Coumarin Probe for Selective Detection of Fluoride Ions in Aqueous Solution and Its Bioimaging in Live Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
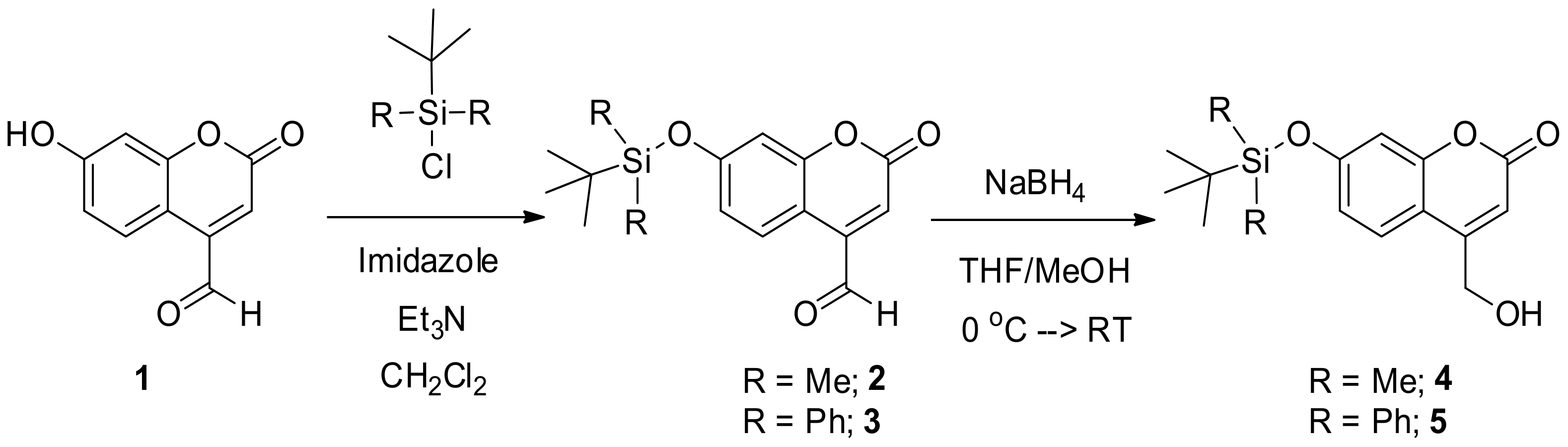
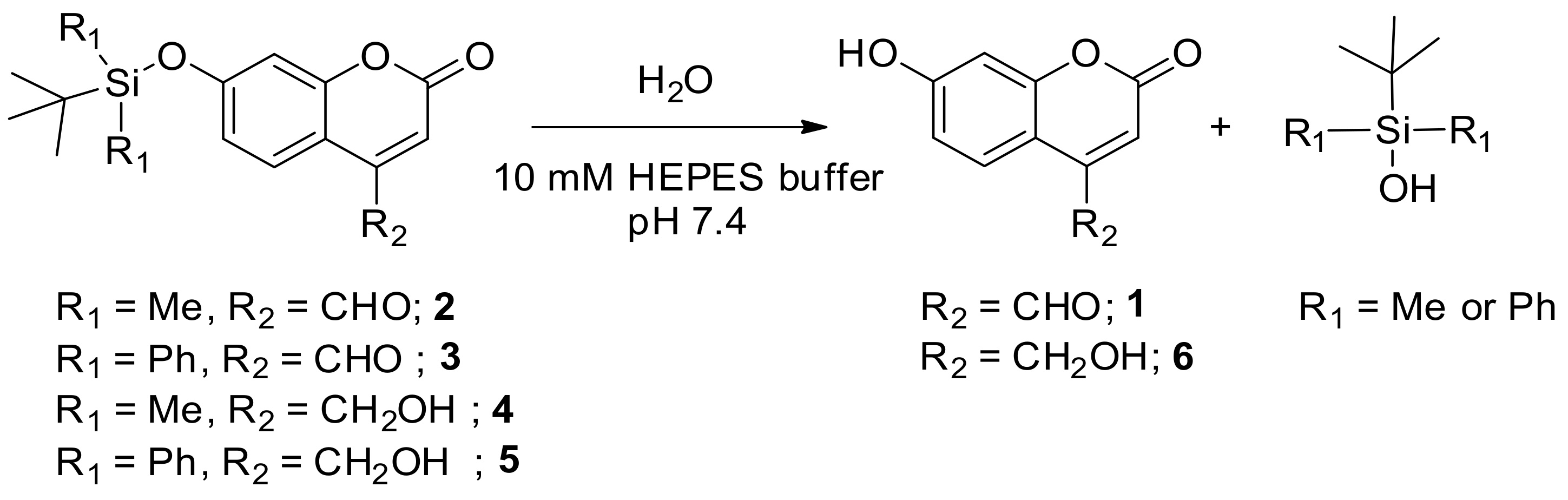
2.2. Synthesis of 7-((Tert-butyldimethylsilyl)oxy)-2-oxo-2H-chromene-4-carbaldehyde (2)
2.3. Synthesis of 7-((Tert-butyldiphenylsilyl)oxy)-2-oxo-2H-chromene-4-carbaldehyde (3)
2.4. Synthesis of 7-((Tert-butyldimethylsilyl)oxy)-4-(hydroxyl methyl)-2H-chromen-2-one (4)
2.5. Synthesis of 7-((Tert-butyldiphenylsilyl)oxy)-4-(hydroxymethyl)-2H-chromene-2-one (5)
2.6. HPLC Analysis
2.7. General Details for UV-Vis and Fluorescence Measurement
2.8. Hydrolytic Stability Study
2.9. Cell Imaging Experiments
3. Results and Discussion
3.1. The Synthesis of Silyl-Capped Coumarins
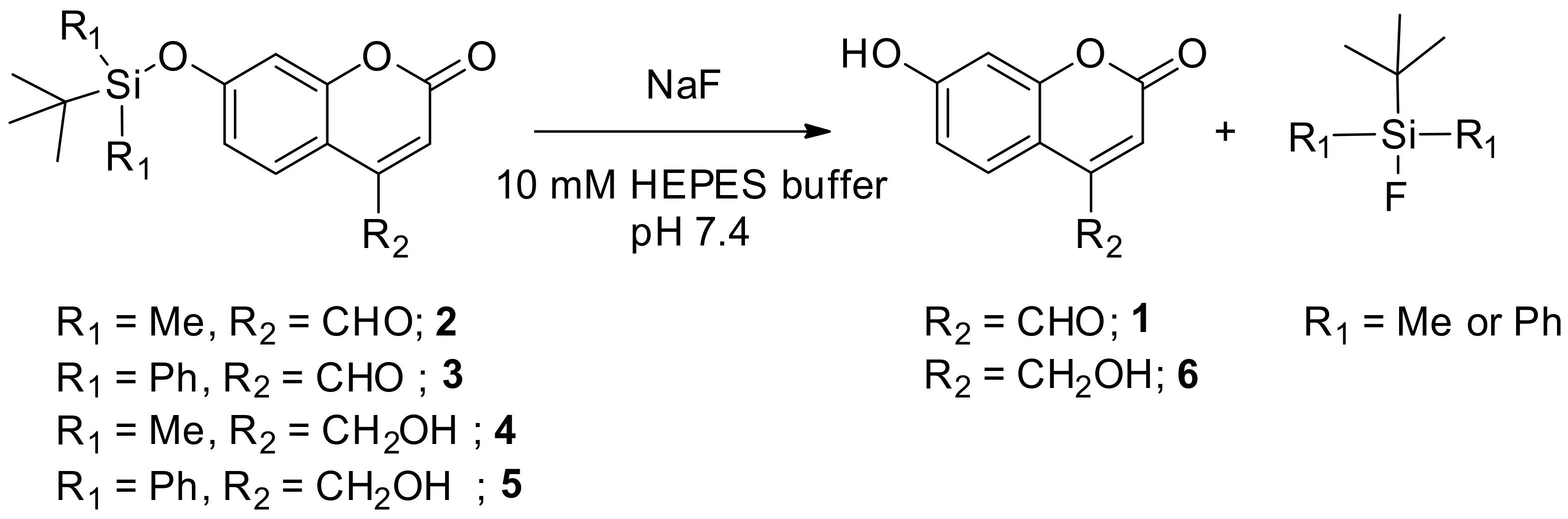
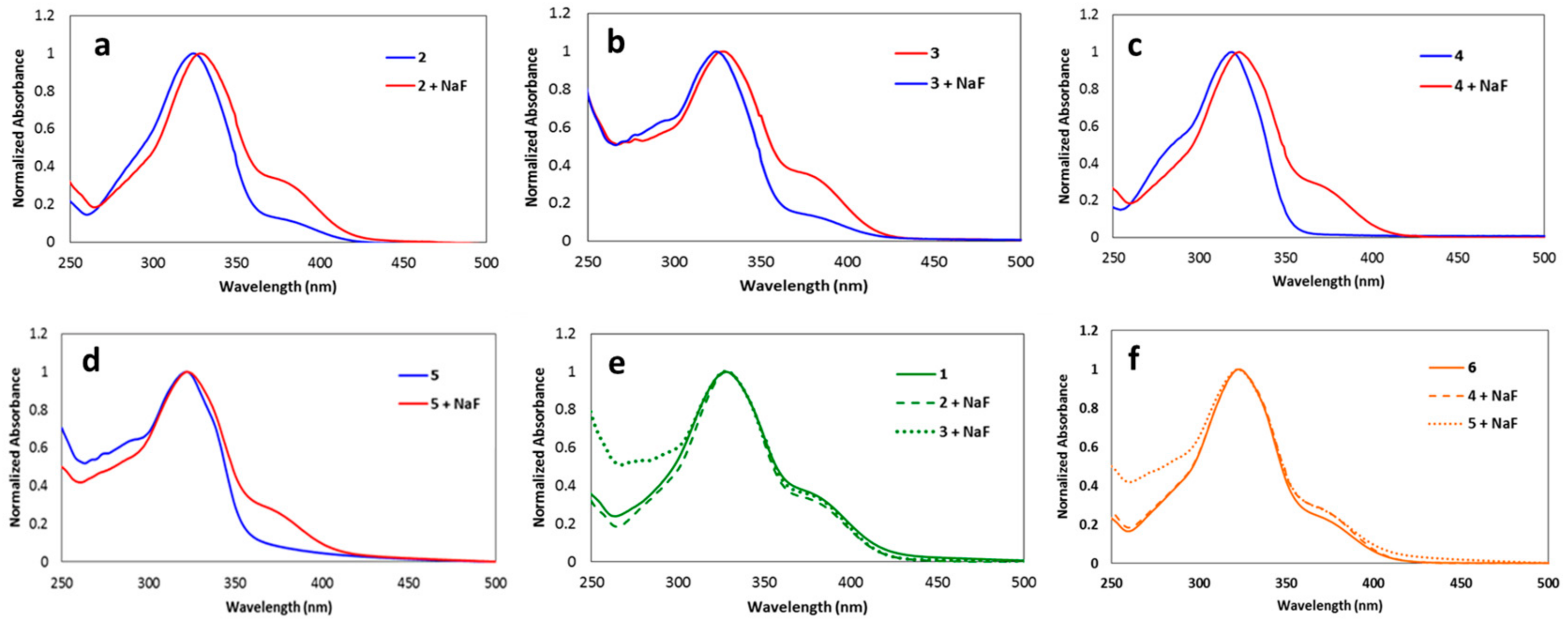
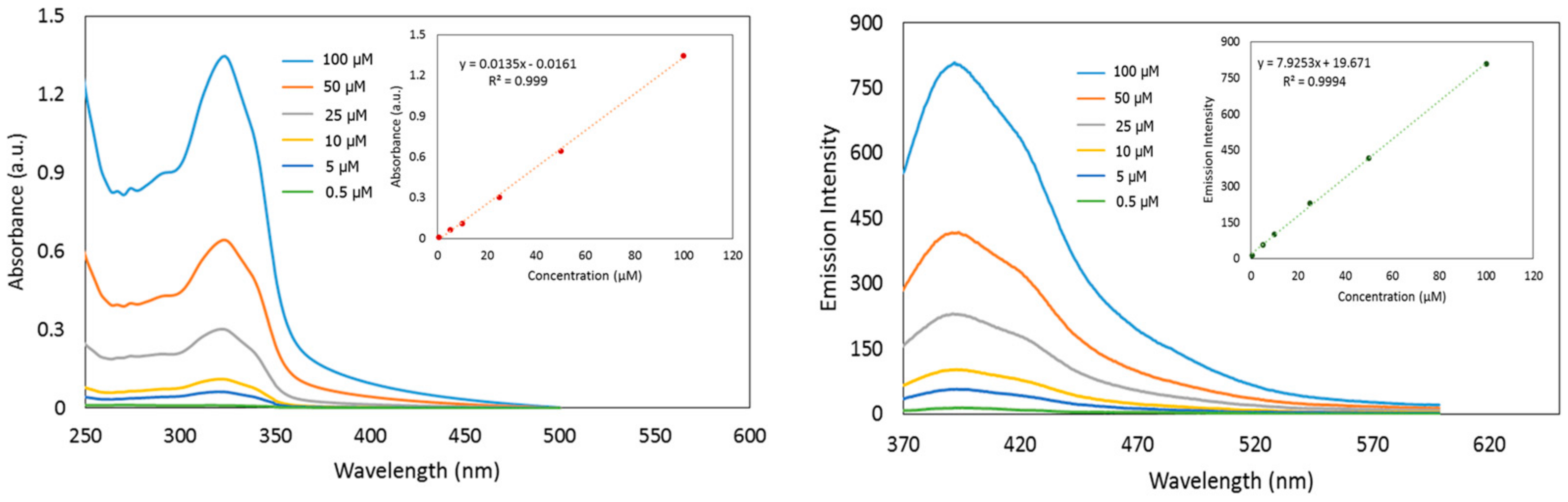
3.2. UV-Visible Spectra Responses of the Synthesized Coumarins toward Fluoride
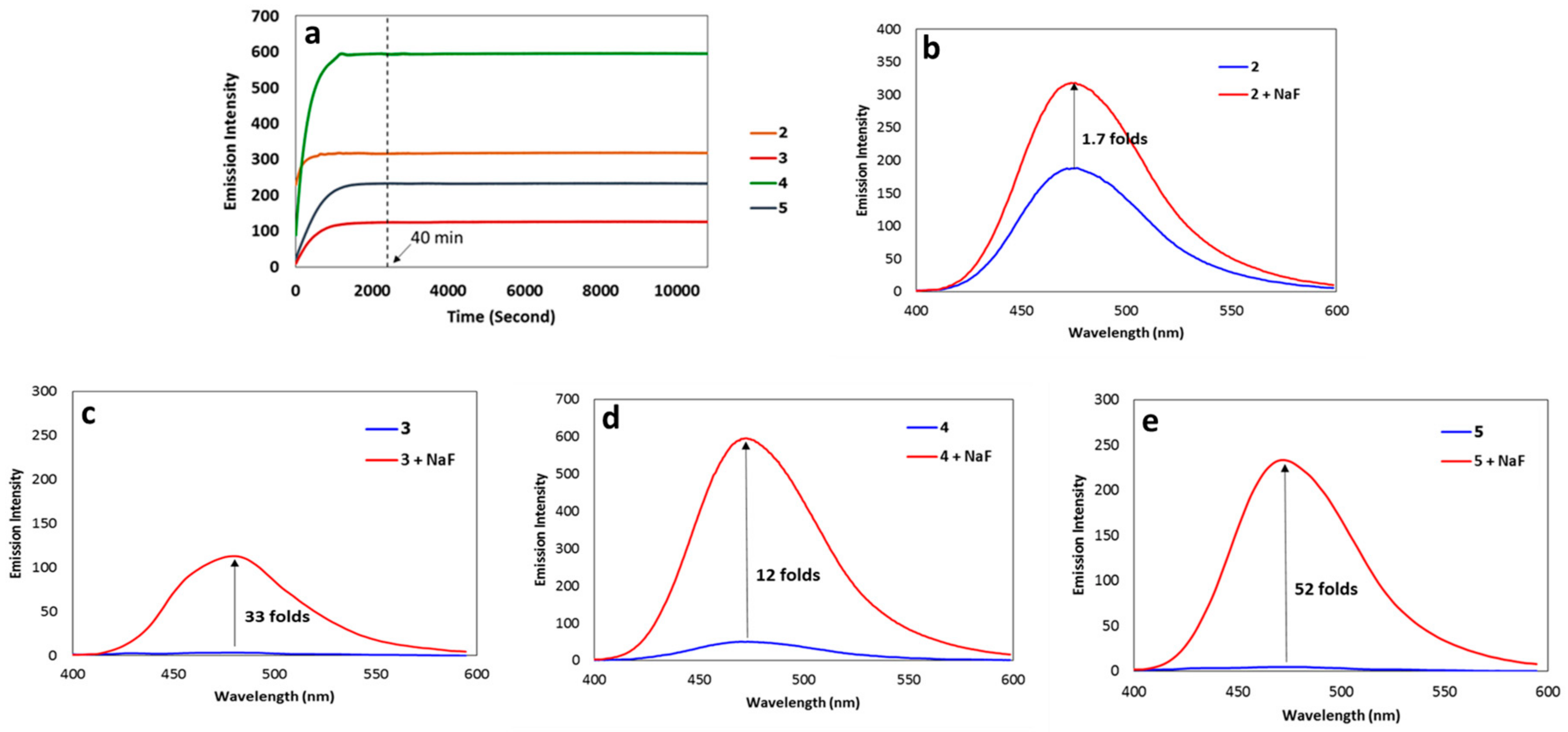
3.3. Fluorescence Response of the Synthesized Coumarins toward Fluoride
3.4. Hydrolytic Stabilities of the Silyl-Capped Coumarins
3.5. Solubility, Selectivity, and Interference Studies of Probe 5
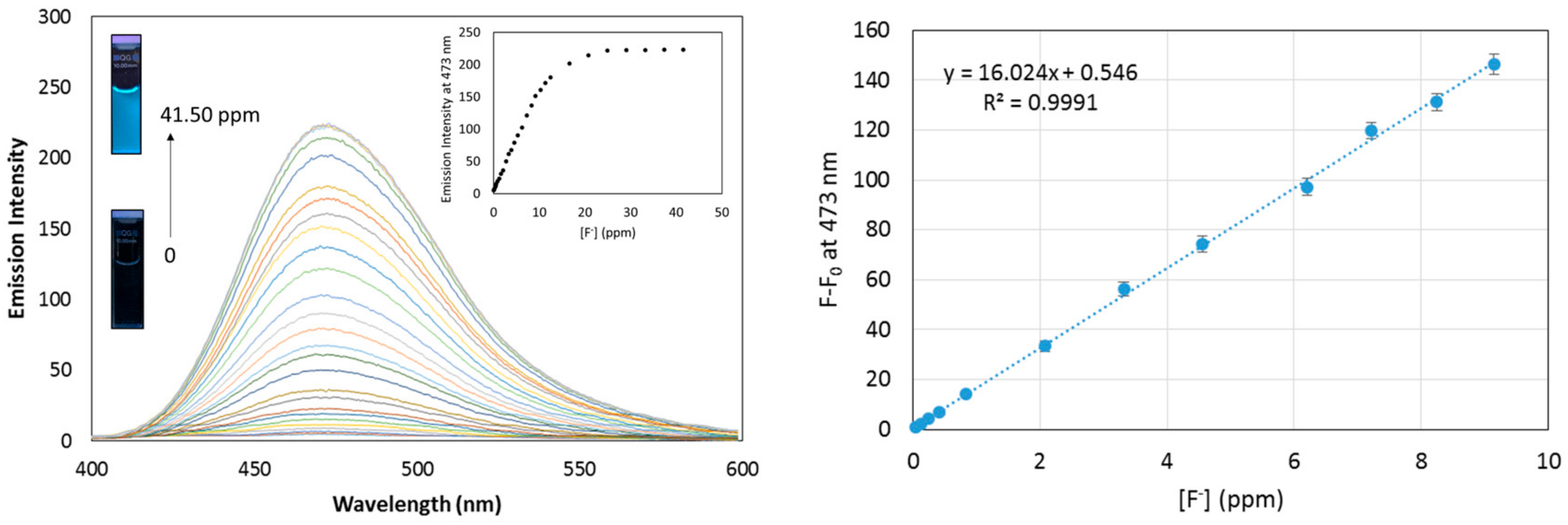
3.6. Fluorescence Response and Limit of Detection of Probe 5 Toward Fluoride
3.7. Fluoride Sensing in Collected Water Samples
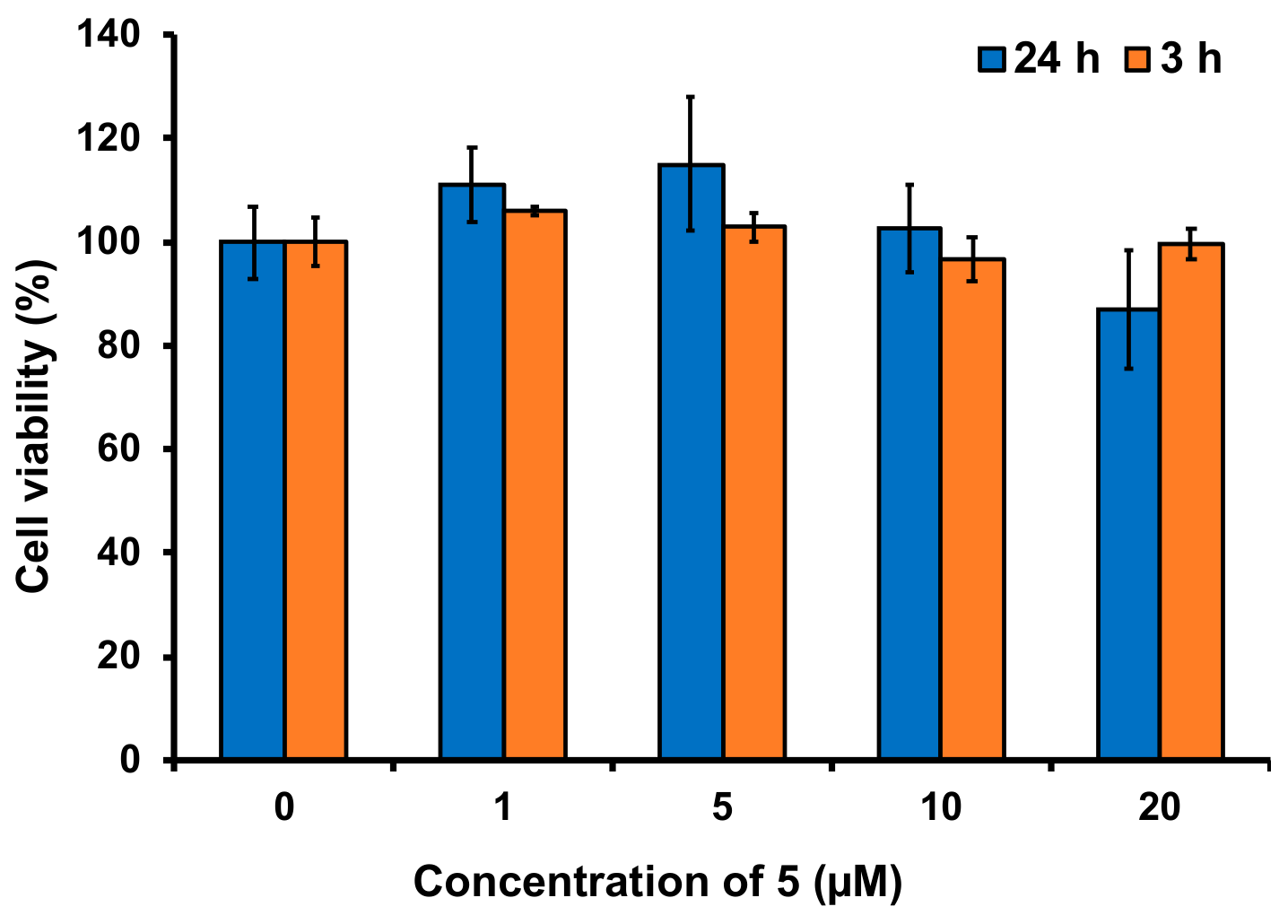
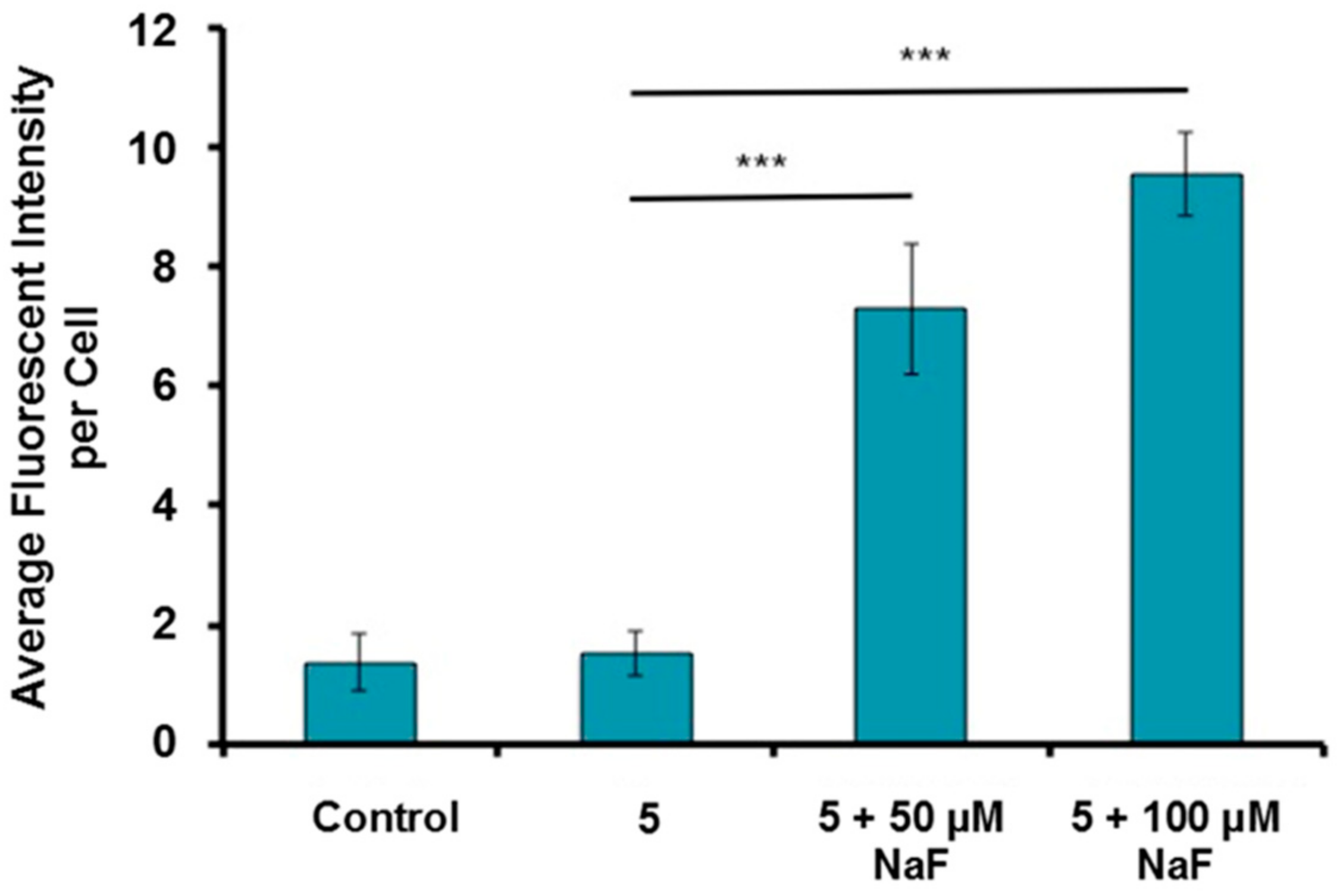
3.8. Imaging of Fluoride in Live Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirk, K.L. Biochemistry of the Elemental Halogens and Inorganic Halides; Plenum Press: New York, NY, USA, 1991; Volume 9, pp. 19–68. [Google Scholar]
- Kleerekoper, M. The role of fluoride in the prevention of osteoporosis. Endocrinol. Metab. Clin. N. Am. 1998, 27, 441–452. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Vabre, B.; Gabbai, F.P. [18F]-Group 13 fluoride derivatives as radiotracers for positron emission tomography. Chem. Soc. Rev. 2016, 45, 954–971. [Google Scholar] [CrossRef] [PubMed]
- Chansaenpak, K.; Wang, M.; Liu, S.; Wu, Z.; Yuan, H.; Conti, P.S.; Li, Z.; Gabbai, F.P. Synthesis and in vivo stability studies of [18F]-zwitterionic phosphonium aryltrifluoroborate/indomethacin conjugates. RSC Adv. 2016, 6, 23126–23133. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Wang, M.; Wang, H.; Giglio, B.C.; Gabbai, F.P.; Wu, Z.; Li, Z. Preparation of [18F]-NHC-BF3 conjugates and their applications in PET imaging. RSC Adv. 2017, 7, 17748–17751. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Wang, H.; Wang, M.; Giglio, B.; Ma, X.; Yuan, H.; Hu, S.; Wu, Z.; Li, Z. Synthesis and Evaluation of [18F]-Ammonium BODIPY Dyes as Potential Positron Emission Tomography Agents for Myocardial Perfusion Imaging. Chem. A Eur. J. 2016, 22, 12122–12129. [Google Scholar] [CrossRef] [PubMed]
- Dreisbuch, R.H. Handbook of Poisoning; Lange Medical Publishers: Los Altos, CA, USA, 1980. [Google Scholar]
- Weatherall, J.A. Handbook of Experimental Pharmacology XX/1; Springer: Berlin, Germany, 1969. [Google Scholar]
- Michigami, Y.; Kuroda, Y.; Ueda, K.; Yamamoto, Y. Determination of urinary fluoride by ion chromatography. Anal. Chim. Acta 1993, 274, 299–302. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and Colorimetric Chemosensors for Fluoride-Ion Detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Saini, R.; Kumar, A.; Luxami, V.; Kaur, N.; Singh, P.; Kumar, S. Chemodosimeters: An approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols. Coord. Chem. Rev. 2012, 256, 1992–2028. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, J.-I. Chromogenic and Fluorescent Chemodosimeter for Detection of Fluoride in Aqueous Solution. Organ. Lett. 2007, 9, 3109–3112. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, Z.; Zhou, Y.; Li, Y.; Xu, Z. Colorimetric fluoride sensor based on 1,8-naphthalimide derivatives. Dyes Pigments 2011, 91, 442–445. [Google Scholar] [CrossRef]
- Zhang, J.F.; Lim, C.S.; Bhuniya, S.; Cho, B.R.; Kim, J.S. A Highly Selective Colorimetric and Ratiometric Two-Photon Fluorescent Probe for Fluoride Ion Detection. Organ. Lett. 2011, 13, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Bamesberger, A.; Yang, L.; Houtwed, H.; Cao, H. Excimer-monomer switch: A reaction-based approach for selective detection of fluoride. Analyst 2014, 139, 3588–3592. [Google Scholar] [CrossRef] [PubMed]
- Bamesberger, A.; Schwartz, C.; Song, Q.; Han, W.; Wang, Z.; Cao, H. Rational design of a rapid fluorescent approach for detection of inorganic fluoride in MeCN-H2O: A new fluorescence switch based on N-aryl-1,8-naphthalimide. New J. Chem. 2014, 38, 884–888. [Google Scholar] [CrossRef]
- Woo, J.; Kim, G.; Quintero, K.; Hanrahan, M.P.; Palencia, H.; Cao, H. Investigation of desilylation in the recognition mechanism to fluoride by a 1,8-naphthalimide derivative. Organ. Biomol. Chem. 2014, 12, 8275–8279. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tang, X. Visualizing Fluoride Ion in Mitochondria and Lysosome of Living Cells and in Living Mice with Positively Charged Ratiometric Probes. Anal. Chem. 2015, 87, 8613–8617. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yin, J.; Ma, X.; Yu, S.; Wei, D.; Du, Y. A carbohydrate modified fluoride ion sensor and its applications. Anal. Chim. Acta 2011, 703, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zeng, F.; Yu, C.; Hou, X.; Wu, S. A PEGylated Fluorescent Turn-On Sensor for Detecting Fluoride Ions in Totally Aqueous Media and Its Imaging in Live Cells. Chem. A Eur. J. 2013, 19, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Bozdemir, O.A.; Sozmen, F.; Buyukcakir, O.; Guliyev, R.; Cakmak, Y.; Akkaya, E.U. Reaction-Based Sensing of Fluoride Ions Using Built-In Triggers for Intramolecular Charge Transfer and Photoinduced Electron Transfer. Organ. Lett. 2010, 12, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Liu, H.; Mack, J.; Wang, S.; Tian, J.; Lu, H.; Li, Z.; Shen, Z. A new aza-BODIPY based NIR region colorimetric and fluorescent chemodosimeter for fluoride. RSC Adv. 2014, 4, 53864–53869. [Google Scholar] [CrossRef]
- Zhu, B.; Yuan, F.; Li, R.; Li, Y.; Wei, Q.; Ma, Z.; Du, B.; Zhang, X. A highly selective colorimetric and ratiometric fluorescent chemodosimeter for imaging fluoride ions in living cells. Chem. Commun. 2011, 47, 7098–7100. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Feng, J.; Hu, D.; Wang, S.; Li, S.; Li, Y.; Yang, G. A Rapid Aqueous Fluoride Ion Sensor with Dual Output Modes. Angew. Chem. Int. Ed. 2010, 49, 4915–4918. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, Y.; Tang, X. Quaternary Ammonium Promoted Ultra Selective and Sensitive Fluorescence Detection of Fluoride Ion in Water and Living Cells. Anal. Chem. 2014, 86, 10006–10009. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.; Koh, M.; Park, S.B.; Hong, J.-I. Fluorescent probe for detection of fluoride in water and bioimaging in A549 human lung carcinoma cells. Chem. Commun. 2009, 31, 4735–4737. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F. Novel fluorogenic probe for fluoride ion based on the fluoride-induced cleavage of tert-butyldimethylsilyl ether. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Chavali, R.; Gunda, N.S.K.; Naicker, S.; Mitra, S.K. Rapid detection of fluoride in potable water using a novel fluorogenic compound 7-O-tert-butyldiphenylsilyl-4-methylcoumarin. Anal. Chem. Res. 2015, 6 (Suppl. C), 26–31. [Google Scholar] [CrossRef]
- Sokkalingam, P.; Lee, C.-H. Highly Sensitive Fluorescence “Turn-On” Indicator for Fluoride Anion with Remarkable Selectivity in Organic and Aqueous Media. J. Org. Chem. 2011, 76, 3820–3828. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jang, D.; Kim, S.Y.; Hong, J.-I. A chemodosimetric gelation system showing fluorescence and sol-to-gel transition for fluoride anions in aqueous media. New J. Chem. 2012, 36, 1145–1148. [Google Scholar] [CrossRef]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Štengl, V.; Králová, D.; Opluštil, F.; Němec, T. Mesoporous manganese oxide for warfare agents degradation. Microporous Mesoporous Mater. 2012, 156 (Suppl. C), 224–232. [Google Scholar] [CrossRef]
- Li, Z.; Chansaenpak, K.; Liu, S.; Wade, C.R.; Zhao, H.; Conti, P.S.; Gabbaï, F.P. Harvesting [18F]-fluoride ions in water via direct 18F-19F isotopic exchange: Radiofluorination of zwitterionic aryltrifluoroborates and in vivo stability studies. Med. Chem. Commun. 2012, 3, 1305–1308. [Google Scholar] [CrossRef]
- Vabre, B.; Chansaenpak, K.; Wang, M.; Wang, H.; Li, Z.; Gabbai, F.P. Radiofluorination of a NHC-PF5 adduct: Toward new probes for 18F PET imaging. Chem. Commun. 2017, 53, 8657–8659. [Google Scholar] [CrossRef] [PubMed]
- Sebelius, K. Proposed HHS Recommendation for Fluoride Concentration in Drinking Water for Prevention of Dental Caries. Fed. Regist. 2011, 76, 2383–2388. [Google Scholar]
- Maurer, J.K.; Cheng, M.C.; Boysen, B.G.; Anderson, R.L. Two-Year Carcinogenicity Study of Sodium Fluoride in Rats. JNCI J. Nat. Cancer Inst. 1990, 82, 1118–1126. [Google Scholar] [CrossRef] [PubMed]





| Probe | Solvent System | Limit of Detection | Reference |
|---|---|---|---|
| I | Acetone-water (70% Acetone v/v) | 0.8 ppb | [28] |
| II | THF-water (4% THF v/v) | 200 ppb | [29] |
| III | MeCN | 2.1 ppb | [30] |
| IV | MeOH-HEPES buffer solution (50% MeOH v/v) | N/D | [31] |
| VI | HEPES buffer solution | N/D | [27] |
| 5 | MeCN-HEPES buffer solution (0.8% MeCN v/v) | 43 ppb | This work |
| Samples [b] | Amount of Fluoride (ppm) | Recovery (%) | |
|---|---|---|---|
| Added | Found | ||
| Tap water | 0.50 | 0.51 ± 0.02 | 102.7 ± 4.2 |
| 5.0 | 4.82 ± 0.08 | 96.4 ± 1.5 | |
| River water [c] | 0.50 | 0.52 ± 0.03 | 104.0 ± 5.3 |
| 5.0 | 4.87 ± 0.07 | 97.3 ± 1.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chansaenpak, K.; Kamkaew, A.; Weeranantanapan, O.; Suttisintong, K.; Tumcharern, G. Coumarin Probe for Selective Detection of Fluoride Ions in Aqueous Solution and Its Bioimaging in Live Cells. Sensors 2018, 18, 2042. https://doi.org/10.3390/s18072042
Chansaenpak K, Kamkaew A, Weeranantanapan O, Suttisintong K, Tumcharern G. Coumarin Probe for Selective Detection of Fluoride Ions in Aqueous Solution and Its Bioimaging in Live Cells. Sensors. 2018; 18(7):2042. https://doi.org/10.3390/s18072042
Chicago/Turabian StyleChansaenpak, Kantapat, Anyanee Kamkaew, Oratai Weeranantanapan, Khomson Suttisintong, and Gamolwan Tumcharern. 2018. "Coumarin Probe for Selective Detection of Fluoride Ions in Aqueous Solution and Its Bioimaging in Live Cells" Sensors 18, no. 7: 2042. https://doi.org/10.3390/s18072042
APA StyleChansaenpak, K., Kamkaew, A., Weeranantanapan, O., Suttisintong, K., & Tumcharern, G. (2018). Coumarin Probe for Selective Detection of Fluoride Ions in Aqueous Solution and Its Bioimaging in Live Cells. Sensors, 18(7), 2042. https://doi.org/10.3390/s18072042





