Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection and Quality Assessment
3. Results
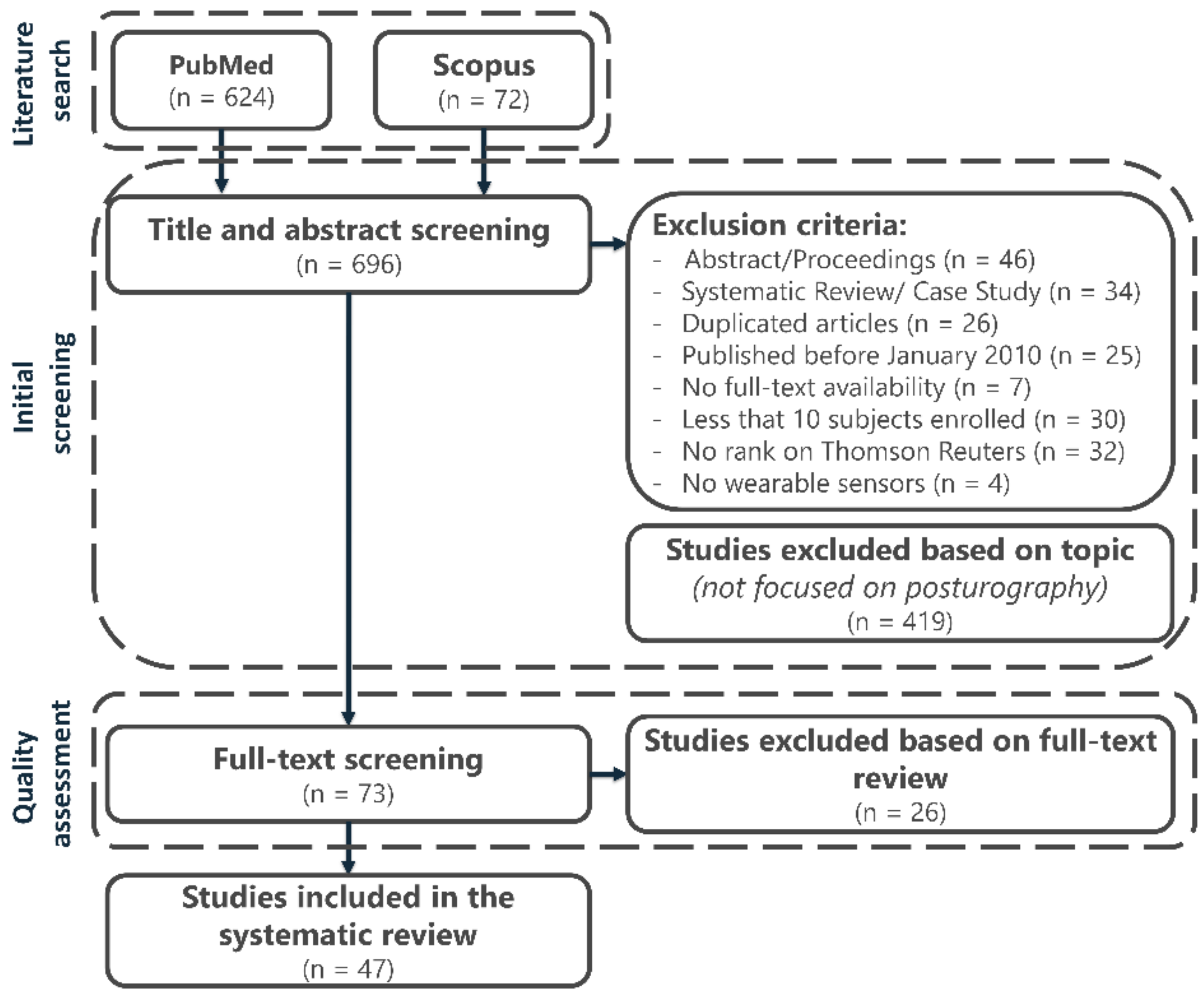
3.1. Searching Results and Study Selection
3.2. Quality Assessment Results
3.3. Sample Population Characteristics
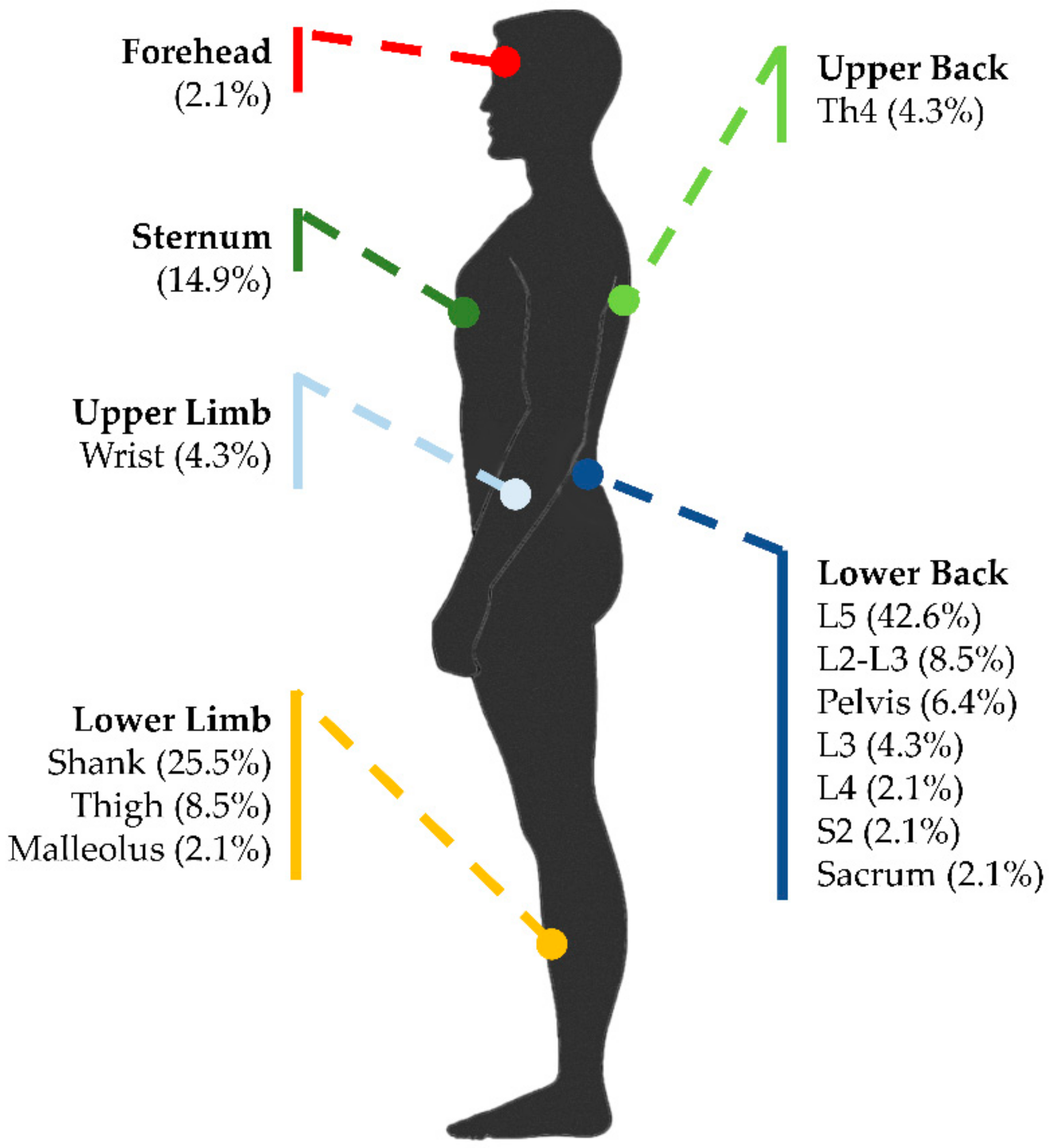
3.4. Sensor Type and Placement
3.5. Parameters for Standing-Balance Assessment
3.6. Validation Against a Gold Standard
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | Accelerometer |
| AP | Anteroposterior |
| BBS | Berg Balance Scale |
| BESS | Balance Error Scoring System |
| CF | Centroidal frequency |
| COM | Center of Mass |
| COP | Center of Pression |
| DPN | Diabetic Peripheral Neuropathy |
| EO | Eyes Open |
| EC | Eyes Closed |
| EV | External Validity |
| FD | Frequency Dispersion |
| FGD | Frontal Gait Disorder |
| FOG | Freezing of Gait |
| GYR | Gyroscope |
| IMU | Inertial Measurement Unit |
| iPD | Idiopathic Parkinson’s Disease |
| IV | Internal Validity |
| JI | Jerk Index |
| MAG | Magnetometer |
| MD | Mean Distance |
| MIMU | Magneto Inertial Measurement Unit |
| ML | Mediolateral |
| MS | Multiple Sclerosis |
| MV | Mean Sway Velocity |
| N/A | Not Available |
| nJI | Normalized Jerk Index |
| PD | Parkinson’s Disease |
| PIGD | Postural Instability Gait Difficulty |
| RMS | Root Mean Square |
| SA | Sway Area |
| SAW | Stand and Walk |
| SOT | Sensory Organization Test |
| SPL | Sway Path Length |
| TBI | Traumatic Brain Injury |
| TD | Tremor Dominant |
| vPD | Time-Up-and-GO Test |
| TUG | Vascular Parkinson’s Disease |
References
- Piirtola, M.; Era, P. Force platform measurements as predictors of falls among older people—A review. Gerontology 2006, 52, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, S.; Guidi, M.; Ladislao, L.; Ghetti, G. Analysis and reliability of posturographic parameters in Parkinson patients at an early stage. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Shanghai, China, 1–4 September 2005; pp. 651–654. [Google Scholar]
- Agostini, V.; Chiaramello, E.; Bredariol, C.; Cavallini, C.; Knaflitz, M. Postural control after traumatic brain injury in patients with neuro-ophthalmic deficits. Gait Posture 2011, 34, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, E.; Ghetti, G.; Rabini, R.A.; Fioretti, S. Functional reach test: Movement strategies in diabetic subjects. Gait Posture 2014, 39, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Sbrollini, A.; Cavallini, C.; Busso, A.; Pignata, G.; Knaflitz, M. The role of central vision in posture: Postural sway adaptations in Stargardt patients. Gait Posture 2016, 43, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Chiaramello, E.; Canavese, L.; Bredariol, C.; Knaflitz, M. Postural sway in volleyball players. Hum. Mov. Sci. 2013, 32, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Bukiet, B.; Ji, Z.; Findley, T. Measurement of balance in computer posturography: Comparison of methods—A brief review. J. Bodyw. Mov. Ther. 2011, 15, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Neville, C.; Ludlow, C.; Rieger, B. Measuring postural stability with an inertial sensor: Validity and sensitivity. Med. Devices Evid. Res. 2015, 8, 447–455. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Plotnik, M.; Brozgol, M.; Maidan, I.; Giladi, N.; Gurevich, T.; Hausdorff, J.M. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson’s disease? Med. Eng. Phys. 2010, 32, 119–125. [Google Scholar]
- Kim, J.H.; Sienko, K.H. The Design of a Cell-Phone Based Balance-Training Device. J. Med. Devices 2009, 3, 027510. [Google Scholar] [CrossRef]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. Neuroeng. Rehabilit. 2014, 11, 1–10. [Google Scholar] [CrossRef]
- Leardini, A.; Lullini, G.; Giannini, S.; Berti, L.; Ortolani, M.; Caravaggi, P. Validation of the angular measurements of a new inertial-measurement-unit based rehabilitation system: Comparison with state-of-the-art gait analysis. J. Neuroeng. Rehabilit. 2014, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grimm, B.; Bolink, S. Evaluating physical function and activity in the elderly patient using wearable motion sensors. EFORT Open Rev. 2016, 1, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.; King, L.; Mancini, M. Role of Body-Worn Movement Monitor Technology for Balance and Gait Rehabilitation. Phys. Ther. 2015, 95, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Mileti, I.; Taborri, J.; Rossi, S.; Prete, Z.D.; Paoloni, M.; Suppa, A.; Palermo, E. Measuring age-related differences in kinematic postural strategies under yaw perturbation. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabilit. Med. 2010, 46, 239–248. [Google Scholar]
- Özdemir, A.T.; Barshan, B. Detecting falls with wearable sensors using machine learning techniques. Sensors 2014, 14, 10691–10708. [Google Scholar] [CrossRef]
- Shany, T.; Redmond, S.J.; Narayanan, M.R.; Lovell, N.H. Sensors-based wearable systems for monitoring of human movement and falls. IEEE Sens. J. 2012, 12, 658–670. [Google Scholar] [CrossRef]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Review of fall risk assessment in geriatric populations using inertial sensors. J. Neuroeng. Rehabilit. 2013, 10, 91. [Google Scholar] [CrossRef]
- Roeing, K.L.; Hsieh, K.L.; Sosnoff, J.J. A systematic review of balance and fall risk assessments with mobile phone technology. Arch. Gerontol. Geriatr. 2017, 73, 222–226. [Google Scholar] [CrossRef]
- Sun, R.; Sosnoff, J.J. Novel sensing technology in fall risk assessment in older adults: A systematic review. BMC Geriatr. 2018, 18, 14. [Google Scholar] [CrossRef]
- Pang, I.; Okubo, Y.; Sturnieks, D.; Lord, S.R.; Brodie, M.A. Detection of Near Falls Using Wearable Devices: A Systematic Review. J. Geriatr. Phys. Ther. 2019, 42, 48–56. [Google Scholar] [CrossRef]
- Tedesco, S.; Barton, J.; O’Flynn, B. A Review of Activity Trackers for Senior Citizens: Research Perspectives, Commercial Landscape and the Role of the Insurance Industry. Sensors 2017, 17, 1277. [Google Scholar] [CrossRef] [PubMed]
- Maetzler, W.; Domingos, J.; Srulijes, K.; Ferreira, J.J.; Bloem, B.R. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov. Disord. 2013, 28, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Wearable Sensor Use for Assessing Standing Balance and Walking Stability in People with Parkinson’s Disease: A Systematic Review. PLoS ONE 2015, 10, e0123705. [Google Scholar] [CrossRef]
- Godinho, C.; Domingos, J.; Cunha, G.; Santos, A.T.; Fernandes, R.M.; Abreu, D.; Goncalves, N.; Matthews, H.; Isaacs, T.; Duffen, J.; et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J. Neuroeng. Rehabilit. 2016, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; McGinnis, R.; Sosnoff, J.J. Novel technology for mobility and balance tracking in patients with multiple sclerosis: A systematic review. Expert Rev. Neurother. 2018, 18, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.-H.; Wong, D.W.-C.; Lam, W.K.; Wan, A.H.-P.; Lee, W.C.-C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Gordt, K.; Gerhardy, T.; Najafi, B.; Schwenk, M. Effects of Wearable Sensor-Based Balance and Gait Training on Balance, Gait, and Functional Performance in Healthy and Patient Populations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gerontology 2018, 64, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Moral-Munoz, J.A.; Esteban-Moreno, B.; Herrera-Viedma, E.; Cobo, M.J.; Perez, I.J. Smartphone Applications to Perform Body Balance Assessment: A Standardized Review. J. Med. Syst. 2018, 42, 119. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Cooper, H.M. Research Synthesis and Meta-Analysis: A Step-by-Step Approach; Publication, S., Ed.; Sage publications: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Slack, M.K.; Draugalis, J.R. Establishing the internal and external validity of experimental studies. Am. J. Heal. Pharm. 2001, 58, 2173–2181. [Google Scholar] [CrossRef]
- Kuijpers, T.; van der Windt, D.A.W.M.; van der Heijden, G.J.M.G.; Bouter, L.M. Systematic review of prognostic cohort studies on shoulder disorders. Pain 2004, 109, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, K.I.; Roushias, A.; Varitimidis, S.E.; Malizos, K.N. Quality of life and psychological consequences in elderly patients after a hip fracture: A review. Clin. Interv. Aging 2018, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooy, K.; van Hout, H.; Marwijk, H.; Marten, H.; Stehouwer, C.; Beekman, A. Depression and the risk for cardiovascular diseases: Systematic review and meta analysis. Int. J. Geriatr. Psychiatry 2007, 22, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, M.; Ainsworth, B.E.; Kwak, L.; Bowles, H.R. A checklist for evaluating the methodological quality of validation studies on self-report instruments for physical activity and sedentary behavior. J. Phys. Act. Health 2012, 9 (Suppl. 1), S29–S36. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Abe, Y.; Sugaya, T.; Sakamoto, M. Postural Control Characteristics during Single Leg Standing of Individuals with a History of Ankle Sprain: Measurements Obtained Using a Gravicorder and Head and Foot Accelerometry. J. Phys. Ther. Sci. 2014, 26, 447–450. [Google Scholar] [CrossRef]
- Adamová, B.; Kutilek, P.; Cakrt, O.; Svoboda, Z.; Viteckova, S.; Smrcka, P. Quantifying postural stability of patients with cerebellar disorder during quiet stance using three-axis accelerometer. Biomed. Signal Process. Control 2018, 40, 378–384. [Google Scholar] [CrossRef]
- Alkathiry, A.A.; Sparto, P.J.; Freund, B.; Whitney, S.L.; Mucha, A.; Furman, J.M.; Collins, M.W.; Kontos, A.P. Using Accelerometers to Record Postural Sway in Adolescents With Concussion: A Cross-Sectional Study. J. Athl. Train. 2018, 53, 1166–1172. [Google Scholar] [CrossRef]
- Baracks, J.; Casa, D.J.; Covassin, T.; Sacko, R.; Scarneo, S.E.; Schnyer, D.; Yeargin, S.W.; Neville, C. Acute Sport-Related Concussion Screening for Collegiate Athletes Using an Instrumented Balance Assessment. J. Athl. Train. 2018, 53, 597–605. [Google Scholar] [CrossRef]
- Baston, C.; Mancini, M.; Rocchi, L.; Horak, F. Effects of Levodopa on Postural Strategies in Parkinson’s disease. Gait Posture 2016, 46, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Bonora, G.; Mancini, M.; Carpinella, I.; Chiari, L.; Ferrarin, M.; Nutt, J.G.; Horak, F.B. Investigation of Anticipatory Postural Adjustments during One-Leg Stance Using Inertial Sensors: Evidence from Subjects with Parkinsonism. Front. Neurol. 2017, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.J.; Siegmund, G.P.; Guskiewicz, K.M.; van den doel, K.; Cretu, E.; Blouin, J.-S. Development and Validation of an Objective Balance Error Scoring System. Med. Sci. Sports Exerc. 2014, 46, 1610–1616. [Google Scholar] [CrossRef]
- Bzduskova, D.; Valkovic, P.; Hirjakova, Z.; Kimijanova, J.; Hlavacka, F. Parkinson’s disease versus ageing: Different postural responses to soleus muscle vibration. Gait Posture 2018, 65, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fan, Y.; Zhuang, X.; Feng, D.; Chen, Y.; Chan, P.; Du, Y. Postural sway in patients with early Parkinson’s disease performing cognitive tasks while standing. Neurol. Res. 2018, 40, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-L.; Tsai, Y.-J.; Lin, C.-H.; Hou, Y.-R.; Sung, W.-H. Evaluation of a smartphone-based assessment system in subjects with chronic ankle instability. Comput. Methods Programs Biomed. 2017, 139, 191–195. [Google Scholar] [CrossRef]
- Craig, J.J.; Bruetsch, A.P.; Lynch, S.G.; Horak, F.B.; Huisinga, J.M. Instrumented balance and walking assessments in persons with multiple sclerosis show strong test-retest reliability. J. Neuroeng. Rehabilit. 2017, 14, 43. [Google Scholar] [CrossRef]
- Cruz-Montecinos, C.; De la Fuente, C.; Rivera-Lillo, G.; Morales-Castillo, S.; Soto-Arellano, V.; Querol, F.; Pérez-Alenda, S. Sensory strategies of postural sway during quiet stance in patients with haemophilic arthropathy. Haemophilia 2017, 23, e419–e426. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Objective Gait and Balance Impairments Relate to Balance Confidence and Perceived Mobility in People With Parkinson Disease. Phys. Ther. 2016, 96, 1734–1743. [Google Scholar] [CrossRef]
- De Souza Fortaleza, A.C.; Mancini, M.; Carlson-Kuhta, P.; King, L.A.; Nutt, J.G.; Chagas, E.F.; Freitas, I.F.; Horak, F.B. Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture 2017, 56, 76–81. [Google Scholar] [CrossRef]
- Doherty, C.; Zhao, L.; Ryan, J.; Komaba, Y.; Inomata, A.; Caulfield, B. Quantification of postural control deficits in patients with recent concussion: An inertial-sensor based approach. Clin. Biomech. 2017, 42, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, H.; Mohler, J.; Marlinski, V.; Rashedi, E.; Toosizadeh, N. The influence of mechanical vibration on local and central balance control. J. Biomech. 2018, 71, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gago, M.F.; Fernandes, V.; Ferreira, J.; Silva, H.; Rodrigues, M.L.; Rocha, L.; Bicho, E.; Sousa, N. The effect of levodopa on postural stability evaluated by wearable inertial measurement units for idiopathic and vascular Parkinson’s disease. Gait Posture 2015, 41, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Gera, G.; Chesnutt, J.; Mancini, M.; Horak, F.B.; King, L.A. Inertial Sensor-Based Assessment of Central Sensory Integration for Balance after Mild Traumatic Brain Injury. Proc. Mil. Med. 2018, 183, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.R.; McGrath, D.; Walsh, L.; Doheny, E.P.; McKeown, D.; Garattini, C.; Cunningham, C.; Crosby, L.; Caulfield, B.; Kenny, R.A. Quantitative falls risk estimation through multi-sensor assessment of standing balance. Physiol. Meas. 2012, 33, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Sayeed, R.; Schwenk, M.; Bharara, M.; Menzies, R.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Balance rehabilitation: Promoting the role of virtual reality in patients with diabetic peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2013, 103, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-Based Interactive Balance Training with Visual Joint Movement Feedback for Improving Postural Stability in Diabetics with Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2015, 61, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xiong, S. Accuracy of Base of Support Using an Inertial Sensor Based Motion Capture System. Sensors 2017, 17, 2091. [Google Scholar] [CrossRef]
- Halická, Z.; Lobotková, J.; Bučková, K.; Hlavačka, F. Effectiveness of different visual biofeedback signals for human balance improvement. Gait Posture 2014, 39, 410–414. [Google Scholar] [CrossRef]
- Heebner, N.R.; Akins, J.S.; Lephart, S.M.; Sell, T.C. Reliability and validity of an accelerometry based measure of static and dynamic postural stability in healthy and active individuals. Gait Posture 2015, 41, 535–539. [Google Scholar] [CrossRef]
- Hejda, J.; Cakrt, O.; Socha, V.; Schlenker, J.; Kutilek, P. 3-D trajectory of body sway angles: A technique for quantifying postural stability. Biocybern. Biomed. Eng. 2015, 35, 185–191. [Google Scholar] [CrossRef]
- Hou, Y.-R.; Chiu, Y.-L.; Chiang, S.-L.; Chen, H.-Y.; Sung, W.-H. Feasibility of a smartphone-based balance assessment system for subjects with chronic stroke. Comput. Methods Programs Biomed. 2018, 161, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.L.; Roach, K.L.; Wajda, D.A.; Sosnoff, J.J. Smartphone technology can measure postural stability and discriminate fall risk in older adults. Gait Posture 2019, 67, 160–165. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Horak, F.B.; Mancini, M.; Pierce, D.; Priest, K.C.; Chesnutt, J.; Sullivan, P.; Chapman, J.C. Instrumenting the Balance Error Scoring System for Use With Patients Reporting Persistent Balance Problems After Mild Traumatic Brain Injury. Arch. Phys. Med. Rehabilit. 2014, 95, 353–359. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Mancini, M.; Fino, P.C.; Chesnutt, J.; Swanson, C.W.; Markwardt, S.; Chapman, J.C. Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion. Ann. Biomed. Eng. 2017, 45, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Lipsmeier, F.; Taylor, K.I.; Kilchenmann, T.; Wolf, D.; Scotland, A.; Schjodt-Eriksen, J.; Cheng, W.-Y.; Fernandez-Garcia, I.; Siebourg-Polster, J.; Jin, L.; et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov. Disord. 2018, 33, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabilit. 2012, 9, 59. [Google Scholar] [CrossRef]
- Matheron, E.; Yang, Q.; Delpit-Baraut, V.; Dailly, O.; Kapoula, Z. Active ocular vergence improves postural control in elderly as close viewing distance with or without a single cognitive task. Neurosci. Lett. 2016, 610, 24–29. [Google Scholar] [CrossRef]
- Melecky, R.; Socha, V.; Kutilek, P.; Hanakova, L.; Takac, P.; Schlenker, J.; Svoboda, Z. Quantification of Trunk Postural Stability Using Convex Polyhedron of the Time-Series Accelerometer Data. J. Healthc. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Mellone, S.; Palmerini, L.; Cappello, A.; Chiari, L. Hilbert-Huang-based tremor removal to assess postural properties from accelerometers. IEEE Trans. Biomed. Eng. 2011, 58, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Phan, D.; Pathirana, P.N.; Horne, M.; Power, L.; Szmulewicz, D. Quantification of Axial Abnormality Due to Cerebellar Ataxia with Inertial Measurements. Sensors 2018, 18, 2791. [Google Scholar] [CrossRef] [PubMed]
- Ozinga, S.J.; Linder, S.M.; Alberts, J.L. Use of Mobile Device Accelerometry to Enhance Evaluation of Postural Instability in Parkinson Disease. Arch. Phys. Med. Rehabilit. 2017, 98, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, L.; Rocchi, L.; Mellone, S.; Valzania, F.; Chiari, L. Feature selection for accelerometer-based posture analysis in Parkinson’s disease. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Quantifying effects of age on balance and gait with inertial sensors in community-dwelling healthy adults. Exp. Gerontol. 2016, 85, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Palmerini, L.; Weiss, A.; Herman, T.; Hausdorff, J.M. Balance testing with inertial sensors in patients with Parkinson’s disease: Assessment of motor subtypes. IEEE Trans. Neural Syst. Rehabilit. Eng. 2014, 22, 1064–1071. [Google Scholar] [CrossRef]
- Rouis, A.; Rezzoug, N.; Gorce, P. Validity of a low-cost wearable device for body sway parameter evaluation. Comput. Methods Biomech. Biomed. Engin. 2014, 17, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.W.; Koutakis, P.; Kloos, A.D.; Kegelmeyer, D.A.; Dicke, J.D.; Devor, S.T. Reliability and validity of a wireless accelerometer for the assessment of postural sway. J. Appl. Biomech. 2015, 31, 159–163. [Google Scholar] [CrossRef]
- Solomon, A.J.; Jacobs, J.V.; Lomond, K.V.; Henry, S.M. Detection of postural sway abnormalities by wireless inertial sensors in minimally disabled patients with multiple sclerosis: A case–control study. J. Neuroeng. Rehabilit. 2015, 12, 74. [Google Scholar] [CrossRef]
- Spain, R.; George, R.S.; Salarian, A.; Mancini, M.; Wagner, J.M.; Horak, F.B.; Bourdette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Armstrong, D.G.; Talal, T.K.; Najafi, B. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLoS ONE 2015, 10, e0135255. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.; Roche, J.; Marchetti, G.; Lin, C.; Steed, D.; Furman, G.; Musolino, M.; Redfern, M. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait Posture 2011, 33, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Al-Ali, F.; Rahemi, H.; Kulkarni, N.; Hamad, A.; Ibrahim, R.; Talal, T.K.; Najafi, B. Hemodialysis Impact on Motor Function beyond Aging and Diabetes-Objectively Assessing Gait and Balance by Wearable Technology. Sensors 2018, 18, 3939. [Google Scholar] [CrossRef] [PubMed]
- Agostini, V.; Aiello, E.; Fortunato, D.; Gastaldi, L.; Knaflitz, M.; Torino, P. A Wearable Device to Assess Postural Sway. In Proceedings of the 2019 IEEE 23rd International Symposium on Consumer Technologies (ISCT), Ancona, Italy, 19–21 June 2019; pp. 1–4. [Google Scholar]
| Item | Index | Score | ||
|---|---|---|---|---|
| Aim of the work | ||||
| 1 | Description of a specific, clearly stated purpose (IV) | Y | N | Maybe |
| 2 | The research question is scientifically relevant (EV) | Y | N | Maybe |
| Inclusion criteria (selection bias) | ||||
| 3 | Description of inclusion and/or exclusion criteria (IV-EV) | Y | N | Maybe |
| Data collection & processing (performance bias) | ||||
| 4 | Data collection is clearly described and reliable (IV-EV) | Y | N | Maybe |
| 5 | Same data collection method used for all subjects (IV) | Y | N | Maybe |
| 6 | Data processing is clearly described and reliable (IV-EV) | Y | N | Maybe |
| Data loss (attrition bias) | ||||
| 7 | Data loss <20% (EV) | Y | N | Maybe |
| Outcomes (detection bias) | ||||
| 8 | Outcomes are topic relevant (EV) | Y | N | Maybe |
| 9 | Outcomes are the same for all the subjects (IV) | Y | N | Maybe |
| 10 | The work answers the scientific question stated in the aim (IV) | Y | N | Maybe |
| Presentation of the results | ||||
| 11 | Presentation of the results is sufficient to assess the adequacy of the analysis (IV) | Y | N | Maybe |
| Statistical approach | ||||
| 12 | Appropriate statistical analysis techniques (SV) | Y | N | Maybe |
| 13 | Clearly states the statistical test used (SV) | Y | N | Maybe |
| 14 | States and references the analytical software used (SV) | Y | N | Maybe |
| 15 | Sufficient number of subjects (SV) | Y | N | Maybe |
| Quality | N | % of articles |
|---|---|---|
| High (score >10) | 47 | 64.4% |
| Medium (score between 5 and 10) | 24 | 32.9% |
| Low (score <5) | 2 | 2.7% |
| Total | 73 |
| First Author (Reference) | Population (Mean Age in Years ± SD) | Sensors | Sensor Placement | Test Condition(s) | Test Duration (in Each Condition) | Feet Position (Angle and Heel Distance) |
|---|---|---|---|---|---|---|
| Abe et al. [40] |
| 2 3D-accelerometers Freq: 100 Hz ACC range: ± 2 g |
|
| 20 s | N/A |
| Adamovà et al. [41] |
| 1 3D-inertial sensor (ACC and GYR) Freq: N/A |
|
| 60 s | 30°, 0 cm |
| Alkathiry et al. [42] |
| 1 3D-accelerometer Freq: 50 Hz |
|
| 30 s | 0°, 0 cm |
| Baracks et al. [43] |
| 1 3D-inertial sensor (ACC, GYR, and MAG) Freq: N/A |
|
| 30 s | 17°, 3.8 cm |
| Baston et al. [44] |
| 2 3D-inertial sensors (ACC and GYR) Freq: 50 Hz |
|
| 30 s | Footprint template |
| Bonora et al. [45] |
| 3 3D-inertial sensors (ACC, GYR, and MAG) Freq: 128 Hz |
|
| 30 s | N/A, Shoulders |
| Brown et al. [46] |
| 7 3D-inertial sensors (ACC and GYR) Freq: 102.4 Hz |
|
| 20 s | N/A |
| Bzduskova et al. [47] |
| 2 2D-accelerometers Freq: 100 Hz |
|
| 20 s | Self-selected, 15 cm |
| Chen et al. [48] |
| 1 3D-inertial sensor (ACC, GYR, and MAG) Freq: N/A ACC range: ± 6 g |
|
| 30 s | Footprint template |
| Chiu et al. [49] |
| 1 3D-accelerometer Freq: 10 Hz |
|
| 20 s | N/A |
| Craig et al. [50] |
| 6 3D-inertial sensors (ACC, GYR, and MAG) Freq: 128 Hz |
|
| 30 s | Self-selected, 10 cm |
| Cruz-Montecinos et al. [51] |
| 1 3D-accelerometer Freq: 250 Hz ACC range: ± 3 g |
|
| 30 s | N/A |
| Curtze et al. [52] |
| 1 3D-inertial sensors (ACC and GYR) Freq: N/A |
|
| 30 s | 30°, 10 cm |
| De Souza Fortaleza et al. [53] |
| 8 3D-inertial sensors (ACC, GYR, and MAG) Freq: N/A |
|
| 30 s | N/A |
| Doherty et al. [54] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 102.4 Hz ACC range: ± 8 g |
|
| 20 s | N/A |
| Ehsani et al. [55] |
| 2 3D-inertial sensors (ACC and GYR) Freq: N/A |
|
| 30 s | 0°, 0 cm |
| Gago et al. [56] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 113 Hz |
|
| 30 s | 0°, 0 cm |
| Gera et al. [57] |
| 1 3D-inertial sensor (ACC, GYR, and MAG) Freq: N/A |
|
| 30 s | 0°, 0 cm |
| Greene et al. [58] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 102.4 Hz |
|
| 40 s and 30 s | 0°, 0 cm |
| Grewal et al. [59] |
| 2 3D-inertial sensors (ACC, GYR, and MAG) Freq: N/A |
|
| 30 s | N/A, shoulders |
| Grewal et al. [60] |
| 5 3D-inertial sensors (ACC, GYR, and MAG) Freq: 100 Hz |
|
| 30 s | Self-selected, self-selected |
| Guo et al. [61] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 240 Hz |
|
| 20 s | 10°, self-selected |
| Halickà et al. [62] |
| 2 2D-accelerometers Freq: 100 Hz ACC range: ± 1.7 g |
|
| 50 s | 30°, 0 cm |
| Heebner et al. [63] |
| 1 3D-accelerometers Freq: 1000 Hz ACC range: ± 1.6 g |
|
| 30 s | N/A |
| Hejda et al. [64] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 100 Hz |
|
| 60 s | 30°, 0 cm |
| Hou et al. [65] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 50 Hz |
|
| 30 s | Self-selected, shoulders |
| Hsieh et al. [66] |
| 1 3D-accelerometer Freq: 200 Hz |
|
| 30 s | N/A |
| King et al. [67] |
| 1 3D-accelerometer Freq: 120 Hz |
|
| 30 s | 0°, 0 cm |
| King et al. [68] |
| 1 3D-inertial sensor (ACC, GYR, and MAG) Freq: N/A |
|
| 30 s | N/A |
| Lipsmeier et al. [69] |
| 1 3D-inertial sensor (ACC, GYR, and MAG) Freq: N/A |
|
| 30 s | Self-selected, self-selected |
| Mancini et al. [70] |
| 1 3D-inertial sensor (ACC and GYR) Freq: 100 Hz ACC range: ± 1.7 g |
|
| 40 s | Self-selected, 10 cm |
| Mancini et al. [71] |
| 1 3D-accelerometer Freq: 50 Hz ACC range: ± 1.7 g |
|
| 30 s | Self-selected, 10 cm |
| Matheron et al. [72] |
| 1 3D-accelerometer Freq: 100 Hz |
|
| 60 s | 30°, 4 cm |
| Melecky et al. [73] |
| 1 3D-inertial sensor (ACC and GYR) Freq: N/A |
|
| 60 s | 30°, 0 cm |
| Mellone et al. [74] |
| 1 3D-accelerometer Freq: 100 Hz ACC range: ±2 g |
|
| 30 s | Footprint template |
| Nguyen et al. [75] |
| 2 3D-accelerometers Freq: 50 Hz |
|
| 30 s | Self-selected, 0 cm |
| Ozinga et al. [76] |
| 1 3D-accelerometer Freq: 100 Hz |
|
| 20 s | Footprint template |
| Palmerini et al. [77] |
| 1 3D-accelerometer Freq: 100 Hz ACC range: ± 2 g |
|
| 30 s | Footprint template |
| Park et al. [78] |
| 6 3D-inertial sensors (ACC, GYR, and MAG) Freq: 128 Hz |
|
| 30 s | 14°, 10 cm |
| Rocchi et al. [79] |
| 1 3D-accelerometer Freq: 100 Hz |
|
| 60 s | 0°, 0 cm |
| Rouis et al. [80] |
| 1 3D-accelerometer Freq: 50 Hz ACC range: ± 2 g |
|
| 30 s | Self-selected, self-selected |
| Saunders et al. [81] |
| 1 3D-accelerometer Freq: 250 Hz ACC range: ± 2 g |
|
| 30 s | 0°, 0 cm |
| Solomon et al. [82] |
| 6 3D-inertial sensors (ACC, GYR, and MAG) Freq: 120 Hz |
|
| 30 s | 17.3°, 10.48 cm |
| Spain et al. [83] |
| 6 3D-inertial sensors (ACC, GYR, and MAG) Freq: 50 Hz ACC range: ± 1.7 g |
|
| 30 s | Footprint template |
| Toosizadeh et al. [84] |
| 2 3D-inertial sensors (ACC, GYR, and MAG) Freq: N/A |
|
| 15 s | 0°, 0 cm |
| Whitney et al. [85] |
| 1 2D-accelerometer Freq: 100 Hz ACC range: ± 1.2 g |
|
| 40 s | N/A |
| Zhou et al. [86] |
| 2 3D-inertial sensors (ACC and GYR) Freq: 100 Hz ACC range: ± 2 g |
|
| 30 s | 0°, 0 cm |
| Balance Disorder | N | % of Articles | Reference(s) |
|---|---|---|---|
| Parkinson’s Disease (PD) | 14 | 29.8% | [44,45,47,48,52,53,56,69,70,71,74,76,77,79] |
| Degenerative Cerebellar Ataxia | 4 | 8.5% | [41,64,73,75] |
| Concussion | 4 | 8.5% | [42,43,54,68] |
| Diabetic Peripheral Neuropathy (DPN) | 4 | 8.5% | [55,59,75,86] |
| Multiple Sclerosis (MS) | 3 | 6.4% | [50,82,83] |
| High fall risk | 2 | 4.3% | [55,66] |
| Traumatic Brain Injury (TBI) | 2 | 4.3% | [57,67] |
| Ankle sprain | 1 | 2.1% | [40] |
| Stroke | 1 | 2.1% | [65] |
| Haemophilia | 1 | 2.1% | [51] |
| Total | 36 | 76.6% |
| Balance Measure (Acceleration) | Domain | Definition of Measure | References |
|---|---|---|---|
| Range | Time | Range of acceleration signals in AP and/or ML directions (m/s2) | [50,71,74,76,78,80,82,85] |
| Root Mean Square (RMS) | Time | RMS of the accelerations in AP and/or ML directions (m/s2) | [40,43,44,48,50,51,52,53,58,63,66,71,74,75,78,80,81,82,83,85] |
| Mean Acceleration | Time | Average of the AP and/or ML accelerations (m/s2) | [49,80] |
| Mean Distance | Time | Mean distance from the center of acceleration trajectory normalized with respect to the duration of the measurement (m/s2) | [50,71,78] |
| Sway Path Length (SPL) | Time | Total accelerometer trajectory length (m/s2) | [41,42,50,71,76,80,82,85] |
| Sway Area (SA) | Time | Area spanned from the acceleration signals normalized with respect to the duration of the measurement (mm2/s5) | [50,57,71,78,80] |
| 95% Ellipse Sway Area | Time | Elliptical area that encapsulates the sway path derived from the AP and ML accelerations (m2/s4) | [43,76,82] |
| 95% Ellipse Sway Normalized Area | Time | Elliptical area that encapsulates the sway path derived from the AP and ML accelerations normalized with respect to the duration of the measurement (m2/s5) | [71,78] |
| Jerk Index (JI) | Time | Function of the time derivative of the acceleration: it is an index of sway smoothness (m2/s5). | [48,50,53,71,74,77,78,82] |
| Normalized Jerk Index (nJI) | Time | Jerk index normalized to range of acceleration excursion and duration (dimensionless) | [52,77,78,83] |
| F50 | Frequency | Frequency containing 50% of the total power (Hz) | [71,77,80] |
| F95 | Frequency | Frequency containing 95% of the total power (Hz) | [50,70,71,74,77,80] |
| Total Power | Frequency | Total power of the spectrum of accelerations (m2/s4) | [68,71,80,82] |
| Frequency Dispersion (FD) | Frequency | Measure of the variability of the frequency content of the power spectral density (0 for a pure sinusoid: it increases with spectral bandwidth to 1) (dimensionless) | [50,52,70,71,77,78] |
| Centroidal Frequency (CF) | Frequency | Frequency at which spectral mass is concentrated: the power of the acceleration signals above and below CF are exactly balanced (Hz). | [52,71,74,77,78,79,83] |
| Mean Frequency | Frequency | Mean frequency of the acceleration power spectrum (Hz) | [50,58,71] |
| Entropy | Frequency | Power spectrum entropy of accelerations (dimensionless) | [58,75,77] |
| Mean Sway Velocity (MV) | Time | First integral of the acceleration signals in AP and/or ML directions (m/s) | [52,58,69,70,71,74,77,78,79,80,82,83] |
| Root Mean Square (RMS) | Time | RMS of the displacements in AP and/or ML directions (mm). | [44,51,58,62,72,77] |
| Mean Distance (MD) | Time | Mean distance from the center of COM (mm) | [56,58,77,79] |
| Range | Time | Range of COM displacement (mm) | [56,77,84] |
| Sway Path Length (SPL) | Time | Total COM trajectory length (mm) | [56,58,77,79] |
| Sway Area (SA) | Time | Area included in the COM displacement (mm2 or cm2) | [59,60,77,84,86] |
| 95% Ellipse Sway Normalized Area | Time | Elliptical area that encapsulates the sway path derived from the AP and ML displacement normalized with respect to the duration of the measurement (mm2/s) | [58,72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. https://doi.org/10.3390/s19194075
Ghislieri M, Gastaldi L, Pastorelli S, Tadano S, Agostini V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors. 2019; 19(19):4075. https://doi.org/10.3390/s19194075
Chicago/Turabian StyleGhislieri, Marco, Laura Gastaldi, Stefano Pastorelli, Shigeru Tadano, and Valentina Agostini. 2019. "Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review" Sensors 19, no. 19: 4075. https://doi.org/10.3390/s19194075
APA StyleGhislieri, M., Gastaldi, L., Pastorelli, S., Tadano, S., & Agostini, V. (2019). Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors, 19(19), 4075. https://doi.org/10.3390/s19194075






