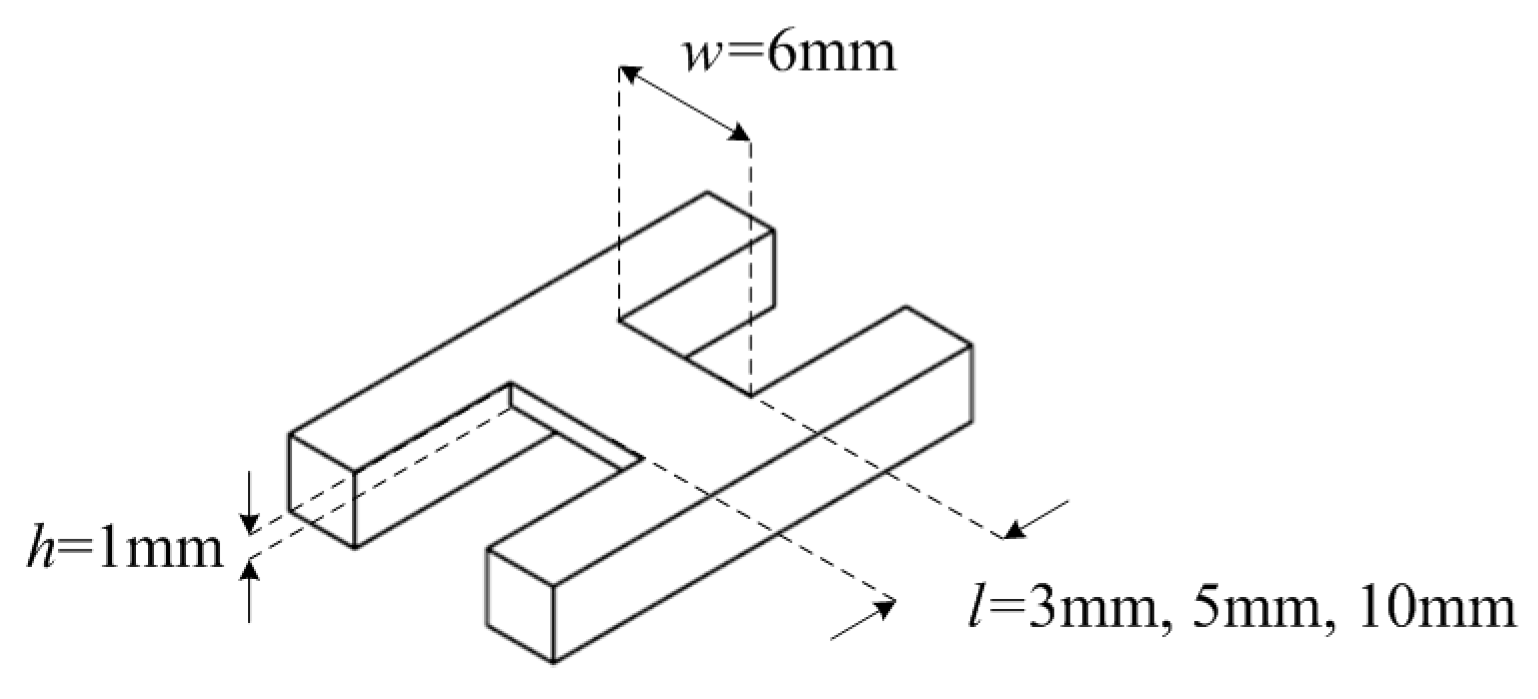
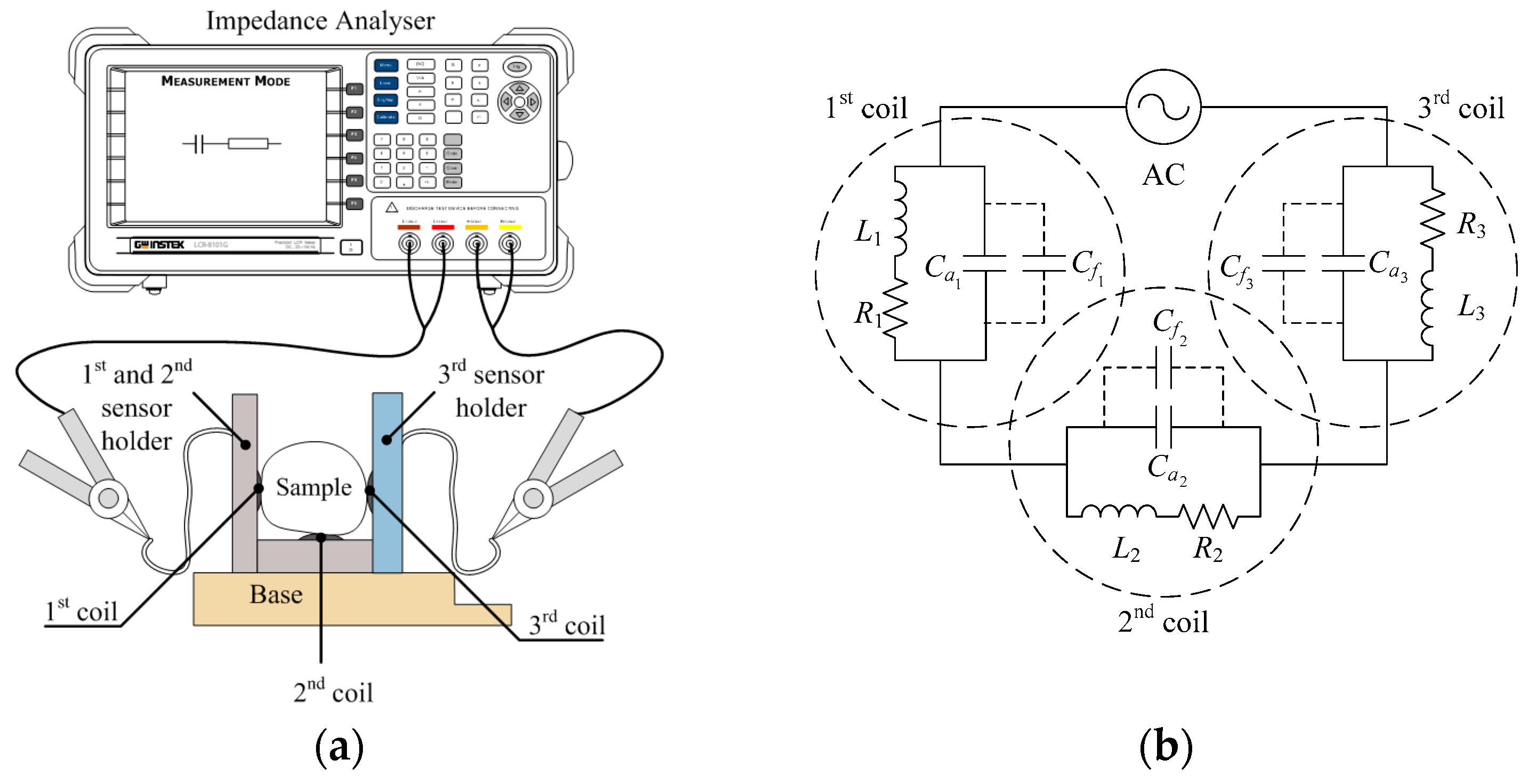
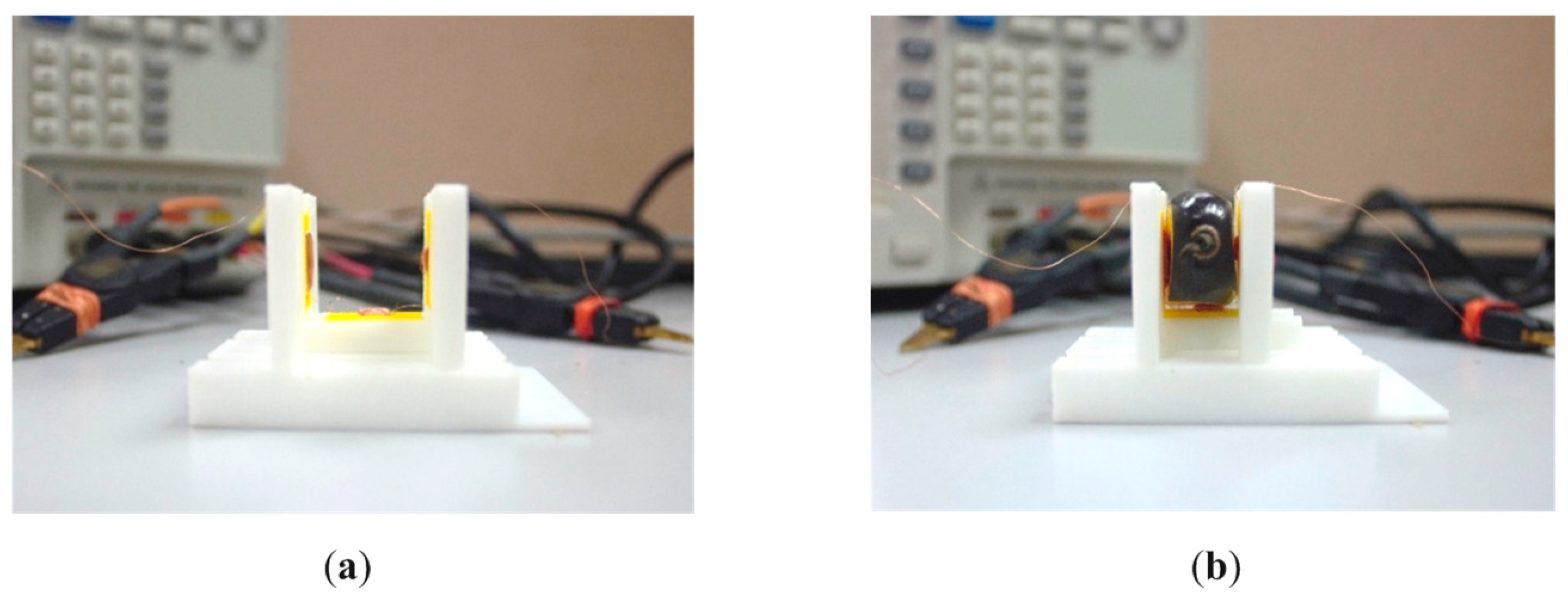
3.1. Self-Capacitance and Fruitlet Capacitance
The value of self-capacitance can be estimated using the general resonance frequency formula as follows:
where
(Hz) is the resonance frequency,
L (H) is the inductance, and
C (F) is the capacitance. There are two different resonances principles that are present in this research, the self-resonance frequency (SRF) and resonance frequency, which was obtained through the maximum peak in the inductance–frequency (
Ls–f) curve from the impedance analyser. Both of the resonances used the same formula as shown in Equation (1), but had a different value of the inductance,
L, and resonance frequency,
.
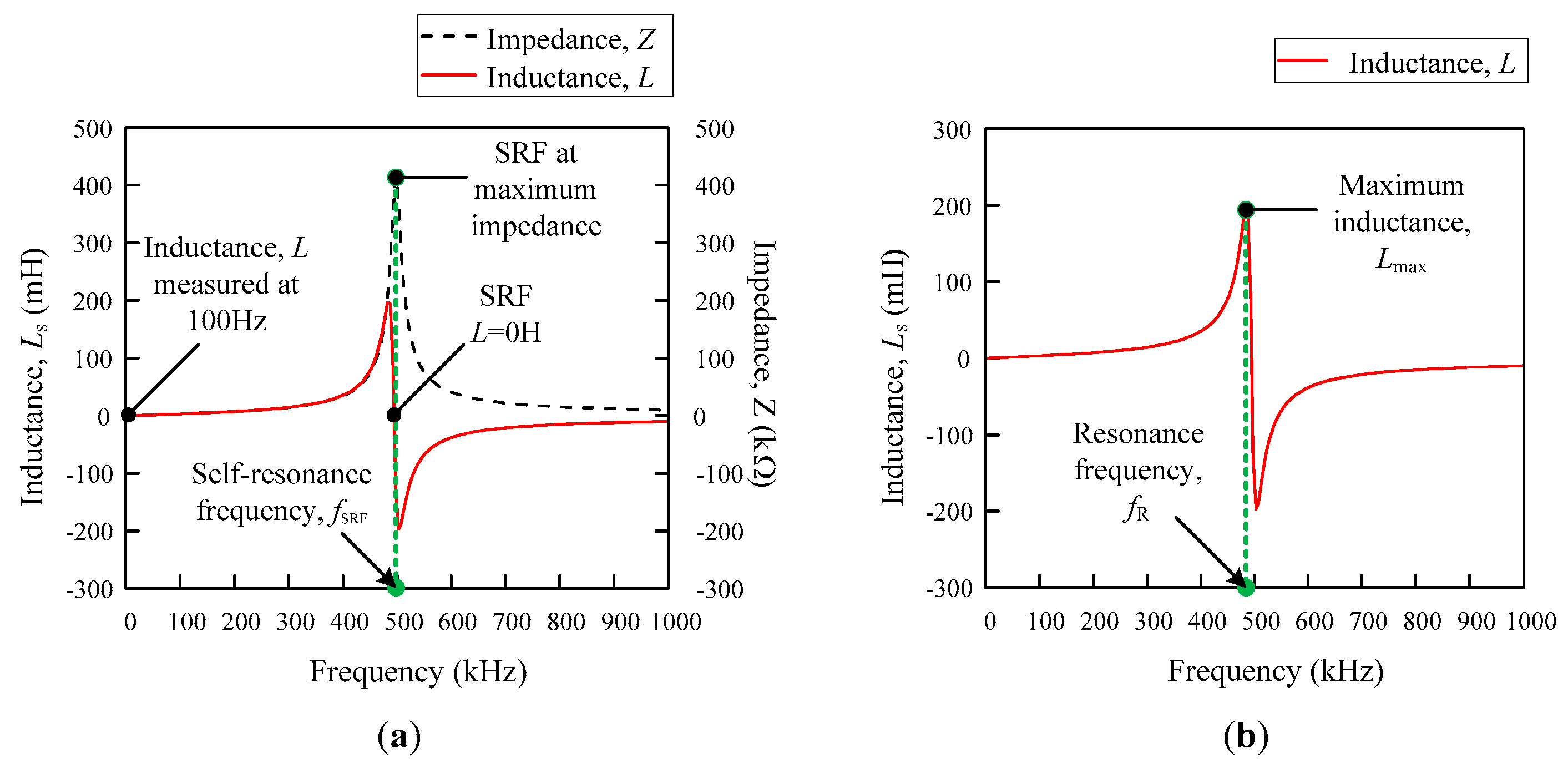
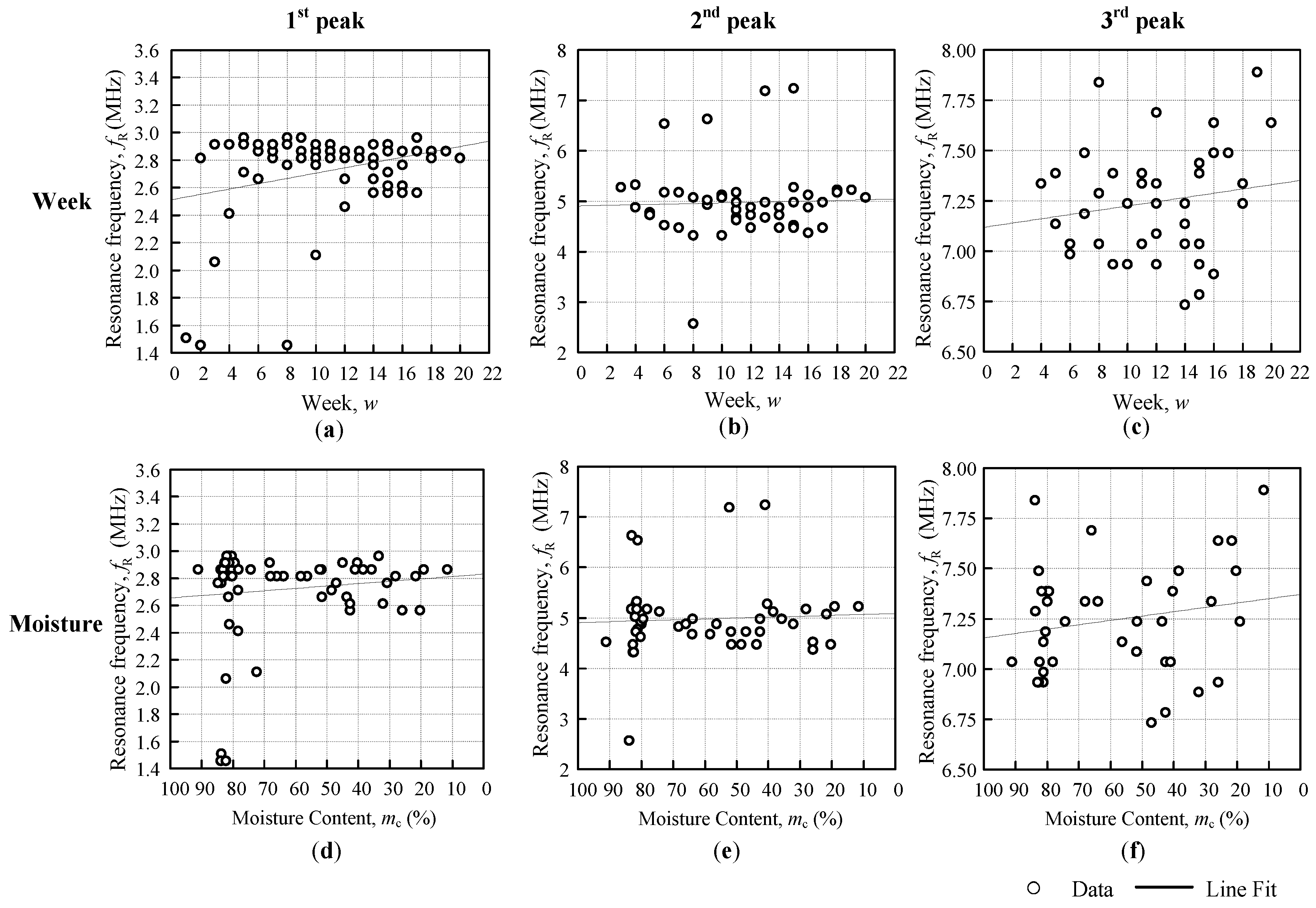
Figure 5a,b illustrate the differences between them. The SRF is the resonance frequency that occurs at
= 0 H and the standard value of the inductance that is used is measured at 100 Hz [
24]. On the other hand, the resonance frequency that is used for the analysis in this study is referred to as the maximum inductance peak of the
Ls–f curve, as shown in
Figure 4b. From this information, the self-capacitance can be estimated from both methods, but the approach that has been used throughout this research is based on the information that was gained from
Figure 5b.
The self-capacitance and fruitlet capacitance calculation are rather straightforward from Equation (1), and the parameter taken is, as shown in
Figure 4b, and rearranged as follows:
where the
(F) is the calculated by the capacitance at the resonance, by substituting the maximum peak inductance,
(H) and the resonance frequency
(Hz) at
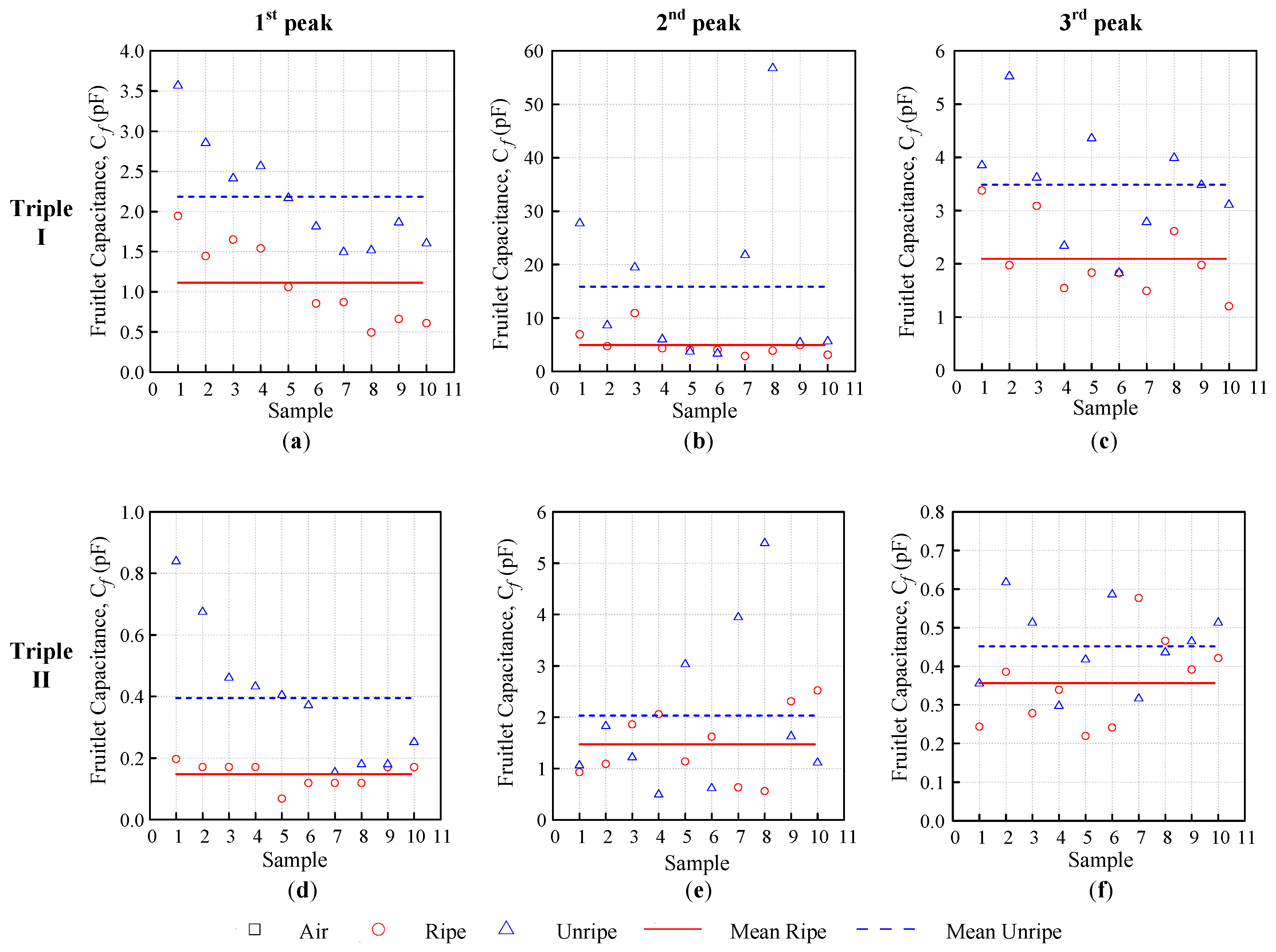
. The fruitlet capacitance
is determined from equation below, as follows:
where
(F) is the fruitlet capacitance,
(F) is the total self-capacitance that is obtained through Equation (2), and
(F) is the capacitance that is calculated using the measured resonance frequency of air, with no sample, it was considered measuring the air literally. The capacitance at the peak resonance frequency
is introduced especially so as to avoid confusion with the total self-capacitance that can be obtained at any given frequency using Equation (1).
3.2. Comparison Analysis Method
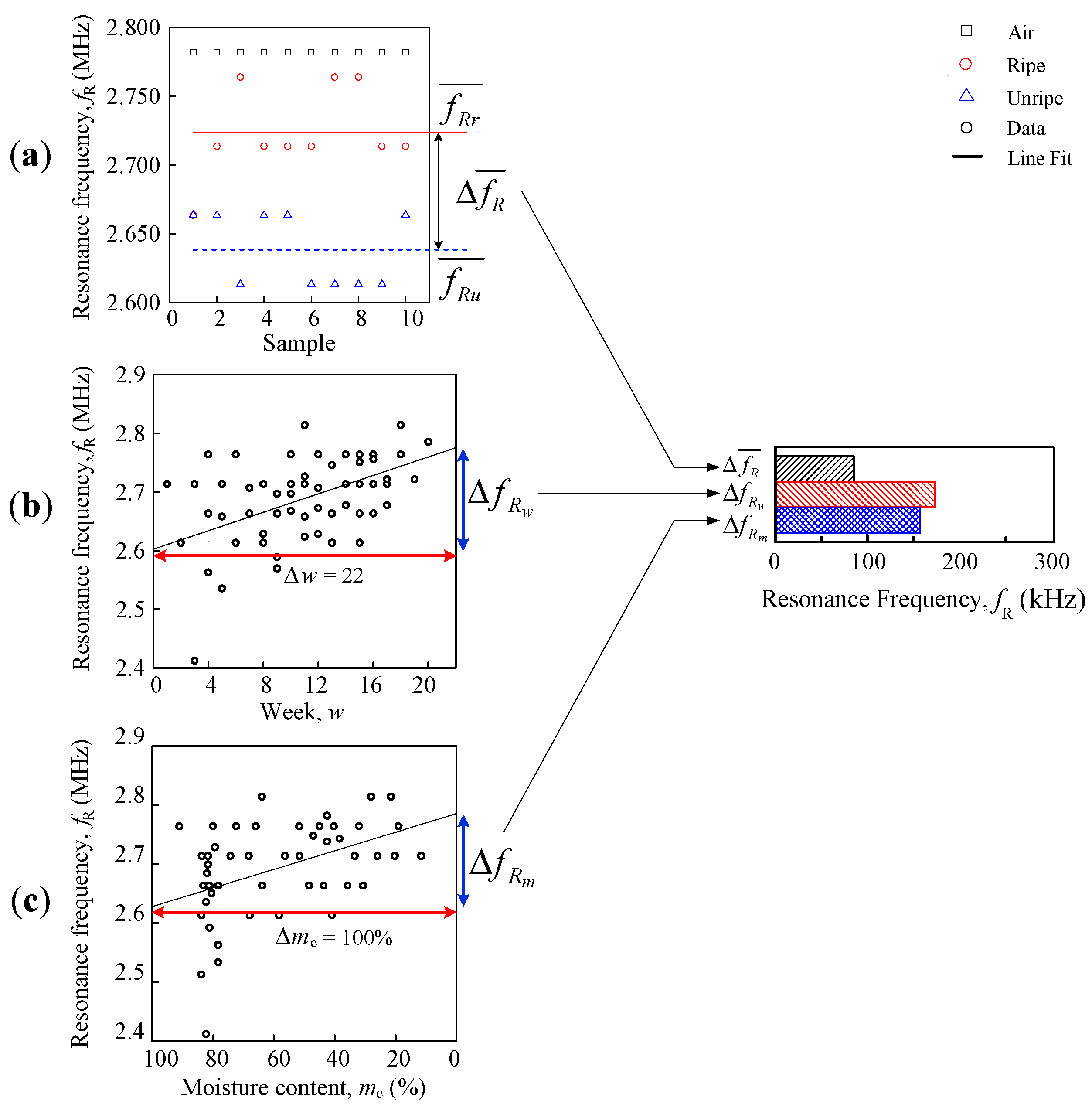
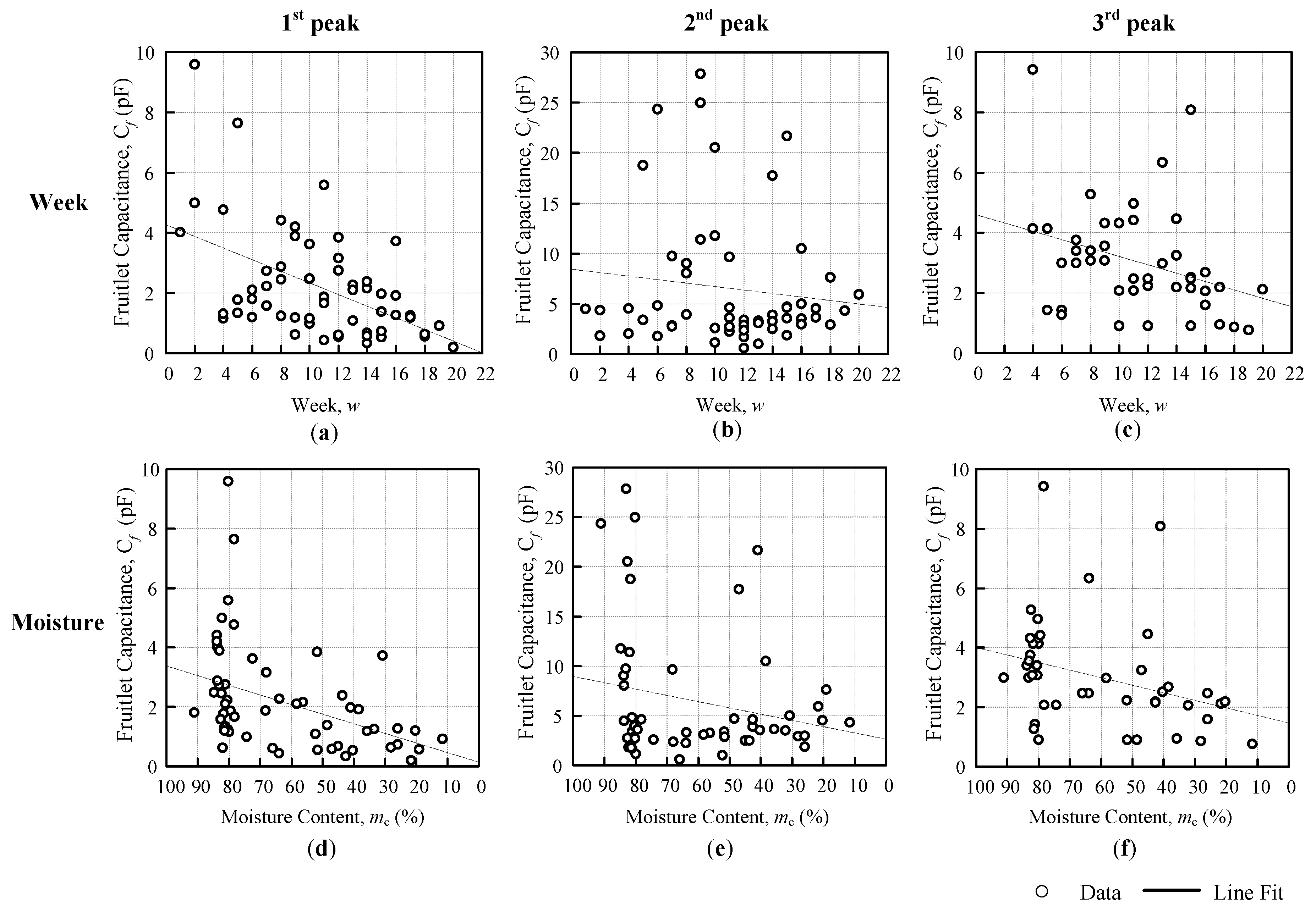
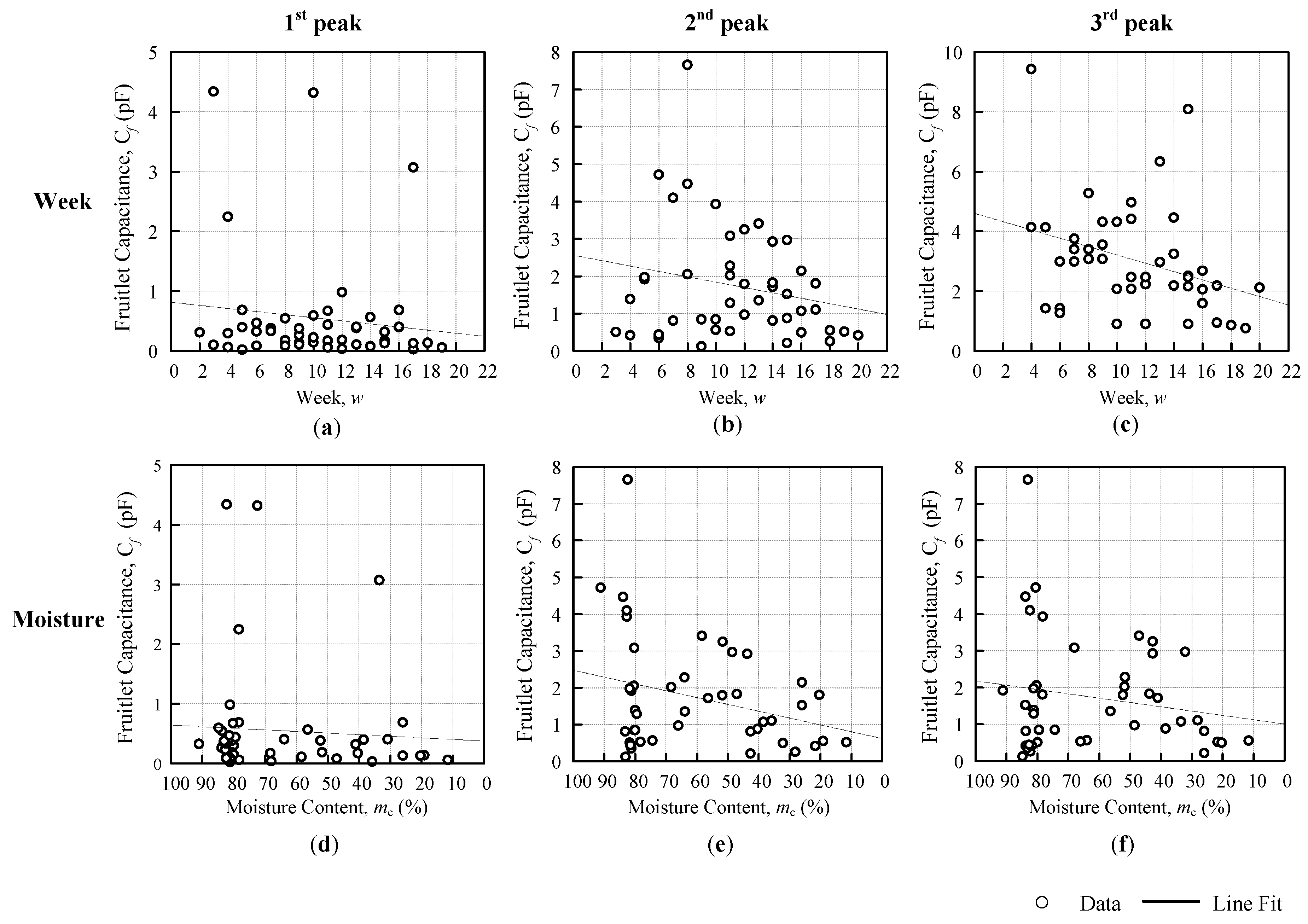
The comparison analyses are divided into three, ripe-unripe direct comparison, comparison against a week, and moisture content, as shown in
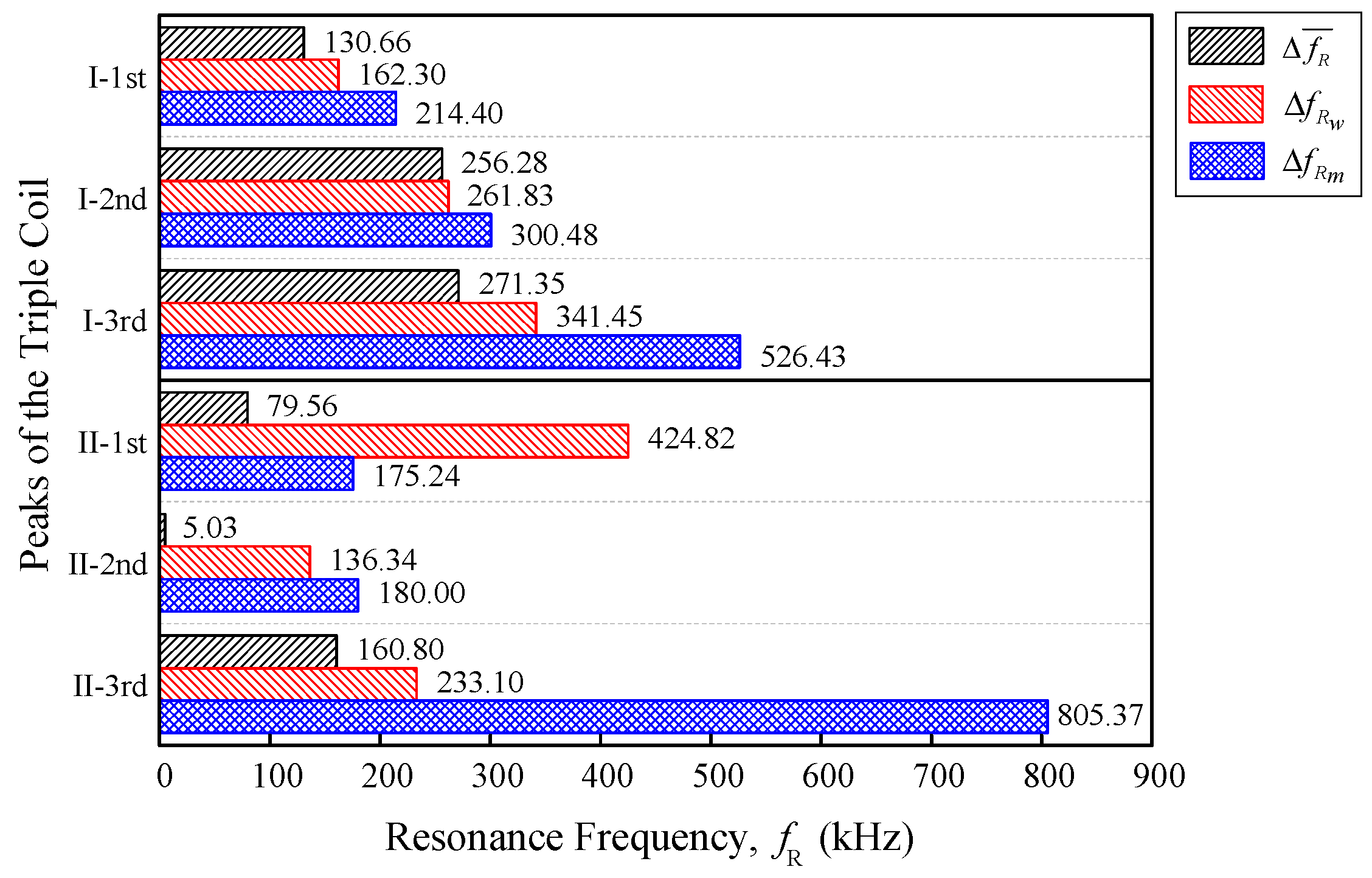
Figure 6. The differences are further summarized into a horizontal bar graph. There were two types of data sets that were used for the analysis in this paper, resonance frequency and fruitlet capacitance.
The first evaluation used is direct ripe-unripe comparison analysis as illustrated in
Figure 6a where the mean difference for peak resonance frequency
(Hz) and fruitlet capacitance
(F) are calculated using formula:
where
(Hz) is mean ripe resonance frequency,
(Hz) is the mean unripe resonance frequency,
(F) is the mean ripe fruitlet capacitance, and
(F) is the mean unripe fruitlet capacitance.
The approximation regression line is fit for a week, and the moisture content evaluation for both the coil resonance frequency and the fruitlet capacitance are shown in
Figure 6b,c, respectively, which follows the general line equation below, as follows:
where
y is the
y-axis component, such as the resonance frequency or fruitlet capacitance; and
x is the
x-axis component, either the weeks or the moisture content according to the graph. The
value is the sensitivity of the coil sensor.
For the second evaluation analysis against a week, the common notation for both the resonance frequency
(Hz) and the fruitlet capacitance
(F) data setsis as follows:
w is the number of weeks and
is fixed at 22 weeks. Equation (6) has been further defined for the resonance frequency and fruitlet capacitance against the week, as follows:
where
(Hz) is the frequency at
w = 0 on the resonance frequency against the weeks graph,
(Hz/week) is the sensitivity of the coil sensor resonance frequency with respect to the week, and
(Hz) is the resonance frequency of the weeks difference.
where
(F) is the fruitlet capacitance at
w = 0 on the fruitlet capacitance against the weeks graph,
(F/week) is the sensitivity of the coil sensor of the fruitlet capacitance with respect to the week, and
(F) is the fruitlet capacitance of the weeks difference.
Furthermore, for the third evaluation analysis against the moisture content, the common notations that were used for both sets of data are as follows:
(%) is the moisture content in percentage and
is fixed at 100%. Hence, the equation for the resonance frequency
(Hz) and fruitlet capacitance
(F) against the moisture content are as follows:
where
(Hz) is the resonance frequency at
= 0% on the resonance frequency against the moisture graph,
(Hz/%) is the sensitivity of the coil sensor with respect to moisture content,
(Hz) is the resonance frequency moisture content difference.
where
(F) is the fruitlet capacitance at
= 0% on the fruitlet capacitance against the moisture graph,
(F/%) is the sensitivity of the coil sensor with respect to moisture content, and
(Hz) is the fruitlet capacitance moisture content difference. Note that the resonance frequency against the moisture content begins with 100%, in
Figure 6c, with the purpose of following the time vector (week) pattern so as to observe its trend and therefore, its gradient value is actually negative when compared to the weeks graph in
Figure 6b.
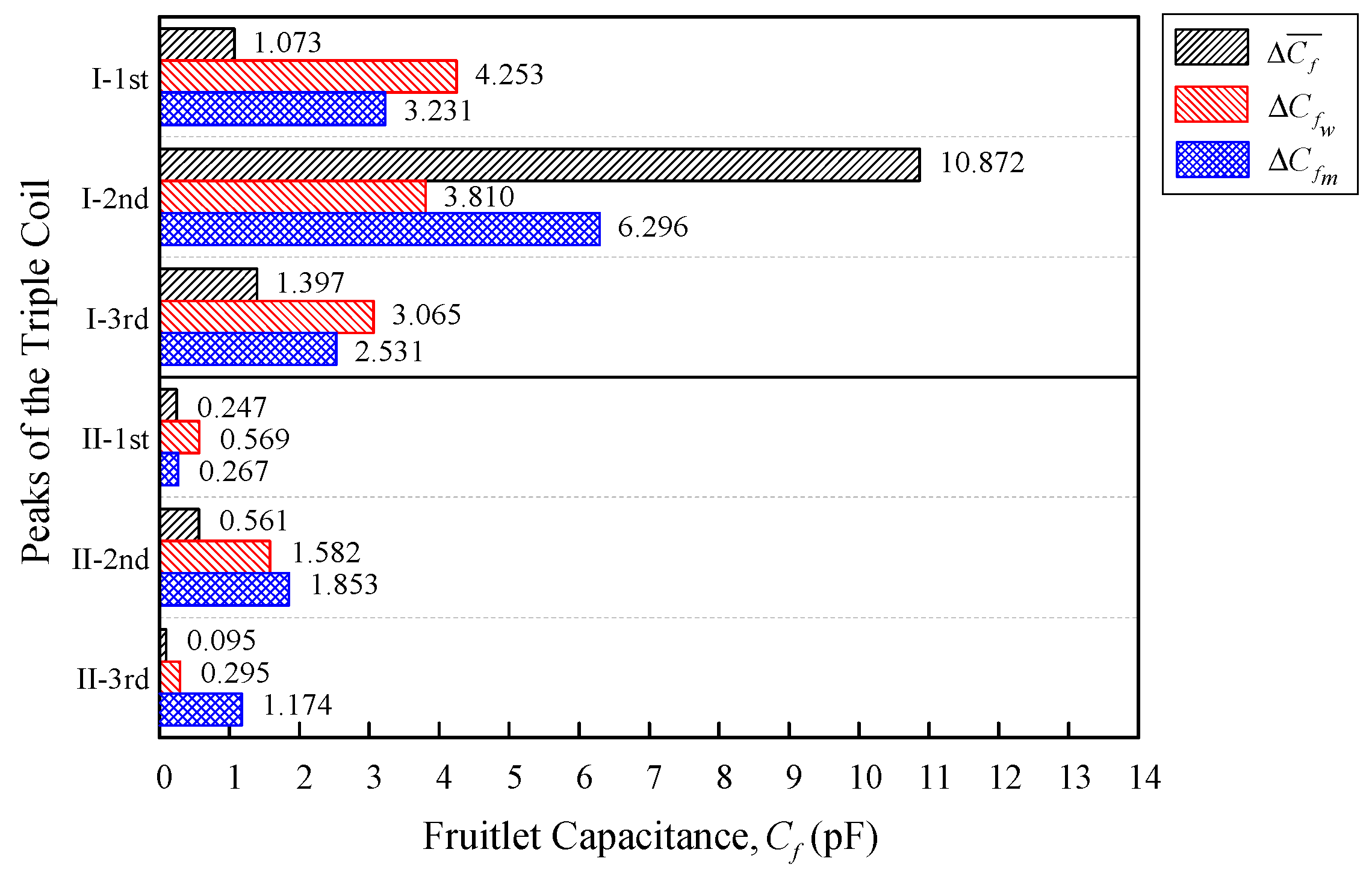
Further analysis was conducted on the data in order to compare the ripe–unripe, week, and moisture differences. A simple statistical method was introduced to observe the variability and stability of the data. In order for the coil configuration to be selected, the coil needs to have a small variability as well as a high output sensitivity for the best performance.
Differences in the mean
and standard deviation
were introduced as well as the coefficient of variation
, to compare both of the triple flat-type air coil performances. The coefficient of the variation
is a standardized measure of dispersion, which is defined as the ratio of the standard deviation to the mean.
is widely used to express the precision and repeatability of the data [
25].
where
σ is the standard deviation and
is the average of the differences for the resonance frequency and fruitlet capacitance, as shown in Equations (20) and (21), respectively.
where
(Hz) is the resonance frequency differences mean, which consist of
,
and
from Equations (4), (9), and (15), respectively. Whereas
(F) is the fruitlet capacitance differences mean that consist of
,
, and
from Equations (5), (12), and (18), respectively. For a comparison between the data sets with different means, the coefficient of the variation is preferred instead of the standard deviation. Since the value of
is a dimensionless number independent of the unit in which the measurement is calculated, the sensor needed to be designed so that the coefficient of the variation
was close to zero, where the data yields a constant absolute error over the operational range.