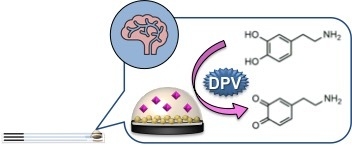
Electrochemical Nanocomposite Single-Use Sensor for Dopamine Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
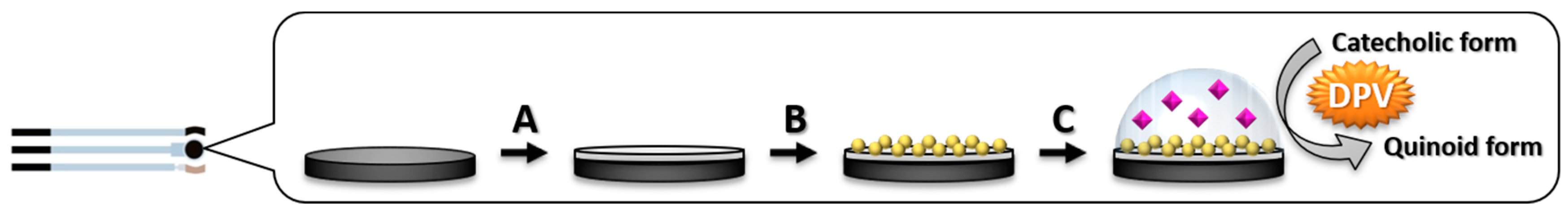
2.3. Sensor Development
2.3.1. Gold Nanoparticles @ Polyaniline Electrodeposition
2.3.2. Electrochemical Characterization of the Modified GSPEs
2.3.3. Dopamine Detection
2.3.4. Real Samples Analysis
3. Results and Discussion
3.1. Modification of GSPEs
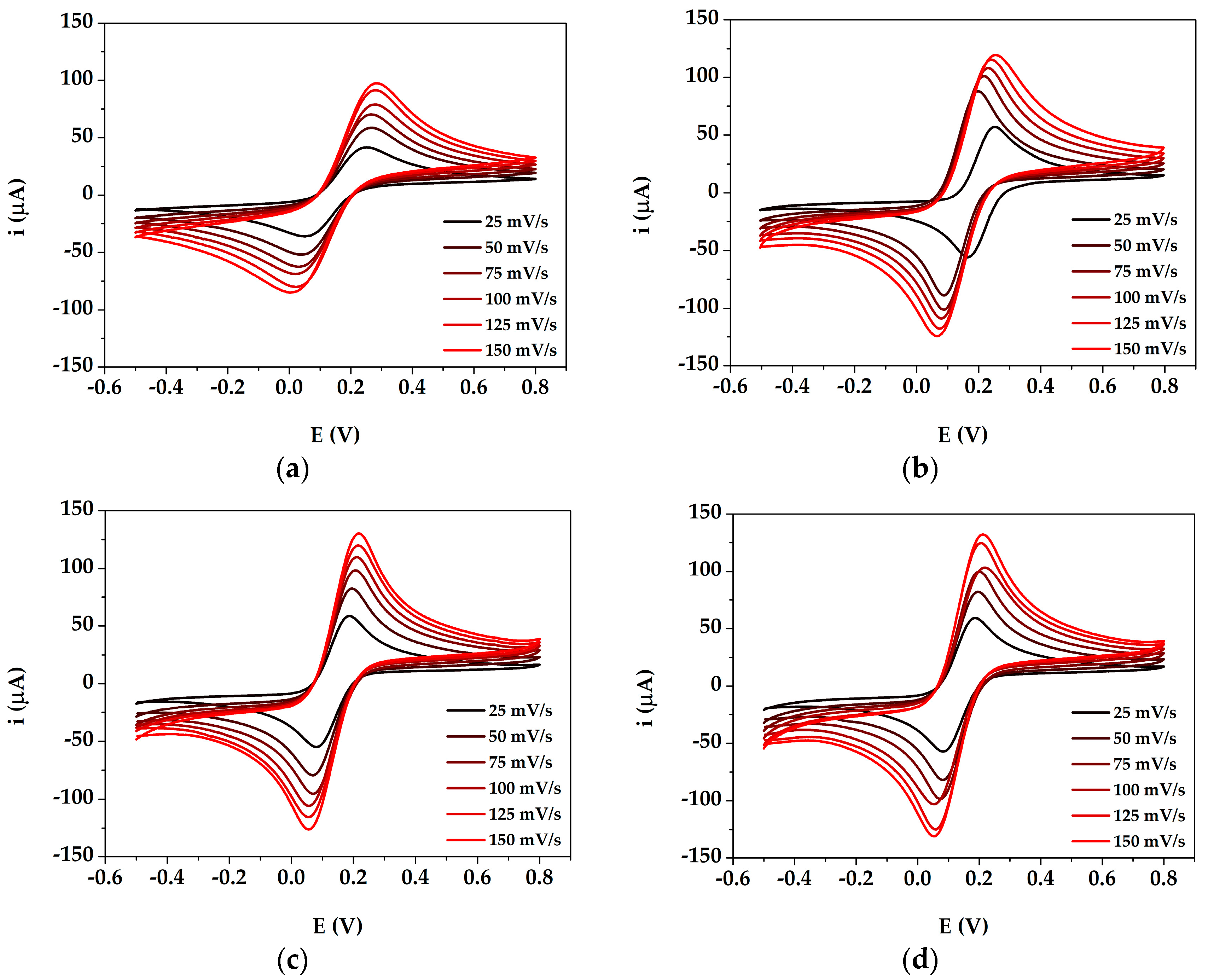
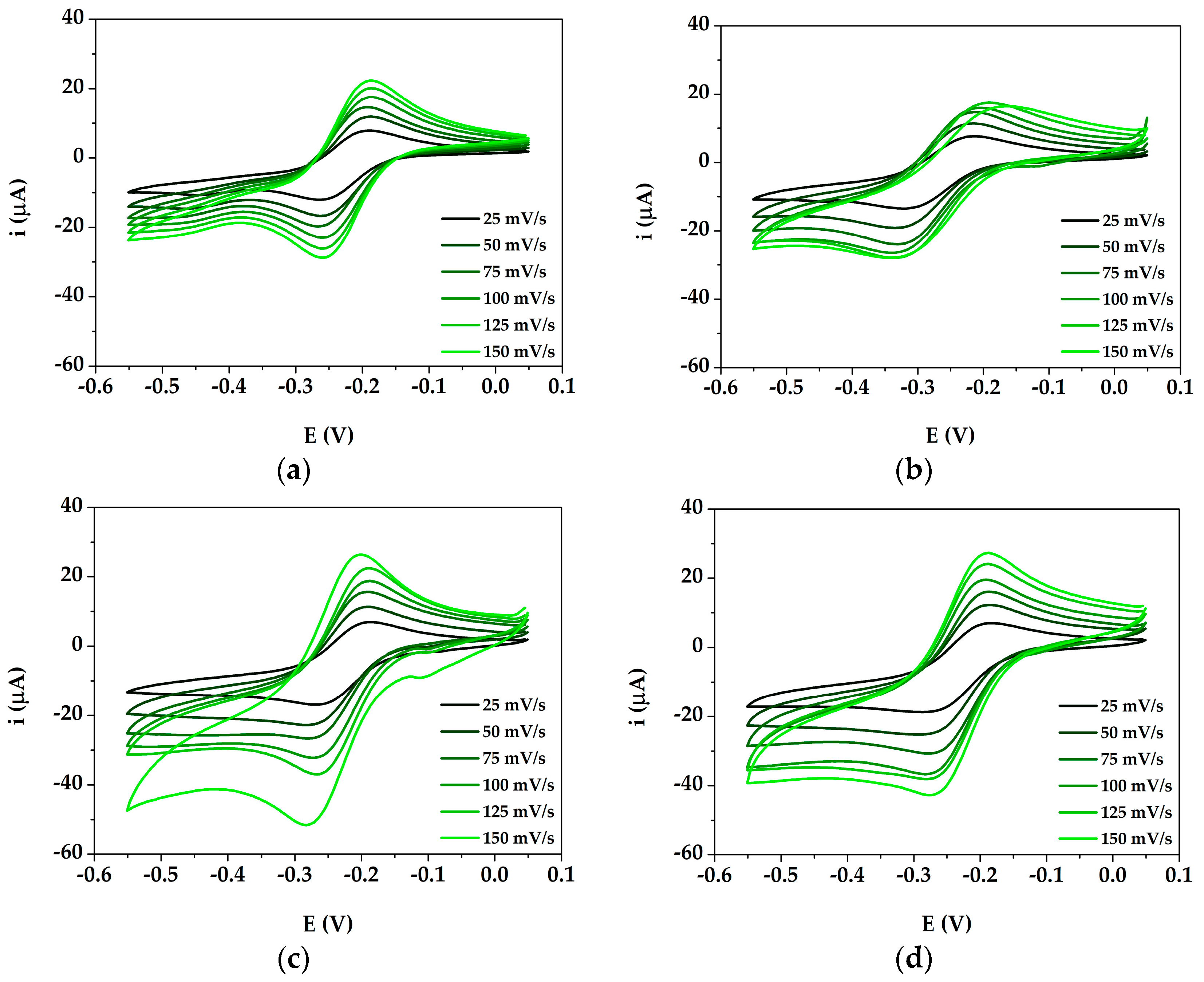
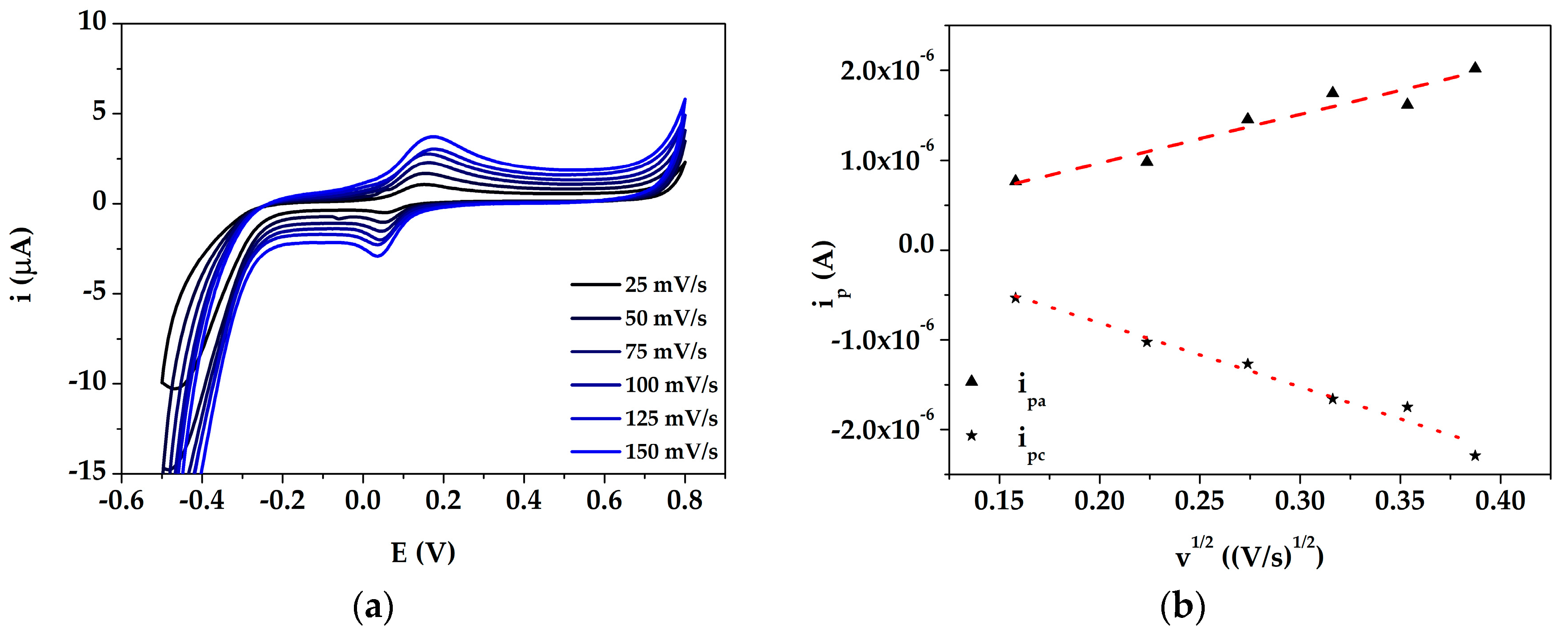
3.2. Study of DA Oxidation by Cyclic Voltammetry at AuNPs@PANI/GSPE
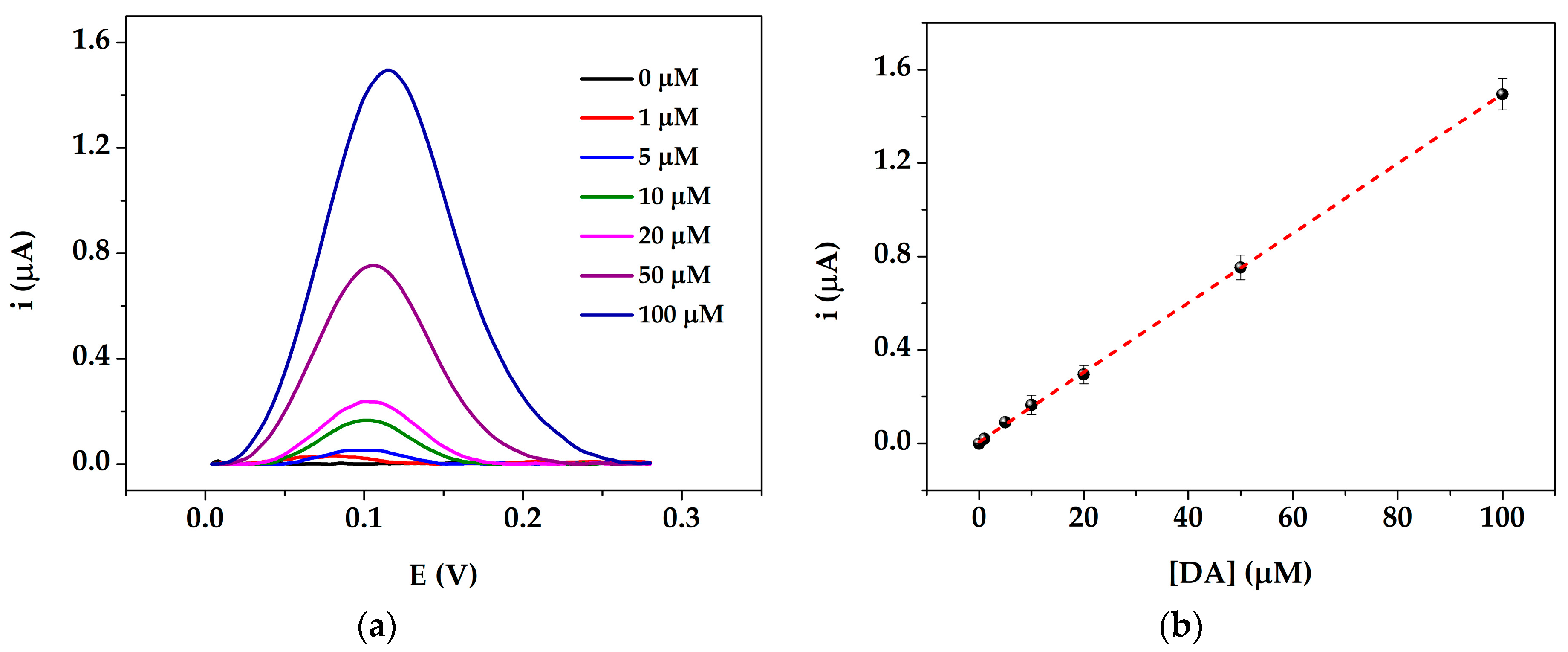
3.3. Dopamine Calibration Curve
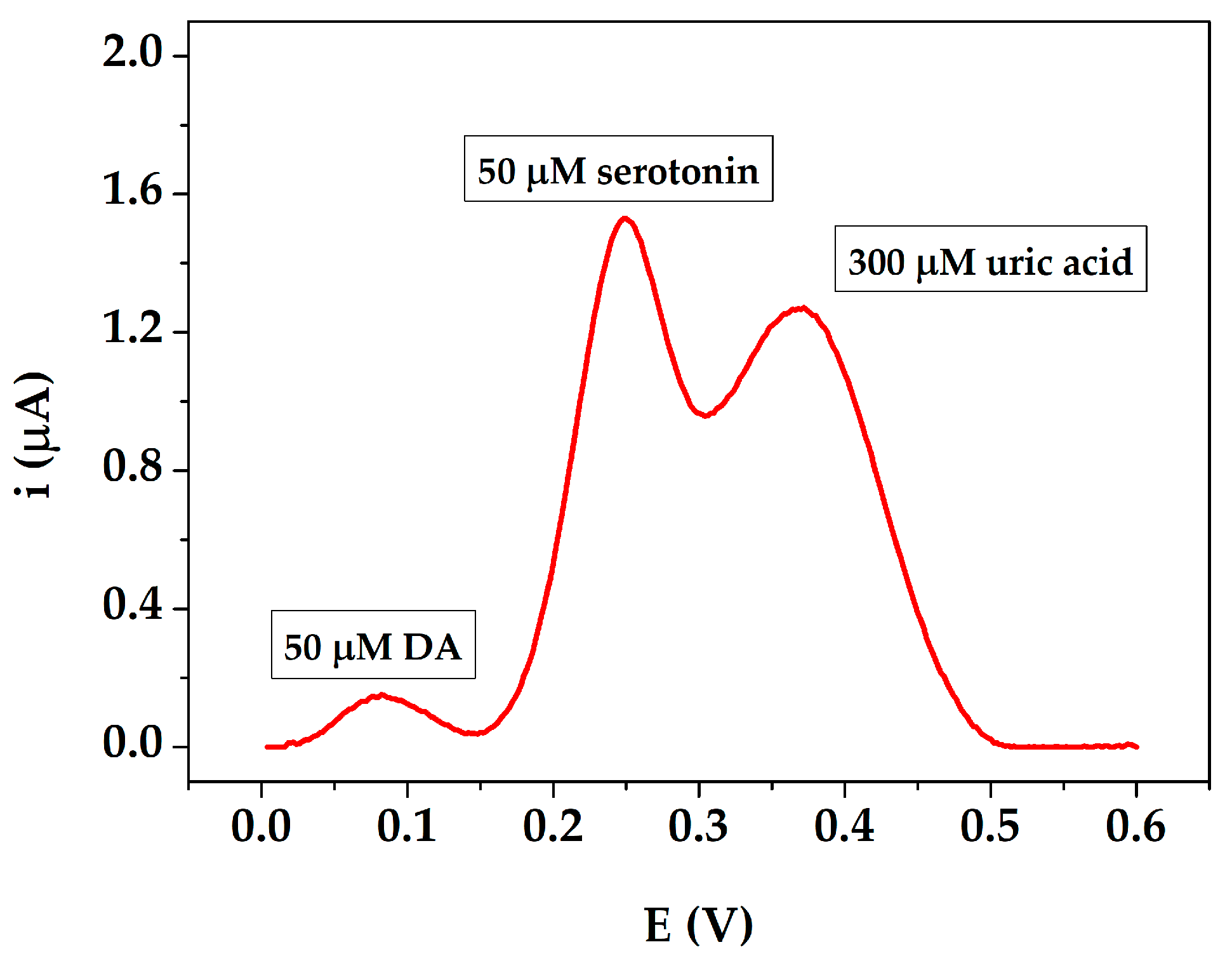
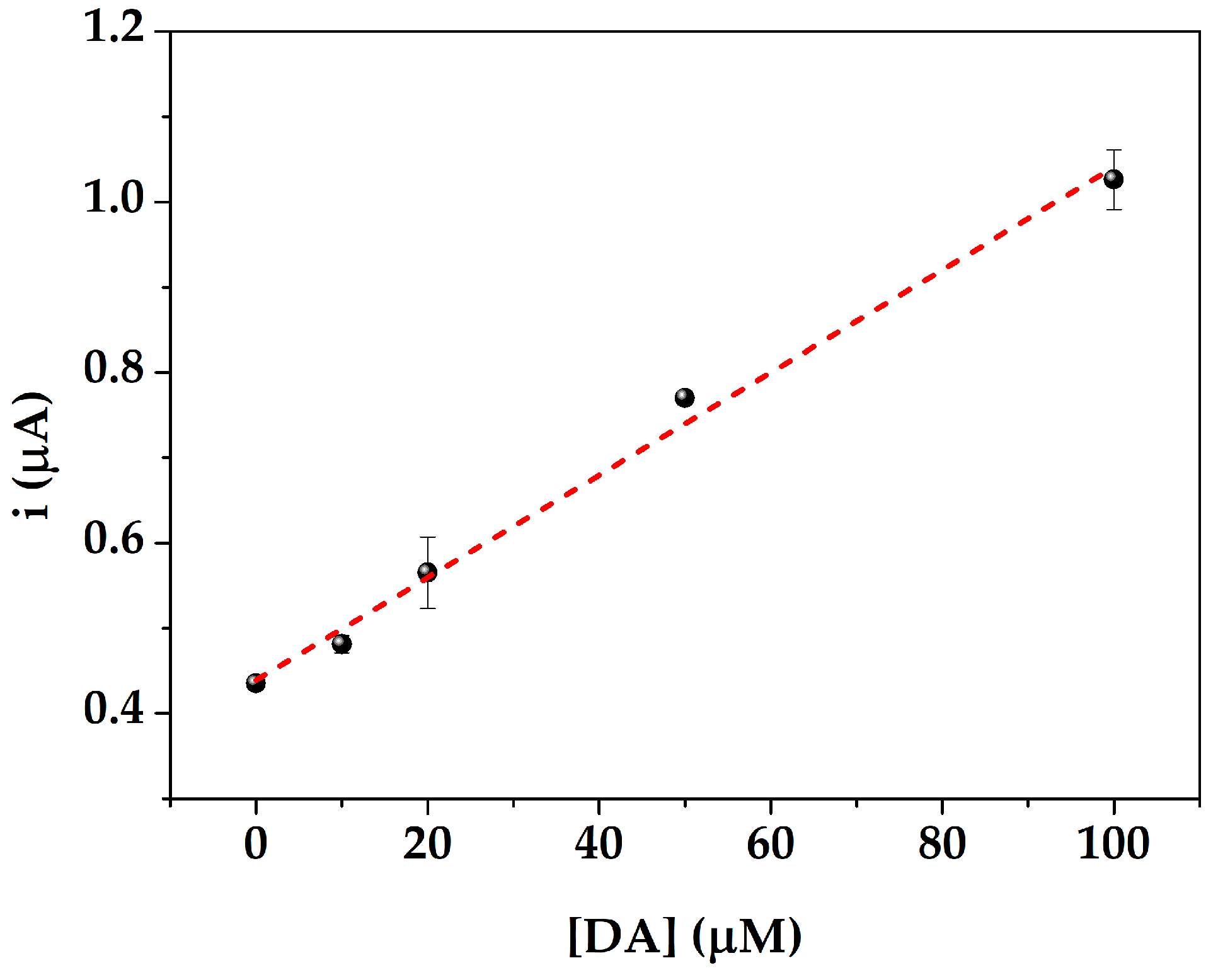
3.4. Serum Samples Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zemková, H.; Stojilkovic, S.S. Neurotransmitter receptors as signaling platforms in anterior pituitary cells. Mol. Cell. Endocrinol. 2018, 463, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Sangubotla, R.; Kim, J. Recent trends in analytical approaches for detecting neurotransmitters in Alzheimer’s disease. Trends Anal. Chem. 2018, 105, 240–250. [Google Scholar] [CrossRef]
- Baranwal, A.; Chandra, P. Clinical implications and electrochemical biosensing of monoamine neurotransmitters in body fluids, in vitro, in vivo, and ex vivo models. Biosens. Bioelectron. 2018, 121, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical analysis of neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Si, B.; Song, E. Recent advances in the detection of neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, S.; Song, Y.; Zhang, Y.; Li, Z.; Gao, F.; Xie, J.; Sha, L.; Xu, Q.; Shen, Y.; et al. In situ detection of neurotransmitters and epileptiform electrophysiology activity in awake mice brains using a nanocomposites modified microelectrode array. Sens. Actuators B Chem. 2019, 288, 601–610. [Google Scholar] [CrossRef]
- Tavakolian-Ardakani, Z.; Hosu, O.; Cristea, C.; Mazloum-Ardakani, M.; Marrazza, G. Latest trends in electrochemical sensors for neurotransmitters: A review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Mekawy, M.; Azzam, A.M.; Akhtar, N.; Gomaa, H.; Selim, M.M.; Faheem, A.; El-Safty, S.A. Ultrasensitive in-vitro monitoring of monoamine neurotransmitters from dopaminergic cells. Sens. Actuators B Chem. 2018, 259, 114–124. [Google Scholar] [CrossRef]
- Ramachandran, A.; Panda, S.; Karunakaran Yesodha, S. Physiological level and selective electrochemical sensing of dopamine by a solution processable graphene and its enhanced sensing property in general. Sens. Actuators B Chem. 2018, 256, 488–497. [Google Scholar] [CrossRef]
- Dinesh, B.; Saraswathi, R.; Senthil Kumar, A. Water based homogenous carbon ink modified electrode as an efficient sensor system for simultaneous detection of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2017, 233, 92–104. [Google Scholar] [CrossRef]
- Tsierkezos, N.G.; Ritter, U.; Nugraha Thaha, Y.; Knauer, A.; Fernandes, D.; Kelarakis, A.; McCarthy, E.K. Boron-doped multi-walled carbon nanotubes as sensing material for analysis of dopamine and epinephrine in presence of uric acid. Chem. Phys. Lett. 2018, 710, 157–167. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Wilson, D.; Gonçalves, D.; Oliveira, O.N. Low-cost screen-printed electrodes based on electrochemically reduced graphene oxide-carbon black nanocomposites for dopamine, epinephrine and paracetamol detection. J. Colloid Interface Sci. 2018, 515, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Diestra, D.; Thapa, B.; Beltran-Huarac, J.; Weiner, B.R.; Morell, G. L-cysteine capped ZnS: Mn quantum dots for room-temperature detection of dopamine with high sensitivity and selectivity. Biosens. Bioelectron. 2017, 87, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. Morphology-controlled synthesis of Bi2S3 nanorods-reduced graphene oxide composites with high-performance for electrochemical detection of dopamine. Sens. Actuators B Chem. 2018, 257, 936–943. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Kang, K.; Niu, L.M.; Kang, W.J. Electroanalysis of neurotransmitters via 3D gold nanoparticles and a graphene composite coupled with a microdialysis device. J. Electroanal. Chem. 2019, 834, 249–257. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef]
- Tertiş, M.; Florea, A.; Adumitrăchioaie, A.; Cernat, A.; Bogdan, D.; Barbu-Tudoran, L.; Jaffrezic Renault, N.; Săndulescu, R.; Cristea, C. Detection of dopamine by a biomimetic electrochemical sensor based on polythioaniline-bridged gold nanoparticles. Chempluschem 2017, 82, 561–569. [Google Scholar] [CrossRef]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Cherrington, R.; Liang, J. Materials and Deposition Processes for Multifunctionality. In Design and Manufacture of Plastic Components for Multifunctionality: Structural Composites, Injection Molding, and 3D Printing; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 19–21. [Google Scholar]
- Yilmaz, F.; Kukukyavuz, Z. Solution properties of polyaniline. Polym. Int. 2010, 59, 552–556. [Google Scholar] [CrossRef]
- Saberi, R.-S.; Shahrokhian, S.; Marrazza, G. Amplified electrochemical DNA sensor based on polyaniline film and gold nanoparticles. Electroanalysis 2013, 25, 1373–1380. [Google Scholar] [CrossRef]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Rapini, R.; Cincinelli, A.; Marrazza, G. Acetamiprid multidetection by disposable electrochemical DNA aptasensor. Talanta 2016, 161, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Selvolini, G.; Băjan, I.; Hosu, O.; Cristea, C.; Săndulescu, R.; Marrazza, G. DNA-based sensor for the detection of an organophosphorus pesticide: Profenofos. Sensors 2018, 18, 2035. [Google Scholar] [CrossRef] [PubMed]
- Zablocka, I.; Wysocka-Zolopa, M.; Winkler, K. Electrochemical detection of dopamine at a gold electrode modified with a polypyrrole–mesoporous silica molecular sieves (MCM-48) film. Int. J. Mol. Sci. 2019, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Ma, Y.; Parajuli, R.R.; Balogun, Y.; Lai, W.Y.C.; He, H. A nonoxidative sensor based on a self-doped polyaniline/carbon nanotube composite for sensitive and selective detection of the neurotransmitter dopamine. Anal. Chem. 2007, 79, 2583–2587. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Ma, W.; Zhang, Y.; Xhang, D. Preparation of a glassy carbon electrode modified with reduced graphene oxide and overoxidized electropolymerized polypyrrole, and its application to the determination of dopamine in the presence of ascorbic acid and uric acid. Microchim. Acta 2019, 186, 407. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Kumara Swamy, B.E.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine in real samples using polyaniline/NiO, ZnO, and Fe3O4 nanocomposites on glassy carbon electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S. Simultaneous detection of ascorbic acid, dopamine, uric acid and tryptophan with Azure A-interlinked multi-walled carbon nanotube/gold nanoparticles composite modified electrode. Arab. J. Chem. 2016, 9, 471–480. [Google Scholar] [CrossRef]
- Muratova, I.S.; Mikhelson, K.N. Voltammetric sensing of dopamine in urine samples with electrochemically activated commercially available screen-printed carbon electrodes. Int. J. Biosens. Bioelectron. 2018, 4, 169–173. [Google Scholar] [CrossRef]
- Raoof, J.B.; Kiani, A.; Ojani, R.; Valiollahi, R. Electrochemical determination of dopamine using banana-MWCNTs modified carbon paste electrodes. Anal. Chim. Actaytical Bioanal. Electrochem. 2011, 3, 59–66. [Google Scholar]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
| GSPE | PANI/GSPE | AuNPs/GSPE | AuNPs@PANI/GSPE | ||
|---|---|---|---|---|---|
| [Fe(CN)6]4−/3− | Aanodic | 6.8 | 7.8 | 9.1 | 9.3 |
| Acathodic | 6.2 | 8.3 | 8.9 | 9.2 | |
| Ā | 6.5 | 8.0 | 9.0 | 9.2 | |
| %RSD | 7 | 5 | 2 | 1 | |
| [Ru(NH3)6]2+/3+ | Aanodic | 1.8 | 0.8 | 2.3 | 2.1 |
| Acathodic | 2.1 | 1.4 | 3.1 | 2.4 | |
| Ā | 2.0 | 1.1 | 2.7 | 2.3 | |
| %RSD | 10 | 36 | 22 | 8 |
| Dilution Ratio | isample/i1 | %RSD |
|---|---|---|
| 1:5 | 0.10 | 1.15 |
| 1:10 | 0.07 | 1.48 |
| 1:20 | 0.16 | 0.84 |
| 1:40 | 0.23 | 0.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvolini, G.; Lazzarini, C.; Marrazza, G. Electrochemical Nanocomposite Single-Use Sensor for Dopamine Detection. Sensors 2019, 19, 3097. https://doi.org/10.3390/s19143097
Selvolini G, Lazzarini C, Marrazza G. Electrochemical Nanocomposite Single-Use Sensor for Dopamine Detection. Sensors. 2019; 19(14):3097. https://doi.org/10.3390/s19143097
Chicago/Turabian StyleSelvolini, Giulia, Cinzia Lazzarini, and Giovanna Marrazza. 2019. "Electrochemical Nanocomposite Single-Use Sensor for Dopamine Detection" Sensors 19, no. 14: 3097. https://doi.org/10.3390/s19143097
APA StyleSelvolini, G., Lazzarini, C., & Marrazza, G. (2019). Electrochemical Nanocomposite Single-Use Sensor for Dopamine Detection. Sensors, 19(14), 3097. https://doi.org/10.3390/s19143097