanofibers for High-Performance Cortisol Biosensors. Biosens. Bioelectron. 2019, 131, 30–36. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Pu, H.; Chang, J.; Mao, S.; Chen, J. Field-Effect Transistor Biosensors with Two-Dimensional Black Phosphorus Nanosheets. Biosens. Bioelectron. 2017, 89, 505–510. [Google Scholar] [CrossRef]
- Seshadri, P.; Manoli, K.; Schneiderhan-Marra, N.; Anthes, U.; Wierzchowiec, P.; Bonrad, K.; Franco, C.D.; Torsi, L. Low-Picomolar, Label-Free Procalcitonin Analytical Detection with an Electrolyte-Gated Organic Field-Effect Transistor Based Electronic Immunosensor. Biosens. Bioelectron. 2018, 104, 113–119. [Google Scholar] [CrossRef]
- Shi, W.; Yu, J.; Katz, H.E. Sensitive and Selective Pentacene-Guanine Field-Effect Transistor Sensing of Nitrogen Dioxide and Interferent Vapor Analytes. Sens. Actuators B Chem. 2018, 254, 940–948. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Pu, H.; Guo, X.; Chang, J.; Zhou, G.; Mao, S.; Kron, M.; Chen, J. Field-Effect Transistor Biosensor for Rapid Detection of Ebola Antigen. Sci. Rep. 2017, 7, 10974. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Lin, W.-S.; Wong, J.-C.; Hsu, W.-C.; Peng, Y.-S.; Chen, C.-L. Bottom-Up Assembly of Silicon Nanowire Conductometric Sensors for the Detection of Apolipoprotein A1, a Biomarker for Bladder Cancer. Microchim. Acta 2017, 184, 2419–2428. [Google Scholar] [CrossRef]
- Presnova, G.; Presnov, D.; Krupenin, V.; Grigorenko, V.; Trifonov, A.; Andreeva, I.; Ignatenko, O.; Egorov, A.; Rubtsova, M. Biosensor Based on a Silicon Nanowire Field-Effect Transistor Functionalized by Gold Nanoparticles for the Highly Sensitive Determination of Prostate Specific Antigen. Biosens. Bioelectron. 2017, 88, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, M.; Presnova, G.; Presnov, D.; Krupenin, V.; Grigorenko, V.; Egorov, A. Biosensor Based on a Nanowire Field-Effect Transistor for the Determination of Prostate Specific Antigen. Procedia Technol. 2017, 27, 234–235. [Google Scholar] [CrossRef]
- Lei, Y.-M.; Xiao, M.-M.; Li, Y.-T.; Xu, L.; Zhang, H.; Zhang, Z.-Y.; Zhang, G.-J. Detection of Heart Failure-Related Biomarker in Whole Blood with Graphene Field Effect Transistor Biosensor. Biosens. Bioelectron. 2017, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Munief, W.-M.; Lu, X.; Teucke, T.; Wilhelm, J.; Britz, A.; Hempel, F.; Lanche, R.; Schwartz, M.; Law, J.K.Y.; Grandthyll, S.; et al. Reduced Graphene Oxide Biosensor Platform for the Detection of NT-ProBNP Biomarker in Its Clinical Range. Biosens. Bioelectron. 2019, 126, 136–142. [Google Scholar] [CrossRef]
- Islam, S.; Shukla, S.; Bajpaj, V.K.; Han, Y.-K.; Huh, Y.S.; Kumar, A.; Ghosh, A.; Gandhi, S. A Smart Nanosensor for the Detection of Human Immunodeficiency Virus and Associated Cardiovascular and Arthritis Diseases Using Functionalized Graphene-Based Transistors. Biosens. Bioelectron. 2019, 126, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Si, K.; Cheng, S.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Multianalyte Detection of Cancer Biomarkers in Human Serum Using Label-Free Field Effect Transistor Biosensor. Sens. Mater. 2018, 30, 991–999. [Google Scholar]
- Islam, S.; Shukla, S.; Bajpaj, V.K.; Han, Y.-K.; Huh, Y.S.; Ghosh, A.; Gandhi, S. Microfluidic-Based Graphene Field Effect Transistor for Femtomolar Detection of Chlorpyrifos. Sci. Rep. 2019, 9, 276. [Google Scholar] [CrossRef]
- Berto, M.; Vecchi, E.; Baiamonte, L.; Condo, C.; Sensi, M.; Lauro, M.D.; Sola, M.; Stradis, A.D.; Biscarini, F.; Minafra, A.; et al. Label Free Detection of Plant Viruses with Organic Transistor Biosensors. Sens. Actuators B Chem. 2019, 281, 150–156. [Google Scholar] [CrossRef]
- Napoli, C.; Lai, S.; Giannetti, A.; Tombelli, S.; Baldini, F.; Barbaro, M.; Bonfiglio, A. Electronic Detection of DNA Hybridization by Coupling Organic Field-Effect Transistor-Based Sensors and Hairpin-Shaped Probes. Sensors 2018, 18, 990. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xia, H.; Zauberman, J.; Tomaiuolo, M.; Ping, J.; Zhang, Q.; Ducos, P.; Ye, H.; Wang, S.; Yang, X.; et al. Detection of Sub-fM DNA with Target Recycling and Self-Assembly Amplification on Graphene Field-Effect Biosensors. Nano Lett. 2018, 18, 3509–3515. [Google Scholar] [CrossRef]
- Mei, J.; Li, Y.-T.; Zhang, H.; Xiao, M.-M.; Ning, Y.; Zhang, Z.-Y.; Zhang, G.-J. Molybdenum Disulfide Field-Effect Transistor Biosensor for Ultrasensitive Detection of DNA by Employing Morpholino as Probe. Biosens. Bioelectron. 2018, 110, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Wang, Q.; Xiao, M.; Zhong, D.; Sun, W.; Zhang, G.; Zhang, Z. Ultrasensitive Monolayer MoS2 Field-Effect Transistor Based DNA Sensors for Screening of Down Syndrome. Nano Lett. 2019, 19, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.; Borme, J.; Guerreiro, J.R.; Machado, G., Jr.; Cerqueira, M.F.; Petrovykh, D.Y.; Alpuim, P. Attomolar Label-Free Detection of DNA Hybridization with Electrolyte-Gated Graphene Field-Effect Transistors. ACS Sens. 2019, 4, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Fatin, M.F.; Ruslinda, A.R.; Gopinath, S.C.B.; Arshad, M.K.M. High-Performance Interactive Analysis of Split Aptamer and HIV-1 Tat on Multiwall Carbon Nanotube-Modified Field-Effect Transistor. Int. J. Biol. Macromol. 2019, 125, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Majd, S.M.; Salimi, A.; Astinchap, B. The Development of Radio Frequency Magnetron Sputtered P-Type Nickel Oxide Thin Film Field-Effect Transistor Device Combined with Nucleic Acid Probe for Ultrasensitive Label-Free HIV-1 Gene Detection. Sens. Actuators B Chem. 2018, 266, 178–186. [Google Scholar] [CrossRef]
- Singh, N.K.; Thungon, P.D.; Estrela, P.; Goswami, P. Development of an Aptamer-Based Field Effect Transistor Biosensor for Quantitative Detection of Plasmodium Falciparum Glutamate Dehydrogenase in Serum Samples. Biosens. Bioelectron. 2019, 123, 30–35. [Google Scholar] [CrossRef]
- Hao, Z.; Zhu, Y.; Wang, X.; Rotti, P.G.; DiMarco, C.; Tyler, S.R.; Zhao, X.; Engelhardt, J.F.; Hone, J.; Lin, Q. Real-Time Monitoring of Insulin Using a Graphene Field-Effect Transistor Aptameric Nanosensor. ACS Appl. Mater. Interfaces 2017, 9, 27504–27511. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.-A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer–Field-Effect Transistors Overcome Debye Length Limitations for Small-Molecule Sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, S.; Wustoni, S.; Kobayashi, M.; Hayashi, H.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Effect of Human Serum on the Electrical Detection of Amyloid-β Fibrils in Biological Environments Using Azo-Dye Immobilized Field Effect Transistor (FET) Biosensor. Sens. Biosens. Res. 2018, 17, 25–29. [Google Scholar] [CrossRef]
- Gutierrez-Sanz, O.; Andoy, N.M.; Filipiak, M.S.; Haustein, N.; Tarasov, A. Direct, Label-Free, and Rapid Transistor-Based Immunodetection in Whole Serum. ACS Sens. 2017, 2, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Andoy, N.M.; Filipiak, M.S.; Vettel, D.; Gutierrez-Sanz, O.; Tarasov, A. Graphene-Based Electronic Immunosensor with Femtomolar Detection Limit in Whole Serum. Adv. Mater. Technol. 2018, 3, 1800186. [Google Scholar] [CrossRef]
- Filipiak, M.S.; Rother, M.; Andoy, N.M.; Knudsen, A.C.; Grimm, S.; Bachran, C.; Swee, L.K.; Zaumseil, J.; Tarasov, A. Highly Sensitive, Selective and Label-Free Protein Detection in Physiological Solutions Using Carbon Nanotube Transistors with Nanobody Receptors. Sens. Actuators B Chem. 2018, 255, 1507–1516. [Google Scholar] [CrossRef]
- Hideshima, S.; Saito, M.; Fujita, K.; Harada, Y.; Tsuna, M.; Sekiguchi, S.; Kuroiwa, S.; Nakanishi, T.; Osaka, T. Label-free Detection of Allergens in Food via Surfactant-Induced Signal Amplification Using a Field Effect Transistor-Based Biosensor. Sens. Actuators B Chem. 2018, 254, 1011–1016. [Google Scholar] [CrossRef]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.-M.; Lieber, C.-M. General Strategy for Biodetection in High Ionic Strength Solutions Using Transistor-Based Nanoelectronic Sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haustein, N.; Gutierrez-Sanz, O.; Tarasov, A. Analytical Model to Describe the Effect of Polyethylene Glycol on Ionic Screening of Analyte Charges in Transistor-Based Immusisssnosensing. ACS Sens. 2019, 4, 874–882. [Google Scholar] [CrossRef]


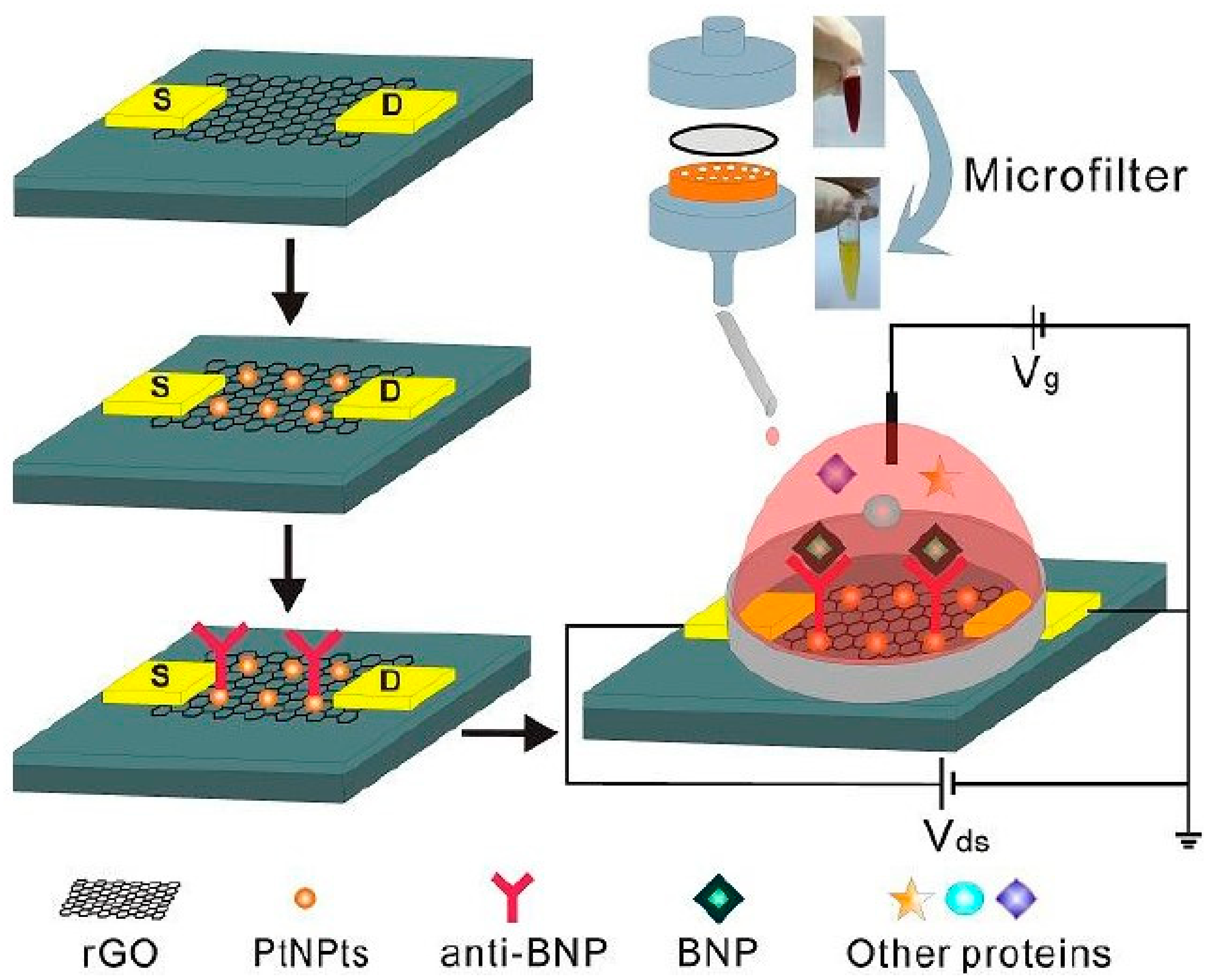
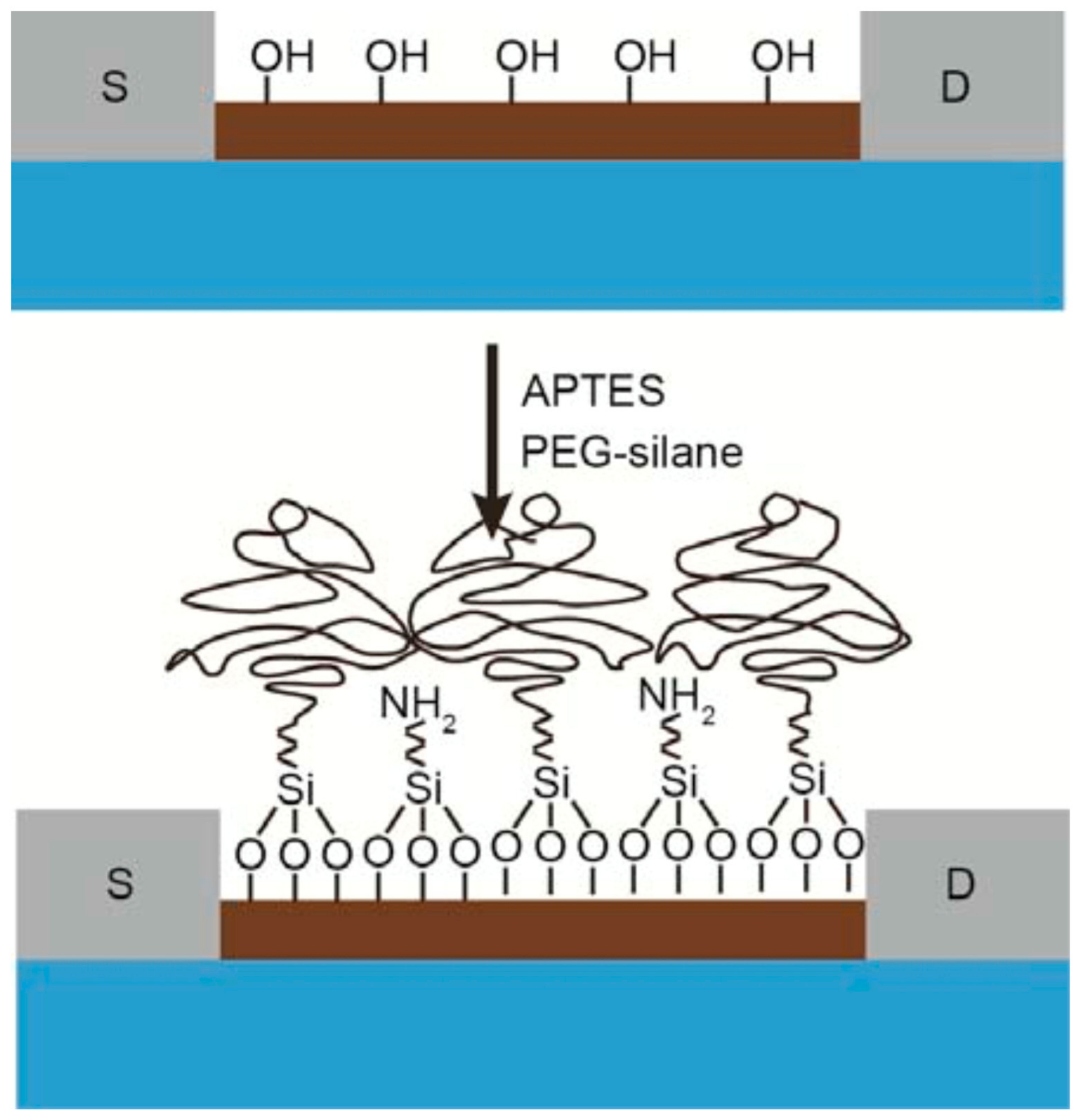
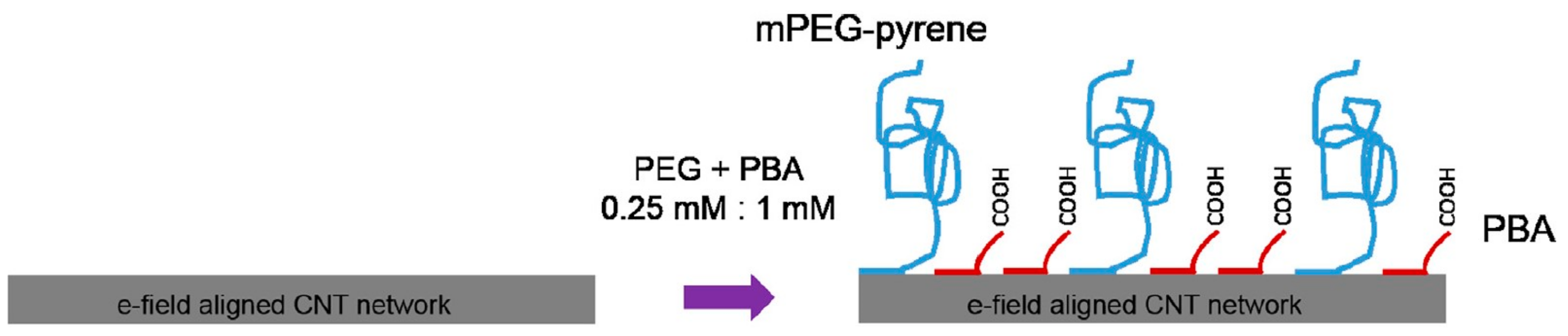
| Transducer Material | Bioprobe | Target Molecule | Analyte | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Graphene foam | ATP Aptamer | ATP | 0.1 × HS | 0.5 pM | 0.5 pM–50 µM | [63] |
| Reduced Graphene Oxide | Anti-E. coli | E. coli | Deionized water River water | 103 CFU/mL 104 CFU/mL | 103–105 CFU/mL | [64] |
| Indium Tin Oxide Nanowire | DNA | HBV DNA | 100 mM PBS | 1 fM | 1 fM–10 µM | [66] |
| Zinc Oxide Nanoribbon | GOx | Glucose | 100 mM PBS | 70 µM | 0.0–80 mM | [67] |
| Zinc Oxide Nanoribbon | GOx | Glucose | 10 mM PBS | 3.8 µM | 10 µM–5 mM | [68] |
| Zinc Oxide Nanoribbon | PyO | Phosphate | 0.02 M HEPES | 0.5 µM | 0.1 µM–7 mM | [69] |
| Silicon Nanoribbon | Anti-CEA | CEA | 0.01 × PBS | 0.01 ng/mL | 0.01–100 ng/mL | [70] |
| Silicon Nanoribbon | Anti-PSA | PSA | 100 mM PBS HS | 10 pM 100 pM | 10 pM–1 µM 100 pM–1 µM | [71] |
| Silicon Nanoribbon | DNA | DNA of CorS | 0.1 × PBS | 50 pM | 50 pM–1 μM | [72] |
| Aluminum Gallium Nitride | HIV-1 Aptamer Half anti-CEA CRP Aptamer CRP Aptamer Anti-NT-proBNP | HIV-1 RT CEA CRP CRP NT-proBNP | 1 × PBS 1 × PBS 1 × PBS HS HS | 1 fM–10 pM 100 fM–1 nM 1 fM–100 nM 0.34–23.2 mg/L 180.9 pg/mL– 5 ng/mL | [74] | |
| Indium Tin Oxide | Anti-Cortisol | Cortisol | 1 × PBS | 1 pg/mL | 10 fg/mL–10 ng/mL | [79] |
| Needle-like Carbon Nanofiber | Anti-Cortisol | Cortisol | PBS | 100 aM | 100 aM–10 nM | [80] |
| Black Phosphorus | Anti-IgG | Human IgG | 0.01 × PBS | 10 ng/mL | 10–500 ng/mL | [81] |
| Poly-3-hexyl-thiophene | Anti-PCT | PCT | PBS | 2.2 pM | 0.8 pM–4.7 nM | [82] |
| Graphene | Human anti-EGP | EGP | 0.01 × PBS, HS, Human Plasma | 1 ng/mL | 1–444 ng/mL | [84] |
| Silicon Nanowire | Anti-APOA1 | hAPOA1 | 0.01 × PBS | 1 ng/mL | 100 pg/mL–10 μg/mL | [85] |
| Silicon Nanoribbon | Anti-PSA | PSA | 0.01 × PBS | 23 fg/mL | 23 fg/mL–500 ng/mL | [86] |
| Reduced Graphene Oxide | Anti-BNP | BNP | 0.001 × PBS | 100 fM | 100 fM–1 nM | [88] |
| Reduced Graphene Oxide | Anti-NT-proBNP | NT-proBNP | HS | 10 pg/mL | [89] | |
| Graphene | Anti-p24 Anti-cTn1 Anti-CCP | p24-HIV cTn1 CCP | 50 mM PBS 50 mM PBS 50 mM PBS | 100 fg/mL 10 fg/mL 10 fg/mL | 1 fg/mL–1 μg/mL 1 fg/mL–1 μg/mL 1 fg/mL–1 μg/mL | [90] |
| Silicon | Anti-AFP Anti-CYFRA 21-1 | AFP CYFRA 21-1 | HS HS | 10 ng/mL 1 ng/mL | 1–100 ng/mL 1–100 ng/mL | [91] |
| Graphene | Anti-Chlorpyrifos | Chlorpyrifos | Standard PB | 1.8 fM | 1 fM–1 μM | [92] |
| Pentacene | Anti-PPV | PPV | 50 mM PBS | 180 pg/mL | 5 ng/mL–50 μg/mL | [93] |
| Organic | Hairpin-shaped DNA | DNA | TE 1 M NaCl | 100 pM | 100 pM–10 nM | [94] |
| Graphene | Hairpin-shaped DNA | DNA | 5 × saline-sodium citrate | 5 fM | [95] | |
| Molybdenum Disulfide | Phosphorodiamidate Morpholino Oligos | DNA | 0.5 × PBS 10 × diluted HS | 6 fM | 10 fM–1 nM 10 fM–1 pM | [96] |
| Molybdenum Disulfide | DNA | DNA | 0.1 × PBS | 100 aM | 100 aM–1 fM | [97] |
| Graphene | DNA | DNA | 10 mM PB, 150 mM NaCl, 50 mM MgCl2 | 25 aM | 1 aM–100 fM | [98] |
| Multiwall Carbon Nanotubes | HIV-1 Aptamer | HIV-1 Tat | Blood sample | 600 pM | 0.2 nM–1 µM | [99] |
| Nickel Oxide | DNA | HIV DNA | 0.01 M PBS | 0.3 aM | 1 aM–10 nM | [100] |
| Silicon | PfGDH Aptamer | PfGDH | HS 50 mM K3PO4, 50 mM NaCl, 5 mM KCl, 2.5 mM MgCl2 | 48.6 pM 16.7 pM | 100 fM–10 nM 100 fM–10 nM | [101] |
| Graphene | Insulin Aptamer | Insulin | PBS | 35 pM | 100 pM–1 µM | [102] |
| Indium (III) Oxide | Dopamine Aptamer Serotonin Aptamer S1P Aptamer Glucose Aptamer Glucose Aptamer | Dopamine Serotonin Lipid S1P Glucose Glucose | 1 × PBS, 1 × aCSF 1 × PBS, 1 × aCSF 1 × HEPES 1 × Ringer buffer Whole blood diluted with 1 × Ringer buffer | 10−14–10−9 M 10−14–10−9 M 10 pM–100 nM 10 pM–10 nM 10 μM–1 mM | [103] | |
| Silicon | Congo Red | Aβ fibrils | HS | 100 pM–10 μM | [104] | |
| Metal Oxide | F(ab’)2 of anti-TSH | TSH | Serum | 500 fM | 500 fM–10 nM | [105] |
| Graphene | F(ab’)2 of anti-TSH | TSH | Serum | 10 fM | 0.8 fM–1 nM | [106] |
| Singlewall Carbon Nanotubes | Nanobody | GFP | 100 mM Tris | <1 pM–10 nM | [107] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, C.-A.; Chen, W.-Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors 2019, 19, 4214. https://doi.org/10.3390/s19194214
Vu C-A, Chen W-Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors. 2019; 19(19):4214. https://doi.org/10.3390/s19194214
Chicago/Turabian StyleVu, Cao-An, and Wen-Yih Chen. 2019. "Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects" Sensors 19, no. 19: 4214. https://doi.org/10.3390/s19194214
APA StyleVu, C.-A., & Chen, W.-Y. (2019). Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors, 19(19), 4214. https://doi.org/10.3390/s19194214





