Autonomic Nervous System Response during Light Physical Activity in Adolescents with Anorexia Nervosa Measured by Wearable Devices
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Baseline 12-Lead ECG
2.3. Echocardiographic Measurements
2.4. ECG Monitoring by Wearable Sensors
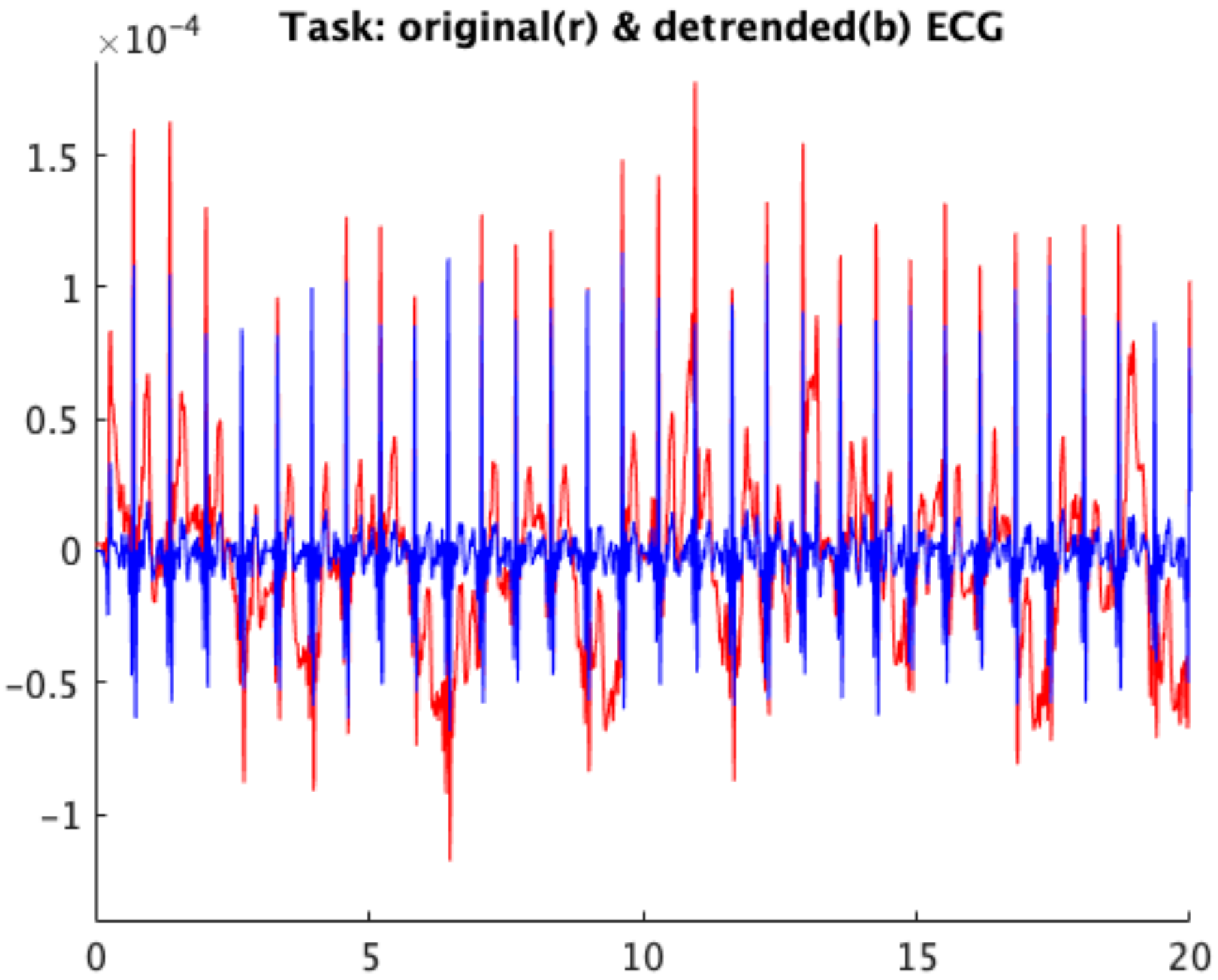
2.5. ECG Signal Analysis
2.6. Statistical Analysis
3. Results
3.1. Group Characteristics
3.2. Basal 12-Lead ECG
3.3. 2-D Doppler Echocardiography
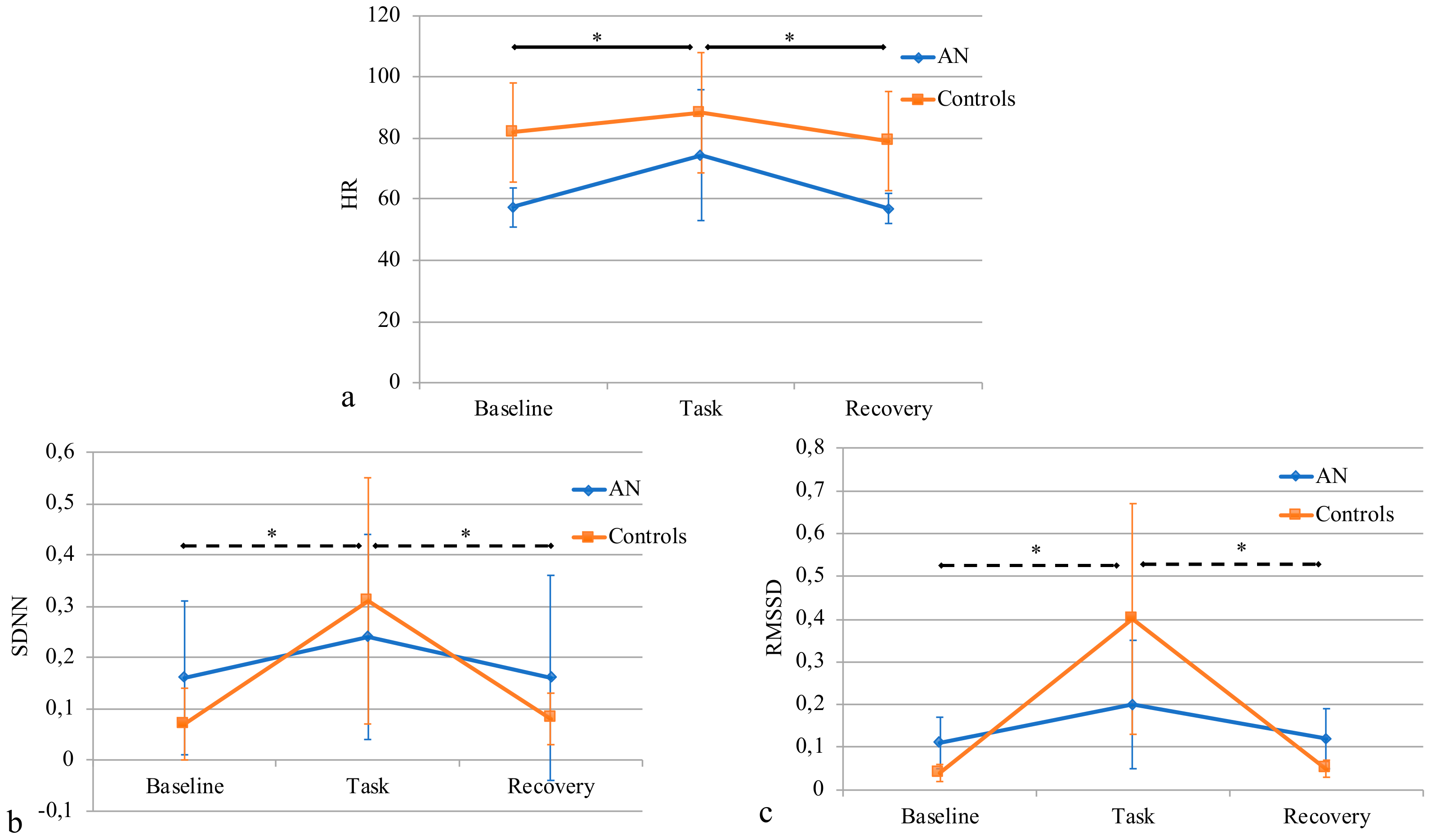
3.4. HR and HRV Analysis
3.5. Correlations
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual Mental Disorders, 5th ed.; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar]
- Birmingham, C.L.; Su, J.; Hlynsky, J.A.; Goldner, E.M.; Gao, M. The mortality rate from anorexia nervosa. Int. J. Eat. Disord. 2005, 38, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Sukkar, S.G.; Rosa, G.M.; Zappi, A.; Bezante, G.P.; Balbi, M.; Brunelli, C. Anorexia nervosa and heart disease: a systematic review. Eat. Weight Disord. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kollai, M.; Bonyhay, I.; Jokkel, G.; Szonyi, L. Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur. Heart J. 1994, 15, 1113–1138. [Google Scholar] [CrossRef] [PubMed]
- Kreipe, R.E.; Goldstein, B.; De King, D.E.; Tipton, R.; Kempski, M.H. Heart rate power spectrum analysis of autonomic dysfunction in adolescents with anorexia nervosa. Int. J. Eat. Disord. 1994, 16, 159–165. [Google Scholar] [CrossRef]
- Petretta, M.; Bonaduce, D.; Scalfi, L.; de Filippo, E.; Marciano, F.; Migaux, M.L.; Themistoclakis, S.; Ianniciello, A.; Contaldo, F. Heart rate variability as a measure of autonomic nervous system function in anorexia nervosa. Clin. Cardiol. 1997, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Rechlin, T.; Weis, M.; Ott, C.; Blechner, F.; Joraschky, P. Alterations of autonomic cardiac control in anorexia nervosa. Biol. Psychiatry 1998, 43, 358–363. [Google Scholar] [CrossRef]
- Casu, M.; Patrone, V.; Gianelli, M.V.; Marchegiani, A.; Ragni, G.; Murialdo, G.; Polleri, A. Spectral analysis of R–R interval variability by short-term recording in anorexia nervosa. Eat. Weight Disord. 2007, 7, 239–243. [Google Scholar] [CrossRef]
- Galetta, F.; Franzoni, F.; Prattichizzo, F.; Rolla, M.; Santoro, G.; Pentimone, F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J. Adolesc. 2003, 32, 416–421. [Google Scholar] [CrossRef]
- Cong, N.D.; Saikawa, R.; Ogawa, R.; Hara, M.; Takahashi, N.; Sakata, T. Reduced 24 hour ambulatory blood pressure and abnormal heart rate variability in patients with dysorexia nervosa. Heart 2004, 90, 563–564. [Google Scholar] [CrossRef]
- Melanson, E.L.; Donahoo, W.T.; Krantz, M.J.; Poirier, P.; Mehler, P.S. Resting and ambulatory heart rate variability in chronic anorexia nervosa. Am. J. Cardiol. 2004, 94, 1217–1220. [Google Scholar] [CrossRef]
- Roche, F.; Barthelemy, J.C.; Garet, M.; Costes, F.; Pichot, V.; Duverney, D.; Kadem, M.; Millot, L.; Estour, B. Chronotropic incompetence to exercise separates low body weight from established anorexia nervosa. Clin. Physiol. Funct. Imaging 2004, 24, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jacoangeli, F.; Mezzasalma, F.S.; Canto, G.; Jacoangeli, F.; Colica, C.; de Lorenzo, A.; Iellamo, F. Baroreflex sensitivity and heart rate variability are enhanced in patients with anorexia nervosa. Int. J. Cardiol. 2013, 162, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Bomba, M.; Corbetta, F.; Gambera, A.; Nicosia, F.; Bonini, L.; Neri, F.; Tremolizzo, L.; Nacinovich, R. Heart rate variability in adolescents with functional hypothalamic amenorrhea and anorexia nervosa. Psychiatry Res. 2014, 215, 406–409. [Google Scholar] [CrossRef]
- Takimoto, Y.; Yoshiuchi, K.; Ishizawa, T.; Yamamoto, Y.; Akabayashi, A. Autonomic dysfunction responses to head-up tilt in anorexia nervosa. Clin. Auton. Res. 2014, 24, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Fujita, M.; Nin, K.; Noma, S.; Teramukai, S. Relationship between duration of illness and cardiac autonomic nervous activity in anorexia nervosa. Biopsychosoc. Med. 2015, 9, 12. [Google Scholar] [CrossRef]
- Mazurak, N.; Enck, P.; Muth, E.; Teufel, M.; Zipfel, S. Heart Rate Variability as a Measure of Cardiac Autonomic Function in Anorexia Nervosa: A Review of the Literature. Eur. Eat. Disorders Rev. 2011, 19, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Yoshiuchi, K.; Takimoto, Y.; Yamamoto, Y.; Akabayashi, A. Heart rate and blood pressure variability and baroreflex sensitivity in patients with anorexia nervosa. Psychosom Med. 2008, 70, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Perini, R.; Veicsteinas, A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Kriemler, S.; Eser, P.; Saner, H.; Wilhelm, M. Physical activity intensity and surrogate markers for cardiovascular health in adolescents. Eur. J. Appl. Physiol. 2013, 113, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Boullosa, D.A.; Barros, E.S.; Del Rosso, S.; Nakamura, F.Y.; Leicht, A.S. Reliability of Heart Rate Measures during Walking before and after Running Maximal Efforts. Int. J. Sports Med. 2014, 35, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Daanen, H.A.; Lamberts, R.P.; Kallen, V.L.; Jin, A.; Van Meeteren, N.L. A systematic review on heart-rate recovery to monitor changes in training status in athletes. Int. J. Sports Physiol. Perform. 2012, 7, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Danieli, A.; Lusa, L.; Potočnik, N.; Meglič, B.; Grad, A.; Bajrović, F.F. Resting heart rate variability and heart rate recovery after submaximal exercise. Clin. Auton. Res. 2014, 24, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Le, F.K.; Lahiri, M.; Kannankeril, P.J.; Ng, J.; Kadish, A.H. Assessment of parasympathetic reactivation after exercise. Am. J. Physiol. Heart Circ. Physiol. 2006, 2290, H2446–H2452. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Levine, B.D. Exercise and the autonomic nervous system. Handb Clin Neurol. 2013, 117, 147–160. [Google Scholar] [PubMed]
- Lipski, J.; Cohen, L.; Espinoza, J.; Motro, M.; Dack, S.; Donoso, E. Value of Holter monitoring in assessing cardiac arrhythmias in symptomatic patients. Am J Cardiol. 1976, 37, 102–107. [Google Scholar] [CrossRef]
- Uznańska-Loch, B.; Trzos, E.; Wierzbowska-Drabik, K.; Smigielski, J.; Rechciński, T.; Cieślik-Guerra, U.; Kasprzak, J.D.; Kurpesa, M. Usefulness of extended holter ECG monitoring for serious arrhythmia detection in patients with heart failure and sleep apnea. Ann Noninvasive Electrocardiol. 2013, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Torfs, T.; Smeets, C.J.; Geng, D.; Berset, T.; Van der Auwera, J.; Vandervoort, P.; Grieten, L. Clinical validation of a low-power and wearable ECG patch for long term full-disclosure monitoring. J. Electrocardiol. 2014, 47, 881–889. [Google Scholar] [CrossRef]
- Billeci, L.; Brunori, E.; Scardigli, S.; Curzio, O.; Calderoni, S.; Maestro, S.; Morales, M.A. Excessive physical activity in young girls with restrictive-type anorexia nervosa: its role on cardiac structure and performance. Eat. Weight Disord. 2018, 23, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Tartarisco, G.; Brunori, E.; Crifaci, G.; Scardigli, S.; Balocchi, R.; Pioggia, G.; Maestro, S.; Morales, M.A. The role of wearable sensors and wireless technologies for the assessment of heart rate variability in anorexia nervosa. Eat. Weight Disord. 2015, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Tonacci, A.; Tartarisco, G.; Narzisi, A.; Di Palma, S.; Corda, D.; Baldus, G.; Cruciani, F.; Anzalone, S.M.; Calderoni, S.; et al. An Integrated Approach for the Monitoring of Brain and Autonomic Response of Children with Autism Spectrum Disorders during Treatment by Wearable Technologies. Front. Neurosci. 2016, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Tonacci, A.; Narzisi, A.; Manigrasso, Z.; Varanini, M.; Fulceri, F.; Lattarulo, C.; Calderoni, S.; Muratori, F. Heart Rate Variability during a joint attention task in toddlers with Autism Spectrum Disorders. Front. Physiol. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, S.; Tonacci, A.; Narzisi, A.; Domenici, C.; Pioggia, G.; Muratori, F.; Billeci, L.; The MICHELANGELO Study Group. Monitoring of autonomic response to sociocognitive tasks during treatment in children with autism spectrum disorders by wearable technologies: A feasibility study. Comput. Biol. Med 2017, 85, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Greene, B.R.; McGrath, M.J.; O’Shea, T.J.; Kuris, B.; Ayer, S.M.; Stroiescu, F.; Cionca, V. SHIMMERTM – A Wireless Sensor Platform for Noninvasive Biomedical Research. IEEE Sens. J. 2010, 10, 1527–1534. [Google Scholar] [CrossRef]
- Crifaci, G.; Billeci, L.; Tartarisco, G.; Balocchi, R.; Pioggia, G.; Brunori, E.; Maestro, S.; Morales, M.A. ECG and GSR measure and analysis using wearable systems: Application in anorexia nervosa adolescents. In Proceedings of the 8th International Symposium on Image and Signal Processing and Analysis (ISPA), Trieste, Italy, 4–6 September 2013; pp. 499–504. [Google Scholar]
- Halmi, K.A. Anorexia nervosa: demographic and clinical features in 94 cases. Psychosom. Med. 1974, 36, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Coëffier, M.; Déchelotte, P. Physical activity in patients with anorexia nervosa. Nutr. Rev. 2016, 74, 301–311. [Google Scholar] [CrossRef]
- Sternheim, L.; Danner, U.; Adan, R.; van Elburg, A. Drive for activity in patients with anorexia nervosa. Int. J. Eat. Disord. 2015, 48, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Keyes, A.; Woerwag-Mehta, S.; Bartholdy, S.; Koskina, A.; Middleton, B.; Connan, F.; Webster, P.; Schmidt, U.; Campbell, I.C. Physical activity and the drive to exercise in anorexia nervosa. Int. J. Eat. Disord. 2015, 48, 46–54. [Google Scholar] [CrossRef]
- Kostrzewa, E.; van Elburg, A.A.; Sanders, N.; Sternheim, L.; Adan, R.A.; Kas, M.J. Longitudinal changes in the physical activity of adolescents with anorexia nervosa and their influence on body composition and leptin serum levels after recovery. PLoS ONE 2013, 8, e78251. [Google Scholar] [CrossRef]
- Rizk, M.; Kern, L.; Lalanne, C.; Hanachi, M.; Melchior, J.C.; Pichard, C.; Mattar, L.; EVHAN Group; Berthoz, S.; Godart, N. High-intensity exercise is associated with a better nutritional status in anorexia nervosa. Eur. Eat. Disord. Rev 2018. [Google Scholar] [CrossRef]
- El Ghoch, M.; Soave, F.; Calugi, S.; Dalle Grave, R. Eating disorders, physical fitness and sport performance: a systematic review. Nutrients 2013, 5, 5140–5160. [Google Scholar] [CrossRef]
- Bratland-Sanda, S.; Sundgot-Borgen, J.; Rosenvinge, J.H.; Ro, O.; Hoffart, A.; Martinsen, E.W. Physical fitness, bone mineral density and associations with physical activity in females with longstanding eating disorders and non-clinical controls. J. Sports Med. Phys. Fit. 2010, 50, 303–310. [Google Scholar]
- Bratland-Sanda, S.; Sundgot-Borgen, J.; Ro, O.; Rosenvinge, J.H.; Hoffart, A.; Martinsen, E.W. “I’m not physically active—I only go for walks”: Physical activity in patients with longstanding eating disorders. Int. J. Eat. Disord. 2010, 43, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Fohlin, L.; Freyschuss, U.; Bjarke, B.; Davies, C.T.; Thoren, C. Function and dimensions of the circulatory system in anorexia nervosa. Acta Paediatr. Scand. 1978, 67, 11–16. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, D.M.; Spargo, E.; Wassif, W.S.; Newham, D.J.; Peters, T.J.; Lantos, P.L.; Russell, G.F. Structural and functional changes in skeletal muscle in anorexia nervosa. Acta Neuropathol. 1998, 95, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Nudel, D.B.; Gootman, N.; Nussbaum, M.P.; Shenker, I.R. Altered exercise performance and abnormal sympathetic responses to exercise in patients with anorexia nervosa. J. Pediatr. 1984, 105, 34–37. [Google Scholar] [CrossRef]
- Alberti, M.; Galvani, C.; Capelli, C.; Lanza, M.; El Ghoch, M.; Calugi, S.; Dalle Grave, R. Physical fitness before and after weight restoration in anorexia nervosa. J. Sports Med. Phys. Fit. 2013, 53, 396–402. [Google Scholar]
- Fogelholm, M. Effects of bodyweight reduction on sports performance. Sports Med. 1994, 18, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Biadi, O.; Rossini, R.; Musumeci, G.; Frediani, L.; Masullo, M.; Ramacciotti, C.E.; Dell’Osso, L.; Paoli, R.; Mariotti, R.; Cassano, G.B.; Mariani, M. Cardiopulmonary exercise test in young women affected by anorexia nervosa. Ital. Heart J. 2001, 2, 462–467. [Google Scholar] [PubMed]
- Rowland, T.; Koenigs, L.; Miller, N. Myocardial performance during maximal exercise in adolescents with anorexia nervosa. J. Sports Med. Phys. Fitness 2003, 43, 202–208. [Google Scholar]
- Dippacher, S.; Willaschek, C.; Buchhorn, R. Different nutritional states and autonomic imbalance in childhood. Eur J Clin Nutr. 2014, 68, 1271–1273. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual Mental Disorders, 4th ed.; American Psychiatric Press: Washington, DC, USA, 1994. [Google Scholar]
- Bazett, H. An analysis of the time relationship of electrocardiograms. Heart 1920, 7, 353–370. [Google Scholar]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. American Society of Echocardiography recommendations for chamber quantification: A report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Solar, H.; Fernandez, E.; Tartarisco, G.; Pioggia, G.; Cvetkovic, B.; Kozina, S.; Lustrek, M.; Lampe, J. A non invasive, wearable sensor platform for multi-parametric remote monitoring in CHF patients impact analysis of solutions for chronic disease prevention and management. Lect. Notes Comput. Sci. 2012, 7251, 140–147. [Google Scholar]
- Jane, R.; Laguna, P.; Thakor, N.V.; Caminal, P. Adaptive baseline wander removal in the ECG: comparative analysis with cubic spline technique. In Proceedings of the Computer in Cardiology, Durham, NC, USA, 11–14 October 1992; pp. 143–146. [Google Scholar]
- Merri, M.; Farden, D.C.; Mottley, J.G.; Titlebaum, E.L. Sampling frequency of the electrocardiogram for the spectral analysis of heart rate variability. IEEE Trans. Biomed. Eng. 1990, 37, 99–106. [Google Scholar] [CrossRef]
- Bianchi, A.M.; Mainardi, L.T.; Petrucci, E.; Signorini, M.G.; Mainardi, M.; Cerutti, S. Time-variant power spectrum analysis for the detection of transient episodes in HRV signal. IEEE Trans. Biomed. Eng. 1993, 40, 136–144. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Yule, G.U. On a Method of Investigating Periodicities in Disturbed Series, with Special Reference to Wolfer’s Sunspot Numbers. Philoso. Trans. Royal Soc. Lond. Ser. A 1927, 226, 267–298. [Google Scholar] [CrossRef]
- Walker, G. On Periodicity in Series of Related Terms. Proc. Royal Soc. Lond. Ser. A 1931, 131, 518–532. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- National Institutes of Health, & National Heart, Lung, & Blood Institute. Obesity; Department of Health and Human Services: Washington, DC, USA, 1998. [Google Scholar]
- Yahalom, M.; Spitz, M.; Sandler, L.; Heno, N.; Roguin, N.; Turgeman, Y. The significance of bradycardia in anorexia nervosa. Int J Angiol. 2013, 22, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Portilla, M.G. Bradycardia: an important physical finding in anorexia nervosa. J. Ark. Med. Soc. 2011, 107, 206–208. [Google Scholar] [PubMed]
- Sall, H.; Timperley, J. Bradycardia in anorexia nervosa. BMJ Case Rep. 2015, 2015, bcr2015211273. [Google Scholar] [CrossRef] [PubMed]
- Bomba, M.; Tremolizzo, L.; Corbetta, F.; Nicosia, F.; Lanfranconi, F.; Poggioli, G.; Goulene, K.; Stramba-Badiale, M.; Conti, E.; Neri, F.; Nacinovich, R. QT interval and dispersion in drug-free anorexia nervosa adolescents: a case control study. Eur Child Adolesc Psychiatry. 2018, 27, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M. Sensitivity of monthly heart rate and psychometric measures for monitoring physical performance in highly trained young handball players. Int. J. Sports Med. 2015, 36, 351–356. [Google Scholar] [CrossRef] [PubMed]
- al-Ani, M.; Munir, S.M.; White, M.; Townend, J.; Coote, J.H. Changes in R-R variability before and after endurance training measured by power spectral analysis and by the effect of isometric muscle contraction. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 397–403. [Google Scholar]
- Gladwell, V.F.; Fletcher, J.; Patel, N.; Elvidge, L.J.; Lloyd, D.; Chowdhary, S.; Coote, J.H. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J. Physiol. 2005, 567, 713–721. [Google Scholar] [CrossRef]
- Gladwell, V.F.; Coote, J.H. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J. Physiol. 2002, 540, 1095–1102. [Google Scholar] [CrossRef]
- Raven, P.B.; Fadel, P.J.; Ogoh, S. Arterial baroreflex resetting during exercise: a current perspective. Exp. Physiol. 2006, 91, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Wasmund, W.L.; Keller, D.M.; O-Yurvati, A.; Gallagher, K.M.; Mitchell, J.H.; Raven, P.B. Role of central command in carotid baroreflex resetting in humans during static exercise. J. Physiol. 2002, 543, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Lamberts, R.P.; Lambert, M.I. Methods of prescribing relative exercise intensity: physiological and practical considerations. Sports Med. 2013, 43, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Tulppo, M.P.; Mäkikallio, T.H.; Seppänen, T.; Laukkanen, R.T.; Huikuri, H.V. Vagal modulation of heart rate during exercise: effects of age and physical fitness. Am. J. Physiol. 1998, 274, H424–H429. [Google Scholar] [CrossRef] [PubMed]
- Martinmäki, K.; Häkkinen, K.; Mikkola, J.; Rusko, H. Effect of low-dose endurance training on heart rate variability at rest and during an incremental maximal exercise test. Eur. J. Appl. Physiol. 2008, 104, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.S.; Lee, I.; Kim, S.; Lim, C.S.; Joh, H.K.; Park, B.J.; Song, M.K. The Effects of a Campus Forest-Walking Program on Undergraduate and Graduate Students’ Physical and Psychological Health. Int. J. Environ. Res. Public Health 2017, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Effects of Walking in a Forest on Young Women. Int. J. Environ. Res. Public Health 2019, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Bartak, V.; Vybiral, S.; Papezova, H.; Dostalova, I.; Pacak, K.; Nedvidkova, J. Basal and exercise-induced sympathetic nervous activity and lipolysis in adipose tissue of patients with anorexia nervosa. Eur. J. Clin. Invest. 2004, 34, 371–377. [Google Scholar] [CrossRef]
- Birmingham, C.L.; Tan, A.O. Respiratory muscle weakness and anorexia nervosa. Int. J. Eating Disord. 2003, 33, 230–233. [Google Scholar] [CrossRef]
- Escudero, C.A.; Potts, J.E.; Lam, P.Y.; De Souza, A.M.; Duff, K.; Mugford, G.J.; Sandor, G.G.S. Echocardiographic Assessment of Ventricular Function During Exercise in Adolescent Female Patients With Anorexia Nervosa. J. Am. Soc. Echocardiogr. 2019. [CrossRef]


| Subject | Age (y) | Weight (kg) | Height (cm) | BMI (Kg m−2) | z-Score | Age of Onset (y) | Hospitalization (mo) | Duration of Disease (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 16.5 | 47.9 | 177 | 15.3 | −2.83 | 15.1 | 16.4 | 15 |
| 2 | 16.9 | 39.1 | 155 | 16.3 | −2.16 | 15.1 | 15.6 | 5 |
| 3 | 13.7 | 42.8 | 168 | 15.2 | −1.89 | 12.3 | 12.8 | 5 |
| 4 | 16.7 | 44.7 | 162 | 17.0 | −1.66 | 15.3 | 16.9 | 18 |
| 5 | 17.2 | 42.0 | 163 | 15.7 | −2.75 | 12.2 | 17.2 | 60 |
| 6 | 18.6 | 34.3 | 151 | 14.9 | −2.14 | 14.7 | 17.6 | 35 |
| 7 | 16.2 | 41.4 | 170 | 14.3 | −3.65 | 12.0 | 14.8 | 32 |
| 8 | 19.0 | 37.3 | 162 | 17.4 | −1.93 | 16.1 | 17.1 | 12 |
| 9 | 14.7 | 38.9 | 167 | 13.9 | −3.32 | 13.1 | 14.6 | 17 |
| 10 | 13.4 | 43.9 | 160 | 17.1 | −0.56 | 13.1 | 13.5 | 4 |
| 11 | 12.7 | 42.5 | 161 | 16.4 | −0.88 | 12.1 | 13.2 | 13 |
| 12 | 17.0 | 42.6 | 169 | 14.9 | −3.3 | 13.2 | 17.1 | 47 |
| 13 | 17.0 | 47.0 | 166 | 17.1 | −1.7 | 13.6 | 17.1 | 43 |
| 14 | 15.0 | 34.7 | 163 | 13.0 | −4.05 | 14.5 | 14.11 | 6 |
| 15 | 13.2 | 32.0 | 161 | 12.3 | −4.39 | 11.8 | 13.5 | 21 |
| 16 | 15.8 | 42.2 | 151 | 17.9 | −0.95 | 12.1 | 12.11 | 10 |
| 17 | 12.7 | 39.9 | 155 | 16.6 | −0.78 | 11.0 | 11.11 | 11 |
| 18 | 13.3 | 47.3 | 168 | 16.8 | −0.86 | 11.6 | 13.3 | 21 |
| 19 | 16.0 | 48.8 | 166 | 17.7 | −1.12 | 14.2 | 15.8 | 18 |
| 20 | 16.2 | 41.7 | 162 | 15.9 | −2.27 | 14.5 | 15.6 | 13 |
| 21 | 10.4 | 26.4 | 140 | 13.5 | −2.16 | 10.0 | 10.5 | 5 |
| 22 | 16.2 | 39.3 | 160 | 15.3 | −2.65 | 15.2 | 16.3 | 13 |
| 23 | 14.8 | 54.0 | 173 | 18.0 | −0.66 | 13.1 | 14.1 | 12 |
| Feature (mean±SD) | AN | Controls | p-Value |
|---|---|---|---|
| HR (bpm) | 57.3 ± 16.2 | 81.8 ± 6.4 | <0.001 ** |
| SDNN (s) | 0.16 ± 0.15 | 0.07 ± 0.07 | 0.009 ** |
| RMSSD (s) | 0.11 ± 0.06 | 0.04 ± 0.02 | 0.028 * |
| LFn (n.u.) | 0.35 ± 0.14 | 0.45 ± 0.14 | 0.030 * |
| HFn (n.u.) | 0.66 ± 0.15 | 0.47 ± 0.17 | 0.004 ** |
| LF/HF (ratio) | 0.83 ± 0.13 | 1.16 ± 0.45 | 0.030 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billeci, L.; Tonacci, A.; Brunori, E.; Raso, R.; Calderoni, S.; Maestro, S.; Morales, M.A. Autonomic Nervous System Response during Light Physical Activity in Adolescents with Anorexia Nervosa Measured by Wearable Devices. Sensors 2019, 19, 2820. https://doi.org/10.3390/s19122820
Billeci L, Tonacci A, Brunori E, Raso R, Calderoni S, Maestro S, Morales MA. Autonomic Nervous System Response during Light Physical Activity in Adolescents with Anorexia Nervosa Measured by Wearable Devices. Sensors. 2019; 19(12):2820. https://doi.org/10.3390/s19122820
Chicago/Turabian StyleBilleci, Lucia, Alessandro Tonacci, Elena Brunori, Rossella Raso, Sara Calderoni, Sandra Maestro, and Maria Aurora Morales. 2019. "Autonomic Nervous System Response during Light Physical Activity in Adolescents with Anorexia Nervosa Measured by Wearable Devices" Sensors 19, no. 12: 2820. https://doi.org/10.3390/s19122820
APA StyleBilleci, L., Tonacci, A., Brunori, E., Raso, R., Calderoni, S., Maestro, S., & Morales, M. A. (2019). Autonomic Nervous System Response during Light Physical Activity in Adolescents with Anorexia Nervosa Measured by Wearable Devices. Sensors, 19(12), 2820. https://doi.org/10.3390/s19122820







