New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape
Abstract
1. Introduction
2. Experimental
2.1. Materials and Instruments
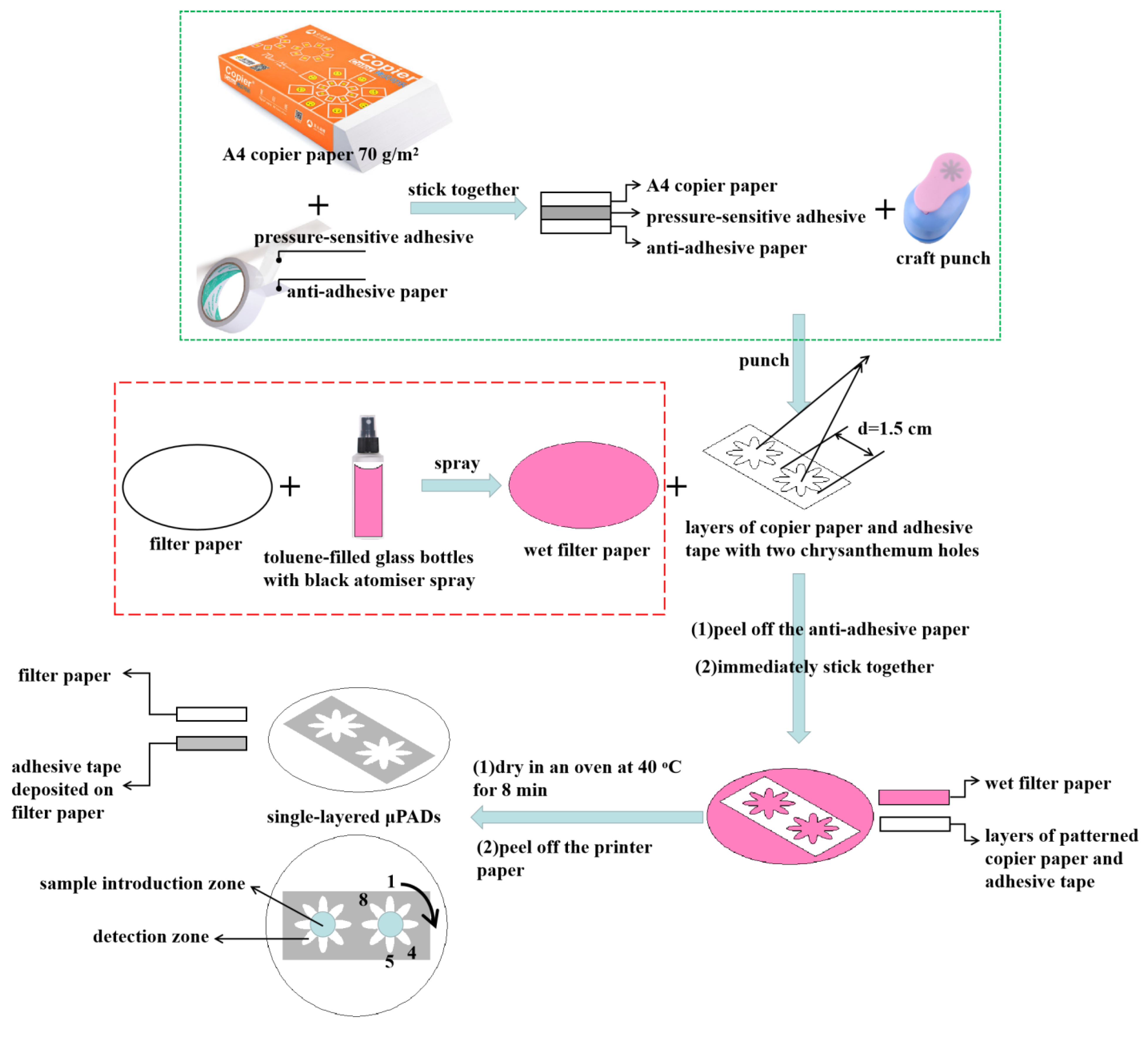
2.2. The Fabrication of Single-Layered μPADs
2.3. Nitrite Detection
2.4. Glucose Detection
2.5. Multiplexed Analysis of Nitrite and Glucose
2.6. Recovery Test
3. Results and Discussion
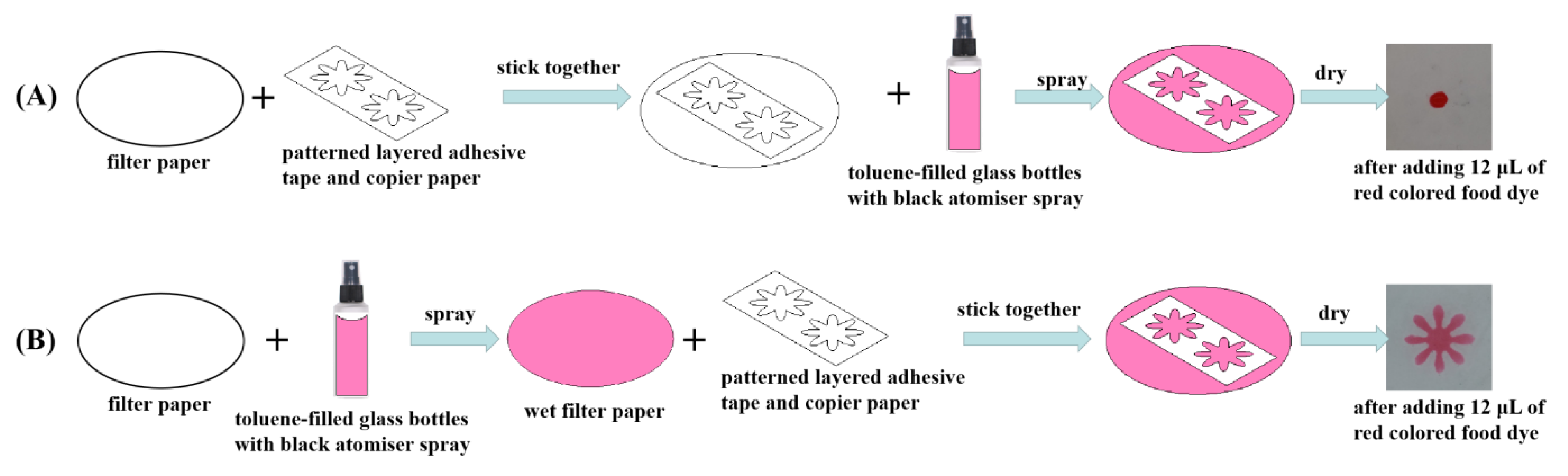
3.1. The Optimization for Fabrication Process
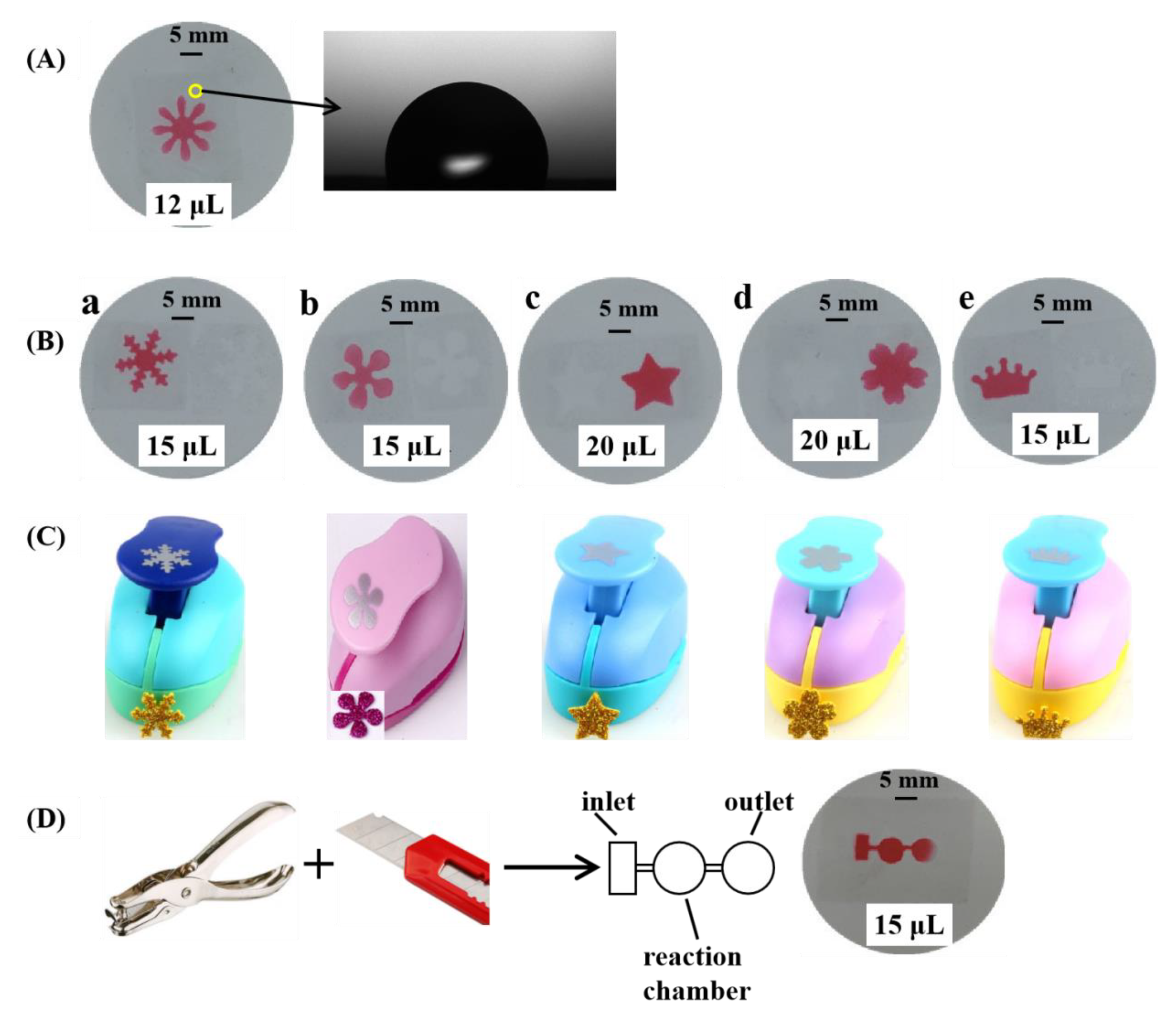
3.2. The Fabrication of μPADs
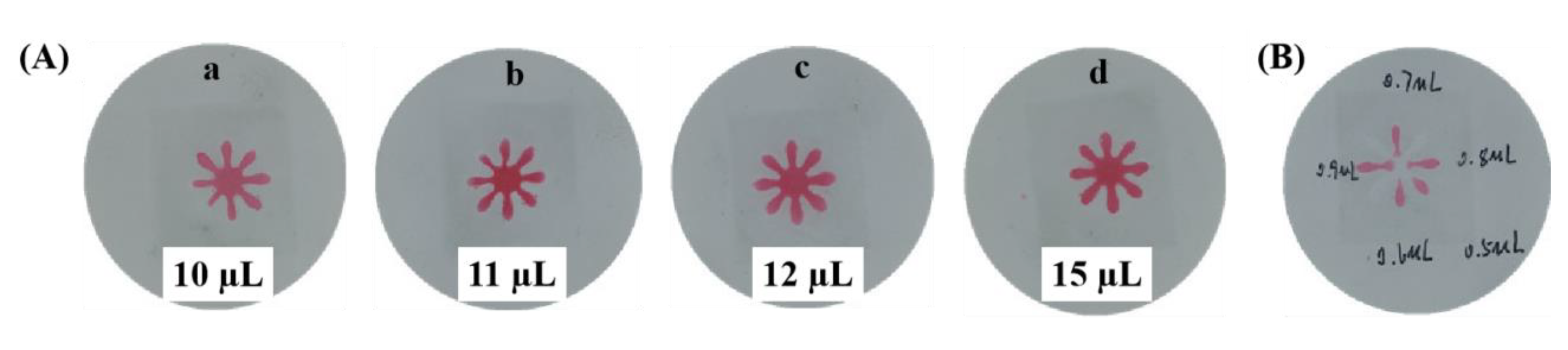
3.3. The Optimization of Sample Volume
3.4. Nitrite Assay Performance
3.5. Glucose Assay Performance
3.6. Multiplexed Analysis of Nitrite and Glucose
3.7. Specificity, Reproducibility and Stability
3.8. Recovery Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low Volume, Portable Bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zi, X.; Zeng, H.; Sun, J.; Xu, L.; Chen, S. Low-cost fabrication of a paper-based microfluidic using a folded pattern paper. Anal. Chim. Acta 2019, 1053, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, H.; Jian, Y.; Lan, F.; Zhang, L.; Liu, H.; Ge, S.; Yu, J. Ultrasensitive microfluidic paper-based electrochemical/visual biosensor based on spherical-like cerium dioxide catalyst for miR-21 detection. Biosens. Bioelectron. 2018, 105, 218–225. [Google Scholar] [CrossRef]
- Wang, L.; Musile, G.; McCord, B.R. An aptamer-based paper microfluidic device for the colorimetric determination of cocaine. Electrophoresis 2018, 39, 470–475. [Google Scholar] [CrossRef]
- Fiedoruk-Pogrebniak, M.; Granica, M.; Koncki, R. Compact detectors made of paired LEDs for photometric and fluorometric measurements on paper. Talanta 2018, 178, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Malic, L.; Veres, T.; Tabrizian, M. Biochip functionalization using electrowetting-on-dielectric digital microfluidics for surface plasmon resonance imaging detection of DNA hybridization. Biosens. Bioelectron. 2009, 24, 2218–2224. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Alam, A.M.; Kim, K.M.; Lee, S.H.; Kim, Y.H.; Kabir, A.H.; Kim, G.M.; Dang, T.D. Chemiluminescence microfluidic system of gold nanoparticles enhanced luminol-silver nitrate for the determination of vitamin B12. Biomed. Microdevices 2013, 15, 195–202. [Google Scholar] [CrossRef]
- Coltro, W.K.T.; De Jesus, D.P.; Da Silva, J.A.F.; Lago, C.L.D.; Carrilho, E.; Silva, J. Toner and paper-based fabrication techniques for microfluidic applications. Electrophoresis 2010, 31, 2487–2498. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (μPADs) and Micro Total Analysis Systems (μTAS): Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef]
- Lee, J.; Soper, S.A.; Murray, K.K. Microfluidic chips for mass spectrometry-based proteomics. J. Mass Spectrom. 2009, 44, 579–593. [Google Scholar] [CrossRef]
- Gerold, C.T.; Bakker, E.; Henry, C.S. Selective distance-based K+ quantification on paper-based microfluidics. Anal. Chem. 2018, 90, 4894–4900. [Google Scholar] [CrossRef]
- Koesdjojo, M.T.; Pengpumkiat, S.; Boonloed, A.; Huynh, D.; Remcho, T.P.; Remcho, V.T.; Wu, Y. Cost Effective Paper-Based Colorimetric Microfluidic Devices and Mobile Phone Camera Readers for the Classroom. J. Chem. Educ. 2015, 92, 737–741. [Google Scholar] [CrossRef]
- Rossini, E.L.; Milani, M.I.; Carrilho, E.; Pezza, L.; Pezza, H.R. Simultaneous determination of renal function biomarkers in urine using a validated paper-based microfluidic analytical device. Anal. Chim. Acta 2018, 997, 16–23. [Google Scholar] [CrossRef]
- Chabaud, K.R.; Thomas, J.L.; Torres, M.N.; Oliveira, S.; McCord, B.R. Simultaneous colorimetric detection of metallic salts contained in low explosives residue using a microfluidic paper-based analytical device (µPAD). Forensic Chem. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Taghizadeh-Behbahani, M.; Hemmateenejad, B.; Shamsipur, M. Colorimetric determination of acidity constant using a paper-based microfluidic analytical device. Chem. Pap. 2018, 72, 1239–1247. [Google Scholar] [CrossRef]
- Liu, C.; Gomez, F.A.; Miao, Y.; Cui, P.; Lee, W. A colorimetric assay system for dopamine using microfluidic paper-based analytical devices. Talanta 2019, 194, 171–176. [Google Scholar] [CrossRef]
- Da Silva, G.O.; De Araujo, W.R.; Paixão, T.R. Portable and low-cost colorimetric office paper-based device for phenacetin detection in seized cocaine samples. Talanta 2018, 176, 674–678. [Google Scholar] [CrossRef]
- Chiang, C.K.; Kurniawan, A.; Kao, C.Y.; Wang, M.J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef]
- Puneeth, S.B.; Goel, S. Novel 3D Printed Microfluidic Paper-Based Analytical Device with Integrated Screen-Printed Electrodes for Automated Viscosity Measurements. IEEE Trans. Electron Devices 2019, 66, 3196–3201. [Google Scholar]
- Baker, C.A.; Casto, L.D.; A Schuster, J.; Neice, C.D. Characterization of low adsorption filter membranes for electrophoresis and electrokinetic sample manipulations in microfluidic paper-based analytical devices. Anal. Methods 2018, 10, 3616–3623. [Google Scholar]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef]
- Kao, P.-K.; Hsu, C.-C.; Hsu, J.C.-C. One-step rapid fabrication of paper-based microfluidic devices using fluorocarbon plasma polymerization. Microfluid. Nanofluidics 2014, 16, 811–818. [Google Scholar] [CrossRef]
- Demirel, G.; Babur, E. Vapor-phase deposition of polymers as a simple and versatile technique to generate paper-based microfluidic platforms for bioassay applications. Analyst 2014, 139, 2326–2331. [Google Scholar] [CrossRef]
- Cardoso, T.M.G.; Garcia, P.T.; Coltro, W.K.T. Colorimetric determination of nitrite in clinical, food and environmental samples using microfluidic devices stamped in paper platforms. Anal. Methods 2015, 7, 7311–7317. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Paper-based analytical device for instrumental-free detection of thiocyanate in saliva as a biomarker of tobacco smoke exposure. Talanta 2016, 147, 390–396. [Google Scholar] [CrossRef]
- Nurak, T.; Praphairaksit, N.; Chailapakul, O. Fabrication of paper-based devices by lacquer spraying method for the determination of nickel (II) ion in waste water. Talanta 2013, 114, 291–296. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Ge, L.; Ge, S. A novel chemiluminescence paper microfluidic biosensor based on enzymatic reaction for uric acid determination. Biosens. Bioelectron. 2011, 26, 3284–3289. [Google Scholar] [CrossRef]
- Mentele, M.M.; Cunningham, J.; Koehler, K.; Volckens, J.; Henry, C.S. Microfluidic paper-based analytical device for particulate metals. Anal. Chem. 2012, 84, 4474–4480. [Google Scholar] [CrossRef]
- Ren, Y.; Ray, S.; Liu, Y. Reconfigurable Acrylic-tape Hybrid Microfluidics. Sci. Rep. 2019, 9, 4824. [Google Scholar] [CrossRef]
- Shiva, S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Boil. 2013, 1, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Viboonratanasri, D.; Pabchanda, S.; Prompinit, P. Rapid and simple preparation of rhodamine 6G loaded HY zeolite for highly selective nitrite detection. Appl. Surf. Sci. 2018, 440, 1261–1268. [Google Scholar] [CrossRef]
- Powell, H.R.; A McCredie, D.; A Ritchie, M. Urinary nitrite in symptomatic and asymptomatic urinary infection. Arch. Dis. Child. 1987, 62, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; Garcia, P.T.; Cardoso, T.M.G.; Lopes, F.M.; Martins, F.T.; Coltro, W.K.T. Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst 2016, 141, 4749–4756. [Google Scholar] [CrossRef]
- Liu, W.; Cassano, C.L.; Xu, X.; Fan, Z.H. Laminated Paper-Based Analytical Devices (LPAD) with Origami-Enabled Chemiluminescence Immunoassay for Cotinine Detection in Mouse Serum. Anal. Chem. 2013, 85, 10270–10276. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef]
- Vosmanská, V.; Kolářová, K.; Rimpelová, S.; Kolská, Z.; Švorčík, V. Antibacterial wound dressing: Plasma treatment effect on chitosan impregnation and in situ synthesis of silver chloride on cellulose surface. RSC Adv. 2015, 5, 17690–17699. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- Allegrini, F.; Olivieri, A.C. IUPAC-consistent approach to the limit of detection in partial least-squares calibration. Anal. Chem. 2014, 86, 7858–7866. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz-Duval, A.S.; Misra, S.K.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2019, 144, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Wang, H.; Huang, Y.; Tan, Z.; Hu, X. Fabrication and characterization of copper nanoparticle thin-films and the electrocatalytic behavior. Anal. Chim. Acta 2004, 526, 13–17. [Google Scholar] [CrossRef]
- Nam, Y.S.; Noh, K.C.; Kim, N.K.; Lee, Y.; Park, H.K.; Lee, K.B. Sensitive and selective determination of NO2− ion in aqueous samples using modified gold nanoparticle as a colorimetric probe. Talanta 2014, 125, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.L.; Han, M.S.; Lee, J.S.; Mirkin, C.A. Colorimetric nitrite and nitrate detection with gold nanoparticle probes and kinetic end points. J. Am. Chem. Soc. 2009, 131, 6362–6363. [Google Scholar] [CrossRef] [PubMed]
- Nilghaz, A.; Bagherbaigi, S.; Lam, C.L.; Mousavi, S.M.; Cόrcoles, E.P.; Wicaksono, D.H. Multiple semi-quantitative colorimetric assays in compact embeddable microfluidic cloth-based analytical device (μCAD) for effective point-of-care diagnostic. Microfluid. Nanofluid. 2015, 19, 317–333. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Z.; Wang, M. Fabrication of a paper-based microfluidic device to readily determine nitrite ion concentration by simple colorimetric assay. J. Chem. Educ. 2015, 92, 733–736. [Google Scholar] [CrossRef]
- Qinghai, S.; Bats, J.W.; Schmittel, M. Two closely related iridium (III) complexes as colorimetric and fluorometric chemodosimeters for nitrite in aqueous solution operating along different modes of action. Inorg. Chem. 2011, 50, 10531–10533. [Google Scholar] [CrossRef]
- Strasinger, S.K.; Di Lorenzo, M.S. Urinalysis and Body Fluids; FA Davis: Philadelphia, PA, USA, 2014. [Google Scholar]
- Yu, W.; Seo, W.; Tan, T.; Jung, B.; Ziaie, B. A diaper-embedded disposable nitrite sensor with integrated on-board urine-activated battery for UTI screening. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016; Volume 38, pp. 303–306. [Google Scholar]
- Kuan, C.M.; York, R.L.; Cheng, C.M. Lignocellulose-based analytical devices: Bamboo as an analytical platform for chemical detection. Sci. Rep. 2015, 5, 18570. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Garcia, P.; Lopes, F.; Coltro, W. Based Colorimetric Biosensor for Tear Glucose Measurements. Micromachines 2017, 8, 104. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Wang, F.; Xiang, X.; Luo, M.; Ji, X.; He, Z. Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosens. Bioelectron. 2012, 35, 363–368. [Google Scholar] [CrossRef] [PubMed]
- De Tarso Garcia, P.; Cardoso, T.M.G.; Garcia, C.D.; Carrilho, E.; Coltro, W.K.T. A handheld stamping process to fabricate microfluidic paper-based analytical devices with chemically modified surface for clinical assays. Rsc Adv. 2014, 4, 37637–37644. [Google Scholar] [CrossRef]
- Im, S.H.; Kim, K.R.; Park, Y.M.; Yoon, J.H.; Hong, J.W.; Yoon, H.C. An animal cell culture monitoring system using a smartphone-mountable paper-based analytical device. Sens. Actuat. B Chem. 2016, 229, 166–173. [Google Scholar] [CrossRef]
- Evans, E.; Gabriel, E.F.M.; Benavidez, T.E.; Coltro, W.K.T.; Garcia, C.D. Modification of microfluidic paper-based devices with silica nanoparticles. Analyst 2014, 139, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Ornatska, M.; Sharpe, E.; Andreescu, D.; Andreescu, S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal. Chem. 2011, 83, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Gómez, I.; Salinas-Castillo, A.; García, A.G.; Álvarez-Bermejo, J.A.; de Orbe-Payá, I.; Rodríguez-Diéguez, A.; Capitán-Vallvey, L.F. Microfluidic paper-based device for colorimetric determination of glucose based on a metal-organic framework acting as peroxidase mimetic. Microchim. Acta 2018, 185, 47. [Google Scholar] [CrossRef] [PubMed]
- Kissner, R.; Koppenol, W.H. Product distribution of peroxynitrite decay as a function of pH, temperature, and concentration. J. Am. Chem. Soc. 2002, 124, 234–239. [Google Scholar] [CrossRef] [PubMed]







| Sensing Reagents/Materials | Method | Response Time (min) | Volume (μL) | Linear Range (mM) | LOD (mM) | Refs |
|---|---|---|---|---|---|---|
| Copper nanoparticles modified electrodes | Electrochemistry | 0.05 | 0.05~30 | 0.02 | [46] | |
| Functionalized gold nanoparticles | colorimetric | 30 | 0~0.11 | 0.022 | [47] | |
| Griess reagent | colorimetric | 2 | 15 | 0.16 | [49] | |
| Griess reagent | colorimetric | 0.087 | [53] | |||
| Griess reagent | Colorimetric | 2 | 1.40 | 0.156~1.25 | [50] | |
| Iridium(III) complexes | Colorimetric | 240 | 0.05~20 | 0.05 | [51] | |
| Gold nanoparticle | Colorimetric | 25 | 100 | 0.022 | [48] | |
| Griess reagent | Colorimetric | 20 | 0.06 | [54] | ||
| Griess reagent | Colorimetric | 10 | 12 | 0.02~0.9 | 0.015 ± 0.004 | This work |
| Sensing Reagents/Materials | Method | Response Time (min) | Sample Volume (μL) | Linear Range (mM) | LOD (mM) | Refs |
|---|---|---|---|---|---|---|
| GOx, HRP, 4-AAP/DHBS | colorimetric | 15 | 70 | 0.1~1.0 | 0.023 | [37] |
| GOx, HRP, TMB | colorimetric | 15 | 70 | 1.0~5.0 | 0.057 | [37] |
| GOx, HRP, TMB | colorimetric | 15 | 5 | 0.1~1 | 0.05 | [55] |
| GOx, HRP, 4-AAP/TOPS | colorimetric | 30 | 2 | 0.3~1 | 0.213 | [56] |
| GOx, HRP, 4-AAP/DHBS | colorimetric | 40 | 2.0~12 | 0.7 | [57] | |
| GOx, HRP, 4-AAP/MAOS | colorimetric | 1 | 15 | 0.3~8.0 | 0.3 | [58] |
| GOx, HRP, KI/trehalose | colorimetric | 30 | 10 | 0.5~10 | 0.5 | [59] |
| GOx, Ceria nanoparticles | colorimetric | 10 | 0.5~100 | 0.5 | [60] | |
| GOx, HRP, KI | colorimetric | 10 | 0~27.8 | 1.4 | [23] | |
| GOx, HRP, TMB | Colorimetric | 10 | 12 | 0.05~0.7 | 0.022 ± 0.006 | This work |
| Solution # | Green Color Intensity | Mean Green Color Intensity | Std. Dev. | CV (%) | ||
| 1 | 136.26 | 144.34 | 140.54 | 140.38 | 4.04 | 2.88 |
| 2 | 121.63 | 117.63 | 122.09 | 120.45 | 2.45 | 2.03 |
| 3 | 105.51 | 103.44 | 97.86 | 102.27 | 3.96 | 3.87 |
| Solution # | Red Color Intensity | Mean Red Color Intensity | Std. Dev. | CV (%) | ||
| 4 | 116.10 | 122.91 | 121.27 | 120.09 | 3.55 | 2.96 |
| 5 | 88.80 | 86.32 | 92.35 | 89.16 | 3.03 | 3.40 |
| 6 | 78.66 | 73.88 | 74.35 | 75.63 | 2.63 | 3.48 |
| Nitrite Conc. (mM) | Batch #/Sensor #/Green Color Intensity | Mean Green Color Intensity | Mean of Means | Std. of Means | CV (%) | ||
| High (0.9) | 1/1/105.51 | 1/2/103.44 | 1/3/97.86 | Batch #1: 102.27 | 100.83 | 4.41 | 4.37 |
| High (0.9) | 2/1/98.22 | 2/2/96.14 | 2/3/93.28 | Batch #2: 95.88 | |||
| High (0.9) | 3/1/102.58 | 3/2/106.79 | 3/3/103.66 | Batch #3: 104.34 | |||
| Medium (0.5) | 1/1/116.63 | 1/2/115.60 | 1/3/122.09 | Batch #1: 118.11 | 120.70 | 5.93 | 4.91 |
| Medium (0.5) | 2/1/129.35 | 2/2/130.21 | 2/3/122.89 | Batch #2: 127.48 | |||
| Medium (0.5) | 3/1/114.58 | 3/2/116.42 | 3/3/118.49 | Batch #3: 116.50 | |||
| Low (0.1) | 1/1/136.26 | 1/2/144.34 | 1/3/140.54 | Batch #1: 140.38 | 141.12 | 4.60 | 3.26 |
| Low (0.1) | 2/1/145.36 | 2/2/146.30 | 2/3/142.11 | Batch #2: 144.59 | |||
| Low (0.1) | 3/1/130.58 | 3/2/139.41 | 3/3/136.22 | Batch #3: 135.40 | |||
| Glucose Conc. (mM) | Batch #/Sensor #/Red Color Intensity | Mean Red Color Intensity | Mean of Means | Std. of Means | CV (%) | ||
| High (0.7) | 1/1/78.66 | 1/2/73.88 | 1/3/74.35 | Batch #1: 75.63 | 76.28 | 4.19 | 5.49 |
| High (0.7) | 2/1/80.25 | 2/2/79.48 | 2/3/82.55 | Batch #2: 80.76 | |||
| High (0.7) | 3/1/72.53 | 3/2/73.88 | 3/3/70.96 | Batch #3: 72.46 | |||
| Medium (0.5) | 1/1/88.80 | 1/2/86.32 | 1/3/92.35 | Batch #1: 89.16 | 88.72 | 3.75 | 4.23 |
| Medium (0.5) | 2/1/85.69 | 2/2/82.11 | 2/3/86.52 | Batch #2: 84.77 | |||
| Medium (0.5) | 3/1/94.26 | 3/2/90.81 | 3/3/91.63 | Batch #3: 92.23 | |||
| Low (0.1) | 1/1/116.1 | 1/2/122.91 | 1/3/121.27 | Batch #1: 120.09 | 119.74 | 5.00 | 4.18 |
| Low (0.1) | 2/1/126.32 | 2/2/122.20 | 2/3/125.16 | Batch #2: 124.56 | |||
| Low (0.1) | 3/1/112.56 | 3/2/114.79 | 3/3/116.36 | Batch #3: 114.57 | |||
| nitrite | Sample No. | Added (mM) | Found (mM) | RSD (%) | Recovery (%) |
| 1 | 0.1 | 0.105 | 1.7 | 105 | |
| 2 | 0.5 | 0.548 | 3.4 | 110 | |
| 3 | 0.7 | 0.784 | 4.6 | 112 | |
| 4 | 0.9 | 0.906 | 1.8 | 101 | |
| glucose | Sample No. | Added (mM) | Found (mM) | RSD (%) | Recovery (%) |
| 1 | 0.05 | 0.0480 | 1.8 | 96 | |
| 2 | 0.1 | 0.0933 | 1.8 | 93 | |
| 3 | 0.15 | 0.137 | 3.0 | 91 | |
| 4 | 0.2 | 0.215 | 4.5 | 108 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Deng, M.; Yang, Y. New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape. Sensors 2019, 19, 4082. https://doi.org/10.3390/s19194082
Yu P, Deng M, Yang Y. New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape. Sensors. 2019; 19(19):4082. https://doi.org/10.3390/s19194082
Chicago/Turabian StyleYu, Peng, Muhan Deng, and Yi Yang. 2019. "New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape" Sensors 19, no. 19: 4082. https://doi.org/10.3390/s19194082
APA StyleYu, P., Deng, M., & Yang, Y. (2019). New Single-Layered Paper-Based Microfluidic Devices for the Analysis of Nitrite and Glucose Built via Deposition of Adhesive Tape. Sensors, 19(19), 4082. https://doi.org/10.3390/s19194082






