Potential Point-of-Care Microfluidic Devices to Diagnose Iron Deficiency Anemia
Abstract
1. Introduction
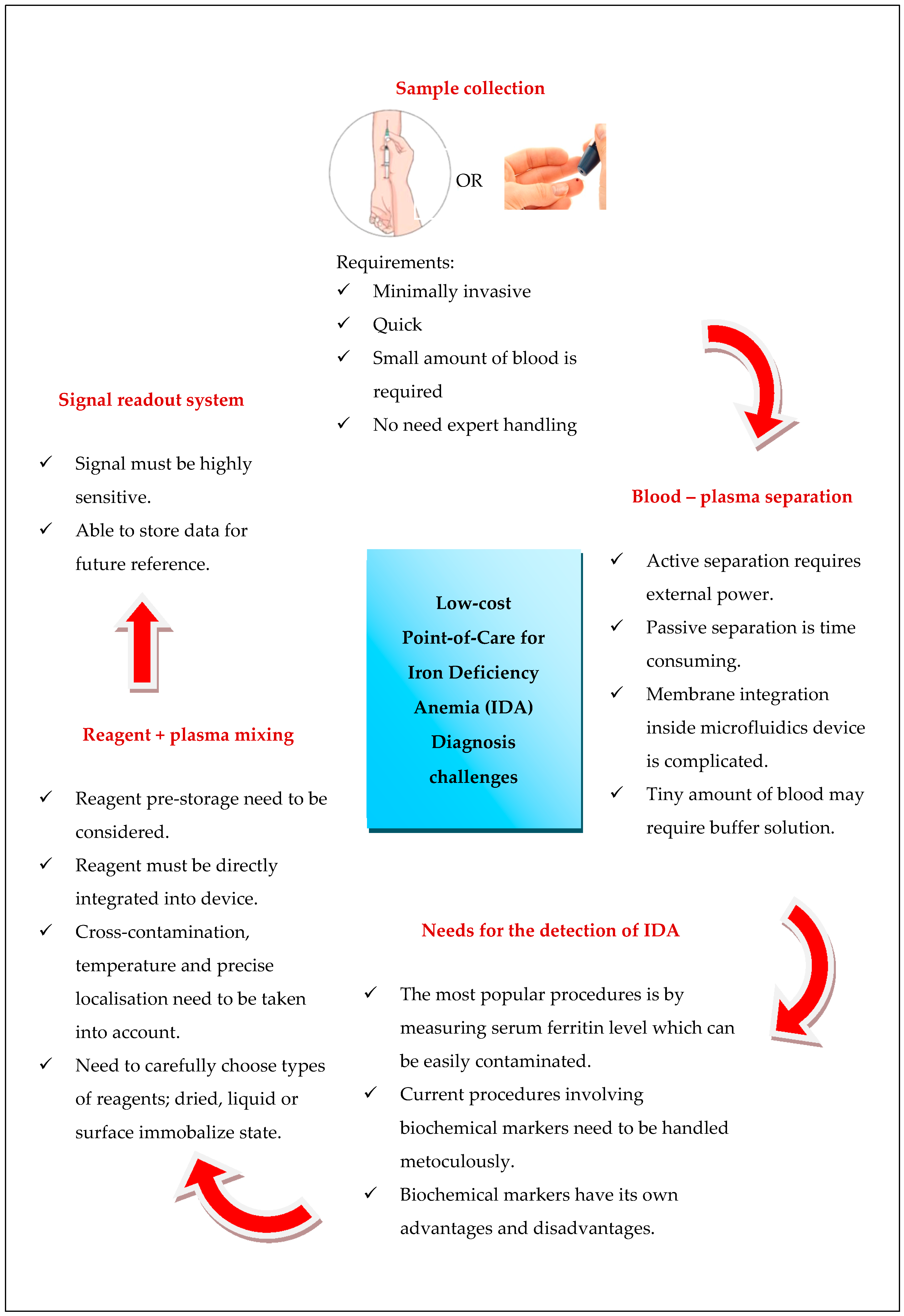
2. Diagnosing Iron Deficiency Anemia
3. State-of-the-Art Microfluidic Devices for Iron Deficiency Anemia Detection
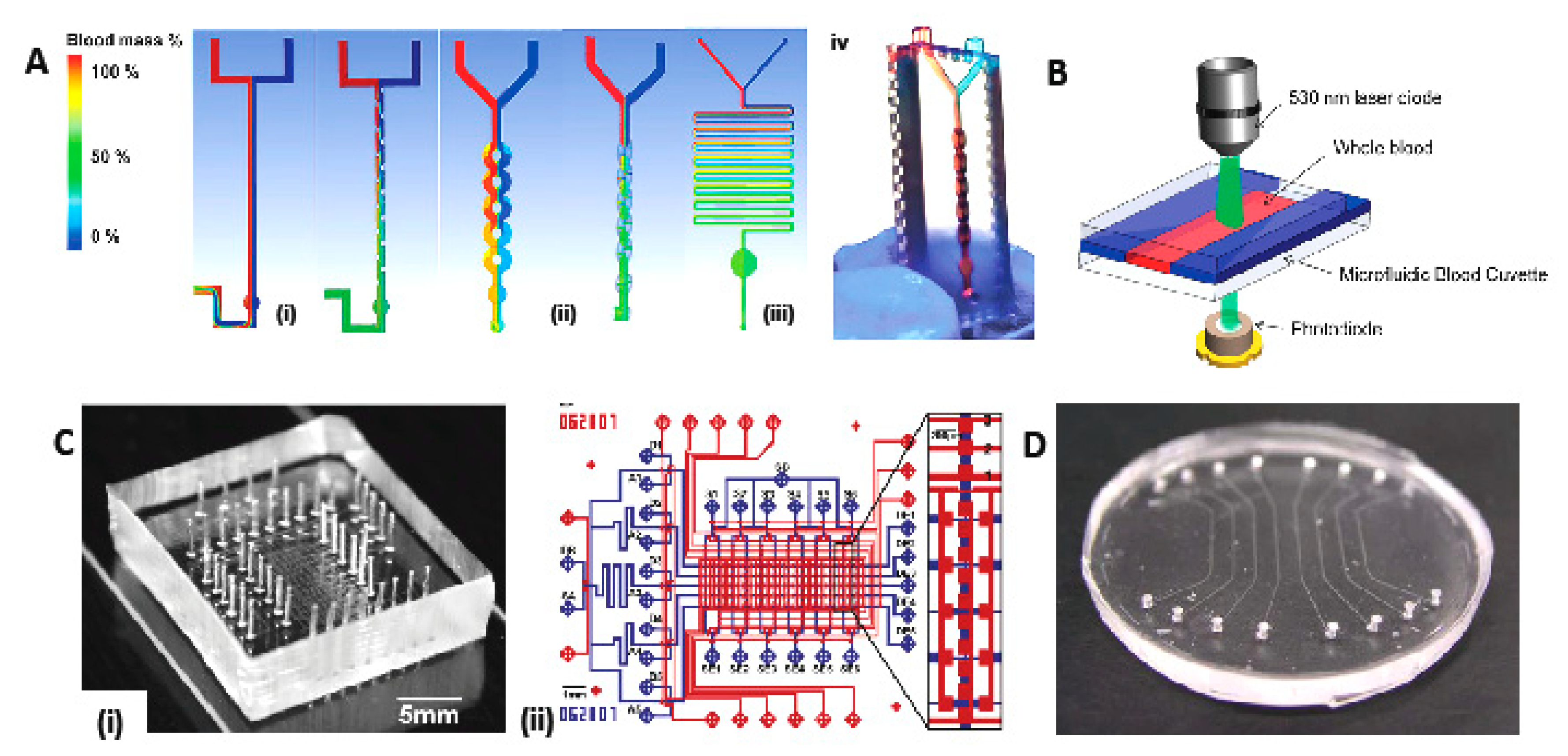
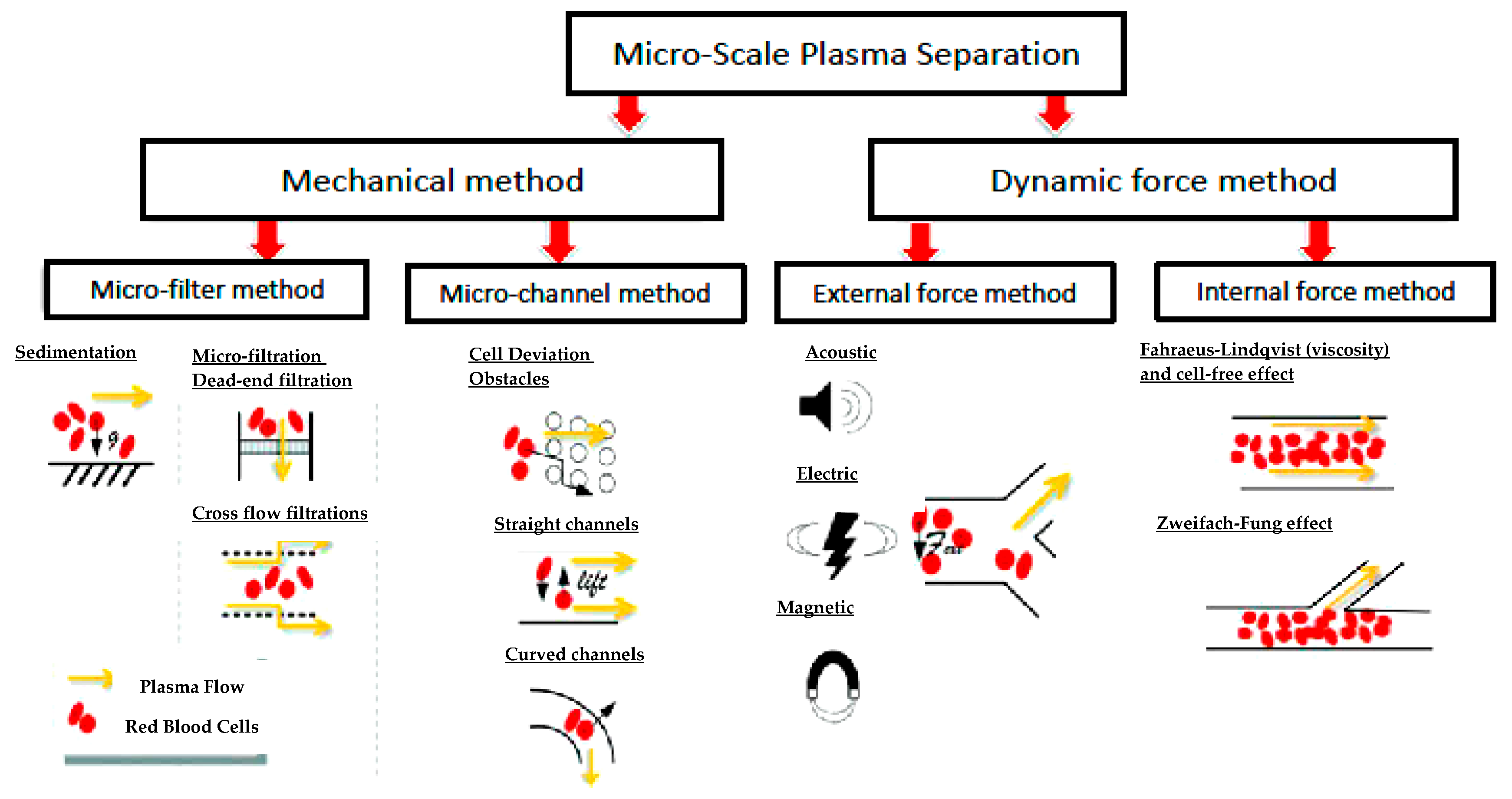
3.1. Filtration and Mixing Micro-Device for Blood-Plasma Separation
3.1.1. State-of-the-Art Blood Plasma Separation and Filtration
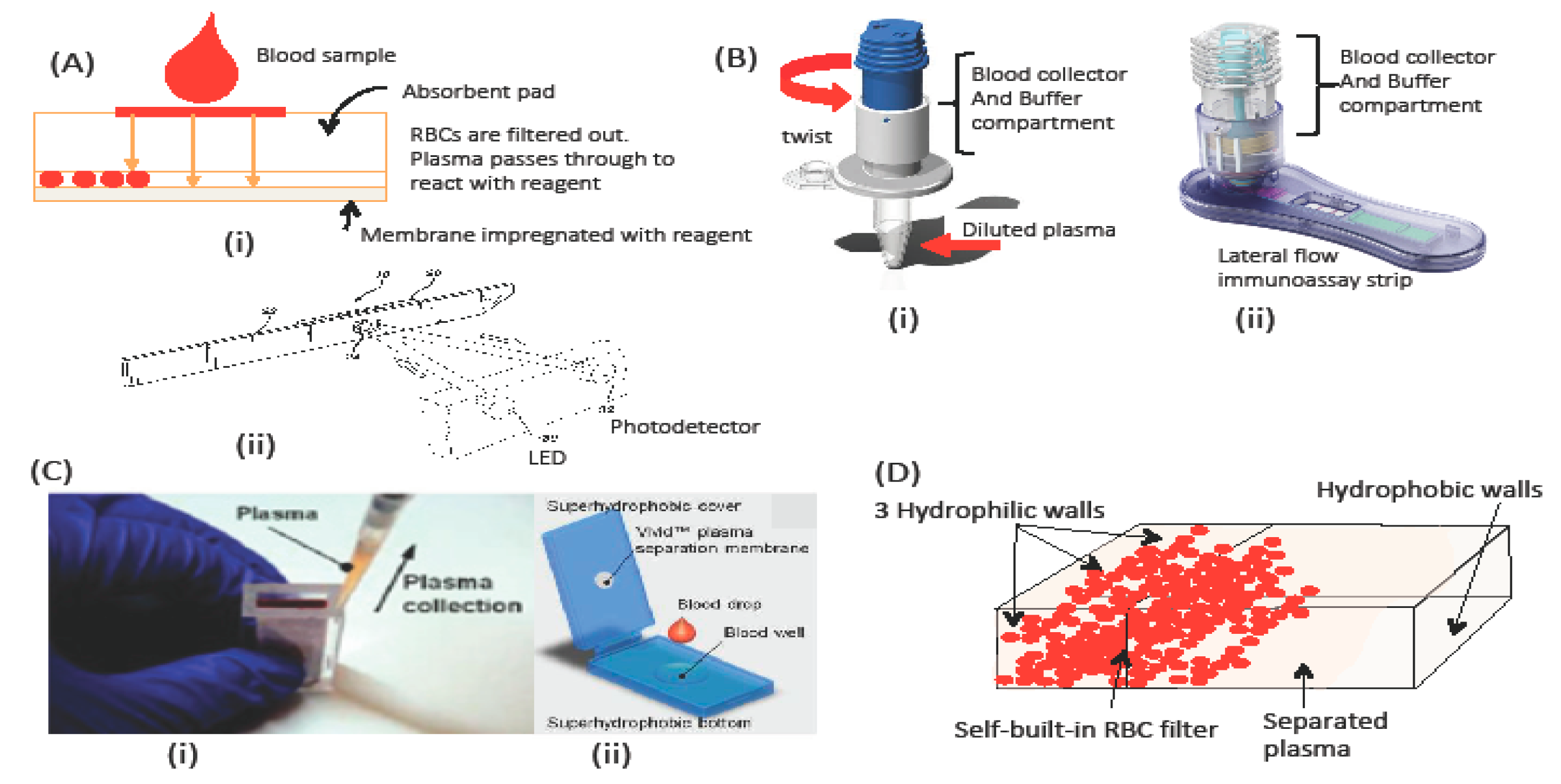
3.1.2. Membrane-based blood-plasma separation
3.1.3. Buffer or No Buffer for Separation?
3.1.4. Reagent-Deposition and Micro-Mixing
4. Outlook and Challenges of Portable Microfluidics System for Iron Deficiency Anemia Detection
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Berger, J.; Dillon, J.-C. Control of iron deficiency in developing countries. Sante 2002, 12, 22–30. [Google Scholar] [PubMed]
- Wu, A.C.; Lesperance, L.; Bernstein, H. Screening for iron deficiency. Pediatr. Rev. 2002, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kohli-Kumar, M. Screening for anemia in children: AAP recommendations—A critique. Pediatrics 2001, 108, E56. [Google Scholar] [CrossRef] [PubMed]
- Sukrat, B.; Sirichotiyakul, S. The prevalence and causes of anemia during pregnancy in Maharaj Nakorn Chiang Mai Hospital. J. Med. Assoc. Thai. 2006, 89 (Suppl 4), S142–S146. [Google Scholar] [PubMed]
- Felt, B.T.; Peirano, P.; Algarín, C.; Chamorro, R.; Sir, T.; Kaciroti, N.; Lozoff, B. Long-term neuroendocrine effects of iron-deficiency anemia in infancy. Pediatr. Res. 2012, 71, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ouf, N.M.; Jan, M.M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 2015, 36, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; DeStefano, A.J.; Cecil, T.L.; Zaidi, K.; Williams, R.L. Metal impurities in food and drugs. Pharm. Res. 2010, 27, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Barbosa, U.; Peña-Vazquez, E.; Barciela-Alonso, M.C.; Costa Ferreira, S.L.; Pinto dos Santos, A.M.; Bermejo-Barrera, P. Simultaneous determination and speciation analysis of arsenic and chromium in iron supplements used for iron-deficiency anemia treatment by HPLC-ICP-MS. Talanta 2017, 170, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Rivet, C.; Lee, H.; Hirsch, A.; Hamilton, S.; Lu, H. Microfluidics for medical diagnostics and biosensors. Chem. Eng. Sci. 2011, 66, 1490–1507. [Google Scholar] [CrossRef]
- Oita, I.; Halewyck, H.; Thys, B.; Rombaut, B.; Vander Heyden, Y.; Mangelings, D. Microfluidics in macro-biomolecules analysis: Macro inside in a nano world. Anal. Bioanal. Chem. 2010, 398, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.T.; Fu, G.; Dou, M.; Xu, F.; Liu, R.; Qi, H.; Li, X. Biomarker detection for disease diagnosis using cost-effective microfluidic platforms. Analyst 2015, 140, 7062–7081. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kiehne, J.; Sinoway, L.I.; Cameron, C.E.; Huang, T.J. Microfluidic opportunities in the field of nutrition. Lab Chip 2013, 13, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, W.; Li, Z.; Li, L.; Gu, H.; Fu, J. Emerging microengineered tools for functional analysis and phenotyping of blood cells. Trends Biotechnol. 2014, 32, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Czugala, M.; Fay, C.; O’Connor, N.E.; Corcoran, B.; Benito-Lopez, F.; Diamond, D. Portable integrated microfluidic analytical platform for the monitoring and detection of nitrite. Talanta 2013, 116, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Crevillén, A.G.; Hervás, M.; López, M.A.; González, M.C.; Escarpa, A. Real sample analysis on microfluidic devices. Talanta 2007, 74, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ip, A.; Xu, F.; Giguel, F.F.; Moon, S.; Akay, A.; Kuritzkes, D.R.; Demirci, U. Development of a microfluidic system for measuring HIV-1 viral load. In Proceedings of the SPIE 7666, Sensors, and Command, Control, Communications, and Intelligence (C3I) Technologies for Homeland Security and Homeland Defense IX, Orlando, FL, USA, 5 May 2010; Volume 7666, p. 76661H. [Google Scholar]
- Warkiani, M.E.; Tay, A.K.P.; Khoo, B.L.; Xiaofeng, X.; Han, J.; Lim, C.T.; Tulloch, S.; Oldfield, F.S.; Hayes, R.; Soo, R.A.; et al. Malaria detection using inertial microfluidics. Lab Chip 2015, 15, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Fook Kong, T.; Ye, W.; Peng, W.K.; Wei Hou, H.; Marcos; Preiser, P.R.; Nguyen, N.-T.; Han, J. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Sci. Rep. 2015, 5, 11425. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-F.; Lien, K.-Y.; Lei, H.-Y.; Lee, G.-B. An integrated microfluidic system for rapid diagnosis of dengue virus infection. Biosens. Bioelectron. 2009, 25, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Pekin, D.; Skhiri, Y.; Baret, J.-C.; Corre, D.L.; Mazutis, L.; Salem, C.B.; Millot, F.; Harrak, A.E.; Hutchison, J.B.; Larson, J.W.; et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 2011, 11, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K.; Burgess, D.C.H.; Lim, Y.W.; Aledort, J.E.; Rafael, M.E.; Ricci, K.A.; Boer, R.; Hilborne, L.; et al. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118. [Google Scholar] [CrossRef] [PubMed]
- WHO Micronutrient Deficiencies: Iron Deficiency Anaemia. Available online: http://www.who.int/nutrition/topics/ida/en/ (accessed on 3 August 2017).
- Plevniak, K.; Campbell, M.; Myers, T.; Hodges, A.; He, M. 3D printed auto-mixing chip enables rapid smartphone diagnosis of anemia. Biomicrofluidics 2016, 10, 054113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Son, J.K.; Seo, Y.H.; Kim, B.H.; Lee, W.H.; Park, K.T.; Nahm, K.B.; Choi, E.Y. Disposable microfluidic blood cuvette for measuring hemoglobin concentration. Microsyst. Technol. 2014, 20, 499–504. [Google Scholar] [CrossRef]
- Tengfei, Z.; Chaohui, W.; Baogang, M.; Zhuangde, J. Paper capillary force driven hollow channel as a platform for multiphase flows bioassays. Sens. Bio-Sens. Res. 2016, 8, 43–46. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. Biomarkers for the differentiation of anemia and their clinical usefulness. J. Blood Med. 2013, 4, 11–22. [Google Scholar] [PubMed]
- Kartalov, E.P.; Zhong, J.F.; Scherer, A.; Quake, S.R.; Taylor, C.R.; Anderson, W.F. High-throughput multi-antigen microfluidic fluorescence immunoassays. Biotechniques 2006, 40, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kartalov, E.P.; Lin, D.H.; Lee, D.T.; Anderson, W.F.; Taylor, C.R.; Scherer, A. Internally calibrated quantification of protein analytes in human serum by fluorescence immunoassays in disposable elastomeric microfluidic devices. Electrophoresis 2008, 29, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Srinivasan, B.; Vemulapati, S.; Mehta, S.; Erickson, D. Personalized nutrition diagnostics at the point-of-need. Lab Chip 2016, 16, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Schrott, W.; Nebyla, M.; Meisterová, L.; Přibyl, M. Fast ferritin immunoassay on PDMS microchips. Chem. Pap. 2010, 65, 246. [Google Scholar] [CrossRef]
- Schrott, W.; Nebyla, M.; Přibyl, M.; Šnita, D. Detection of immunoglobulins in a laser induced fluorescence system utilizing polydimethysiloxane microchips with advanced surface and optical properties. Biomicrofluidics 2011, 5, 014101. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.P.; Yager, P.; Goldstein, R.E.; Austin, R.H. Biotechnology at low Reynolds numbers. Biophys. J. 1996, 71, 3430–3441. [Google Scholar] [CrossRef]
- Wilding, P.; Kricka, L.J.; Cheng, J.; Hvichia, G.; Shoffner, M.A.; Fortina, P. Integrated cell isolation and polymerase chain reaction analysis using silicon microfilter chambers. Anal. Biochem. 1998, 257, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Patel, M.K.; Kersaudy-Kerhoas, M.; Bailey, C.; Desmulliez, M.P.Y. Parametrical modeling and design optimization of blood plasma separation device with microchannel mechanism. In Proceedings of the Electronic Components and Technology Conference, San Diego, CA, USA, 26–29 May 2009; pp. 1970–1976. [Google Scholar]
- Blattert, C.; Jurischka, R.; Tahhan, I.; Schoth, A.; Kerth, P.; Menz, W. Microfluidic blood/plasma separation unit based on microchannel bend structures. In Proceedings of the 2005 3rd IEEE/EMBS Special Topic Conference on Microtechnology in Medicine and Biology, Oahu, HI, USA, 12–15 May 2005; Volume 2005, pp. 38–41. [Google Scholar]
- Xue, X.; Marson, S.; Patel, M.K.; Bailey, C.; O’Neill, W.; Topham, D.; Kay, R.W.; Desmulliez, M.P.Y. Progress towards the design and numerical analysis of a 3D microchannel biochip separator. Int. J. Numer. Method Biomed. Eng. 2011, 27, 1771–1792. [Google Scholar] [CrossRef]
- Tripathi, S.; Varun Kumar, Y.V.B.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Wu, Z.; Hjort, K. Microfluidic Hydrodynamic Cell Separation: A Review. Micro Nanosyst. 2009, 1, 181–192. [Google Scholar] [CrossRef]
- Wyatt Shields, C., IV; Reyes, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluidics Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Maria, M.S.; Kumar, B.S.; Chandra, T.S.; Sen, A.K. Development of a microfluidic device for cell concentration and blood cell-plasma separation. Biomed. Microdevices 2015, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Sollier, E. Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323–3346. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.A.; McGarraugh, G.; Yu, Y.S. Control Solution for a Blood Glucose Monitor. U.S. Patent 5,605,837, 25 February 1997. [Google Scholar]
- Biesbrouck, G.M.; Glatz, J.F.C. Device and Method for Separating and Analyzing Blood. United States Patent Application US 14/882,377, 14 April 2016. [Google Scholar]
- Homsy, A.; van der Wal, P.D.; Doll, W.; Schaller, R.; Korsatko, S.; Ratzer, M.; Ellmerer, M.; Pieber, T.R.; Nicol, A.; de Rooij, N.F. Development and validation of a low cost blood filtration element separating plasma from undiluted whole blood. Biomicrofluidics 2012, 6, 012804. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mauk, M.; Gross, R.; Bushman, F.D.; Edelstein, P.H.; Collman, R.G.; Bau, H.H. Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal. Chem. 2013, 85, 10463–10470. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liao, S.-C.; Song, J.; Mauk, M.G.; Li, X.; Wu, G.; Ge, D.; Greenberg, R.M.; Yang, S.; Bau, H.H. A high-efficiency superhydrophobic plasma separator. Lab Chip 2016, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Aran, K.; Fok, A.; Sasso, L.A.; Kamdar, N.; Guan, Y.; Sun, Q.; Ündar, A.; Zahn, J.D. Microfiltration platform for continuous blood plasma protein extraction from whole blood during cardiac surgery. Lab Chip 2011, 11, 2858–2868. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Choi, Y.H.; Yang, J.-H.; Park, C.W.; Kim, W.-J.; Ah, C.S.; Sung, G.Y. Magnetically-actuated blood filter unit attachable to pre-made biochips. Lab Chip 2012, 12, 3272–3326. [Google Scholar] [CrossRef] [PubMed]
- Sneha Maria, M.; Rakesh, P.E.; Chandra, T.S.; Sen, A.K. Capillary flow of blood in a microchannel with differential wetting for blood plasma separation and on-chip glucose detection. Biomicrofluidics 2016, 10, 054108. [Google Scholar] [CrossRef] [PubMed]
- Aunet, D.L.; Gerogevich, G.G.; Jeng, T.-W.; Oosta, G.M.; Siegel, N.A. Methods and Devices for the Separation of Plasma or Serum from Whole Blood. U.S. Patent 4,933,092, 12 June 1990. [Google Scholar]
- Liu, Y.; Dai, W.; Li, H.; Wu, W.; Wang, W. A 3D filter for plasma separation from whole blood. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 564–567. [Google Scholar]
- Kar, S.; Maiti, T.K.; Chakraborty, S. Capillarity-driven blood plasma separation on paper-based devices. Analyst 2015, 140, 6473–6476. [Google Scholar] [CrossRef] [PubMed]
- Nilghaz, A.; Shen, W. Low-cost blood plasma separation method using salt functionalized paper. RSC Adv. 2015, 5, 53172–53179. [Google Scholar] [CrossRef]
- Osborn, J.L.; Lutz, B.; Fu, E.; Kauffman, P.; Stevens, D.Y.; Yager, P. Microfluidics without pumps: Reinventing the T-sensor and H-filter in paper networks. Lab Chip 2010, 10, 2659–2965. [Google Scholar] [CrossRef] [PubMed]
- Bandara, G.C.; Heist, C.A.; Remcho, V.T. Patterned polycaprolactone-filled glass microfiber microfluidic devices for total protein content analysis. Talanta 2018, 176, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Kersaudy-Kerhoas, M.; Kavanagh, D.M.; Dhariwal, R.S.; Campbell, C.J.; Desmulliez, M.P.Y. Validation of a blood plasma separation system by biomarker detection. Lab Chip 2010, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.; Marchalot, J.; Fouillet, Y.; Digianantonio, L.; Claustre, P.; Cubizolles, M.; Achard, J. A passive microfluidic fragmentation system for continuous fluid-particles separation. In Bio-MEMS and Medical Microdevices; Tserepi, A., Delgado-Restituto, M., Makarona, E., Eds.; SPIE Publications: Bellingham, WA, USA, 2013; p. 87650M. [Google Scholar]
- Pamme, N. Continuous flow separations in microfluidic devices. Lab Chip 2007, 7, 1644. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-S.; Chang, H.-Y. A simple microfluidics for real-time plasma separation and hCG detection from whole blood. J. Chin. Inst. Eng. 2015, 38, 685–691. [Google Scholar] [CrossRef]
- Yang, S.; Ündar, A.; Zahn, J.D. A microfluidic device for continuous, real time blood plasma separation. Lab Chip 2006, 6, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef] [PubMed]
- Rigler, A.; Wagner, C.; Svasek, P.; Jachimowicz, A.; Hudek, P.; Kraft, M.; Vellekoop, M. Improved Lamination Micromixer with Wedge Shaped Inlet Channels for IR Spectroscopy. In Proceedings of the 22nd International Eurosensors Conference, Dresden, Germany, 7–10 September 2008; pp. 577–580. [Google Scholar]
- Ashiba, H.; Fujimaki, M.; Awazu, K.; Tanaka, T.; Makishima, M. Microfluidic chips for forward blood typing performed with a multichannel waveguide-mode sensor. Sens. Bio-Sens. Res. 2016, 7, 121–126. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic mixing: A review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef] [PubMed]
- Samborski, A.; Jankowski, P.; Wȩgrzyn, J.; Michalski, J.A.; Pawłowska, S.; Jakieła, S.; Garstecki, P. Blood diagnostics using sedimentation to extract plasma on a fully integrated point-of-care microfluidic system. Eng. Life Sci. 2015, 15, 333–339. [Google Scholar] [CrossRef]
- Dimov, I.K.; Basabe-Desmonts, L.; Garcia-Cordero, J.L.; Ross, B.M.; Park, Y.; Ricco, A.J.; Lee, L.P. Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS). Lab Chip 2011, 11, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Hitzbleck, M.; Delamarche, E. Reagents in microfluidics: An ‘in’ and ‘out’ challenge. Chem. Soc. Rev. 2013, 42, 8494. [Google Scholar] [CrossRef] [PubMed]
- Berthier, J.; Brakke, K.A.; Berthier, E. Fiber-based microfluidics. In Open Microfluidics; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 336. [Google Scholar]
- Abner, D.J. Inkjet Reagent Deposition for Biosensor Manufacturing. United States Patent Application US 12/944,817, 17 May 2012. [Google Scholar]
- Krauss, S.T.; Remcho, T.P.; Monazami, E.; Thompson, B.L.; Reinke, P.; Begley, M.R.; Landers, J.P. Inkjet printing on transparency films for reagent storage with polyester–toner microdevices. Anal. Methods 2016, 8, 7061–7068. [Google Scholar] [CrossRef]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-based inkjet-printed microfluidic analytical devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Maejima, K.; Tomikawa, S.; Suzuki, K.; Citterio, D. Inkjet printing: An integrated and green chemical approach to microfluidic paper-based analytical devices. RSC Adv. 2013, 3, 9258. [Google Scholar] [CrossRef]
- Abe, K.; Suzuki, K.; Citterio, D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal. Chem. 2008, 80, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cai, L.; Zhong, M.; Zheng, S. Low-cost and rapid prototyping of microfluidic paper-based analytical devices by inkjet printing of permanent marker ink. RSC Adv. 2015, 5, 4770–4773. [Google Scholar] [CrossRef]
- Komuro, N.; Takaki, S.; Suzuki, K.; Citterio, D. Inkjet printed (bio)chemical sensing devices. Anal. Bioanal. Chem. 2013, 405, 5785–5805. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet printing for biosensor fabrication: Combining chemistry and technology for advanced manufacturing. Lab Chip 2015, 15, 2538–2558. [Google Scholar] [CrossRef] [PubMed]
- Fridley, G.E.; Le, H.; Yager, P. Highly sensitive immunoassay based on controlled rehydration of patterned reagents in a 2-dimensional paper network. Anal. Chem. 2014, 86, 6447–6453. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.Y.; Petri, C.R.; Osborn, J.L.; Spicar-Mihalic, P.; McKenzie, K.G.; Yager, P. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip 2008, 8, 2038. [Google Scholar] [CrossRef] [PubMed]
- Brivio, M.; Li, Y.; Ahlford, A.; Kjeldsen, B.G.; Reimers, J.L.; Bu, M.; Syvänen, A.; Bang, D.D.; Wolff, A. A simple and efficient method for on-chip storage of reagents: Towards Lab-on-a-Chip system for point-of-care DNA diagnostics. In Micro Total Analysis Systems 2007; Springer: Paris, France, 2007; pp. 59–61. [Google Scholar]
- Hitzbleck, M.; Gervais, L.; Delamarche, E. Controlled release of reagents in capillary-driven microfluidics using reagent integrators. Lab Chip 2011, 11, 2680. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.A.; Bellotti, V.; Stray, S.; Lipschitz, D.A.; Cook, J.D.; Pippard, M.J.; Huebers, H.A. Plasma ferritin determination as a diagnostic tool. West. J. Med. 1986, 145, 657–663. [Google Scholar] [PubMed]


| Biochemical Marker Serum Ferritin | Normal N | Iron Depletion D | Iron Deficiency Without Anemia N | Iron Deficiency Anemia D |
|---|---|---|---|---|
| (mcg/dL) (mcg/L) | 100 ± 60 | <20 (200) | ≤10 (100) | <10 (100) |
| Serum iron | (1000 ± 600) | |||
| N | N | D | D | |
| (mcg/dL) (mcg/L) | 115 ± 50 | <115 (20.6) | <60 (10.7) | <40 (7.2) |
| Total iron-binding | (20.6 ± 9.0) | |||
| N | N | N/I | I | |
| Capacity | 330 ± 30 | 360 to 390 | 390 to 410 | ≥410 (73.4) |
| (59.0 ± 5.4) | (64.6 to 69.8) | (69.8 to 73.4) | ||
| Transferrin | N | N | D | D |
| Saturation (%) | 35 ± 15 | <30 | <20 | <10 |
| Serum transferring | N | I | I | I |
| Receptor (nmol/L) | <35 | <35 | ≥35 | ≥35 |
| Zinc | N | N | I | I |
| Protoporphyrin/He | <40 | <40 | ≥40 | ≥70 |
| me (µmol/mol) | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, B.K.; M.Soair, S.N.; Talik, N.A.; Lim, W.F.; Mei I, L. Potential Point-of-Care Microfluidic Devices to Diagnose Iron Deficiency Anemia. Sensors 2018, 18, 2625. https://doi.org/10.3390/s18082625
Yap BK, M.Soair SN, Talik NA, Lim WF, Mei I L. Potential Point-of-Care Microfluidic Devices to Diagnose Iron Deficiency Anemia. Sensors. 2018; 18(8):2625. https://doi.org/10.3390/s18082625
Chicago/Turabian StyleYap, Boon Kar, Siti Nur’Arifah M.Soair, Noor Azrina Talik, Wai Feng Lim, and Lai Mei I. 2018. "Potential Point-of-Care Microfluidic Devices to Diagnose Iron Deficiency Anemia" Sensors 18, no. 8: 2625. https://doi.org/10.3390/s18082625
APA StyleYap, B. K., M.Soair, S. N., Talik, N. A., Lim, W. F., & Mei I, L. (2018). Potential Point-of-Care Microfluidic Devices to Diagnose Iron Deficiency Anemia. Sensors, 18(8), 2625. https://doi.org/10.3390/s18082625






