How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors—Separating the Trees from the Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
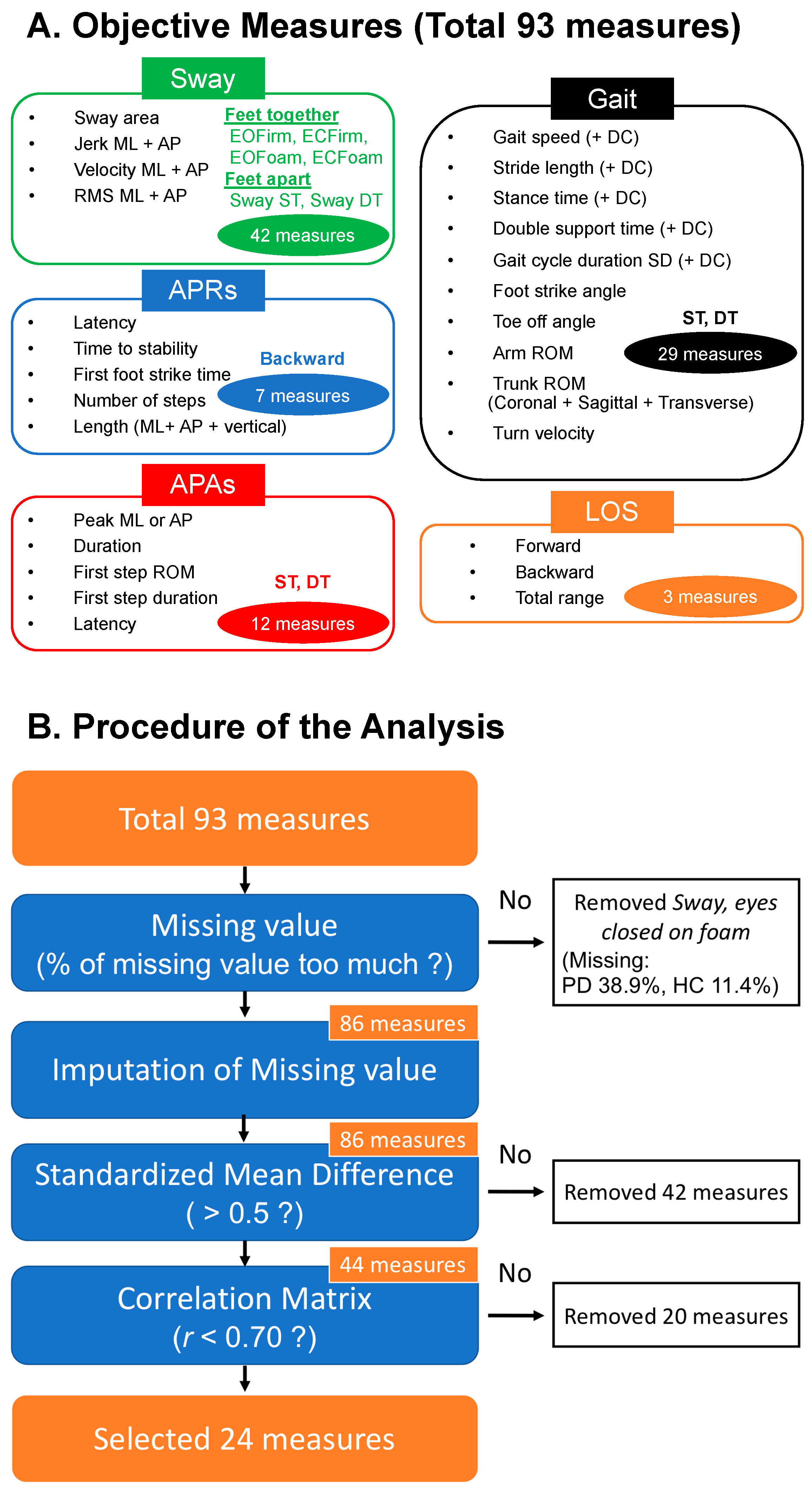
2.3. Outcome Measures
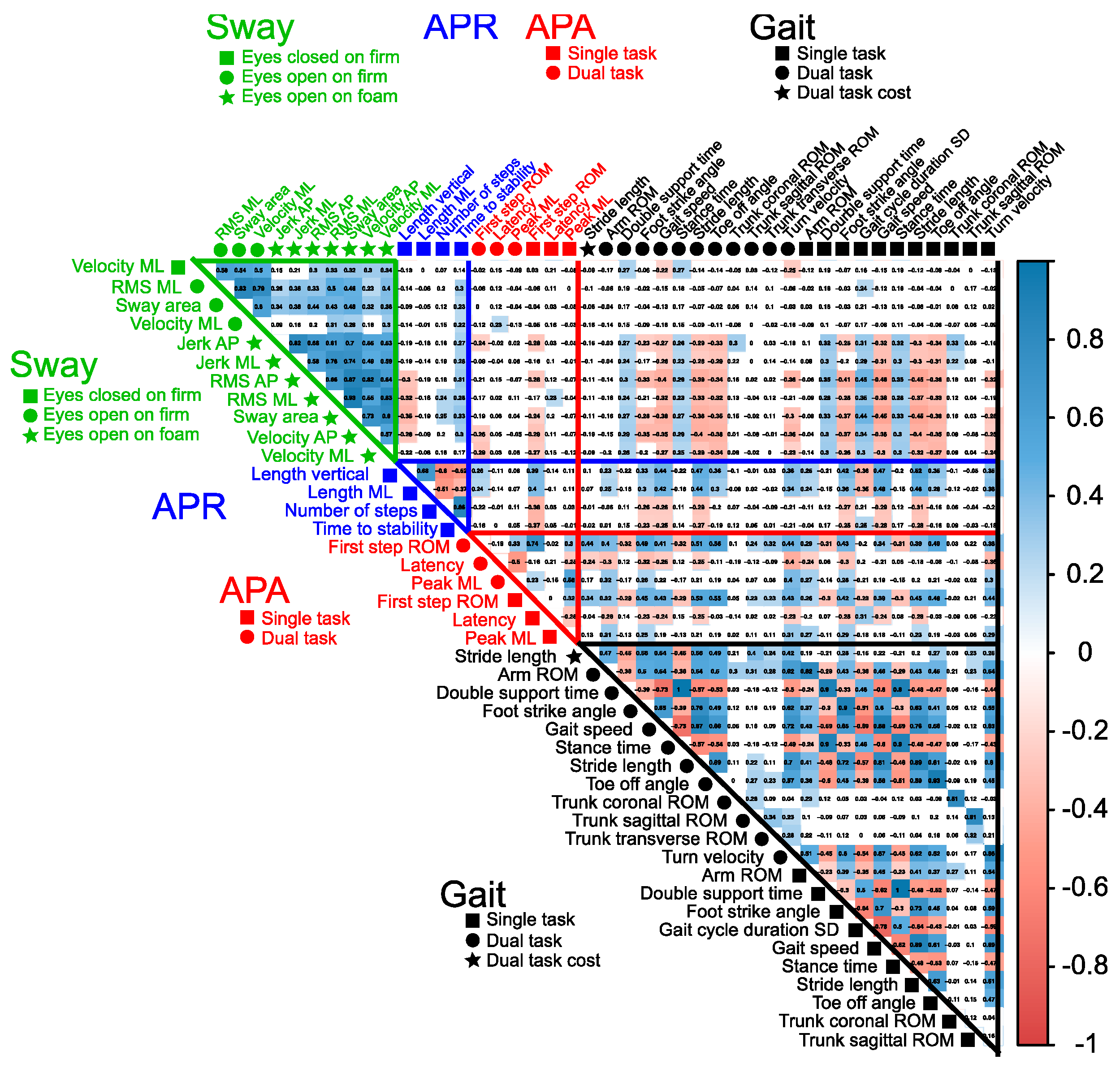
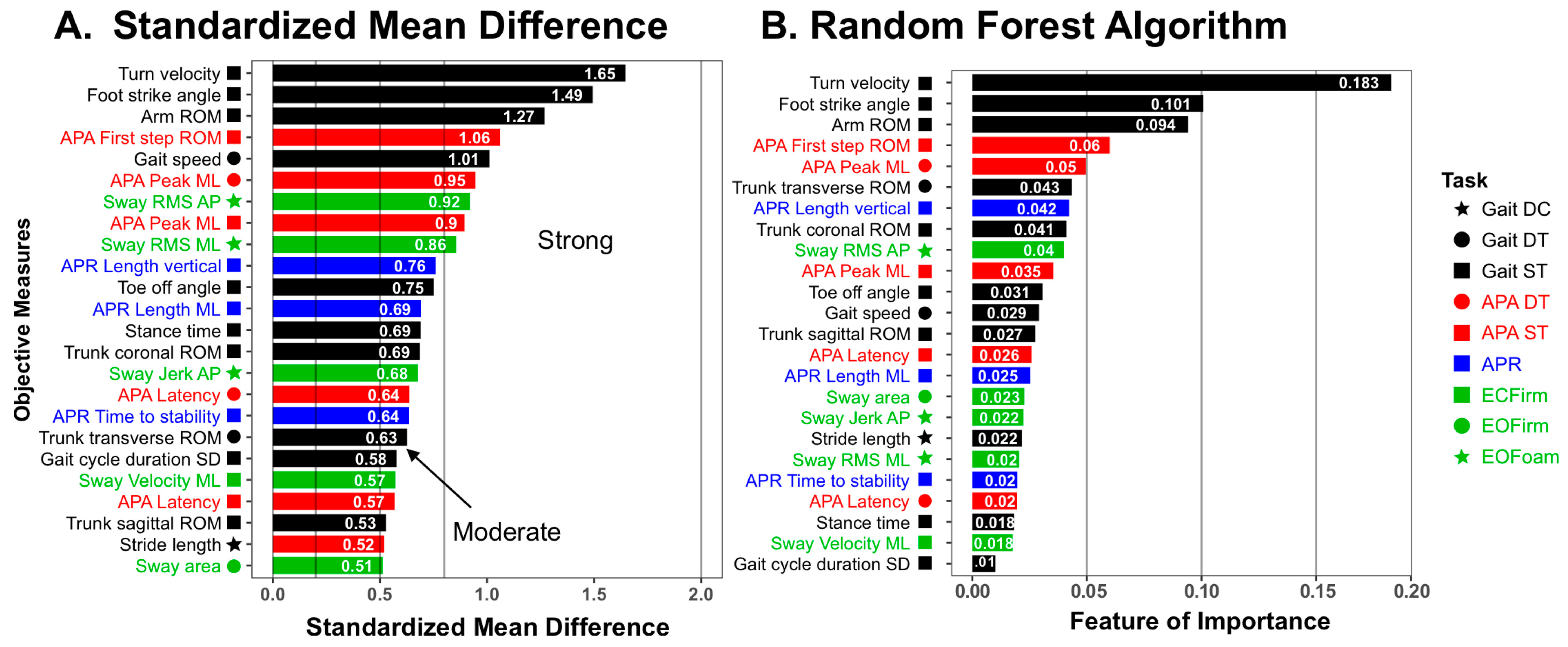
2.4. Statistical Analysis
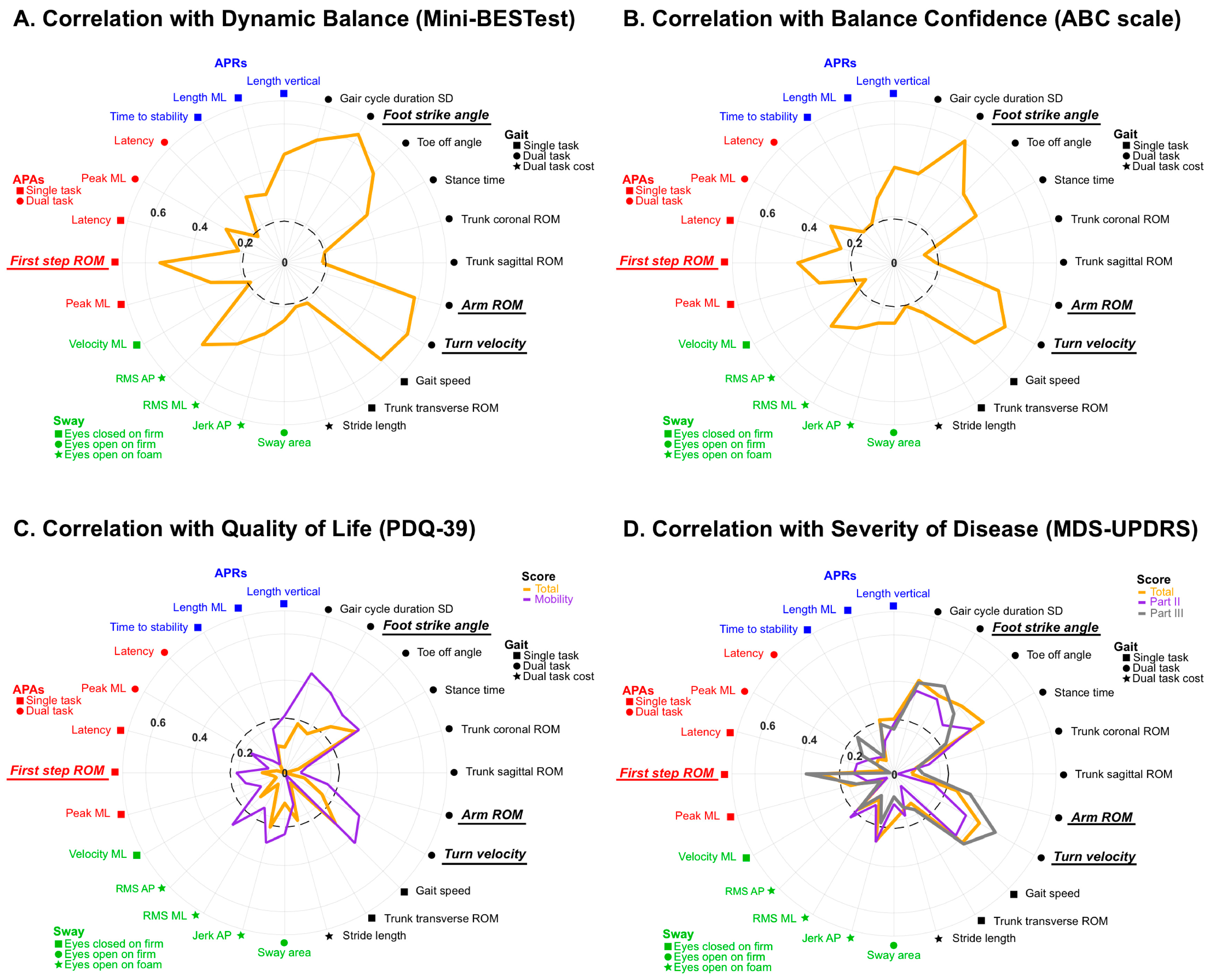
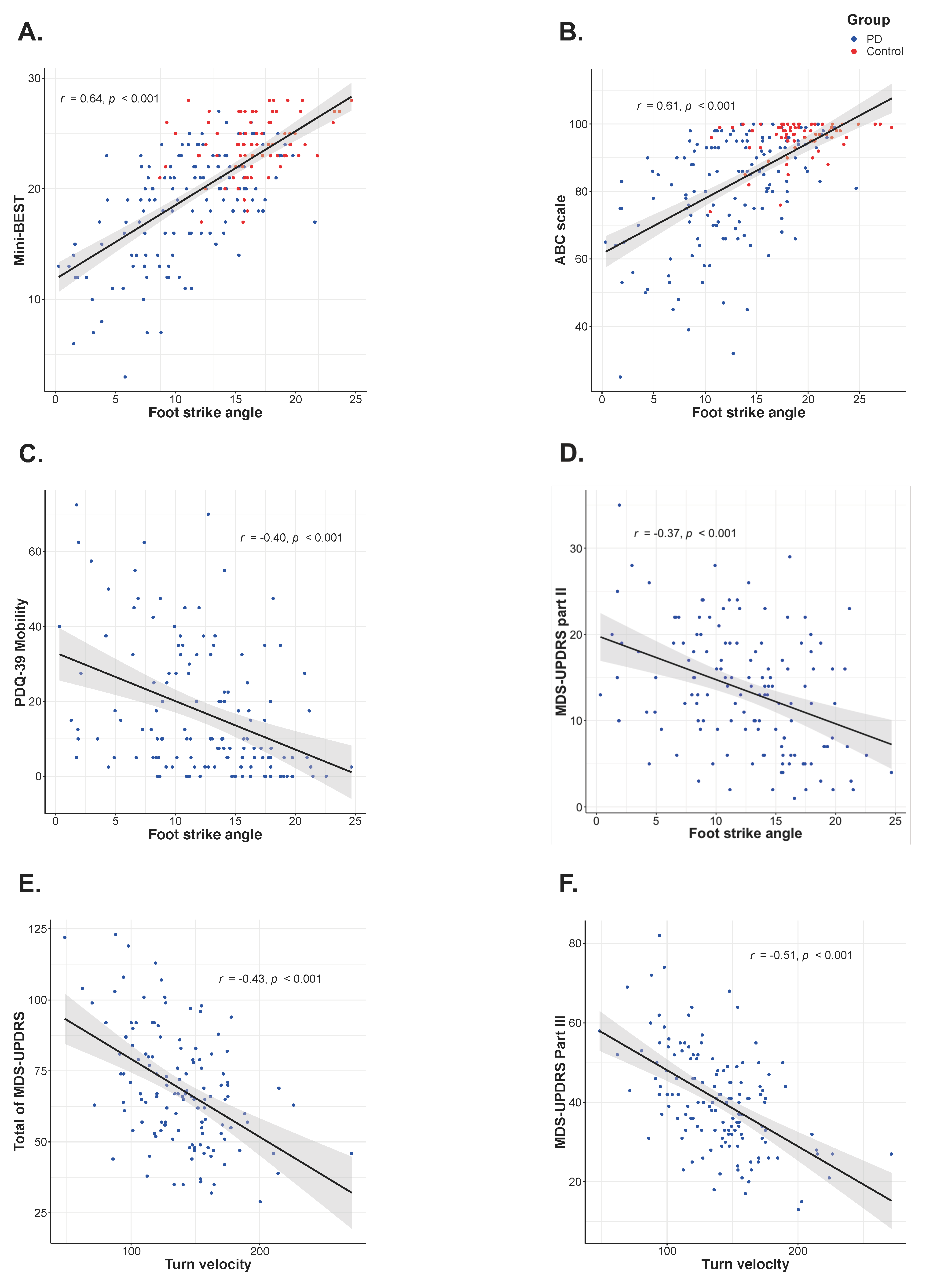
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
Appendix A
| Objective Measures | Unit | Definition |
|---|---|---|
| Sway (seven metrics) | ||
| Sway area | m2/s4 | Area of an ellipse covering 95% of the sway angle in the horizontal plane |
| Jerk | m2/s5 | Smoothness of sway from the time derivative of the sway path in each direction (ML or AP) |
| Velocity | m/s | Mean velocity of derivative of acceleration in each direction (ML or AP) |
| RMS | m/s2 | Root mean square of acceleration time series in each direction (ML or AP) |
| APRs (seven metrics) | ||
| Latency | s | Time from release to onset of the first step |
| Time to stability | s | Time from release to the point of trunk acceleration becoming stationary |
| First foot strike time | s | Time from release to heel strike of the first moving leg (approximation of the first step length in the horizontal plane) |
| Number of steps | N | Number of left and right foot steps to reach stability |
| Length | m | Length of the first step of derivative of foot acceleration (ML: ML direction, AP: AP direction, Vertical: height) |
| APAs (six metrics) | ||
| peak ML | m/s2 | Peak trunk acceleration toward the stance foot of the lateral trunk acceleration |
| peak AP | m/s2 | Peak trunk acceleration forward from baseline |
| Duration | s | Time from APA onset to end |
| First step ROM | degree | Range of motion of the leg (calculated from the integrated sagittal angular velocity, approximation of first step length) |
| First step duration | s | Time from toe-off to first heel strike |
| Latency | s | Time from APA onset to first heel strike (approximation of the first step velocity) |
| Gait (12 metrics) | ||
| Gait cycle duration | s | Duration of a complete gait cycle |
| Gait speed | m/s | The forward speed of the subject |
| Stride length | m/s | Distance between two consecutive heel strikes |
| Foot strike angle | degree | Average angle of the foot at the point of initial contact |
| Toe off angle | degree | Average angle of the foot at the point of push off |
| Stance time | % of gait cycle | Percentage of a gait cycle that either foot is on the ground |
| Double support time | % of gait cycle | Percentage of a gait cycle that both feet are on the ground |
| Arm ROM | degree | Average of range of motion of both arms during arm-swing |
| Trunk ROM | degree | Average range of motion of trunk (coronal: in frontal plane, sagittal: in sagittal plane, transverse: in horizontal plane) |
| Domain | Task | Kept Measures (N = 44) | Removed Measures (N = 42) | ||
|---|---|---|---|---|---|
| Sway | EOFirm | Sway area | Jerk AP | Jerk ML | |
| Velocity ML | RMS ML | Velocity AP | RMS AP | ||
| EOFoam | Sway area | ||||
| Jerk AP | Jerk ML | ||||
| Velocity AP | Velocity ML | ||||
| RMS AP | RMS ML | ||||
| ECFirm | Velocity ML | Sway area | |||
| Jerk AP | Jerk ML | ||||
| Velocity AP | |||||
| RMS AP | RMSML | ||||
| ST and DT | Sway area | ||||
| Jerk AP | Jerk ML | ||||
| Velocity AP | Velocity ML | ||||
| RMS AP | RMS ML | ||||
| APRs | Time to stability | Number of steps | Latency | First foot strike time | |
| Length ML | Length vertical | Length AP | |||
| APAs | ST and DT | Peak ML | First step ROM | Peak AP | Duration |
| Latency | First step duration | ||||
| Gait | ST | Gait cycle duration SD | Gait speed | Trunk transverse ROM | |
| Stride length | Foot strike angle | ||||
| Toe off angle | Stance time | ||||
| Double support time | Arm ROM | ||||
| Trunk coronal ROM | Trunk sagittal ROM | ||||
| Turn velocity | |||||
| DT | Gait speed | Stride length | Gait cycle duration SD | ||
| Foot strike angle | Toe off angle | ||||
| Stance time | Double support time | ||||
| Arm ROM | Trunk coronal ROM | ||||
| Trunk sagittal ROM | Trunk transverse ROM | ||||
| Turn velocity | |||||
| DC | Stride length | Gait cycle duration SD | Gait speed | ||
| Stance time | Double support time | ||||
| LOS | Backward | Forward | |||
| Total range | |||||
| Task | Measures | N | Test | Re-Test | Test-Retest ICC | SEM | MDC | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||
| Gait ST | Gait cycle duration SD | 43 | 0.04 | 0.02 | 0.03 | 0.01 | 0.72 | 0.01 | 0.03 |
| Foot strike angle | 43 | 11.54 | 5.65 | 11.43 | 5.30 | 0.97 | 1.03 | 2.84 | |
| Toe off angle | 43 | 29.16 | 4.94 | 29.75 | 5.00 | 0.95 | 1.12 | 3.12 | |
| Stance time | 43 | 61.55 | 2.10 | 60.95 | 2.03 | 0.94 | 0.51 | 1.41 | |
| Trunk coronal ROM | 43 | 4.37 | 1.87 | 4.27 | 1.72 | 0.97 | 0.29 | 0.80 | |
| Trunk sagittal ROM | 43 | 3.67 | 0.73 | 3.93 | 0.73 | 0.81 | 0.33 | 0.91 | |
| Arm ROM | 43 | 26.51 | 11.48 | 27.39 | 12.84 | 0.96 | 2.46 | 6.83 | |
| Turn velocity | 41 | 134.24 | 35.52 | 138.59 | 35.93 | 0.95 | 8.39 | 23.26 | |
| Gait DT | Gait speed | 40 | 0.75 | 0.20 | 0.79 | 0.20 | 0.90 | 0.06 | 0.18 |
| Trunk transverse ROM | 40 | 6.88 | 1.94 | 6.85 | 2.25 | 0.84 | 0.85 | 2.34 | |
| Dual task cost | Stride length | 40 | −12.27 | 8.30 | −10.94 | 12.57 | 0.66 | 6.20 | 17.17 |
| EOFirm | Sway area | 37 | 0.09 | 0.05 | 0.10 | 0.07 | 0.57 | 0.04 | 0.11 |
| EOFoam | Jerk AP | 34 | 9.16 | 12.63 | 11.34 | 21.63 | 0.63 | 10.87 | 30.12 |
| RMS ML | 34 | 0.12 | 0.04 | 0.12 | 0.06 | 0.79 | 0.02 | 0.06 | |
| RMS AP | 34 | 0.13 | 0.04 | 0.14 | 0.09 | 0.46 | 0.05 | 0.14 | |
| ECFirm | Velocity ML | 37 | 0.12 | 0.06 | 0.13 | 0.11 | 0.47 | 0.06 | 0.18 |
| APA ST | peak ML | 37 | 0.04 | 0.01 | 0.03 | 0.02 | 0.70 | 0.01 | 0.02 |
| First step ROM | 42 | 29.72 | 9.18 | 27.12 | 11.25 | 0.82 | 4.44 | 12.29 | |
| Latency | 37 | 0.68 | 0.21 | 0.74 | 0.19 | 0.49 | 0.14 | 0.40 | |
| APA DT | peak ML | 36 | 0.03 | 0.02 | 0.03 | 0.02 | 0.83 | 0.01 | 0.02 |
| Latency | 36 | 0.76 | 0.20 | 0.81 | 0.34 | 0.23 | 0.25 | 0.68 | |
| APR | Time to stability | 32 | 1.36 | 0.70 | 1.23 | 0.76 | 0.79 | 0.34 | 0.93 |
| Length ML | 32 | 0.14 | 0.11 | 0.17 | 0.11 | 0.44 | 0.08 | 0.22 | |
| Length vertical | 32 | 0.04 | 0.02 | 0.05 | 0.03 | 0.86 | 0.01 | 0.03 |
References
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, Y.J.; Horak, F.B. What Is Wrong with Balance in Parkinson’s Disease? J. Mov. Disord. 2015, 8, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Park. Dis. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schoneburg, B.; Mancini, M.; Horak, F.; Nutt, J.G. Framework for understanding balance dysfunction in Parkinson’s disease. Mov. Disord. 2013, 28, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, A.M.; Hood, J.D.; Gresty, M.A.; Panagi, C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain 1990, 113, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.K.; Horak, F.B.; Woollacott, M.H. Parkinson’s disease impairs the ability to change set quickly. J. Neurol. Sci. 2000, 175, 57–70. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef]
- Morris, M.; Iansek, R.; Smithson, F.; Huxham, F. Postural instability in Parkinson’s disease: A comparison with and without a concurrent task. Gait Posture 2000, 12, 205–216. [Google Scholar] [CrossRef]
- Stylianou, A.P.; McVey, M.A.; Lyons, K.E.; Pahwa, R.; Luchies, C.W. Postural sway in patients with mild to moderate Parkinson’s disease. Int. J. Neurosci. 2011, 121, 614–621. [Google Scholar] [CrossRef]
- Horak, F.B.; Dimitrova, D.; Nutt, J.G. Direction-specific postural instability in subjects with Parkinson’s disease. Exp. Neurol. 2005, 193, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Horak, F.B.; Van Tran, K.; Nutt, J.G. An alternative clinical postural stability test for patients with Parkinson’s disease. J. Neurol. 2006, 253, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; St George, R.J.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Preparation for compensatory forward stepping in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2010, 91, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Burleigh-Jacobs, A.; Horak, F.B.; Nutt, J.G.; Obeso, J.A. Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov. Disord. 1997, 12, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Chiari, L.; Mancini, M.; Carlson-Kuhta, P.; Gross, A.; Horak, F.B. Step initiation in Parkinson’s disease: Influence of initial stance conditions. Neurosci. Lett. 2006, 406, 128–132. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1532–1534. [Google Scholar] [CrossRef]
- Svehlík, M.; Zwick, E.B.; Steinwender, G.; Linhart, W.E.; Schwingenschuh, P.; Katschnig, P.; Ott, E.; Enzinger, C. Gait analysis in patients with Parkinson’s disease off dopaminergic therapy. Arch. Phys. Med. Rehabil. 2009, 90, 1880–1886. [Google Scholar] [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 171–176. [Google Scholar] [CrossRef]
- Mancini, M.; El-Gohary, M.; Pearson, S.; McNames, J.; Schlueter, H.; Nutt, J.G.; King, L.A.; Horak, F.B. Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential. NeuroRehabilitation 2015, 37, 3–10. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Mancini, M.; Rocchi, L.; Horak, F.B.; Chiari, L. Effects of Parkinson’s disease and levodopa on functional limits of stability. Clin. Biomech. 2008, 23, 450–458. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Muthuraman, M.; Witt, K.; Weisser, B.; Fasano, A.; Deuschl, G. Postural control and freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 24, 107–112. [Google Scholar] [CrossRef]
- Sprager, S.; Juric, M.B. Inertial Sensor-Based Gait Recognition: A Review. Sensors 2015, 15, 22089–22127. [Google Scholar] [CrossRef]
- Del Din, S.; Galna, B.; Godfrey, A.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Mirelman, A.; Hausdorff, J.M.; Rochester, L. Analysis of Free-Living Gait in Older Adults With and Without Parkinson’s Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 500–506. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.M.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e0183989. [Google Scholar] [CrossRef]
- El-Gohary, M.; Peterson, D.; Gera, G.; Horak, F.B.; Huisinga, J.M. Validity of the Instrumented Push and Release Test to Quantify Postural Responses in Persons with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 1325–1331. [Google Scholar] [CrossRef]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2011, Suppl. 1, 007. [Google Scholar] [CrossRef]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabil. 2012, 9, 59. [Google Scholar] [CrossRef]
- Mancini, M.; Chiari, L.; Holmstrom, L.; Salarian, A.; Horak, F.B. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture 2016, 43, 125–131. [Google Scholar] [CrossRef]
- Horak, F.B.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; Salarian, A. Balance and Gait Represent Independent Domains of Mobility in Parkinson Disease. Phys. Ther. 2016, 96, 1364–1371. [Google Scholar] [CrossRef]
- Mancini, M.; Zampieri, C.; Carlson-Kuhta, P.; Chiari, L.; Horak, F.B. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: An accelerometer-based approach. Eur. J. Neurol. 2009, 16, 1028–1034. [Google Scholar] [CrossRef]
- Mancini, M.; Carlson-Kuhta, P.; Zampieri, C.; Nutt, J.G.; Chiari, L.; Horak, F.B. Postural sway as a marker of progression in Parkinson’s disease: A pilot longitudinal study. Gait Posture 2012, 36, 471–476. [Google Scholar] [CrossRef]
- Mariani, B.; Rochat, S.; Büla, C.J.; Aminian, K. Heel and toe clearance estimation for gait analysis using wireless inertial sensors. IEEE Trans. Biomed. Eng. 2012, 59, 3162–3168. [Google Scholar] [CrossRef]
- Rampp, A.; Barth, J.; Schülein, S.; Gaßmann, K.G.; Klucken, J.; Eskofier, B.M. Inertial sensor-based stride parameter calculation from gait sequences in geriatric patients. IEEE Trans. Biomed. Eng. 2015, 62, 1089–1097. [Google Scholar] [CrossRef]
- Salarian, A.; Zampieri, C.; Horak, F.B.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. Analyzing 180 degrees turns using an inertial system reveals early signs of progression of Parkinson’s disease. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 224–227. [Google Scholar] [CrossRef]
- Salarian, A.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Aminian, K. iTUG, A Sensitive and Reliable Measure of Mobility. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 303–310. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Horak, F.; Peterson, D. Anticipatory Postural Adjustment During Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients with Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1316–1324. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Nutt, J.; Hiller, A.P.; Maetzler, W.; Deuschl, G.; Horak, F. Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson’s Disease? Front. Aging Neurosci. 2018, 10, 36. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities—Specific Balance Confidence (ABC) Scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M28–M34. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef]
- King, L.A.; Peterson, D.S.; Mancini, M.; Carlson-Kuhta, P.; Fling, B.W.; Smulders, K.; Nutt, J.G.; Dale, M.; Carter, J.; Winters-Stone, K.M.; et al. Do cognitive measures and brain circuitry predict outcomes of exercise in Parkinson Disease: A randomized clinical trial. BMC Neurol. 2015, 15, 218. [Google Scholar] [CrossRef]
- McIlroy, W.; Maki, B. Preferred placement of the feet during quiet stance: Development of a standardized foot placement for balance testing. Clin. Biomech. 1997, 12, 66–70. [Google Scholar] [CrossRef]
- Palmerini, L.; Rocchi, L.; Mellone, S.; Valzania, F.; Chiari, L. Feature selection for accelerometer-based posture analysis in Parkinson’s disease. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 481–490. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Mikkelsen, E.M.; Cronin-Fenton, D.; Kristensen, N.R.; Pham, T.M.; Pedersen, L.; Petersen, I. Missing data and multiple imputation in clinical epidemiological research. Clin. Epidemiol. 2017, 9, 157–166. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: New York City, NY, USA 2009; pp. 21–32. ISBN 978-04-7005-724-7. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470743386 (accessed on 26 June 2019).
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdales, NJ, USA, 1988; pp. 1–17. ISBN 978-11-3474-270-7. [Google Scholar]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Augustin, T. Unbiased split selection for classification trees based on the Gini Index. Comput. Stat. Data Anal. 2007, 52, 483–501. [Google Scholar] [CrossRef]
- Arora, S.; Baig, F.; Lo, C.; Barber, T.R.; Lawton, M.A.; Zhan, A.; Rolinski, M.; Ruffmann, C.; Klein, J.C.; Rumbold, J.; et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 2018, 91, e1528–e1538. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: Berlin, Germany, 2009; pp. 587–604. ISBN 978-0-387-84858-7. [Google Scholar]
- Little, M.A.; McSharry, P.E.; Hunter, E.J.; Spielman, J.; Ramig, L.O. Suitability of dysphonia measurements for telemonitoring of Parkinson’s disease. IEEE Trans. Biomed. Eng. 2009, 56, 1015. [Google Scholar] [CrossRef]
- Tsanas, A.; Little, M.A.; McSharry, P.E.; Spielman, J.; Ramig, L.O. Novel speech signal processing algorithms for high-accuracy classification of Parkinson’s disease. IEEE Trans. Biomed. Eng. 2012, 59, 1264–1271. [Google Scholar] [CrossRef]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef]
- Lord, S.; Galna, B.; Rochester, L. Moving forward on gait measurement: Toward a more refined approach. Mov. Disord. 2013, 28, 1534–1543. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa is a Double-Edged Sword for Balance and Gait in People with Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Ni, M.; Hazzard, J.B.; Signorile, J.F.; Luca, C. Exercise Guidelines for Gait Function in Parkinson’s Disease: A Systematic Review and Meta-analysis. Neurorehabilit. Neural Repair 2018, 32, 872–886. [Google Scholar] [CrossRef]
- Halliday, S.E.; Winter, D.A.; Frank, J.S.; Patla, A.E.; Prince, F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait Posture 1998, 8, 8–14. [Google Scholar] [CrossRef]
- Delval, A.; Moreau, C.; Bleuse, S.; Tard, C.; Ryckewaert, G.; Devos, D.; Defebvre, L. Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin. Neurophysiol. 2014, 125, 1675–1681. [Google Scholar] [CrossRef]
- Frenklach, A.; Louie, S.; Koop, M.M.; Bronte-Stewart, H. Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. Mov. Disord. 2009, 24, 377–385. [Google Scholar] [CrossRef]
- Konczak, J.; Krawczewski, K.; Tuite, P.; Maschke, M.; Konczak, J. The perception of passive motion in Parkinson’s disease. J. Neurol. 2007, 254, 655–663. [Google Scholar] [CrossRef]
- Gomez, C.M.; Tuite, P.J.; Konczak, J.; Maschke, M. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 2003, 126, 2312–2322. [Google Scholar] [CrossRef]
- Seiss, E.; Praamstra, P.; Hesse, C.W.; Rickards, H. Proprioceptive sensory function in Parkinson’s disease and Huntington’s disease: Evidence from proprioception-related EEG potentials. Exp. Brain Res. 2003, 148, 308–319. [Google Scholar] [CrossRef]
- Feller, K.J.; Peterka, R.J.; Horak, F.B. Sensory Re-weighting for Postural Control in Parkinson’s Disease. Front. Hum. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef]
- Smith, B.A.; Carlson-Kuhta, P.; Horak, F.B. Consistency in Administration and Response for the Backward Push and Release Test: A Clinical Assessment of Postural Responses. Physiother. Res. Int. 2016, 21, 36–46. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Harro, C.C.; Kelch, A.; Hargis, C.; DeWitt, A. Comparing Balance Performance on Force Platform Measures in Individuals with Parkinson’s Disease and Healthy Adults. Park. Dis. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Objective Gait and Balance Impairments Relate to Balance Confidence and Perceived Mobility in People with Parkinson Disease. Phys. Ther. 2016, 96, 1734–1743. [Google Scholar] [CrossRef]
| Controls (N = 79) | PD (N = 144) | p Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Male/Female | 48/31 | 93/51 | 0.571 a | ||
| Age | 68.2 | 8.1 | 68.4 | 8.0 | 0.822 |
| Height (cm) | 171.5 | 10.0 | 173.4 | 9.9 | 0.184 b |
| Weight (kg) | 73.7 | 13.1 | 79.7 | 16.9 | 0.018 b |
| Disease Duration (years) | - | - | 6.2 | 5.0 | - |
| MDS-UPDRS | |||||
| Total | - | - | 68.7 | 20.4 | - |
| Part II | - | - | 13.6 | 7.0 | - |
| Part III | - | - | 40.6 | 12.6 | - |
| Mini-BEST | 24.0 | 2.6 | 18.5 | 4.8 | <0.001 b |
| ABC scale | 95.9 | 5.3 | 80.5 | 16.3 | <0.001 b |
| PDQ-39 | |||||
| Total | - | - | 17.6 | 11.8 | - |
| Mobility | - | - | 17.0 | 17.4 | - |
| MoCA | 26.8 | 2.3 | 25.8 | 3.4 | 0.080b |
| Hoehn & Yahr stage | - | 1/115/15/13 | - | ||
| (I/II/III/IV) | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, N.; Shah, V.V.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B.; Mancini, M. How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors—Separating the Trees from the Forest. Sensors 2019, 19, 3320. https://doi.org/10.3390/s19153320
Hasegawa N, Shah VV, Carlson-Kuhta P, Nutt JG, Horak FB, Mancini M. How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors—Separating the Trees from the Forest. Sensors. 2019; 19(15):3320. https://doi.org/10.3390/s19153320
Chicago/Turabian StyleHasegawa, Naoya, Vrutangkumar V. Shah, Patricia Carlson-Kuhta, John G. Nutt, Fay B. Horak, and Martina Mancini. 2019. "How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors—Separating the Trees from the Forest" Sensors 19, no. 15: 3320. https://doi.org/10.3390/s19153320
APA StyleHasegawa, N., Shah, V. V., Carlson-Kuhta, P., Nutt, J. G., Horak, F. B., & Mancini, M. (2019). How to Select Balance Measures Sensitive to Parkinson’s Disease from Body-Worn Inertial Sensors—Separating the Trees from the Forest. Sensors, 19(15), 3320. https://doi.org/10.3390/s19153320




_Carlson-Kuhta.jpg)


