Cytochrome P450 1A1/2, 2B6 and 3A4 HepaRG Cell-Based Biosensors to Monitor Hepatocyte Differentiation, Drug Metabolism and Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. HepaRG Cell Culture
2.3. GFP Reporter Dene Constructs.
2.4. Lentiviral Infection and Selection of Biosensor HepaRG Cells.
2.5. Immunoblotting Blotting
2.6. RNA Expression by RetroTranscription Quantitative Polymerase Chain Reaction
2.7. Fluorescence Microscopy and Flow Cytometry Analyses.
2.8. Statistical Analyses
3. Results
3.1. Subsection
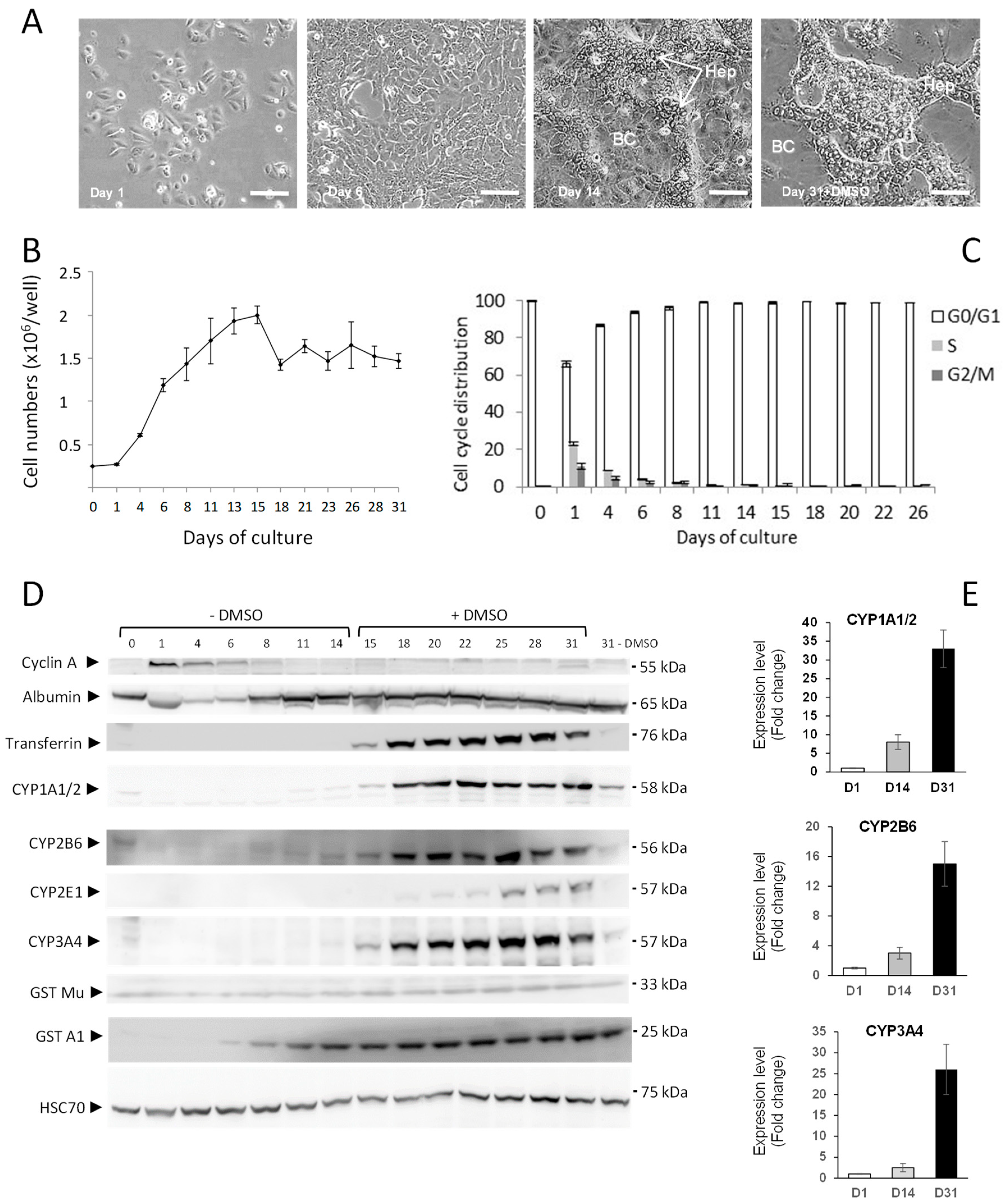
3.1.1. Expression of Phase I and II Enzymes in HepaRG Hepatocyte-Like Cells.
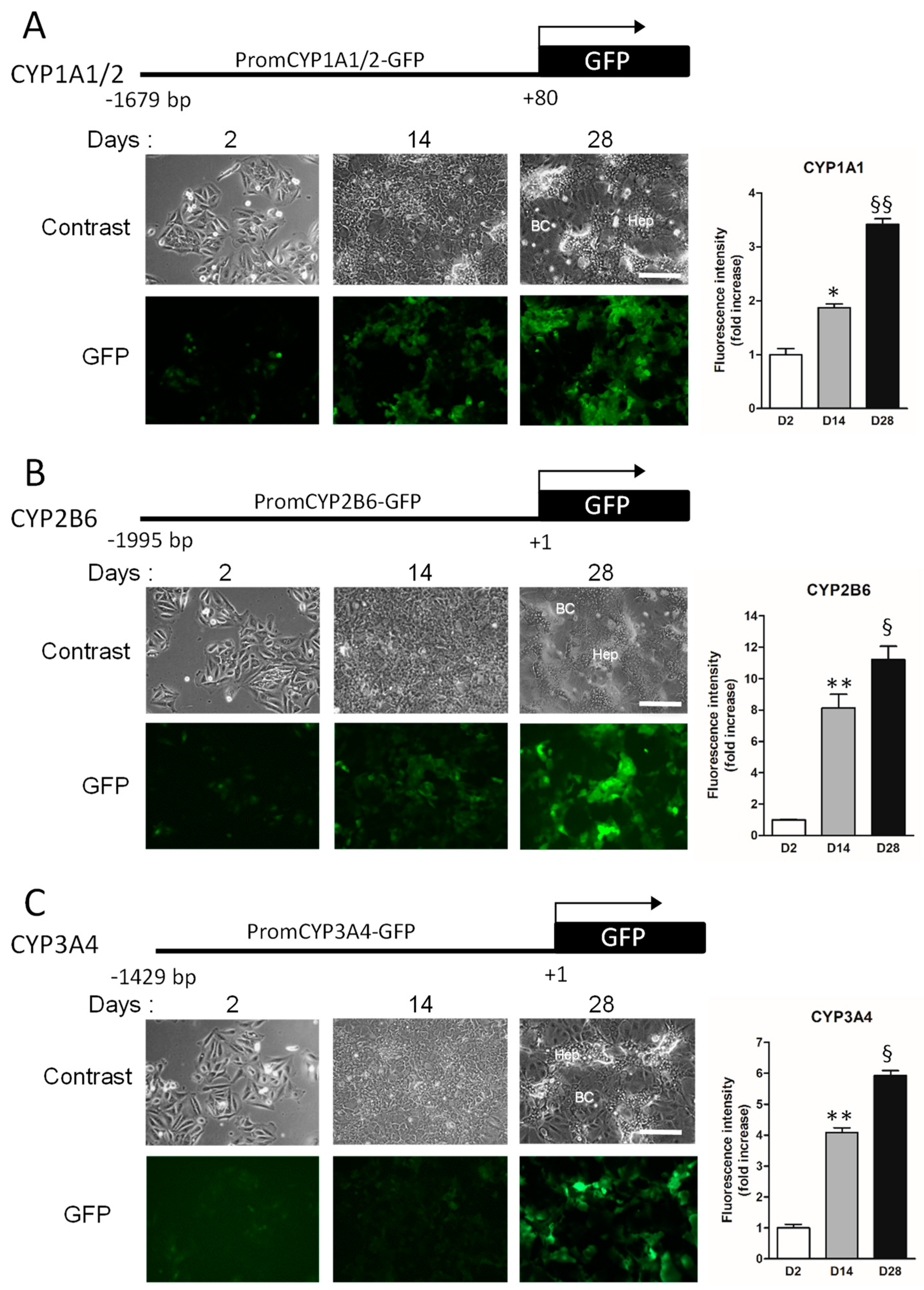
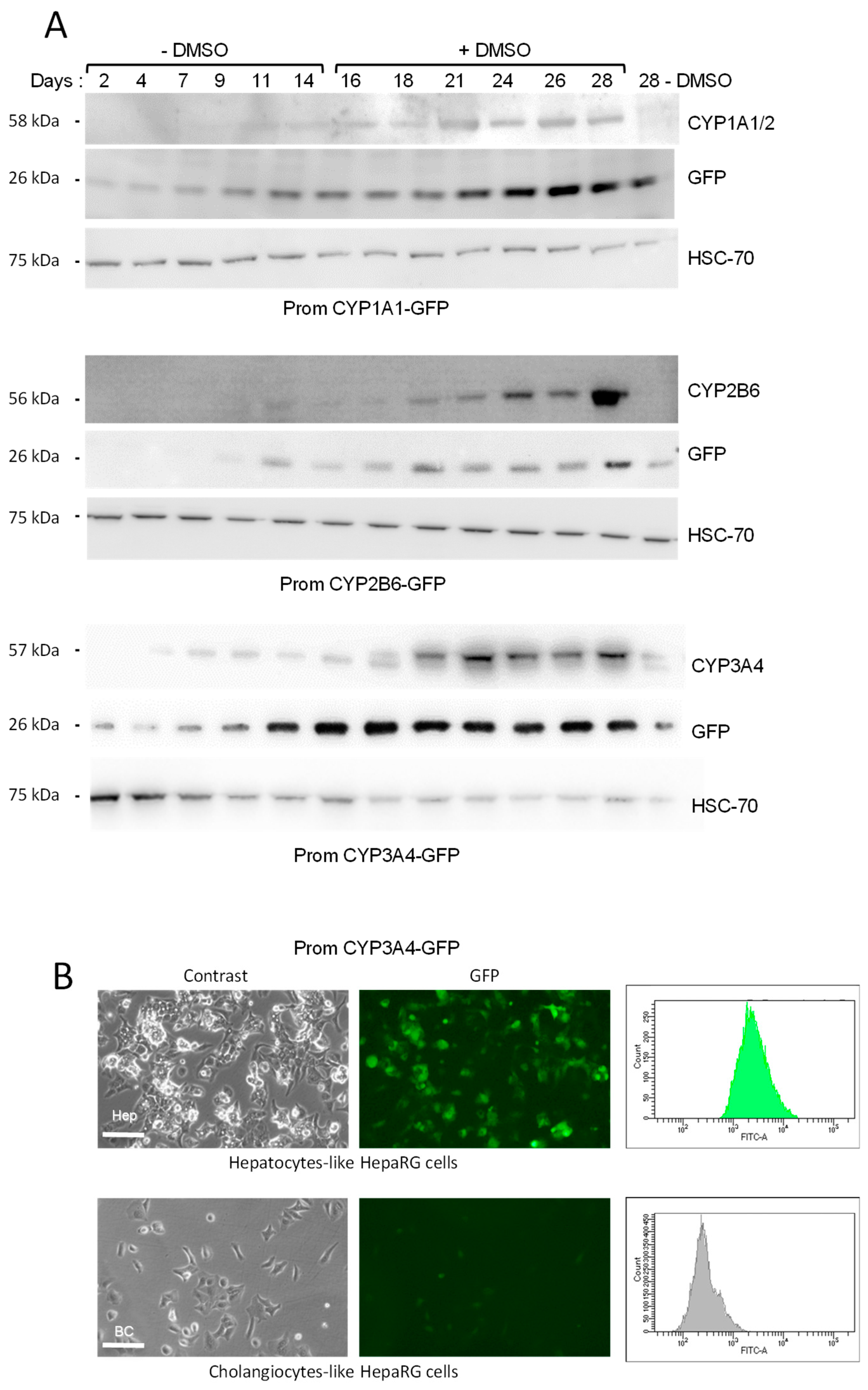
3.1.2. Establishment of Biosensor HepaRG Cells
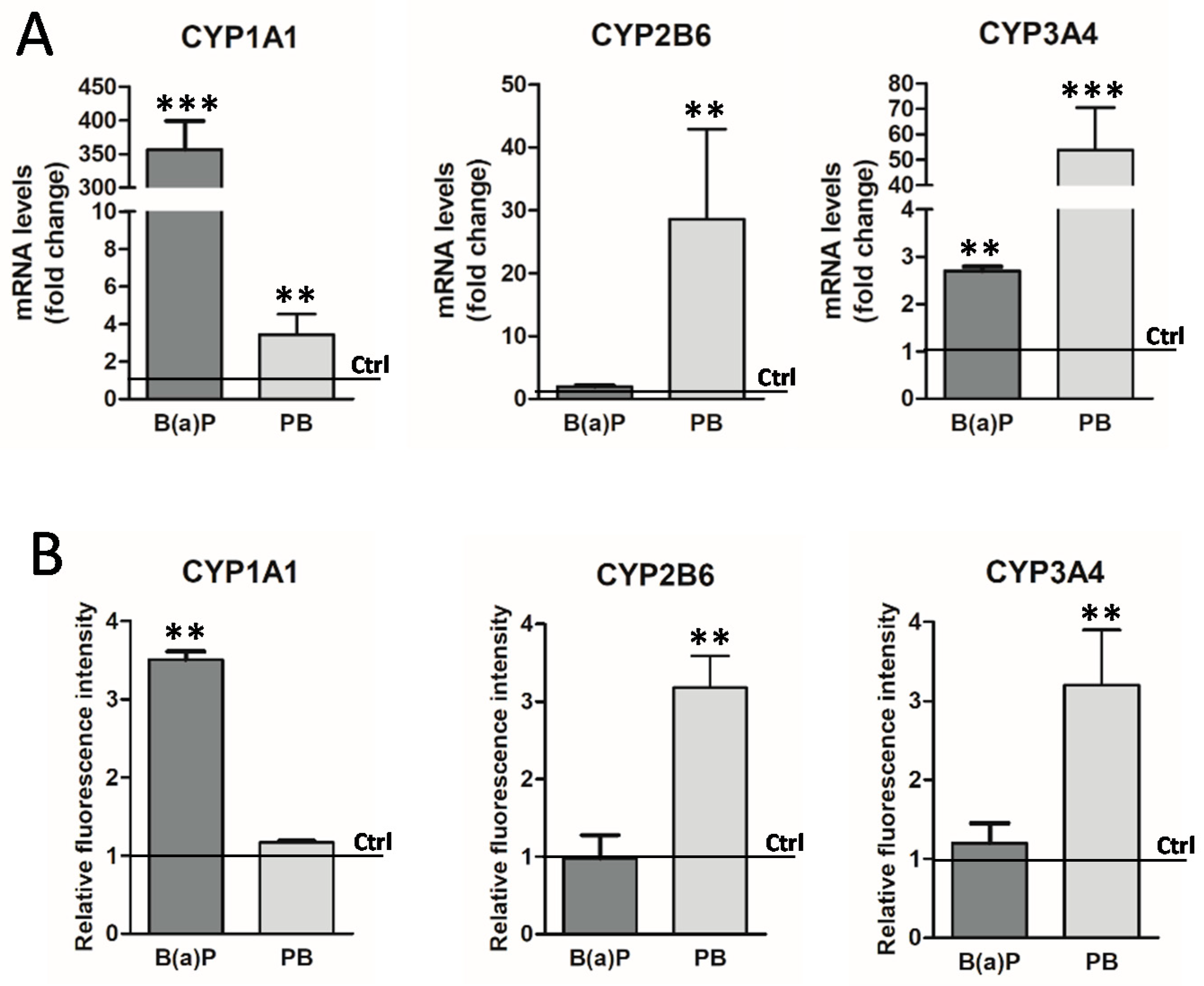
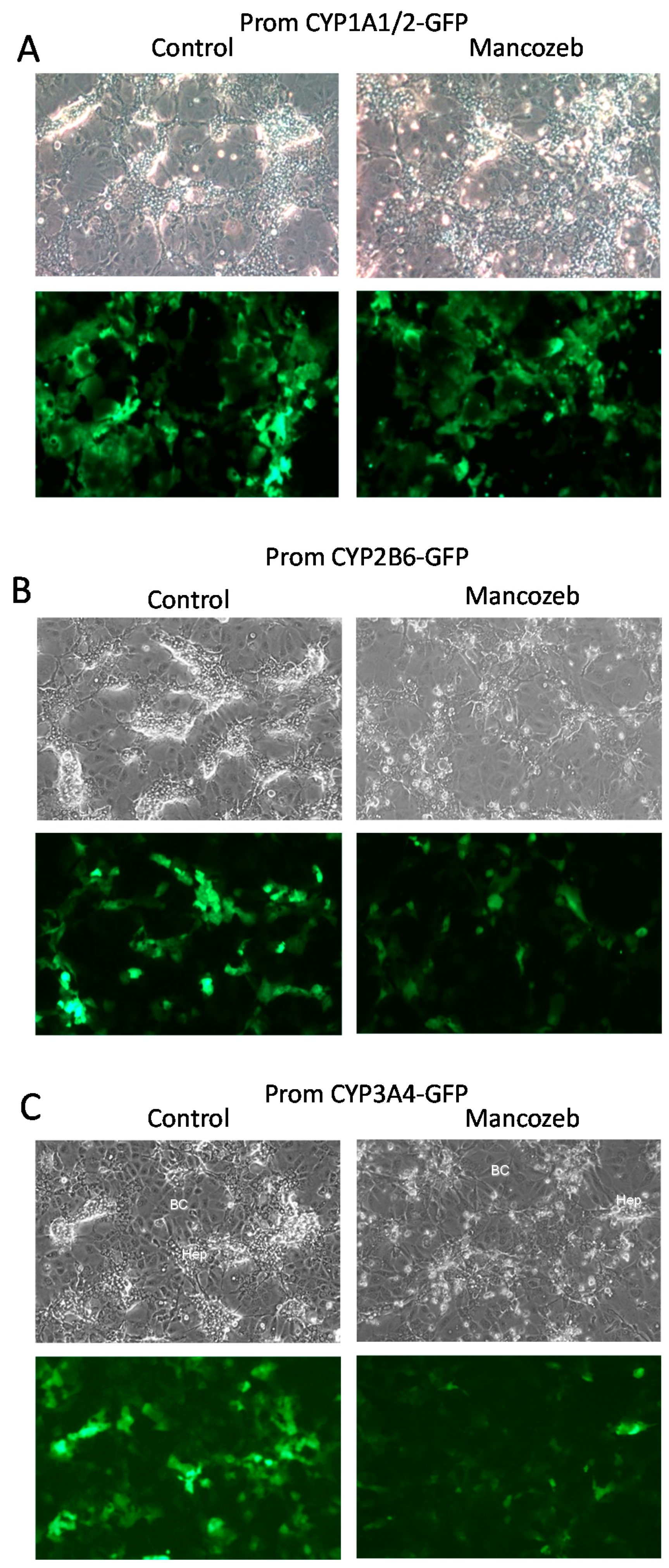
3.1.3. Induction of GFP Expression in Biosensor HepaRG Cells by Xenobiotics
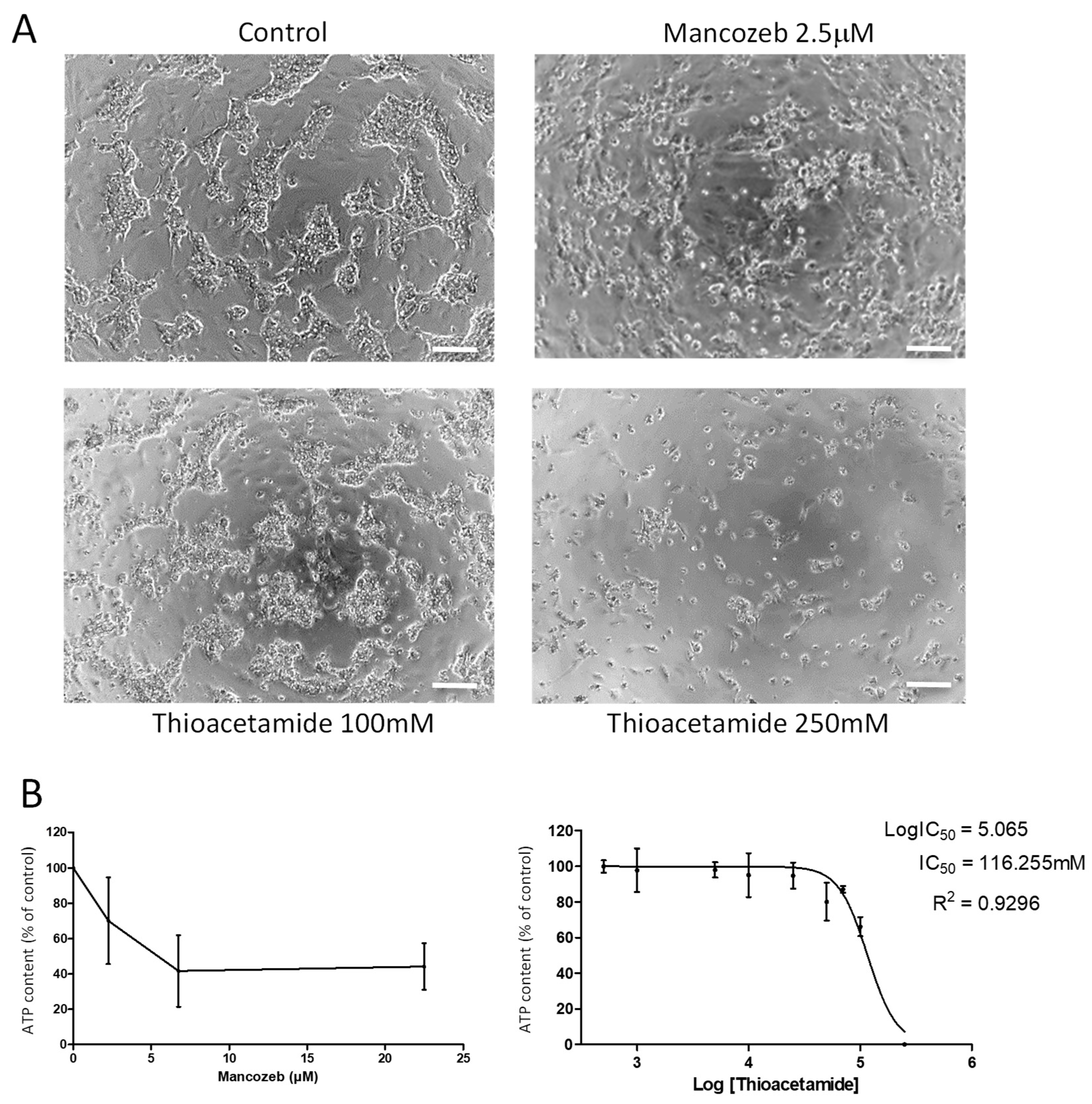
3.1.4. Detection of Selective Hepatocyte-Like Cytotoxicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guillouzo, A.; Guguen-Guillouzo, C. Evolving concepts in liver tissue modeling and implications for in vitro toxicology. Expert Opin. Drug. Metab. Toxicol. 2008, 4, 1279–1294. [Google Scholar] [CrossRef]
- Lasser, K.E.; Allen, P.D.; Woolhandler, S.J.; Himmelstein, D.U.; Wolfe, S.M.; Bor, D.H. Timing of new black box warnings and withdrawal for prescription medications. JAMA 2002, 287, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Suter, W. Predictive value of in vitro safety studies. Curr. Opin. Chem. Biol. 2006, 10, 362–366. [Google Scholar] [CrossRef]
- Quesnot, N. Evaluation de la génotoxicité des contaminants environnementaux, production de lignées bio-senseurs et mesure de l’activité enzymatique du cytochrome P450 2E1 dans les cellules d’hépatome HepaRG. Ph.D. Thesis, Philosophy, University of Rennes 1, Rennes, France, 30 April 2015. [Google Scholar]
- Vinken, M.; Hengstler, J.G. Characterization of hepatocyte-based in vitro systems for reliable toxicity testing. Arch. Toxicol. 2018, 92, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Audebert, M.; Riu, A.; Jacques, C.; Hillenweck, A.; Jamin, E.L.; Zalko, D.; Cravedi, J.-P. Use of the H2AX assay for assessing the genotoxicity of polycyclic aromatic hydrocarbons in human cell lines. Toxicol. Lett. 2010, 199, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line HepG2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Glaise, D.; Ilyin, G.P.; Loyer, P.; Cariou, S.; Bilodeau, M.; Lucas, J.; Puisieux, A.; Ozturk, M.; Guguen-Guillouzo, C. Cell cycle gene regulation in reversibly differentiated new human hepatoma cell line. Cell Growth Differ. 1998, 9, 165–176. [Google Scholar] [PubMed]
- Kelly, J.H. Permanent Human Hepatocyte Cell Line and Its Use in a Liver Assist Device (LAD). U.S. Patent No. 5,290,684, 1 March 1994. [Google Scholar]
- Hashizume, T.; Yoshitomi, S.; Asahi, S.; Uematsu, R.; Matsumura, S.; Chatani, F.; Oda, H. Advantages of human hepatocyte-derived transformants expressing a series of human cytochrome P450 isoforms for genotoxicity examination. Toxicol. Sci. 2010, 116, 488–497. [Google Scholar] [CrossRef]
- Jover, R.; Bort, R.; Gomez-Lechon, M.J.; Castell, J.V. Re-expression of C/EBP alpha induces CYP2B6, CYP2C9 and CYP2D6 genes in HepG2 cells. FEBS Lett. 1998, 431, 227–230. [Google Scholar] [CrossRef]
- Gripon, P.; Rumin, S.; Urban, S.; Le Seyec, J.; Glaise, D.; Cannie, I.; Guyomard, C.; Lucas, J.; Trepo, C.; Guguen-Guillouzo, C. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 2002, 99, 15655–15660. [Google Scholar] [CrossRef]
- Cerec, V.; Glaise, D.; Garnier, D.; Morosan, S.; Turlin, B.; Drenou, B.; Gripon, P.; Kremsdorf, D.; Guguen-Guillouzo, C.; Corlu, A. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology 2007, 45, 957–967. [Google Scholar] [CrossRef]
- Dubois-Pot-Schneider, H.; Fekir, K.; Coulouarn, C.; Glaise, D.; Aninat, C.; Jouarnen, K.; Le Guével, R.; Kubo, T.; Ishida, S.; Morel, F.; et al. Inflammatory cytokines promote the retrodifferentiation of tumor-derived hepatocyte-like cells to progenitor cells. Hepatology 2014, 60, 2077–2090. [Google Scholar] [CrossRef]
- Aninat, C.; Piton, A.; Glaise, D.; Le Charpentier, T.; Langouet, S.; Morel, F.; Guguen-Guillouzo, C.; Guillouzo, A. Expression of cytochrome P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab. Dispos. 2006, 34, 75–83. [Google Scholar] [CrossRef]
- Le Vee, M.; Noel, G.; Jouan, E.; Stieger, B.; Fardel, O. Polarized expression of drug transporters in differentiated human hepatoma HepaRG cells. Toxicol. In Vitro 2013, 27, 1979–1986. [Google Scholar] [CrossRef]
- Quesnot, N.; Bucher, S.; Gade, C.; Vlach, M.; Vène, E.; Valenca, S.; Gicquel, T.; Holst, H.; Robin, M.-A.; Loyer, P. Production of chlorzoxazone glucuronides via cytochrome P4502E1 dependent and independent pathways in human hepatocytes. Arch. Toxicol. 2018, 92, 3077–3091. [Google Scholar] [CrossRef]
- Dumont, J.; Jossé, R.; Lambert, C.; Anthérieu, S.; Laurent, V.; Loyer, P.; Robin, M.-A.; Guillouzo, A. Preferential induction of the AhR gene battery in HepaRG cells after a single or repeated exposure to heterocyclic aromatic amines. Toxicol. Appl. Pharmacol. 2010, 249, 91–100. [Google Scholar] [CrossRef]
- Legendre, C.; Hori, T.; Loyer, P.; Aninat, C.; Ishida, S.; Glaise, D.; Lucas-Clerc, C.; Boudjema, K.; Guguen-Guillouzo, C.; Corlu, A.; et al. Drug-metabolising enzymes are down-regulated by hypoxia in differentiated human hepatoma HepaRG cells: HIF1-alpha involvement in CYP3A4 repression. Eur. J. Cancer 2009, 12, 2882–2892. [Google Scholar] [CrossRef]
- Jossé, R.; Aninat, C.; Glaise, D.; Dumont, J.; Fessard, V.; Morel, F.; Poul, J.M.; Guguen-Guillouzo, C.; Guillouzo, A. Long-term functional stability of HepaRG hepatocytes and use for chronic toxicity and genotoxicity studies. Drug Metab. Dispos. 2008, 36, 1111–1118. [Google Scholar] [CrossRef]
- Pernelle, K.; Le Guevel, R.; Glaise, D.; Stasio, C.G.; Le Charpentier, T.; Bouaita, B.; Corlu, A.; Guguen-Guillouzo, C. Automated detection of hepatotoxic compounds in human hepatocytes using HepaRG cells and image-based analysis of mitochondrial dysfunction using JC-1 dye. Toxicol. Appl. Pharmacol. 2011, 254, 256–266. [Google Scholar] [CrossRef]
- Jetten, M.J.A.; Kleinjans, J.C.S.; Claessen, S.M.; Chesné, C.; van Delft, J.H.M. Baseline and genotoxic compound induced gene expression profiles in HepG2 and HepaRG compared to primary human hepatocytes. Toxicol. In Vitro 2013, 27, 2031–2040. [Google Scholar] [CrossRef]
- Quesnot, N.; Rondel, K.; Martinais, S.; Audebert, M.; Glaise, D.; Morel, F.; Loyer, P.; Robin, M.-A. Evaluation of genotoxicity using automated detection of gammaH2AX in metabolically competent HepaRG cells. Mutagenesis 2016, 31, 43–50. [Google Scholar]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.-C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines down-regulate major cytochrome P450 in primary culture of human hepatocytes. Mol. Pharmacol. 1993, 44, 707–715. [Google Scholar] [PubMed]
- Langouet, S.; Corcos, L.; Abdel-Razzak, Z.; Loyer, P.; Ketterer, B.; Guillouzo, A. Up regulation of glutathione S-transferases alpha by interleukin 4 in human hepatocytes in primary culture. Biochem. Biophys. Res. Commun. 1995, 216, 793–800. [Google Scholar] [CrossRef]
- Tsuji, S.; Kawamura, F.; Hayashi, A.; Ohbayashi, T.; Kazuki, Y.; Chesné, C.; Oshimura, M.; Tada, M. Dual-color fluorescence imaging to monitor CYP3A4 and CYP3A7 expression in human hepatic carcinoma HepG2 and HepaRG cells. PLoS ONE 2014, 9, e104123. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, T.; Tsuji, S.; Tada, M. Fluorometric evaluation of CYP3A4 expression using improved transgenic HepaRG cells carrying a dual-colour reporter for CYP3A4 and CYP3A7. Sci. Rep. 2017, 7, 2874. [Google Scholar] [CrossRef] [PubMed]
- Casabar, R.C.; Das, P.C.; Dekrey, G.K.; Gardiner, C.S.; Cao, Y.; Rose, R.L.; Wallace, A.D. Endosulfan induces CYP2B6 and CYP3A4 by activating the pregnane X receptor. Toxicol. Appl. Pharmacol. 2010, 245, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Blagus, T.; Zager, V.; Cemazar, M.; Sersa, G.; Kamensek, U.; Zegura, B.; Nunic, J.; Filipic, M. A cell-based biosensor system HepG2CDKN1A-DsRed for rapid and simple detection of genotoxic agents. Biosens. Bioelectron. 2014, 64, 102–111. [Google Scholar] [CrossRef]
- Corlu, A.; Loyer, P. Culture conditions promoting hepatocyte proliferation and cell cycle synchronization. In Protocols in In Vitro Hepatocyte Research; Rogiers, V., Vinken, M., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1250, pp. 27–51. [Google Scholar]
- Piton, A.; Rauch, C.; Langouet, S.; Guillouzo, A.; Morel, F. Involvement of pregnane X receptor in the regulation of CYP2B6 gene expression by oltipraz in human hepatocytes. Toxicol. In Vitro 2010, 24, 452–459. [Google Scholar] [CrossRef]
- Ayed-Boussema, I.; Pascussi, J.-M.; Maurel, P.; Bacha, H.; Hassen, W. Zearalenone activates pregnane X receptor, constitutive androstane receptor and aryl hydrocarbon receptor and corresponding phase I target genes mRNA in primary cultures of human hepatocytes. Environ. Toxicol. Pharmacol. 2011, 31, 79–87. [Google Scholar] [CrossRef]
- Gilot, D.; Loyer, P.; Corlu, A.; Glaise, D.; Lagadic-Gossmann, D.; Atfi, A.; Morel, F.; Ichijo, H.; Guguen-Guillouzo, C. Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J. Biol. Chem. 2002, 277, 49220–49229. [Google Scholar] [CrossRef]
- Staňková, P.; Kučera, O.; Lotková, H.; Roušar, T.; Endlicher, R.; Cervinková, Z. The toxic effect of thioacetamide on rat liver in vitro. Toxicol. In Vitro 2010, 24, 2097–2103. [Google Scholar] [CrossRef]
- Hajovsky, H.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963. [Google Scholar] [CrossRef]
- Adjrah, Y.; Karou, S.D.; Agbonon, A.; Eklu-gadegbeku, K.; de Souza, C.; Gbeassor, M. Toxicological assessment of effect of mancozeb-treated lettuce (Lactuca sativa) on wistar rat liver. Ethiop. J. Environ. Stud. Manag. 2013, 6, 67–73. [Google Scholar]
- Pirozzi, A.V.; Stellavato, A.; La Gatta, A.; Lamberti, M.; Schiraldi, C. Mancozeb, a fungicide routinely used in agriculture, worsens nonalcoholic fatty liver disease in the human HepG2 cell model. Toxicol. Lett. 2016, 249, 1–4. [Google Scholar] [CrossRef]
- Rogue, A.; Lambert, C.; Spire, C.; Claude, N.; Guillouzo, A. Interindividual variability in gene expression profiles in human hepatocytes and comparison with HepaRG cells. Drug Metab. Dispos. 2012, 40, 151–158. [Google Scholar] [CrossRef]
- Peyta, L.; Jarnouen, K.; Pinault, M.; Guimaraes, C.; Pais de Barros, J.P.; Chevalier, S.; Dumas, J.F.; Maillot, F.; Hatch, G.M.; Loyer, P.; et al. Reduced cardiolipin content decreases respiratory chain capacities and increases ATP synthesis yield in the human HepaRG cells. Biochim. Biophys. Acta 2016, 1857, 443–453. [Google Scholar] [CrossRef]
- Kanebratt, K.P.; Andersson, T.B. Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab. Dispos. 2008, 36, 1444–1452. [Google Scholar] [CrossRef]
- Anthérieu, S.; Chesné, C.; Li, R.; Guguen-Guillouzo, C.; Guillouzo, A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol. In Vitro 2012, 26, 1278–1285. [Google Scholar] [CrossRef]
- Demazeau, M.; Quesnot, N.; Ripoche, N.; Rauch, C.; Jeftić, J.; Morel, F.; Gauffre, F.; Benvegnu, T.; Loyer, P. Efficient transfection of Xenobiotic Responsive Element-biosensor plasmid using diether lipid and phosphatidylcholine liposomes in differentiated HepaRG cells. Int. J. Pharm. 2017, 524, 268–278. [Google Scholar] [CrossRef]

| Forward | Reverse | |
|---|---|---|
| CYP1A1 | TCTTCCTTCGTCCCCTTCAC | ACACCTTGTCGATAGCACCA |
| CYP2B6 | TTCCTACTGCTTCCGTCTATC | GTGCAGAATCCCACAGCTCA |
| CYP2E1 | TTGAAGCCTCTCGTTGACCC | CGTGGTGGGATACAGCCA |
| CYP3A4 | CTTCATCCAATGGACTGCATAAA | TCCCAAGTATAACACTCTACACACACA |
| CK19 | TTTGAGACGGAACAGGCTCT | AATCCACCTCCACACTGACC |
| GFP | ACAACAGCCACAACGTCTAT | GGGTGTTCTGCTGGTAGTG |
| TBP | GAGCTGTGATGTGAAGTTTCC | TCTGGGTTTGATCATTCTGTA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlach, M.; Quesnot, N.; Dubois-Pot-Schneider, H.; Ribault, C.; Verres, Y.; Petitjean, K.; Rauch, C.; Morel, F.; Robin, M.-A.; Corlu, A.; et al. Cytochrome P450 1A1/2, 2B6 and 3A4 HepaRG Cell-Based Biosensors to Monitor Hepatocyte Differentiation, Drug Metabolism and Toxicity. Sensors 2019, 19, 2245. https://doi.org/10.3390/s19102245
Vlach M, Quesnot N, Dubois-Pot-Schneider H, Ribault C, Verres Y, Petitjean K, Rauch C, Morel F, Robin M-A, Corlu A, et al. Cytochrome P450 1A1/2, 2B6 and 3A4 HepaRG Cell-Based Biosensors to Monitor Hepatocyte Differentiation, Drug Metabolism and Toxicity. Sensors. 2019; 19(10):2245. https://doi.org/10.3390/s19102245
Chicago/Turabian StyleVlach, Manuel, Nicolas Quesnot, Hélène Dubois-Pot-Schneider, Catherine Ribault, Yann Verres, Kilian Petitjean, Claudine Rauch, Fabrice Morel, Marie-Anne Robin, Anne Corlu, and et al. 2019. "Cytochrome P450 1A1/2, 2B6 and 3A4 HepaRG Cell-Based Biosensors to Monitor Hepatocyte Differentiation, Drug Metabolism and Toxicity" Sensors 19, no. 10: 2245. https://doi.org/10.3390/s19102245
APA StyleVlach, M., Quesnot, N., Dubois-Pot-Schneider, H., Ribault, C., Verres, Y., Petitjean, K., Rauch, C., Morel, F., Robin, M.-A., Corlu, A., & Loyer, P. (2019). Cytochrome P450 1A1/2, 2B6 and 3A4 HepaRG Cell-Based Biosensors to Monitor Hepatocyte Differentiation, Drug Metabolism and Toxicity. Sensors, 19(10), 2245. https://doi.org/10.3390/s19102245





