Joint Center Estimation Using Single-Frame Optimization: Part 2: Experimentation
Abstract
1. Introduction
2. Methods: Experimentation
2.1. Review of SFO Methodology
2.2. Experimental Overview
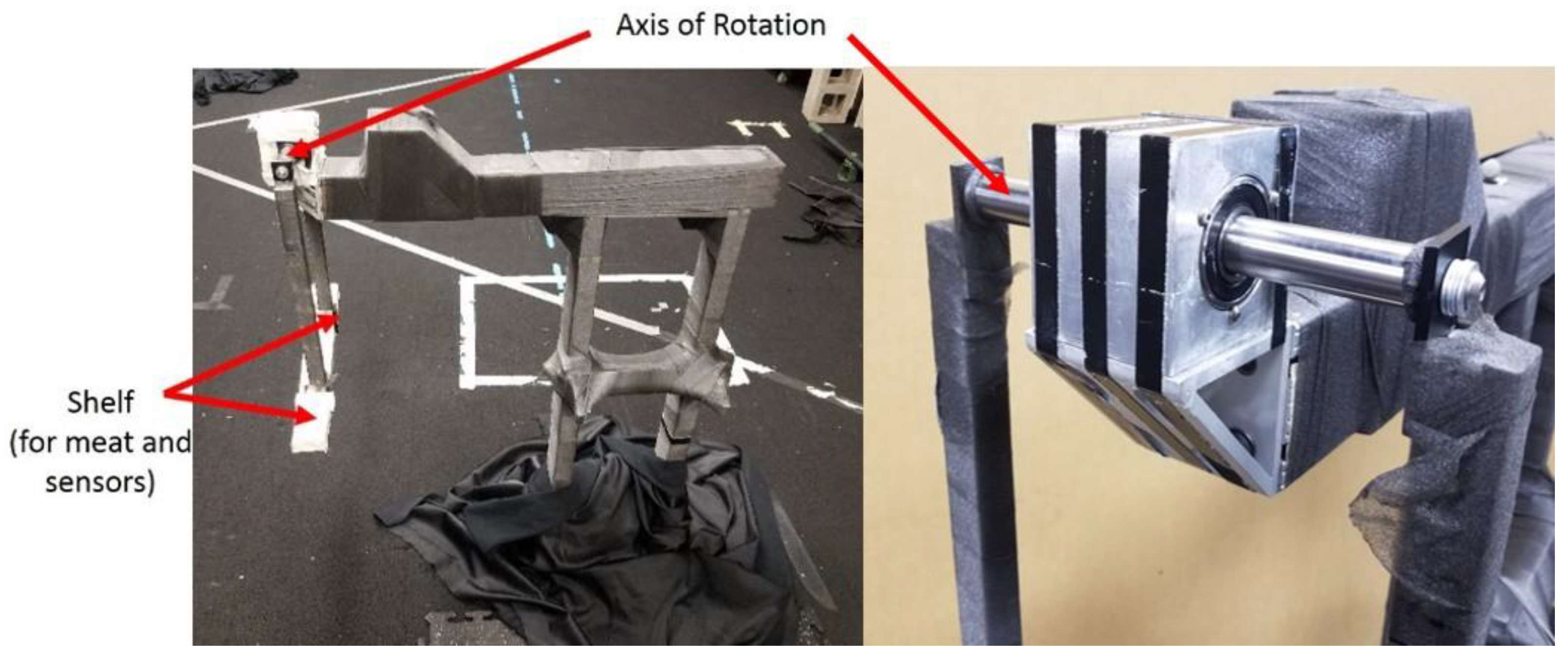
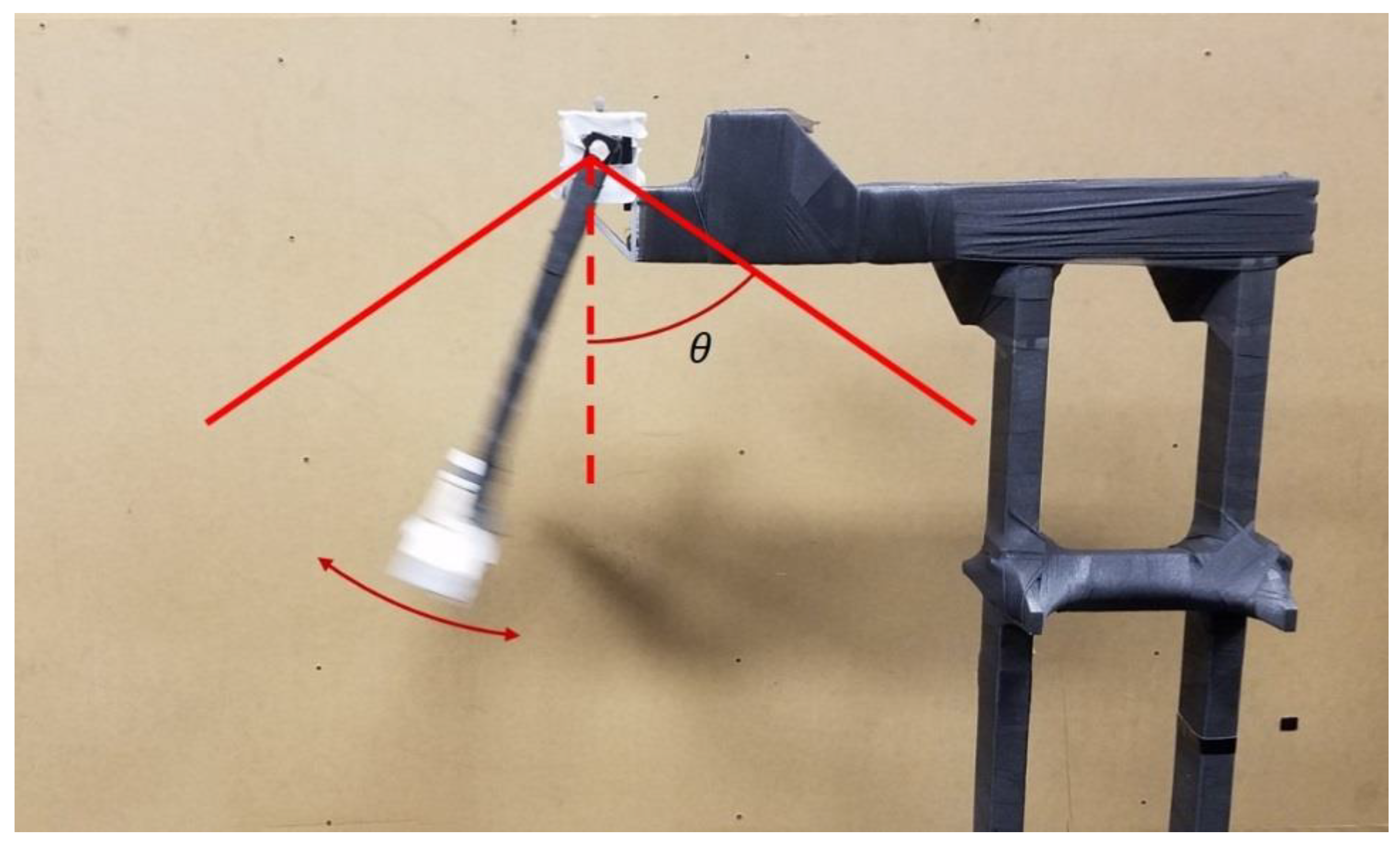
2.3. Pendulum Apparatus
2.4. Simulation of STA
2.5. Pendulum Instrumentation
2.6. Experimental Protocol
3. Methods: Data Processing
3.1. Relating Optical and Inertial Frames
3.2. Data Synchronization
3.3. Marker-Based Joint Axis
3.4. Marker-Based Joint Center
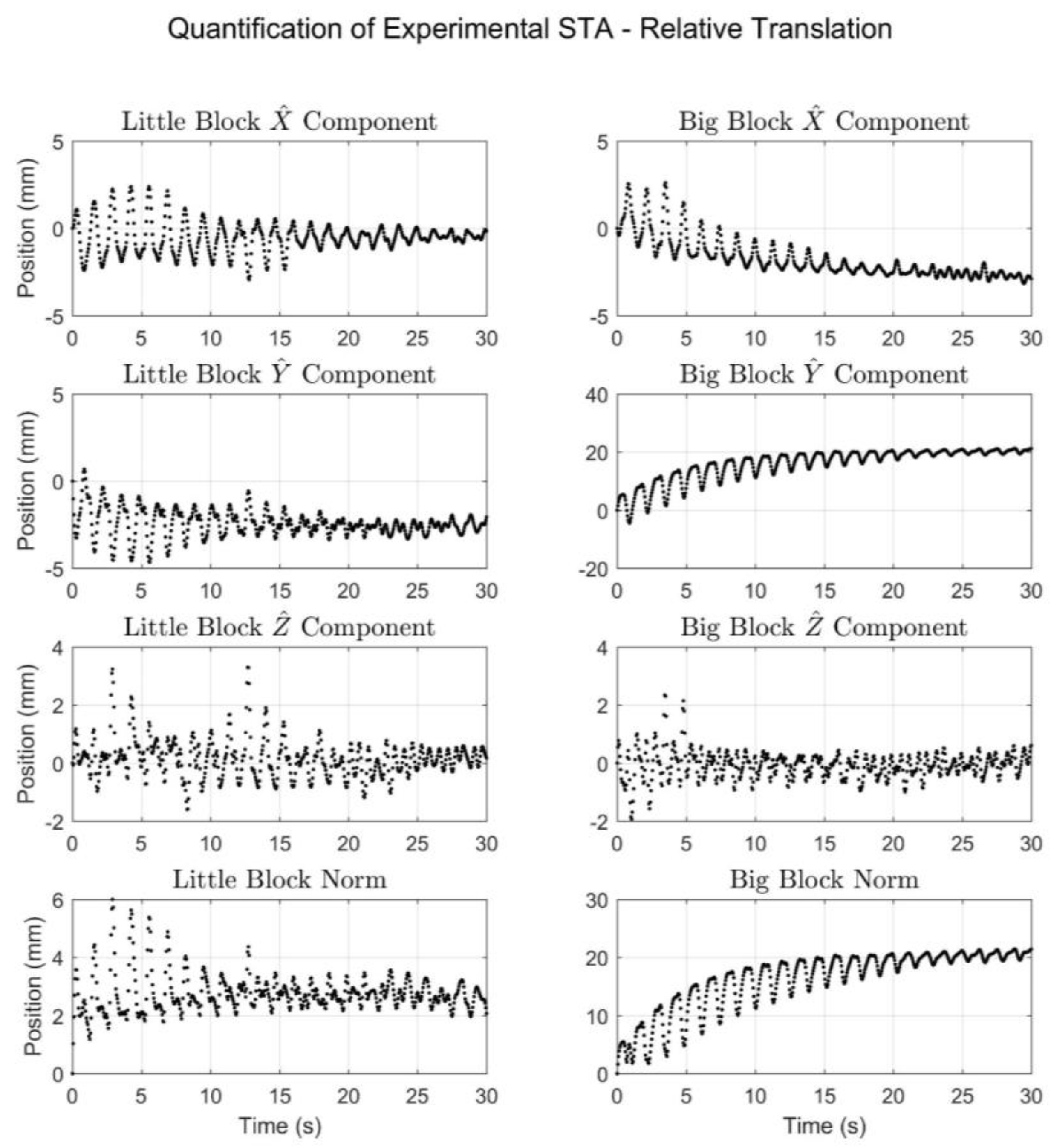
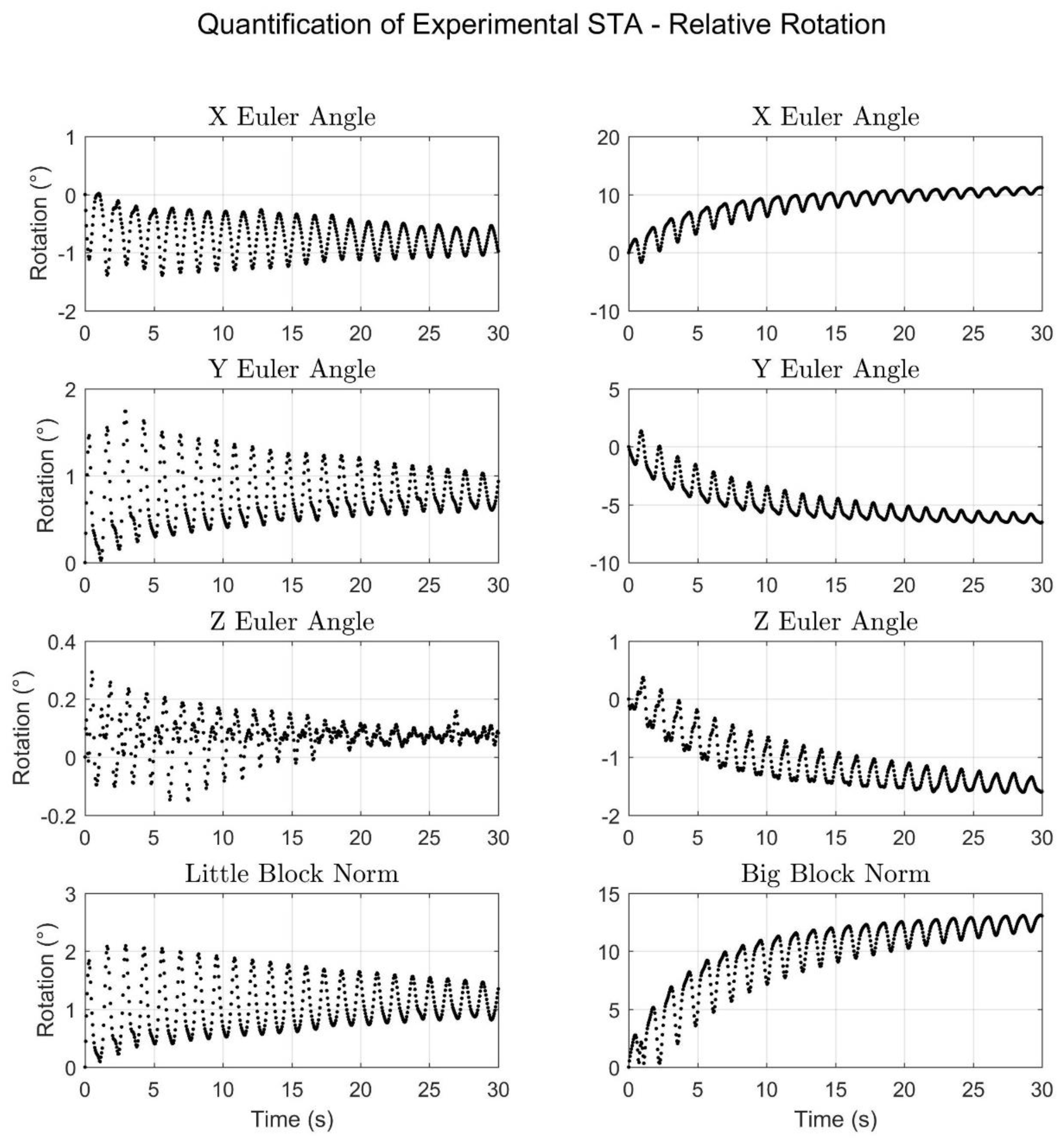
3.5. STA Quantification
3.6. Experiment-Specific Methodology
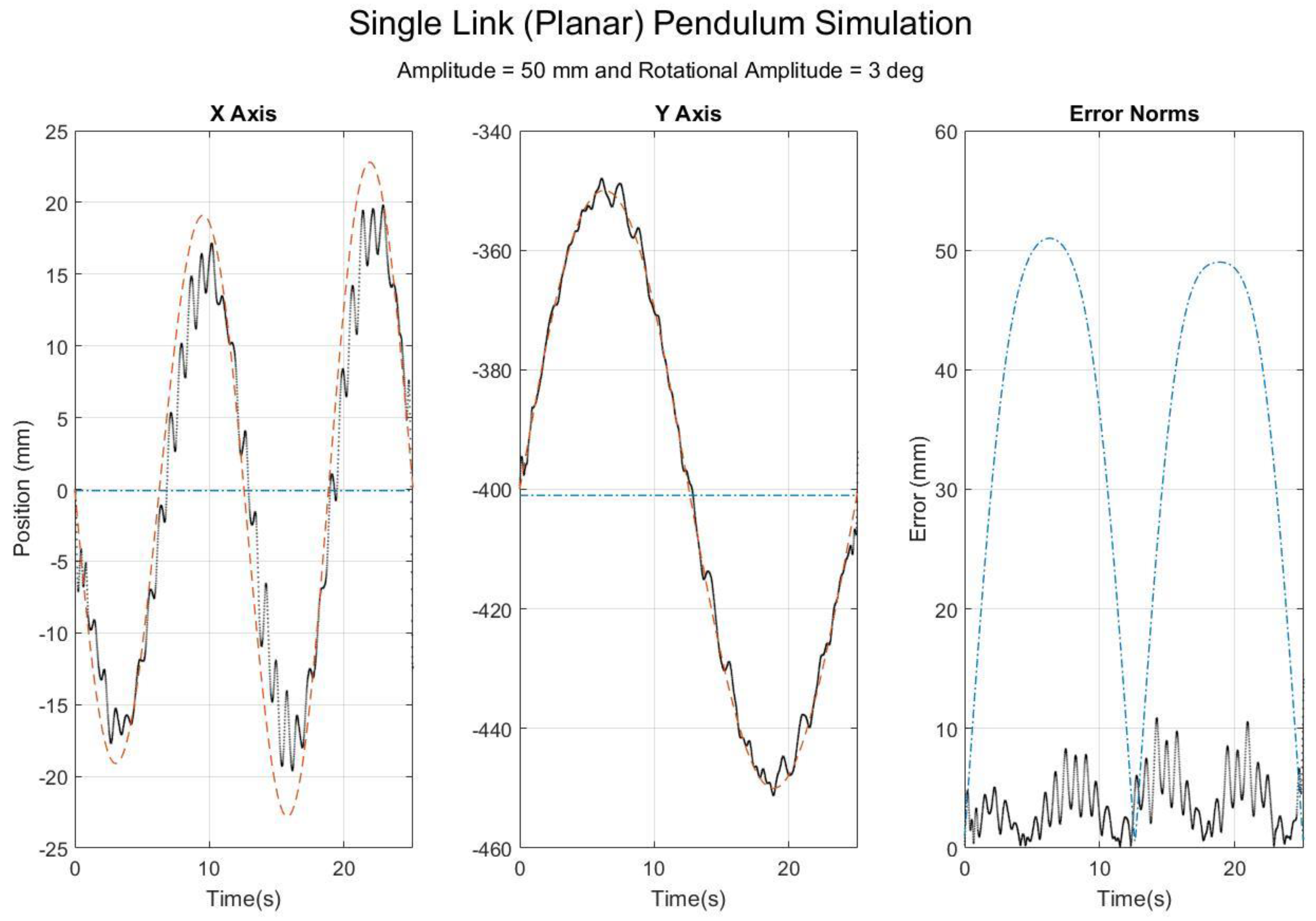
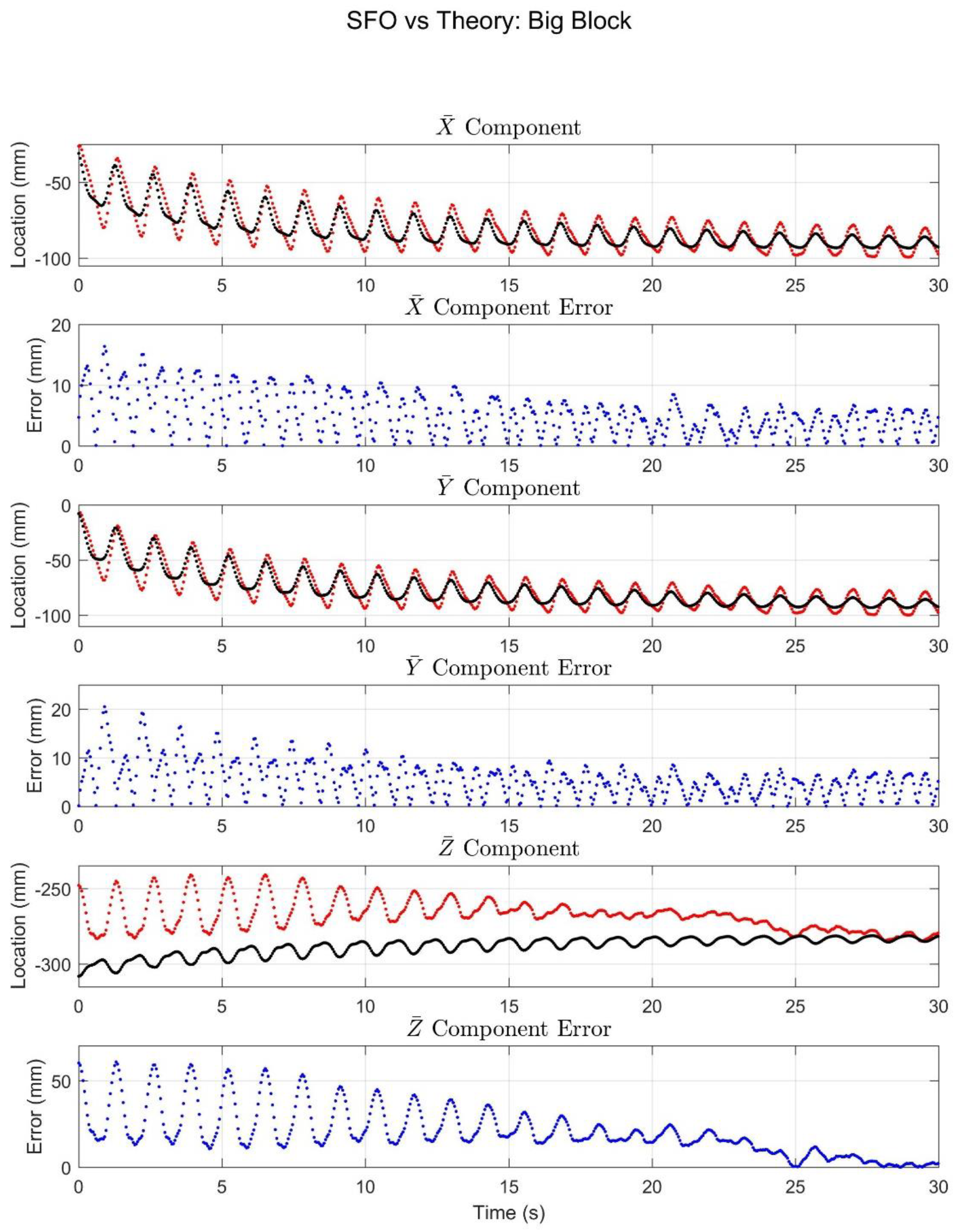
4. Results
5. Discussion
5.1. Interpretations of Results
5.2. Limitations
5.3. Comparison with Simulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seel, T.; Werner, C.; Schauer, T. The adaptive drop foot stimulator—Multivariable learning control of foot pitch and roll motion in paretic gait. Med. Eng. Phys. 2016, 38, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.P.; Cain, S.M.; McGinnis, R.S.; Vitali, R.R.; Perkins, N.C.; McLean, S.G. Quantifying warfighter performance in a target acquisition and aiming task using wireless inertial sensors. Appl. Ergon. 2016, 56, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; McKinnon, C.; Viggiani, D.; Johnson, M.J.; Callaghan, J.P.; Albert, W.J. Biomechanical investigation of prolonged driving in an ergonomically designed truck seat prototype. Ergonomics 2017, 61, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Shell, R.; Robbins, S.M.K.; Dixon, P.C.; Renaud, P.J.; Turcotte, R.A.; Wu, T.; Pearsall, D.J. Skating start propulsion: Three-dimensional kinematic analysis of elite male and female ice hockey players. Sports Biomech. 2017, 16, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Seydel, A.; Farrell, K.; Johnson, R.; Marler, T.; Rahmatalla, S.; Bhatt, R.; Abdel-Malek, K. Improved motion capture processing for high fidelity human models using optimization-based prediction of posture and anthropometry. In International Conference on Applied Human Factors and Ergonomics; Cassenti, D., Ed.; Springer: Cham, Germany, 2017; pp. 549–561. [Google Scholar]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 8383–8392. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.B., III; Ounpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Harrington, M.E.; Zavatsky, A.B.; Lawson, S.E.; Yuan, Z.; Theologis, T.N. Prediction of the hip joint centre in adults, children, and patients with cerebral palsy based on magnetic resonance imaging. J. Biomech. 2007, 40, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, R.M.; Taylor, W.R.; Duda, G.N.; Heller, M.O. A survey of formal methods for determining the centre of rotation of ball joints. J. Biomech. 2006, 39, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Seel, T.; Raisch, J.; Schauer, T. IMU-based joint angle measurement for gait analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, R.S.; Perkins, N.C. Inertial sensor based method for identifying spherical joint center of rotation. J. Biomech. 2013, 46, 2546–2549. [Google Scholar] [CrossRef] [PubMed]
- Frick, E.; Rahmatalla, S. Joint Center Estimation Using Single-Frame Optimization: Part 1: Numerical Simulation. Sensors 2018, 18, 1089. [Google Scholar] [CrossRef] [PubMed]
- Olsson, F.; Halvorsen, K. Experimental evaluation of joint position estimation using inertial sensors. In Proceedings of the 2017 20th International Conference on Information Fusion, Xi’an, China, 10–13 July 2017; pp. 1–8. [Google Scholar] [CrossRef]
- Camomilla, V.; Cereatti, A.; Chèze, L.; Cappozzo, A. A hip joint kinematic driven model for the generation of realistic thigh soft tissue artefacts. J. Biomech. 2013, 46, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, R.M.; Taylor, W.R.; Duda, G.N.; Heller, M.O. A survey of formal methods for determining functional joint axes. J. Biomech. 2007, 40, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Seel, T.; Schauer, T.; Raisch, J. Joint Axis and Position Estimation from Inertial Measurement Data by Exploiting Kinematic Constraints. In Proceedings of the IEEE International Conference on Control Applications (CCA), Dubrovnik, Croatia, 3–5 October 2012. [Google Scholar]
- Müller, P.; Bégin, M.A.; Schauer, T.; Seel, T. Alignment-free, self-calibrating elbow angles measurement using inertial sensors. IEEE J. Biomed. Health Inform. 2017, 21, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.M.; Atkins, P.R.; Kutschke, M.J.; Goebel, J.M.; Foreman, K.B.; Anderson, A.E. Soft tissue artifact causes significant errors in the calculation of joint angles and range of motion at the hip. Gait Posture 2017, 55, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Jolles, B.M.; Theumann, N.; Aminian, K. Soft tissue artifact distribution on lower limbs during treadmill gait: Influence of skin markers’ location on cluster design. J. Biomech. 2015, 48, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Naaim, A.; Moissenet, F.; Duprey, S.; Begon, M.; Chèze, L. Effect of various upper limb multibody models on soft tissue artefact correction: A case study. J. Biomech. 2017, 62, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.S.; Damsgaard, M.; Rasmussen, J.; Ramsey, D.K.; Benoit, D.L. A linear soft tissue artefact model for human movement analysis: Proof of concept using in vivo data. Gait Posture 2012, 35, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.L.; Damsgaard, M.; Andersen, M.S. Surface marker cluster translation, rotation, scaling and deformation: Their contribution to soft tissue artefact and impact on knee joint kinematics. J. Biomech. 2015, 48, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Dumas, R.; Camomilla, V.; Bonci, T.; Chèze, L.; Cappozzo, A. What portion of the soft tissue artefact requires compensation when estimating joint kinematics? J. Biomech. Eng. 2015, 137, 064502. [Google Scholar] [CrossRef] [PubMed]
- Camomilla, V.; Bonci, T.; Dumas, R.; Chèze, L.; Cappozzo, A. A model of the soft tissue artefact rigid component. J. Biomech. 2015, 48, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Aissaoui, R.; Aminian, K.; Dumas, R. Assessment of the lower limb soft tissue artefact at marker-cluster level with a high-density marker set during walking. J. Biomech. 2017, 62, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kapron, A.L.; Aoki, S.K.; Peters, C.L.; Maas, S.A.; Bey, M.J.; Zauel, R.; Anderson, A.E. Accuracy and feasibility of dual fluoroscopy and model-based tracking to quantify in vivo hip kinematics during clinical exams. J. Appl. Biomech. 2014, 30, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Motion Analysis. Cortex Version 1.1 User’s Manual; Motion Analysis Corporation: Santa Rosa, CA, USA, 2008. [Google Scholar]
- 80/20 Inc. The Industrial Erector. 2017. Available online: https://www.8020.net/ (accessed on 14 May 2018).
- Kainz, H.; Carty, C.P.; Modenese, L.; Boyd, R.N.; Lloyd, D.G. Estimation of the hip joint centre in human motion analysis: A systematic review. Clin. Biomech. 2015, 30, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, G.; Dijkstra, F.; Slycke, P. Xsens MTw: Miniature Wireless Inertial Motion Tracker for Highly Accurate 3D Kinematic Applications; Xsens Technologies: Enschede, The Netherlands, 2013. [Google Scholar]
- Chao, E.Y.; Morrey, B.F. Three-dimensional rotation of the elbow. J. Biomech. 1978, 11, 57–71. [Google Scholar] [CrossRef]
- MathWorks, Inc. MATLAB and Signal Processing Toolbox Release 2016a (R2016a); The MathWorks, Inc.: Natick, MA, USA, 2016. [Google Scholar]
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics (ICORR), Zurich, Switzerland, 29 June–1 July 2011; pp. 1–7. [Google Scholar]
- Gamage, S.S.; Lasenby, J. New least squares solutions for estimating the average centre of rotation and the axis of rotation. J. Biomech. 2002, 35, 87–93. [Google Scholar] [CrossRef]
- MathWorks, Inc. MATLAB and Optimization Toolbox Release 2016a (R2016a); The MathWorks, Inc.: Natick, MA, USA, 2016. [Google Scholar]
- Winter, D.A.; Sidwall, H.G.; Hobson, D.A. Measurement and reduction of noise in kinematics of locomotion. J. Biomech. 1974, 7, 157–159. [Google Scholar] [CrossRef]
- Hicks, J.L.; Richards, J.G. Clinical applicability of using spherical fitting to find hip joint centers. Gait Posture 2005, 22, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Baker, R.; Morris, M.E.; Sangeux, M. A comparison of hip joint centre localisation techniques with 3-DUS for clinical gait analysis in children with cerebral palsy. Gait Posture 2012, 36, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Sangeux, M.; Peters, A.; Baker, R. Hip joint centre localization: Evaluation on normal subjects in the context of gait analysis. Gait Posture 2011, 34, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Sangeux, M.; Peters, A.; Baker, R. Corrigendum to “Hip joint centre localization: Evaluation on normal subjects in the context of gait analysis”. Gait Posture 2013, 34, 552–554. [Google Scholar] [CrossRef]
- Sangeux, M.; Pillet, H.; Skalli, W. Which method of hip joint centre localization should be used in gait analysis? Gait Posture 2014, 40, 20–25. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, C.A.; Fowler, J.; Chase, S.; Steeves, K.; Landry, J.; Mohamed, A. Evaluation of anatomical and functional hip joint center methods: The effects of activity type, gender, and proximal reference segment. J. Biomech. Eng. 2016, 138, 011004. [Google Scholar] [CrossRef] [PubMed]
- MacWilliams, B.A. A comparison of four functional methods to determine centers and axes of rotations. Gait Posture 2008, 28, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Grimpampi, E.; Camomilla, V.; Cereatti, A.; de Leva, P.; Cappozzo, A. Metrics for describing soft-tissue artefact and its effect on pose, size, and shape of marker clusters. IEEE Trans. Biomed. Eng. 2014, 61, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Xsens Technologies. MTw User Manual; Xsens Technologies, B.V.: Enschede, The Netherlands, 2013. [Google Scholar]
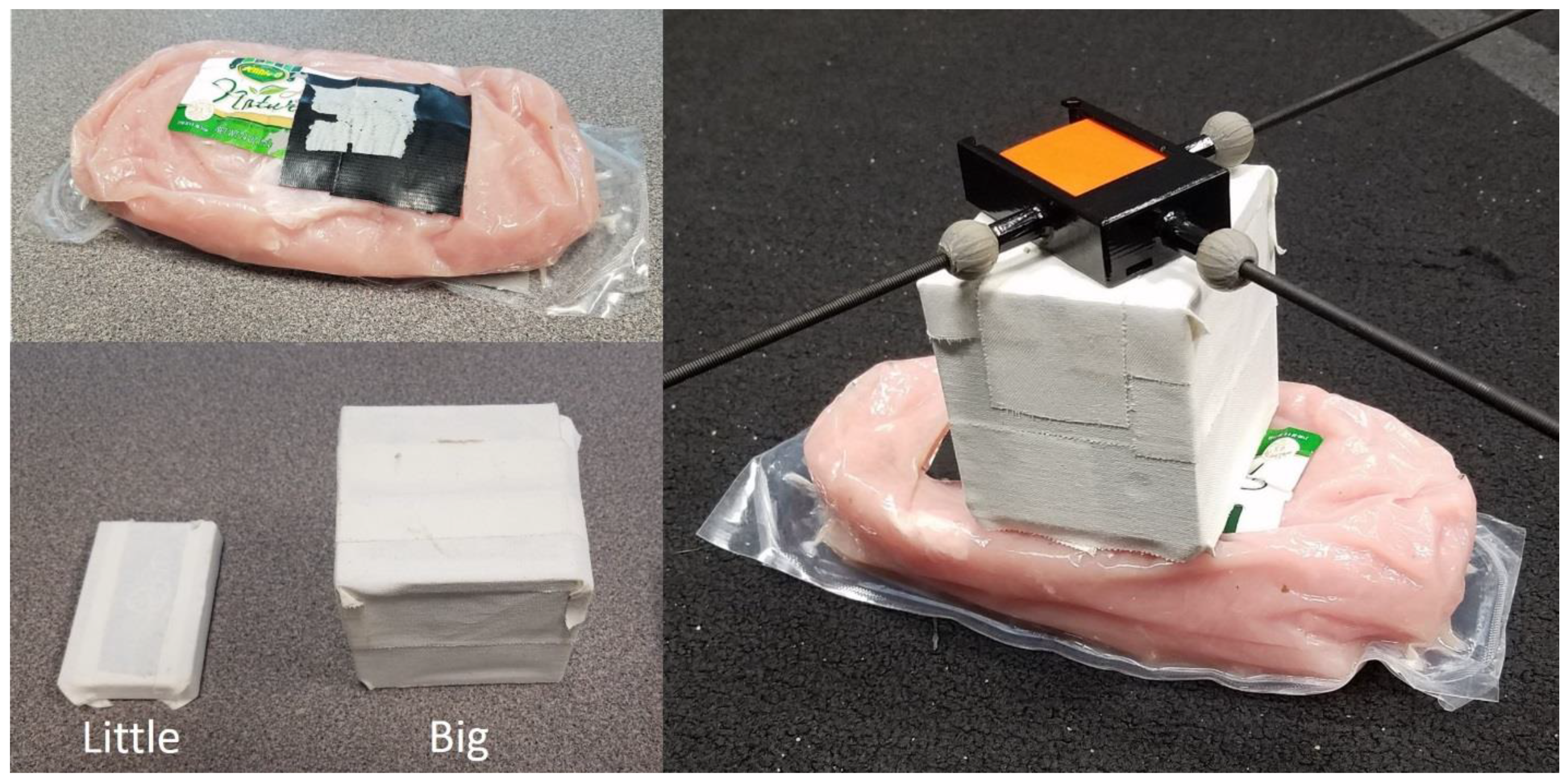
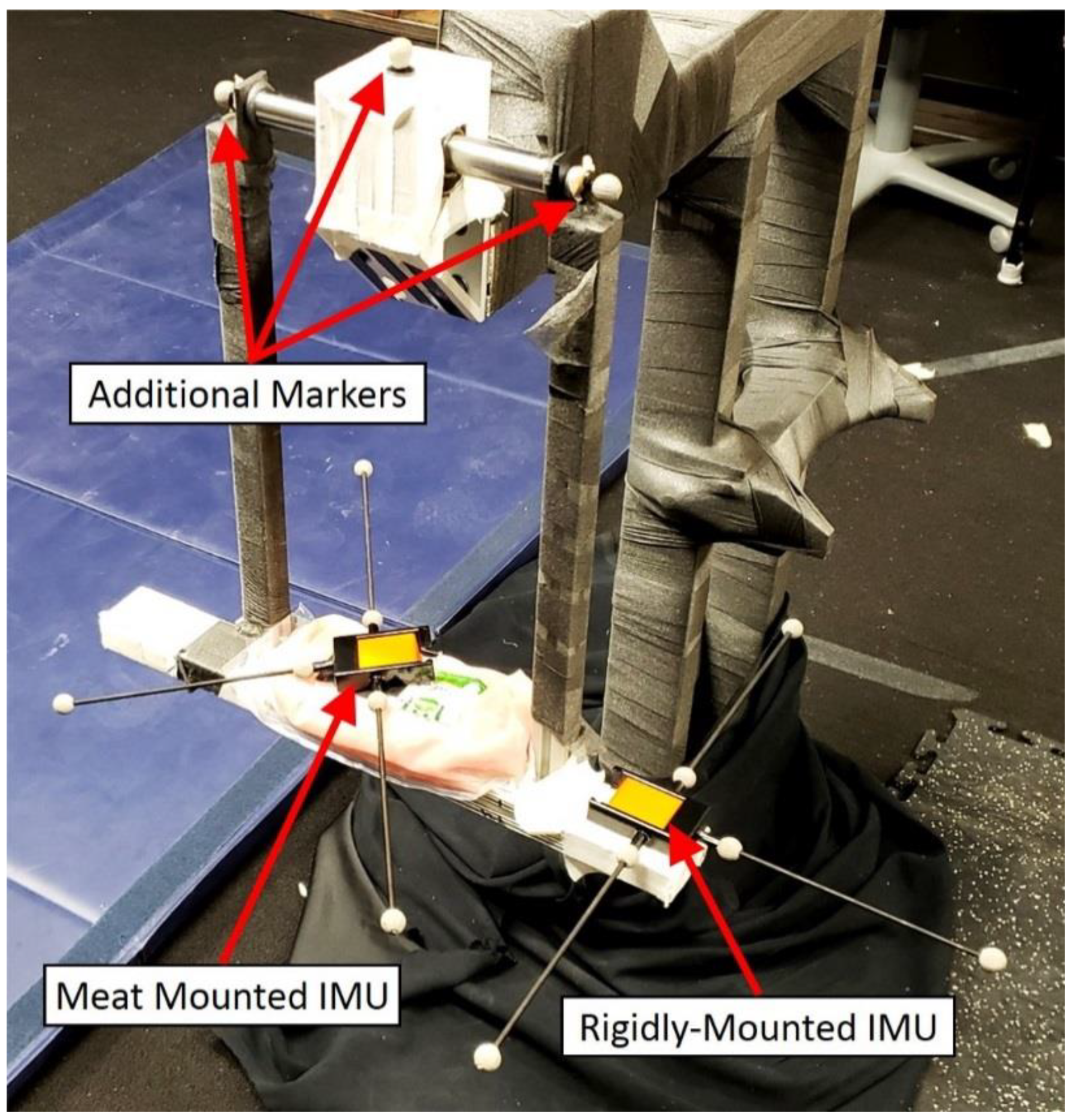
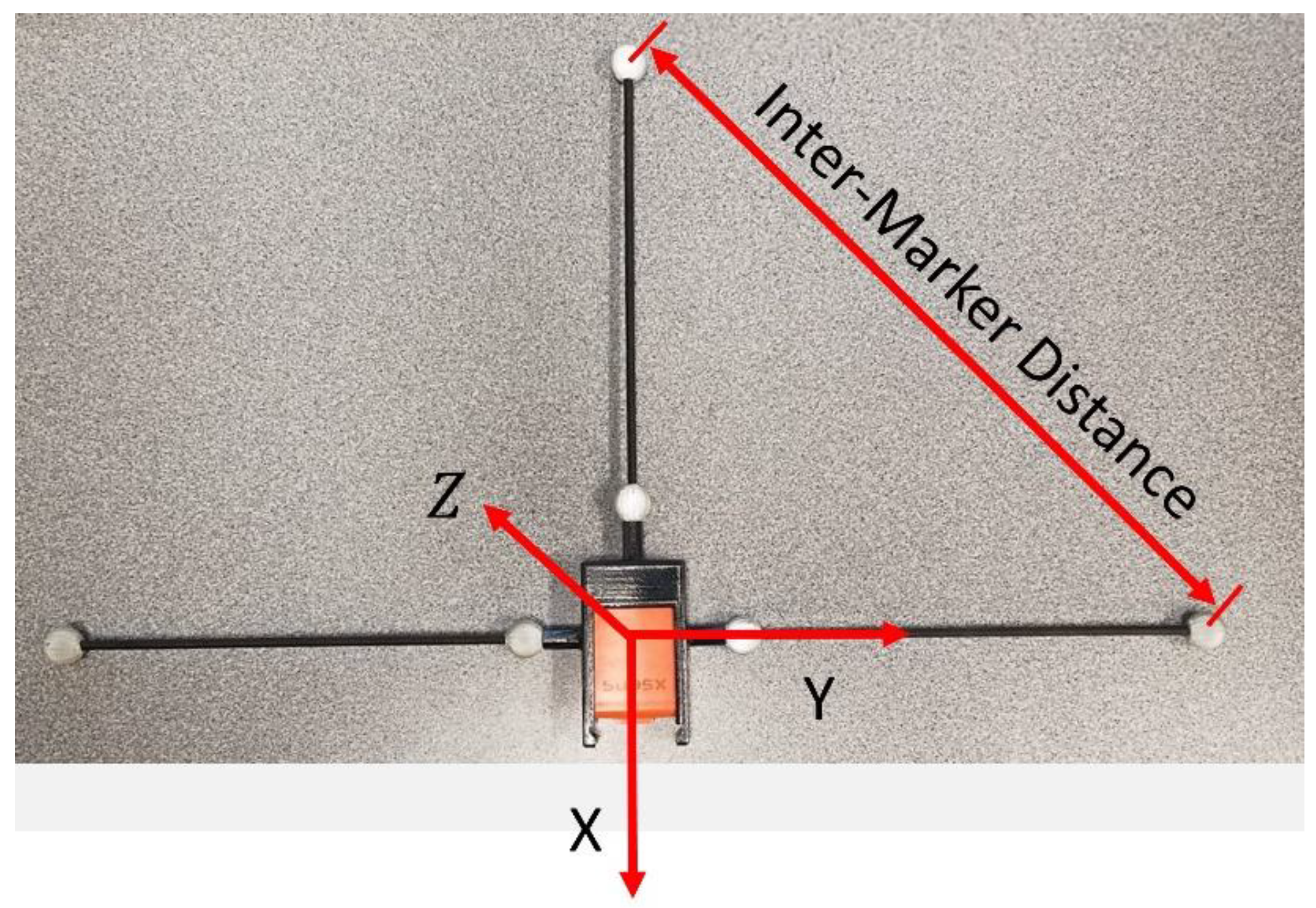


| Block | Mass (g) | Height (mm) | Width (mm) | Depth (mm) |
|---|---|---|---|---|
| Little | 136 | 13 | 50 | 75 |
| Big | 496 | 80 | 80 | 70 |
| Component RMSE (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Block | Time Period (s) | RMSE (mm) | |||||
| Little | 0–30 | 4.95 | 3.26 | 10.58 | 12.13 | 0.73 | 0.78 |
| 0–5 | 6.02 | 3.93 | 15.38 | 16.98 | 0.69 | 0.75 | |
| 10–15 | 3.58 | 2.40 | 9.56 | 10.49 | 0.89 | 0.89 | |
| 25–30 | 7.07 | 4.66 | 8.50 | 12.00 | 0.98 | 0.96 | |
| Big | 0–30 | 6.19 | 6.64 | 25.00 | 26.59 | 0.91 | 0.93 |
| 0–5 | 9.23 | 10.12 | 35.29 | 37.86 | 0.85 | 0.87 | |
| 10–15 | 5.94 | 6.11 | 27.28 | 28.58 | 0.86 | 0.86 | |
| 25–30 | 4.17 | 4.69 | 4.74 | 7.87 | 0.99 | 0.99 | |
| Relative Translation (mm) | Relative Rotation (°) | |||
|---|---|---|---|---|
| Little Block | Big Block | Little Block | Big Block | |
| Overall ROM | ||||
| X Component | 5.3 | 5.8 | 1.4 | 12.9 |
| Y Component | 5.3 | 25.7 | 1.7 | 7.9 |
| Z Component | 4.9 | 4.3 | 0.4 | 2.0 |
| Norm Component | 6.0 | 21.4 | 2.1 | 13.1 |
| Oscillatory ROM | ||||
| X Component | 4.5 | 4.4 | 1.4 | 6.0 |
| Y Component | 4.8 | 13.3 | 1.6 | 4.3 |
| Z Component | 4.2 | 3.9 | 0.4 | 0.9 |
| Norm Component | 4.4 | 10.8 | 2.0 | 6.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frick, E.; Rahmatalla, S. Joint Center Estimation Using Single-Frame Optimization: Part 2: Experimentation. Sensors 2018, 18, 2563. https://doi.org/10.3390/s18082563
Frick E, Rahmatalla S. Joint Center Estimation Using Single-Frame Optimization: Part 2: Experimentation. Sensors. 2018; 18(8):2563. https://doi.org/10.3390/s18082563
Chicago/Turabian StyleFrick, Eric, and Salam Rahmatalla. 2018. "Joint Center Estimation Using Single-Frame Optimization: Part 2: Experimentation" Sensors 18, no. 8: 2563. https://doi.org/10.3390/s18082563
APA StyleFrick, E., & Rahmatalla, S. (2018). Joint Center Estimation Using Single-Frame Optimization: Part 2: Experimentation. Sensors, 18(8), 2563. https://doi.org/10.3390/s18082563





