Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea
Abstract
1. Introduction
2. Materials and Methods
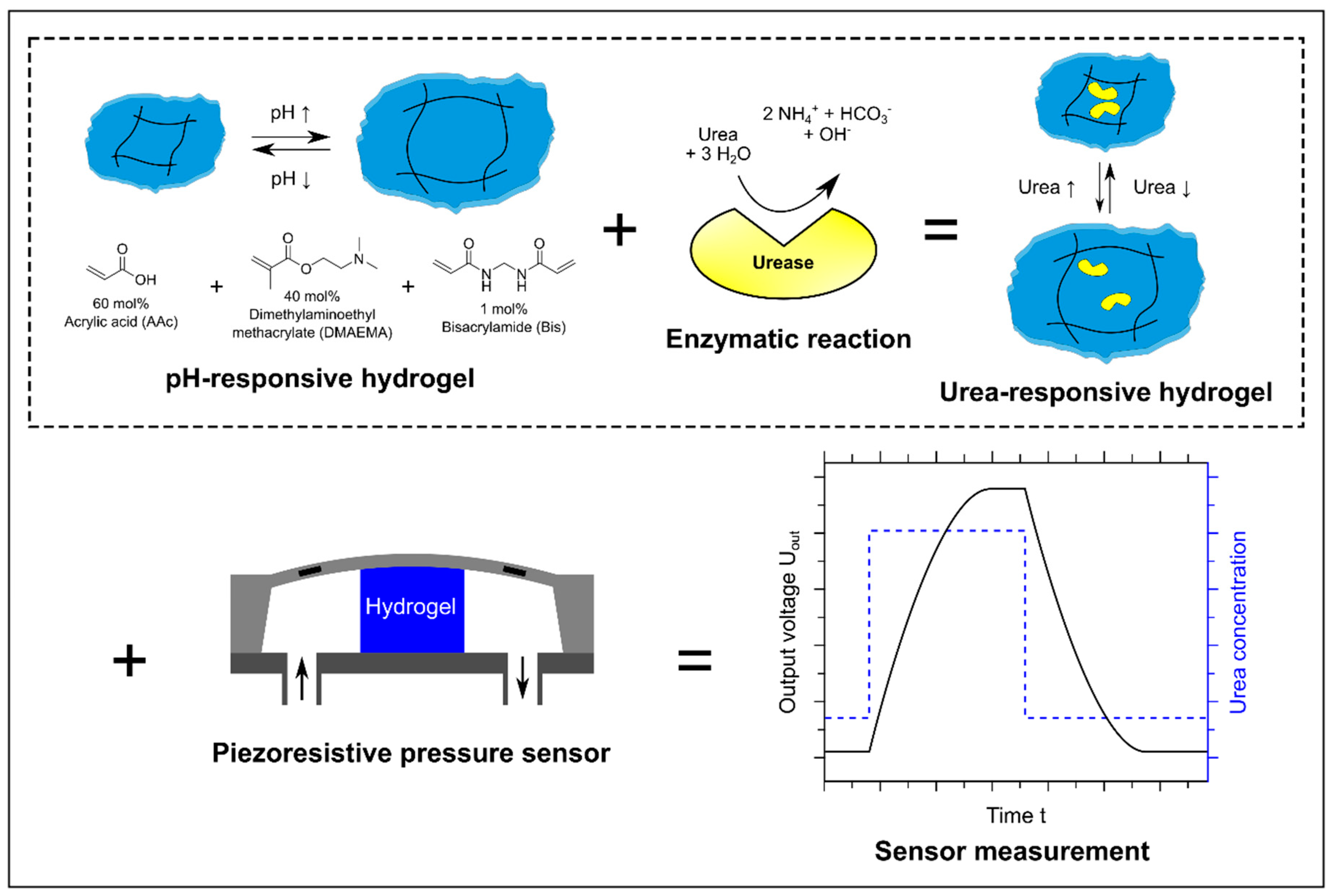
2.1. Synthesis of Urea-Sensitive Hydrogels Based on Poly(Acrylic acid-co-Dimethylaminoethyl methacrylate) and the Functionalized Enzyme Urease
2.2. Swelling Studies in Dependence on the Urea Concentration
2.3. Swelling Kinetics of Urea-Sensitive Hydrogels
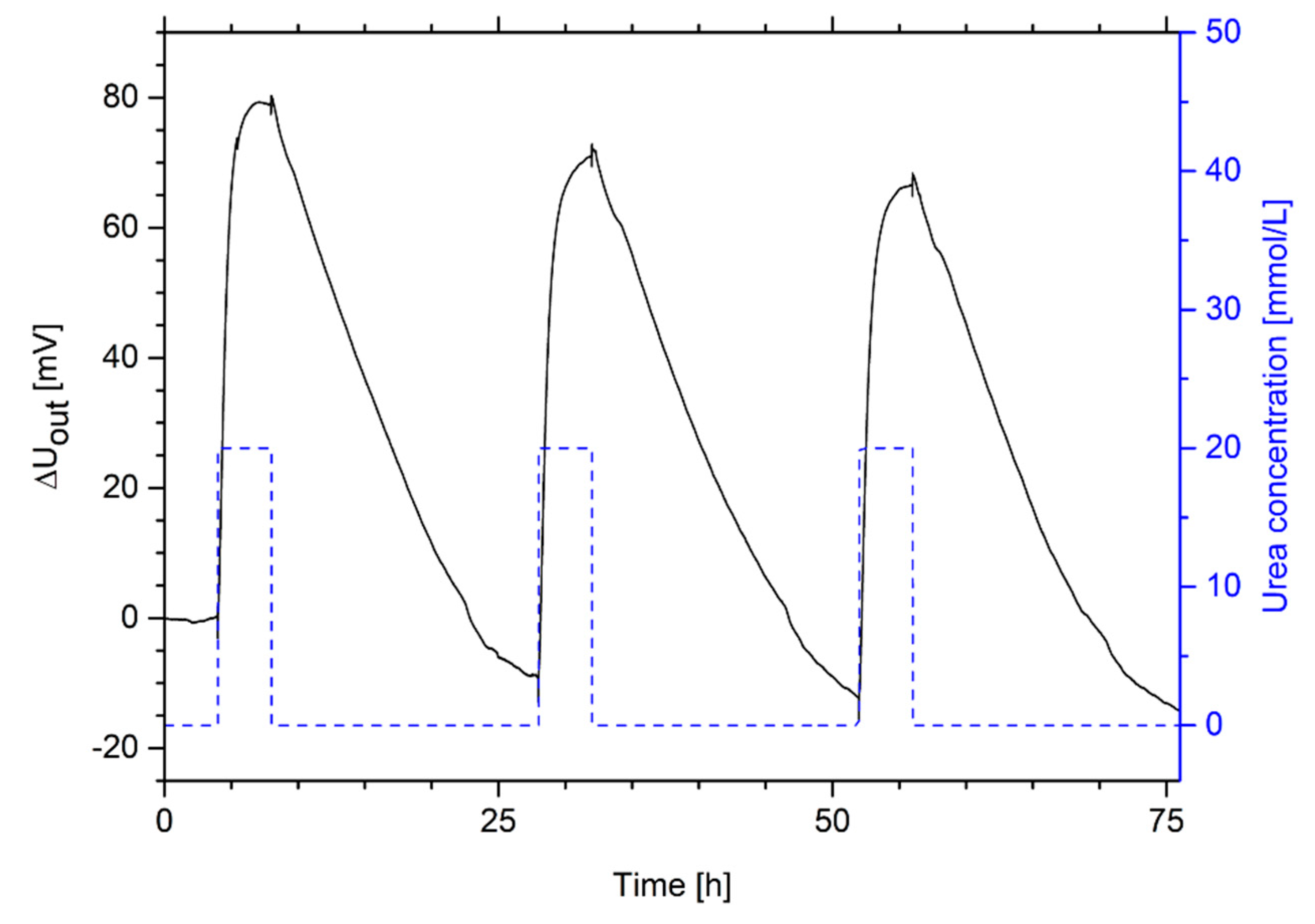
2.4. Repeatability and Long-Term Stability of Urea-Sensitive Hydrogels
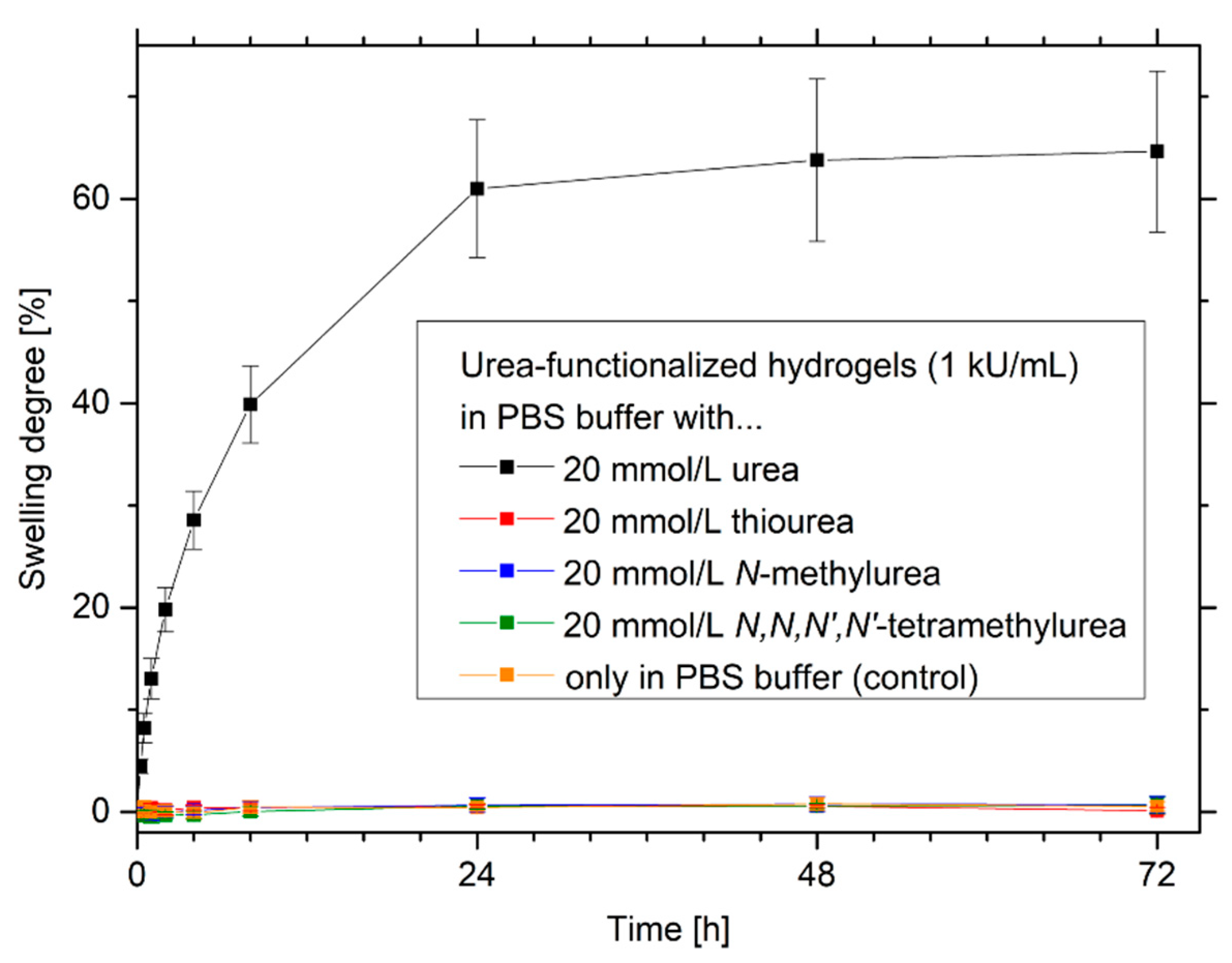
2.5. Selectivity of Urea-Sensitive Hydrogels to Similar Species
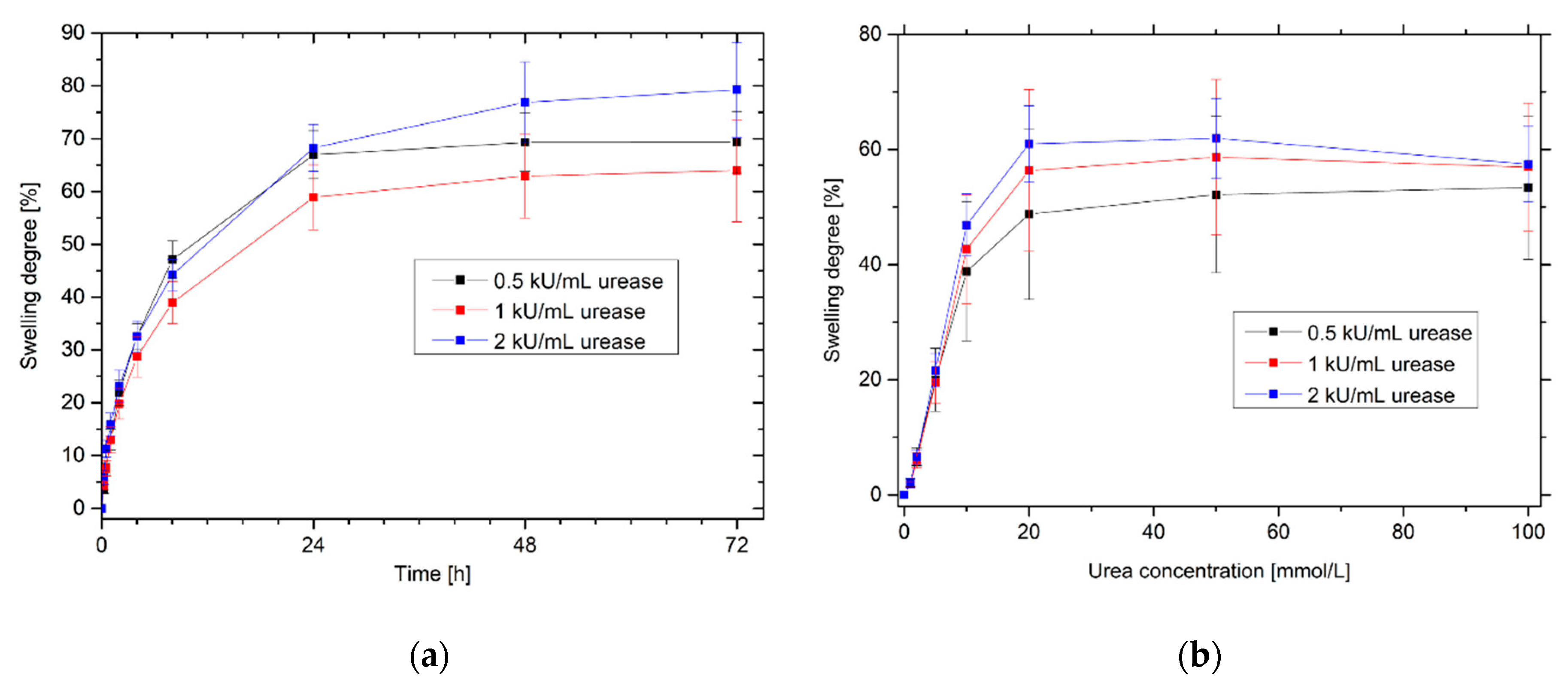
2.6. Effects of Different Amounts of Enzyme on the Sensitivity and Swelling Kinetics of Urea-Sensitive Hydrogels
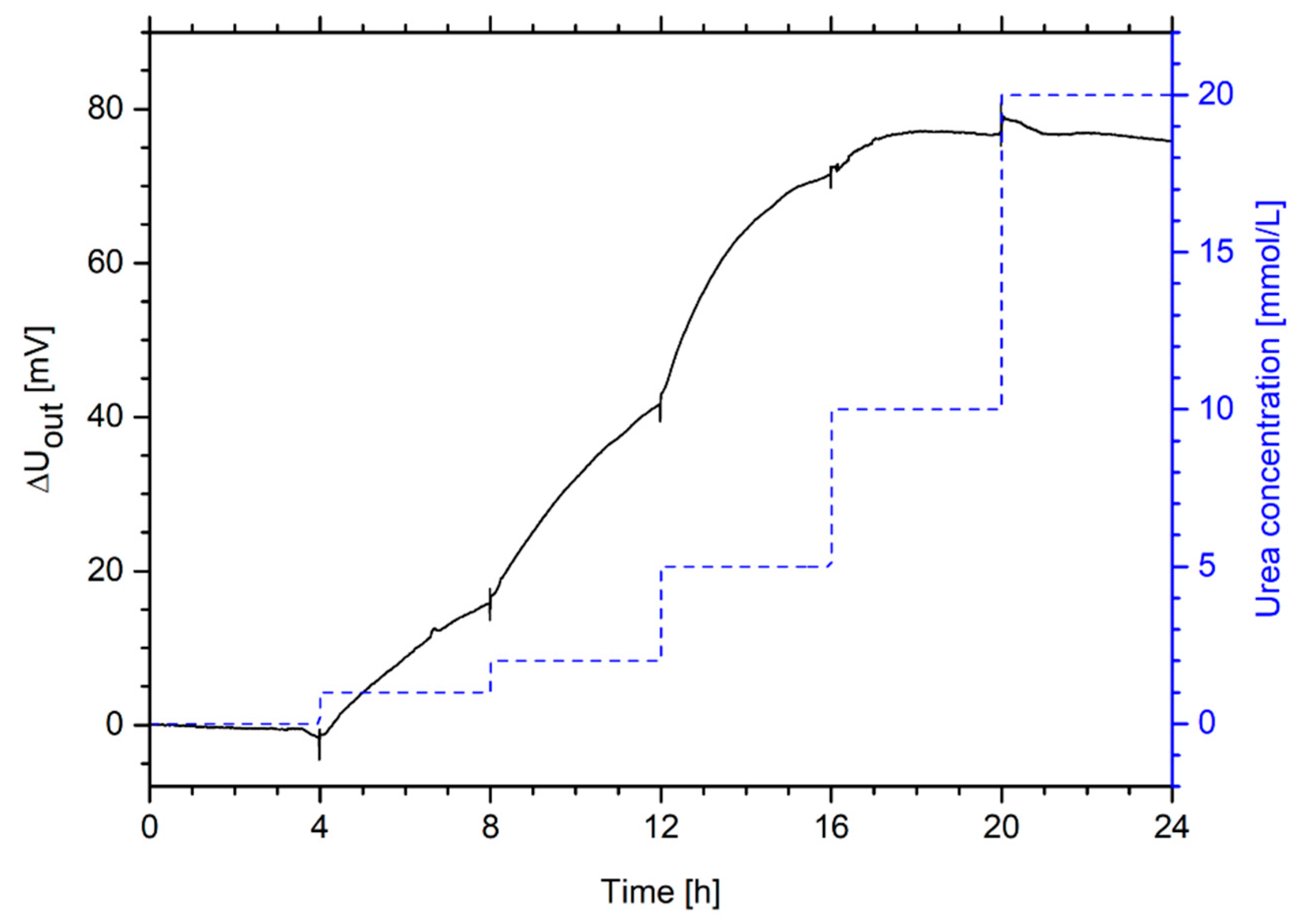
2.7. Hydrogel-Based Piezoresistive Urea Biosensors
3. Results and Discussion
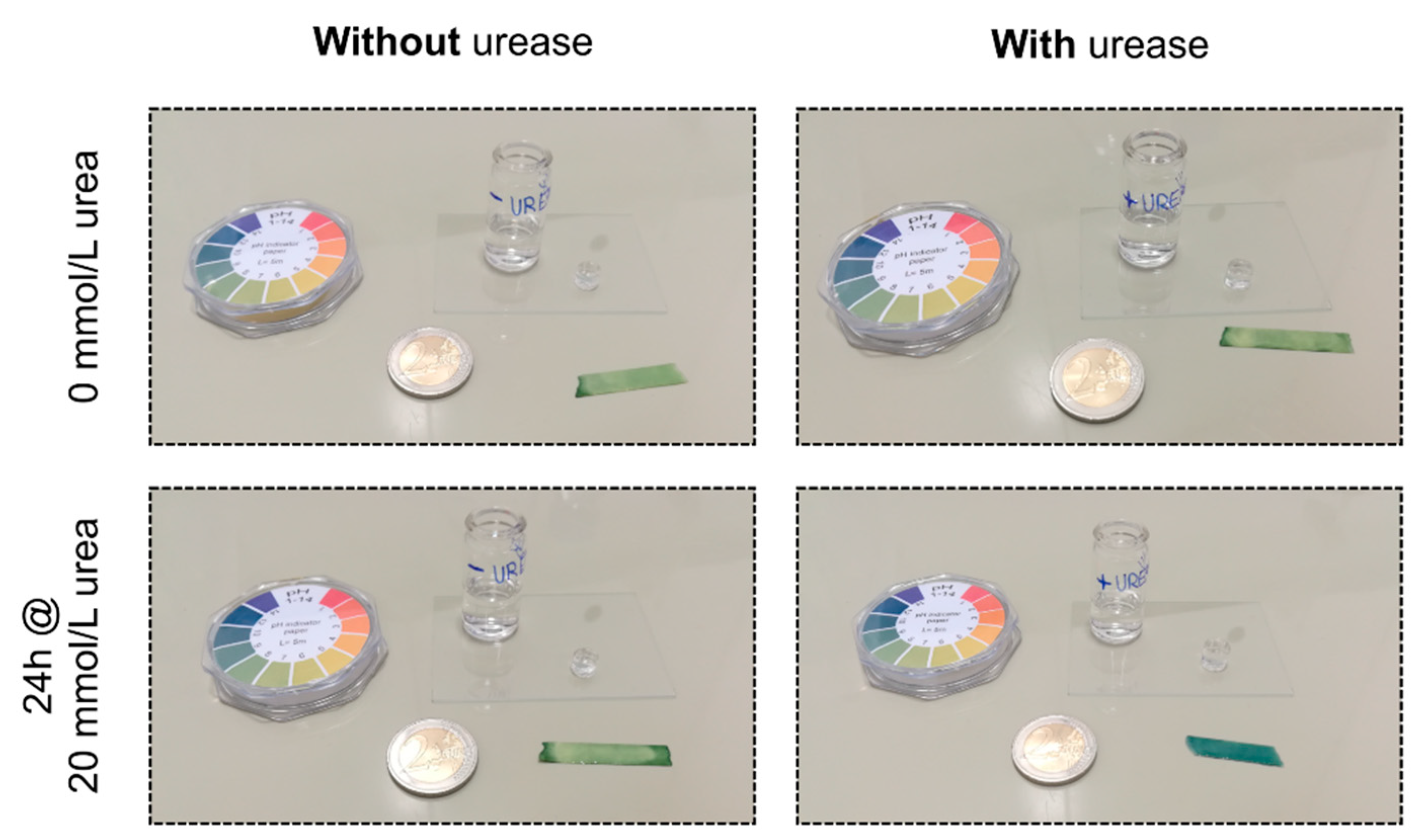
3.1. Verification of the Enzyme Immobilization
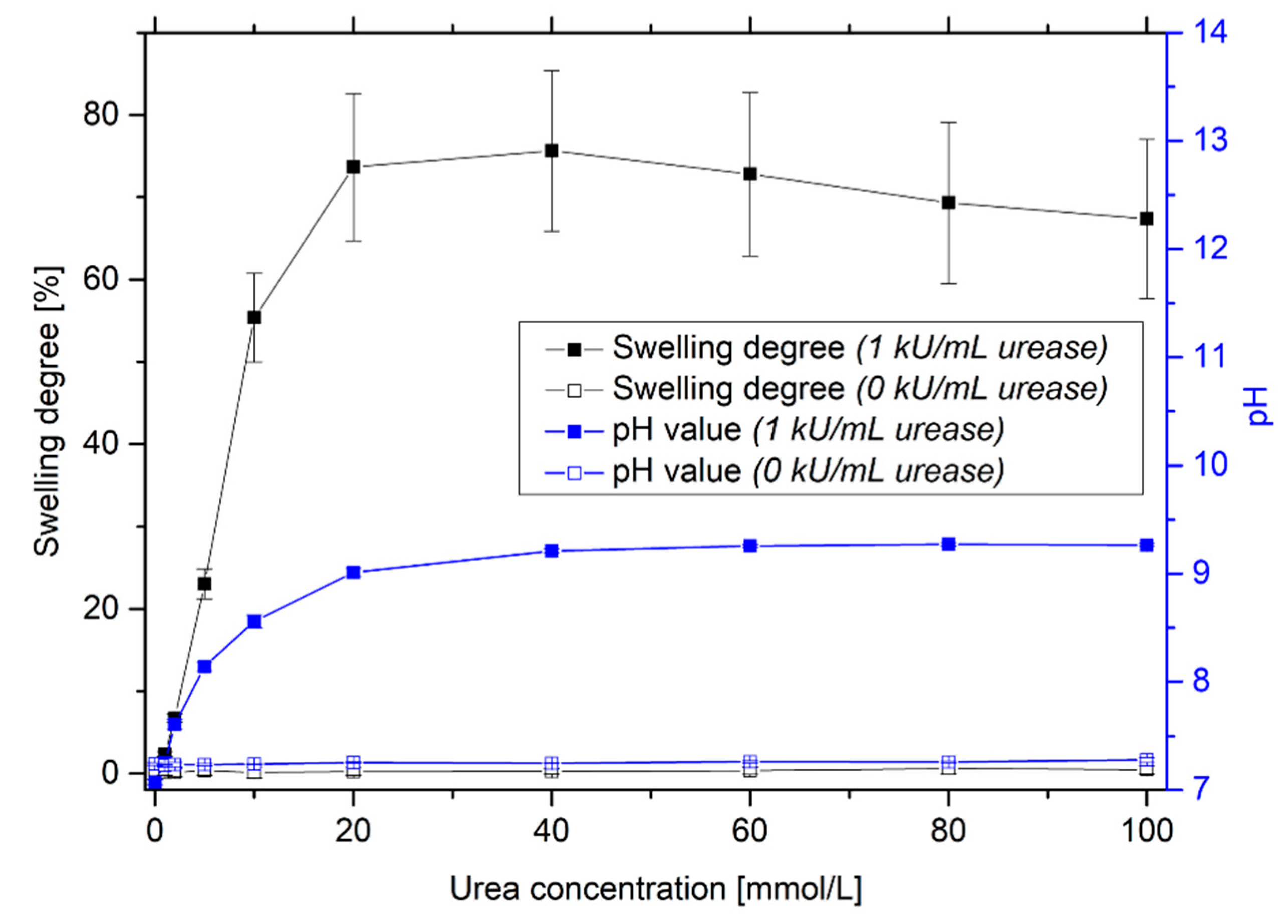
3.2. Swelling Studies in Dependence on the Urea Concentration
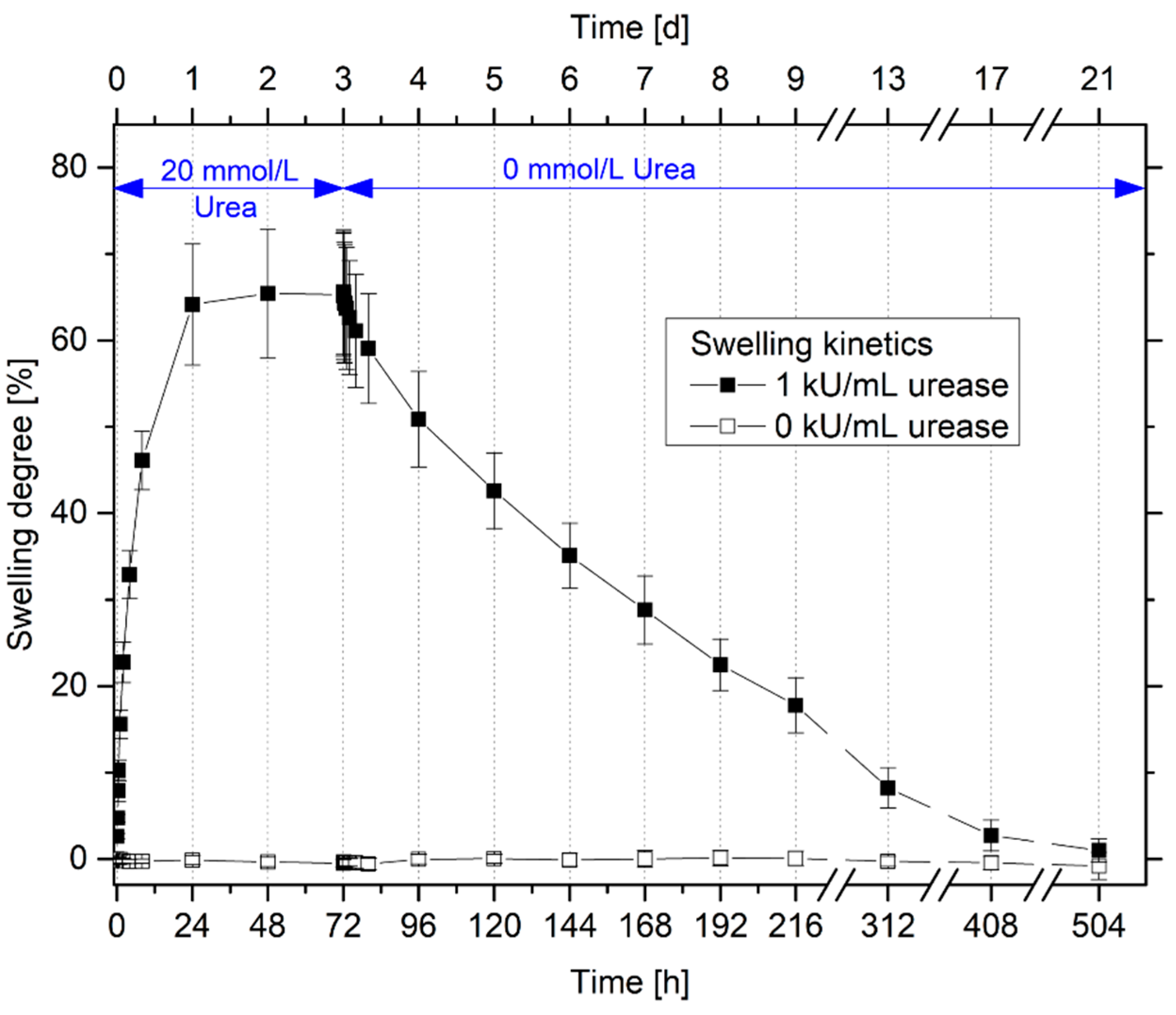
3.3. Swelling Kinetics of Urea-Sensitive Hydrogels
3.4. Repeatability and Long-Term Stability of Urea-Sensitive Hydrogels
3.5. Selectivity of Urea-Sensitive Hydrogels to Similar Species
3.6. Effects of Different Amounts of Enzyme on the Sensitivity and Swelling Kinetics of Urea-Sensitive Hydrogels
3.7. Hydrogel-Based Piezoresistive Urea Biosensors
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; van den Berg, A. Lactate biosensors: current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Icaza, M.; Bilitewski, U. Mass production of biosensors. Anal. Chem. 1993, 65, 525A–533A. [Google Scholar] [CrossRef]
- Turner, A. Biosensors: then and now. Trends Biotechnol. 2013, 31, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Fernandes, S.; Godinho, M.; Borges, J.; Soares, P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Pant, B.D. Design principles and considerations for the ‘ideal’ silicon piezoresistive pressure sensor: A focused review. Microsyst. Technol. 2014, 20, 1213–1247. [Google Scholar] [CrossRef]
- Trinh, Q.T.; Gerlach, G.; Sorber, J.; Arndt, K.-F. Hydrogel-based piezoresistive pH sensors: Design, simulation and output characteristics. Sens. Actuators B Chem. 2006, 117, 17–26. [Google Scholar] [CrossRef]
- Franke, D.; Binder, S.; Gerlach, G. Performance of Fast-Responsive, Porous Crosslinked Poly(N-Isopropylacrylamide) in a Piezoresistive Microsensor. IEEE Sens. Lett. 2017, 1, 1500904. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-based piezoresistive sensor for the detection of ethanol. J. Sens. Sens. Syst. 2018, 7, 219–226. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages. Sensors 2019, 19, 1199. [Google Scholar] [CrossRef]
- Nilsson, H.; Åkerlund, A.-C.; Mosbach, K. Determination of glucose, urea and penicillin using enzyme-pH-electrodes. Biochim. Biophys. Acta BBA - Gen. Subj. 1973, 320, 529–534. [Google Scholar] [CrossRef]
- Schmidt, U.; Jorsch, C.; Guenther, M.; Gerlach, G. Biochemical piezoresistive sensors based on hydrogels for biotechnology and medical applications. J. Sens. Sens. Syst. 2016, 5, 409–417. [Google Scholar] [CrossRef]
- Li, L.; Long, Y.; Gao, J.-M.; Song, K.; Yang, G. Label-free and pH-sensitive colorimetric materials for the sensing of urea. Nanoscale 2016, 8, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, G.; Sumana, G.; Malhotra, B.D. Recent developments in urea biosensors. Biochem. Eng. J. 2009, 44, 42–52. [Google Scholar] [CrossRef]
- Ruzicka, J.; Hansen, E.H.; Ghose, A.K.; Mottola, H.A. Enzymic determination of urea in serum based on pH measurement with the flow injection method. Anal. Chem. 1979, 51, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R.; Leszcyński, P.; Hulanicki, A.; Gła̧b, S. Urea sensors based on glass pH electrodes with physically immobilized urease. Anal. Chim. Acta 1992, 257, 67–72. [Google Scholar] [CrossRef]
- Boubriak, O.A.; Soldatkin, A.P.; Starodub, N.F.; Sandrovsky, A.K.; El’skaya, A.K. Determination of urea in blood serum by a urease biosensor based on an ion-sensitive field-effect transistor. Sens. Actuators B Chem. 1995, 27, 429–431. [Google Scholar] [CrossRef]
- Pijanowska, D.G.; Torbicz, W. pH-ISFET based urea biosensor. Sens. Actuators B Chem. 1997, 44, 370–376. [Google Scholar] [CrossRef]
- Zhai, M.; Chen, Y.; Yi, M.; Ha, H. Swelling behaviour of a new kind of polyampholyte hydrogel composed of dimethylaminoethyl methacrylate and acrylic acid. Polym. Int. 2004, 53, 33–36. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Piezoresistive Hydrogel-Based Sensors for the Detection of Ammonia. Sensors 2019, 19, 971. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Piezoresistive Biosensoren auf der Basis Enzym-funktionalisierter Hydrogele. In Tagungsband zum “19. Heiligenstädter Kolloquium - Technische Systeme für die Lebenswissenschaften”; Institut für Bioprozess- und Analysenmesstechnik e.V.: Heilbad Heiligenstadt, Germany, 2018; pp. 277–284. ISBN 978-3-00-060656-4. [Google Scholar]
- Scouten, W.; Luong, J.; Stephenbrown, R. Enzyme or protein immobilization techniques for applications in biosensor design. Trends Biotechnol. 1995, 13, 178–185. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, F.; Li, M.; Wang, E. pH switching on-off semi-IPN hydrogel based on cross-linked poly(acrylamide-co-acrylic acid) and linear polyallyamine. Polymer 2005, 46, 7695–7700. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Samadi, M.; Ghasemzadeh, H. Fast-swelling Superabsorbent Hydrogels from Poly(2-hydroxy ethyl acrylate-co-sodium acrylate) Grafted on Starch. Starch - Stärke 2008, 60, 79–86. [Google Scholar] [CrossRef]
- Uva, M.; Atrei, A. Surface Morphology at the Microscopic Scale, Swelling/Deswelling, and the Magnetic Properties of PNIPAM/CMC and PNIPAM/CMC/Fe3O4 Hydrogels. Gels 2016, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nakamura, S.; Sakai, K.; Aoyagi, T.; Kikuchi, A.; Sakurai, Y.; Okano, T. Rapid Deswelling Response of Poly( N -isopropylacrylamide) Hydrogels by the Formation of Water Release Channels Using Poly(ethylene oxide) Graft Chains. Macromolecules 1998, 31, 6099–6105. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A.-C. Novel pH-sensitive chitosan hydrogels: swelling behavior and states of water. Polymer 2000, 41, 4589–4598. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Economou, A.; Karapetis, S.K.; Nikoleli, G.-P.; Nikolelis, D.P.; Bratakou, S.; Varzakas, T.H. Enzyme-based Sensors. In Advances in Food Diagnostics; Toldrá, F., Nollet, L.M.L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 231–250. ISBN 978-1-119-10591-6. [Google Scholar]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Synthesis and Characterization of Thermo- and Chemomechanically Responsive Poly(N-isopropylacrylamide-co-methacrylic acid) Hydrogels. Macromolecules 1995, 28, 8016–8020. [Google Scholar] [CrossRef]
- Fares, M.M.; Al-Shboul, A.M. Stimuli pH-responsive (N-vinyl imidazole-co-acryloylmorpholine) Hydrogels; Mesoporous and Nanoporous Scaffolds. J. Biomed. Mater. Res. A 2012, 100A, 863–871. [Google Scholar] [CrossRef]
- Podual, K.; Doyle, F.J.; Peppas, N.A. Preparation and dynamic response of cationic copolymer hydrogels containing glucose oxidase. Polymer 2000, 41, 3975–3983. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Klem, M.T.; Willits, D.; Solis, D.J.; Belcher, A.M.; Young, M.; Douglas, T. Bio-inspired Synthesis of Protein-Encapsulated CoPt Nanoparticles. Adv. Funct. Mater. 2005, 15, 1489–1494. [Google Scholar] [CrossRef]
- Follmer, C.; Pereira, F.V.; da Silveira, N.P.; Carlini, C.R. Jack bean urease (EC 3.5.1.5) aggregation monitored by dynamic and static light scattering. Biophys. Chem. 2004, 111, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.C.; Wang, T.; Derby, B. Inkjet delivery of glucose oxidase. Chem. Commun. 2010, 46, 5452. [Google Scholar] [CrossRef] [PubMed]
- Eggenstein, C.; Borchardt, M.; Diekmann, C.; Gründig, B.; Dumschat, C.; Cammann, K.; Knoll, M.; Spener, F. A disposable biosensor for urea determination in blood based on an ammonium-sensitive transducer. Biosens. Bioelectron. 1999, 14, 33–41. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Lee, K.S.; Kim, T.-H.; Shin, M.-C.; Park, J.-K. Microfabricated Conductometric Urea Biosensor Based on Sol-Gel Immobilized Urease. Electroanalysis 2000, 12, 78–82. [Google Scholar] [CrossRef]
- Das, J.; Sarkar, P. Enzymatic electrochemical biosensor for urea with a polyaniline grafted conducting hydrogel composite modified electrode. RSC Adv. 2016, 6, 92520–92533. [Google Scholar] [CrossRef]
- Singh, M.; Verma, N.; Garg, A.; Redhu, N. Urea biosensors. Sens. Actuators B Chem. 2008, 134, 345–351. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Kroh, C.; Wuchrer, R.; Steinke, N.; Guenther, M.; Gerlach, G.; Härtling, T. Hydrogel-Based Plasmonic Sensor Substrate for the Detection of Ethanol. Sensors 2019, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; Gerlach, G.; Corten, C.; Kuckling, D.; Muller, M.; Shi, Z.; Sorber, J.; Arndt, K.-F. Application of Polyelectrolytic Temperature-Responsive Hydrogels in Chemical Sensors. Macromol. Symp. 2007, 254, 314–321. [Google Scholar] [CrossRef]
- Wang, J.; Gonzalez-Romero, E.; Ozsoz, M. Renewable alcohol biosensors based on alcohol-dehydrogenase/nicotinamide-adenine-dinucleotide graphite epoxy electrodes. Electroanalysis 1992, 4, 539–544. [Google Scholar] [CrossRef]
- Alpat, Ş.; Telefoncu, A. Development of an Alcohol Dehydrogenase Biosensor for Ethanol Determination with Toluidine Blue O Covalently Attached to a Cellulose Acetate Modified Electrode. Sensors 2010, 10, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Murakami, T.; Saito, A.; Kimura, J. Development of an amperometric alcohol sensor based on immobilized alcohol dehydrogenase and entrapped NAD+. Biosens. Bioelectron. 1991, 6, 563–567. [Google Scholar] [CrossRef]
- Krajewska, B. Ureases I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Löffler, G. Basiswissen Biochemie: mit Pathobiochemie; Springer-Lehrbuch; 7., komplett überarbeitete Auflage; Springer: Heidelberg, Germany, 2008; ISBN 978-3-540-76511-0. [Google Scholar]
- Orthner, M.P.; Lin, G.; Avula, M.; Buetefisch, S.; Magda, J.; Rieth, L.W.; Solzbacher, F. Hydrogel based sensor arrays (2×2) with perforated piezoresistive diaphragms for metabolic monitoring (in vitro). Sens. Actuators B Chem. 2010, 145, 807–816. [Google Scholar] [CrossRef]
- Deng, K.; Gerlach, G.; Guenther, M. Force-compensated hydrogel-based pH sensor. Proc. SPIE 2015, 9431, 943112. [Google Scholar] [CrossRef]
- Herber, S.; Berner, J.; Olthuis, W.; Bergveld, P.; van den Berg, A. A micro CO2 gas sensor based on sensing of pH-sensitive hydrogel swelling by means of a pressure sensor. In Proceedings of the the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, 2005. Digest of Technical Papers. TRANSDUCERS ’05, Seoul, Korea, 5–9 June 2005; pp. 1146–1149. [Google Scholar]
- Guenther, M.; Gerlach, G.; Wallmersperger, T. Non-linear Effects in Hydrogel-based Chemical Sensors: Experiment and Modeling. J. Intell. Mater. Syst. Struct. 2009, 20, 949–961. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Kayastha, A.M. Srinivasan Characterization of gelatin-immobilized pigeonpea urease and preparation of a new urea biosensor. Biotechnol. Appl. Biochem. 2001, 34, 55. [Google Scholar] [CrossRef]
- Castillo-Ortega, M.M.; Rodriguez, D.E.; Encinas, J.C.; Plascencia, M.; Méndez-Velarde, F.A.; Olayo, R. Conductometric uric acid and urea biosensor prepared from electroconductive polyaniline–poly(n-butyl methacrylate) composites. Sens. Actuators B Chem. 2002, 85, 19–25. [Google Scholar] [CrossRef]
- Kovács, B.; Nagy, G.; Dombi, R.; Tóth, K. Optical biosensor for urea with improved response time. Biosens. Bioelectron. 2003, 18, 111–118. [Google Scholar] [CrossRef]
- Jiménez, C.; Bartrol, J.; de Rooij, N.F.; Koudelka-Hep, M. Use of photopolymerizable membranes based on polyacrylamide hydrogels for enzymatic microsensor construction. Anal. Chim. Acta 1997, 351, 169–176. [Google Scholar] [CrossRef]
- Puig-Lleixà, C.; Jiménez, C.; Alonso, J.; Bartrolı, J. Polyurethane–acrylate photocurable polymeric membrane for ion-sensitive field-effect transistor based urea biosensors. Anal. Chim. Acta 1999, 389, 179–188. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erfkamp, J.; Guenther, M.; Gerlach, G. Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea. Sensors 2019, 19, 2858. https://doi.org/10.3390/s19132858
Erfkamp J, Guenther M, Gerlach G. Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea. Sensors. 2019; 19(13):2858. https://doi.org/10.3390/s19132858
Chicago/Turabian StyleErfkamp, Jan, Margarita Guenther, and Gerald Gerlach. 2019. "Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea" Sensors 19, no. 13: 2858. https://doi.org/10.3390/s19132858
APA StyleErfkamp, J., Guenther, M., & Gerlach, G. (2019). Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea. Sensors, 19(13), 2858. https://doi.org/10.3390/s19132858




