Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection
Abstract
1. Introduction
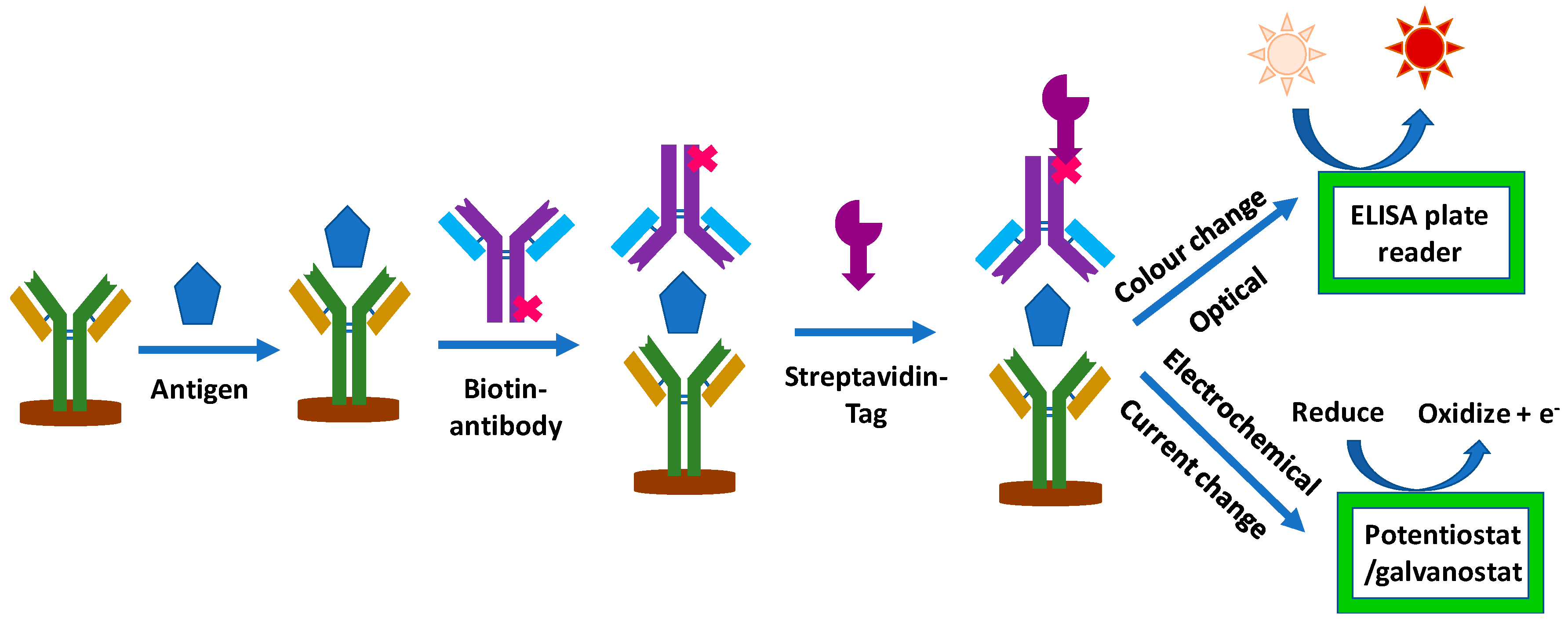
1.1. Electrochemical Sandwich ELISA
1.2. General Mechanism of Enhancement Strategies
2. Matrix Selection, Modification and Development of Immunosensors
3. Electrochemical ELISA Based Detection
3.1. Redox Enzyme Based Detection
3.1.1. Free Redox Enzyme and Redox Enzyme with Nanomaterial Based Enhancement
3.1.2. Redox Enzyme with Carbon Material Based Enhancement
3.2. Redox Marker Based Detection
3.2.1. Free Redox Marker and Redox Marker with Metallic Nanomaterial Based Enhancement
3.2.2. Redox Marker with Carbon Material Based Enhancement
3.2.3. Non-Enzymatic Catalytic Activity and Enzyme-Mimicking Materials Based Signal Amplification Strategies
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Laocharoensuk, R. Development of electrochemical immunosensors towards point-of-care cancer diagnostics: Clinically relevant studies. Electroanalysis 2016, 28, 1716–1729. [Google Scholar] [CrossRef]
- Jayanthi, V.S.P.K.; Sankara, A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Esimbekova, E.N.; Kratasyuk, V.A. Rapid biosensing tools for cancer biomarkers. Biosens. Bioelectron. 2017, 87, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Bansi, D.M.; Saurabh, K.; Chandra Mouli, P. Nanomaterials based biosensors for cancer biomarker detection. J. Phys. Conf. Ser. 2016, 704. [Google Scholar] [CrossRef]
- Ezzati Nazhad Dolatabadi, J.; de la Guardia, M. Nanomaterial-based electrochemical immunosensors as advanced diagnostic tools. Anal. Methods 2014, 6, 3891–3900. [Google Scholar] [CrossRef]
- Moro, L.; Turemis, M.; Marini, B.; Ippodrino, R.; Giardi, M.T. Better together: Strategies based on magnetic particles and quantum dots for improved biosensing. Biotechnol. Adv. 2017, 35, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Rama, E.C.; Costa-García, A. Screen-printed electrochemical immunosensors for the detection of cancer and cardiovascular biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Munge, B.S.; Stracensky, T.; Gamez, K.; DiBiase, D.; Rusling, J.F. Multiplex immunosensor arrays for electrochemical detection of cancer biomarker proteins. Electroanalysis 2016, 28, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent advances in electrochemical immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, S.N.; Azimzadeh, M.; Ozsoz, M. Electrochemical biosensors for cancer biomarkers detection: Recent advances and challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Dixit, C.K.; Kadimisetty, K.; Otieno, B.A.; Tang, C.; Malla, S.; Krause, C.E.; Rusling, J.F. Electrochemistry-based approaches to low cost, high sensitivity, automated, multiplexed protein immunoassays for cancer diagnostics. Analyst 2016, 141, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rong, Q.; Ma, Z. Construction of electrochemical immunosensing interface for multiple cancer biomarkers detection. Electroanalysis 2016, 28, 1692–1699. [Google Scholar] [CrossRef]
- Anik, U.; Timur, S. Towards the electrochemical diagnosis of cancer: Nanomaterial-based immunosensors and cytosensors. RSC Adv. 2016, 6, 111831–111841. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Jiang, L.; Chu, P.K.; Dong, Y.; Wei, Q. A sandwich-type electrochemical immunosensor based on the biotin-streptavidin-biotin structure for detection of human immunoglobulin G. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Kongsuphol, P.; Park, M.K. On-chip electrochemical immunoassay platform for specific protein biomarker estimation in undiluted serum using off-surface membrane matrix. Biosens. Bioelectron. 2017, 91, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Gan, N.; Cao, Y.; Hu, F.; Li, T.; Zheng, L. An ultrasensitive electrochemical immunosensor for alpha-fetoprotein using an envision complex-antibody copolymer as a sensitive label. Materials 2012, 5, 2757–2772. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Gu, H.; Xia, F.; Zuo, X. Recent development of sandwich assay based on the nanobiotechnologies for proteins, nucleic acids, small molecules, and ions. Chem. Rev. 2014, 114, 7631–7677. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, B.; Tang, J.; Liu, B.; Lai, W.; Tang, D. Sandwich-type immunosensors and immunoassays exploiting nanostructure labels: A review. Anal. Chim. Acta 2013, 758, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, Y.; Qiao, X. Recent advances of carbon nanotubes-based electrochemical immunosensors for the detection of protein cancer biomarkers. Electroanalysis 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Magnetic bead-based reverse colorimetric immunoassay strategy for sensing biomolecules. Anal. Chem. 2013, 85, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Wan, Y.; Su, Y.; Zhu, X.; Liu, G.; Fan, C. Development of electrochemical immunosensors towards point of care diagnostics. Biosens. Bioelectron. 2013, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Du, B.; Zhang, X.; Guo, A.; Zhang, Y.; Wu, D.; Ma, H.; Wei, Q. Ultrasensitive enzyme-free immunoassay for squamous cell carcinoma antigen using carbon supported Pd–Au as electrocatalytic labels. Anal. Chim. Acta 2014, 833, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, N.; Ma, Z. Platinum porous nanoparticles hybrid with metal ions as probes for simultaneous detection of multiplex cancer biomarkers. Biosens. Bioelectron. 2014, 53, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wu, D.; Zhang, Y.; Ma, H.; Li, H.; Du, B.; Wei, Q.; Ju, H. Ultrasensitive electrochemical immunosensors for multiplexed determination using mesoporous platinum nanoparticles as nonenzymatic labels. Anal. Chim. Acta 2014, 807, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Li, H.; Wang, H.; Wu, D.; Ma, H.; Cai, Y.; Du, B.; Wei, Q. Nonenzymatic immunosensor for detection of carbohydrate antigen 15-3 based on hierarchical nanoporous PtFe alloy. Biosens. Bioelectron. 2014, 56, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wei, Q.; Wu, H.; Dou, J.; Li, H. Ionic liquid functionalized graphene based immunosensor for sensitive detection of carbohydrate antigen 15-3 integrated with Cd2+-functionalized nanoporous TiO2 as labels. Biosens. Bioelectron. 2014, 59, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Guo, Z.; Yan, T.; Ma, H.; Du, B.; Li, Y.; Wei, Q. Ultrasensitive sandwich-type electrochemical immunosensor based on a novel signal amplification strategy using highly loaded palladium nanoparticles/carbon decorated magnetic microspheres as signal labels. Biosens. Bioelectron. 2015, 68, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sens. Actuators B Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Feng, T.; Qiao, X.; Wang, H.; Sun, Z.; Hong, C. A sandwich-type electrochemical immunosensor for carcinoembryonic antigen based on signal amplification strategy of optimized ferrocene functionalized Fe3O4@SiO2 as labels. Biosens. Bioelectron. 2016, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, L.; Han, X.; Fang, X.; Li, X.; Zhang, Y. Simultaneous electrochemical detection of multiple tumor markers using functionalized graphene nanocomposites as non-enzymatic labels. Sens. Actuators B Chem. 2014, 201, 360–368. [Google Scholar] [CrossRef]
- Gao, J.; Guo, Z.; Su, F.; Gao, L.; Pang, X.; Cao, W.; Du, B.; Wei, Q. Ultrasensitive electrochemical immunoassay for CEA through host–guest interaction of β-cyclodextrin functionalized graphene and Cu@Ag core–shell nanoparticles with adamantine-modified antibody. Biosens. Bioelectron. 2015, 63, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Han, J.; Li, F.; Gao, J.; Li, Y.; Dong, Y.; Wei, Q. A sandwich-type electrochemical immunosensor based on multiple signal amplification for α-fetoprotein labeled by platinum hybrid multiwalled carbon nanotubes adhered copper oxide. Electrochim. Acta 2015, 160, 7–14. [Google Scholar] [CrossRef]
- Li, N.; Ma, H.; Cao, W.; Wu, D.; Yan, T.; Du, B.; Wei, Q. Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens. Bioelectron. 2015, 74, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, R.; Chai, Y.; Zhuo, Y.; Su, H.; Zhang, Y. Horseradish peroxidase-loaded nanospheres attached to hollow gold nanoparticles as signal enhancers in an ultrasensitive immunoassay for alpha-fetoprotein. Microchim. Acta 2014, 181, 679–685. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Su, Y.; Li, F.; Ma, H.; Li, H.; Du, B.; Wei, Q. Ultrasensitive non-mediator electrochemical immunosensors using Au/Ag/Au core/double shell nanoparticles as enzyme-mimetic labels. Talanta 2014, 124, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Amani, K. A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens. Bioelectron. 2014, 52, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, N.; Feng, F.; Ma, Z. Synthesis of cadmium, lead and copper alginate nanobeads as immunosensing probes for the detection of AFP, CEA and PSA. Biosens. Bioelectron. 2015, 70, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ma, Z. Au–ionic liquid functionalized reduced graphene oxide immunosensing platform for simultaneous electrochemical detection of multiple analytes. Biosens. Bioelectron. 2014, 51, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Cao, W.; Li, Y.; Li, H.; Du, B.; Wei, Q. Ultrasensitive sandwich-type electrochemical immunosensor based on a novel signal amplification strategy using highly loaded toluidine blue/gold nanoparticles decorated KIT-6/carboxymethyl chitosan/ionic liquids as signal labels. Biosens. Bioelectron. 2014, 61, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chai, Y.; Zhuo, Y.; Yuan, R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin–streptavidin signal amplification strategy. Biosens. Bioelectron. 2015, 68, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, L.; Fang, X.; Han, X.; Zhang, Y. Dual signal amplification of horseradish peroxidase functionalized nanocomposite as trace label for the electrochemical detection of carcinoembryonic antigen. Electrochim. Acta 2014, 127, 334–341. [Google Scholar] [CrossRef]
- Jia, X.; Chen, X.; Han, J.; Ma, J.; Ma, Z. Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens. Bioelectron. 2014, 53, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, J.; Jiang, L.; Wang, Y.; Li, Y.; Dong, Y.; Wei, Q. An ultrasensitive sandwich-type electrochemical immunosensor based on signal amplification strategy of gold nanoparticles functionalized magnetic multi-walled carbon nanotubes loaded with lead ions. Biosens. Bioelectron. 2015, 68, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Hu, X.; Zhang, S. A signal-enhanced electrochemical immunosensor based on dendrimer functionalized-graphene as a label for the detection of α-1-fetoprotein. J. Electroanal. Chem. 2014, 717–718, 172–176. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Li, N.; Zhang, Y.; Yan, T.; Ma, H.; Wei, Q. Sandwich-type electrochemical immunosensor for the detection of AFP based on Pd octahedral and APTES-M-CeO2-GS as signal labels. Biosens. Bioelectron. 2016, 79, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Z. Multiplexed electrochemical immunoassay of biomarkers using chitosan nanocomposites. Biosens. Bioelectron. 2014, 55, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, X.; Ma, Z. Chitosan coated copper and cadmium hexacyanocobaltate nanocubes as immunosensing probes for the construction of multiple analytes platform. Biosens. Bioelectron. 2014, 61, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jia, X.; Chen, X.; Ma, Z. Simultaneous electrochemical detection of multiple tumor markers using metal ions tagged immunocolloidal gold. Biosens. Bioelectron. 2014, 56, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Guo, Z.; Gao, L.; Zhang, Y.; Fan, D.; Ji, G.; Du, B.; Wei, Q. Ultrasensitive electrochemical immunosensor for carbohydrate antigen 72-4 based on dual signal amplification strategy of nanoporous gold and polyaniline–Au asymmetric multicomponent nanoparticles. Biosens. Bioelectron. 2015, 64, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, C.; Shen, L.; Deng, W.; Yan, M.; Ge, S.; Yu, J.; Song, X. An ultrasensitive electrochemical immunosensor based on the catalytical activity of MoS2-Au composite using Ag nanospheres as labels. Sens. Actuators B Chem. 2015, 206, 30–36. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Cao, W.; Li, Y.; Li, H.; Du, B.; Wei, Q. Facile fabrication of an ultrasensitive sandwich-type electrochemical immunosensor for the quantitative detection of alpha fetoprotein using multifunctional mesoporous silica as platform and label for signal amplification. Talanta 2014, 129, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Qian, C.; Hao, N.; Xu, L.; Yao, C. Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly(o-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag. Biosens. Bioelectron. 2015, 64, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, T.; Gan, N.; Zhang, H.; Long, N.; Hu, F.; Cao, Y.; Jiang, Q.; Jiang, S. Electrochemical coding for multiplexed immunoassays of biomarkers based on bio-based polymer-nanotags. Electrochim. Acta 2015, 163, 238–245. [Google Scholar] [CrossRef]
- Zhou, J.; Lai, W.; Zhuang, J.; Tang, J.; Tang, D. Nanogold-functionalized DNAzyme concatamers with redox-active intercalators for quadruple signal amplification of electrochemical immunoassay. ACS Appl. Mater. Interfaces 2013, 5, 2773–2781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, B.; Chen, G.; Tang, D. Redox and catalysis ‘all-in-one’ infinite coordination polymer for electrochemical immunosensor of tumor markers. Biosens. Bioelectron. 2015, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, X.; Wang, L.; Wu, Q.; Chen, Z.; Lin, X. Sandwich-type amperometric immunosensor for cancer biomarker based on signal amplification strategy of multiple enzyme-linked antibodies as probes modified with carbon nanotubes and concanavalin A. J. Electroanal. Chem. 2014, 732, 38–45. [Google Scholar] [CrossRef]
- Patris, S.; De Pauw, P.; Vandeput, M.; Huet, J.; Van Antwerpen, P.; Muyldermans, S.; Kauffmann, J.-M. Nanoimmunoassay onto a screen printed electrode for HER2 breast cancer biomarker determination. Talanta 2014, 130, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Mao, K.; Li, Y.; Du, B.; Zhang, Y.; Wei, Q. Electrochemical immunosensor for α-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal. Biochem. 2014, 465, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, H.; Li, Y.; Zhang, Y.; Liang, H.; Wei, Q. Ultrasensitive electrochemical immunoassay for squamous cell carcinoma antigen using dumbbell-like Pt–Fe3O4 nanoparticles as signal amplification. Biosens. Bioelectron. 2013, 46, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, A.; Guo, Z.; Xie, L.; Wei, Q.; Du, B. Simultaneous electrochemical detection of cervical cancer markers using reduced graphene oxide-tetraethylene pentamine as electrode materials and distinguishable redox probes as labels. Biosens. Bioelectron. 2014, 54, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, Z.; Liu, Y.; Guo, A.; Lou, W.; Fan, D.; Wei, Q. Sandwich-type electrochemical immunosensor using dumbbell-like nanoparticles for the determination of gastric cancer biomarker CA72-4. Talanta 2015, 134, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wen, W.; Zhang, X.; Wang, S. Electrochemical immunosensor for the prostate specific antigen detection based on carbon nanotube and gold nanoparticle amplification strategy. Microchim. Acta 2015, 182, 1855–1861. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper-based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, B.; Salimi, A.; Hallaj, R.; Moradi, F. Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/PAMAM dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy. Biosens. Bioelectron. 2015, 74, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, J.; Ju, H.; Yan, F. Nanogold/mesoporous carbon foam-mediated silver enhancement for graphene-enhanced electrochemical immunosensing of carcinoembryonic antigen. Biosens. Bioelectron. 2014, 52, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, Z.; Zhuo, Y.; Chai, Y.; Yuan, R. Ultrasensitive electrochemical immunosensor for carbohydrate antigen 19-9 using Au/porous graphene nanocomposites as platform and Au@Pd core/shell bimetallic functionalized graphene nanocomposites as signal enhancers. Biosens. Bioelectron. 2015, 66, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Han, J.; Zhuo, Y.; Yang, Z.; Chai, Y.; Yuan, R. Highly sensitive impedimetric immunosensor based on single-walled carbon nanohorns as labels and bienzyme biocatalyzed precipitation as enhancer for cancer biomarker detection. Biosens. Bioelectron. 2014, 55, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Tang, Y.; Ge, L.; Guo, B.; Yao, C. Amperometric carbohydrate antigen 19-9 immunosensor based on three dimensional ordered macroporous magnetic Au film coupling direct electrochemistry of horseradish peroxidase. Anal. Chim. Acta 2014, 815, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, N.; Yuan, J.; Ma, Z. Triple tumor markers assay based on carbon–gold nanocomposite. Biosens. Bioelectron. 2015, 70, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Yang, H.; Ma, C.; Yu, J.; Yan, M.; Song, X. Sensitive origami dual-analyte electrochemical immunodevice based on polyaniline/Au-paper electrode and multi-labeled 3D graphene sheets. Electrochim. Acta 2014, 120, 102–109. [Google Scholar] [CrossRef]
- Jolly, P.; Damborsky, P.; Madaboosi, N.; Soares, R.R.G.; Chu, V.; Conde, J.P.; Katrlik, J.; Estrela, P. DNA aptamer-based sandwich microfluidic assays for dual quantification and multi-glycan profiling of cancer biomarkers. Biosens. Bioelectron. 2016, 79, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, P.; Li, F.; Chu, Q.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of mesoporous core–shell Pd@Pt nanoparticles/amino group functionalized graphene nanocomposite. Biosens. Bioelectron. 2017, 87, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Jiao, L.; Zhang, J.; Li, H. Amperometric sandwich immunoassay for the carcinoembryonic antigen using a glassy carbon electrode modified with iridium nanoparticles, polydopamine and reduced graphene oxide. Microchim. Acta 2017, 184, 169–175. [Google Scholar] [CrossRef]
- Shan, J.; Ma, Z. Simultaneous detection of five biomarkers of lung cancer by electrochemical immunoassay. Microchim. Acta 2016, 183, 2889–2897. [Google Scholar] [CrossRef]
- Tang, C.K.; Vaze, A.; Shen, M.; Rusling, J.F. High-throughput electrochemical microfluidic immunoarray for multiplexed detection of cancer biomarker proteins. ACS Sens. 2016, 1, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Uludag, Y.; Narter, F.; Sağlam, E.; Köktürk, G.; Gök, M.Y.; Akgün, M.; Barut, S.; Budak, S. An integrated lab-on-a-chip-based electrochemical biosensor for rapid and sensitive detection of cancer biomarkers. Anal. Bioanal. Chem. 2016, 408, 7775–7783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Wang, L.; Zhu, J.; Jiang, W. Cascade signal amplification based on copper nanoparticle-reported rolling circle amplification for ultrasensitive electrochemical detection of the prostate cancer biomarker. ACS Appl. Mater. Interfaces 2016, 8, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Bao, J.; Zhao, Y.; Huo, D.; Chen, M.; Qi, Y.; Yang, M.; Fa, H.; Hou, C. A sandwich-type electrochemical immunoassay for ultrasensitive detection of non-small cell lung cancer biomarker CYFRA21-1. Bioelectrochemistry 2018, 120, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, G.; Wang, H.; Cao, W.; Du, B.; Wei, Q. Sandwich-type electrochemical immunoassay based on Co3O4@MnO2-thionine and pseudo-ELISA method toward sensitive detection of alpha fetoprotein. Biosens. Bioelectron. 2018, 106, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Emami, M.; Farzin, L.; Saber, R. A sandwich-type electrochemical immunosensor based on in situ silver deposition for determination of serum level of HER2 in breast cancer patients. Biosens. Bioelectron. 2018, 103, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; She, Z.; Ma, T.; Tian, S.; Kraatz, H.-B. Electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2018, 102, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Suresh, L.; Brahman, P.K.; Reddy, K.R.; Bondili, J.S. Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzym. Microb. Technol. 2018, 112, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Deng, D.; He, H.; Yan, X.; Wang, Z.; Fan, C.; Luo, L. Effective immobilization of Au nanoparticles on TiO2 loaded graphene for a novel sandwich-type immunosensor. Biosens. Bioelectron. 2018, 102, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Y.; Li, M.; Li, F.; Han, J.; Dong, Y.; Chen, Z.; Wang, P.; Liu, H.; Wei, Q. A novel sandwich-type electrochemical immunosensor for PSA detection based on PtCu bimetallic hybrid (2D/2D) rGO/g-C3N4. Biosens. Bioelectron. 2017, 91, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tian, L.; Zhao, G.; Huang, Y.; Wei, Q.; Cao, W. Ultrasensitive electrochemical immunosensor for alpha fetoprotein detection based on platinum nanoparticles anchored on cobalt oxide/graphene nanosheets for signal amplification. Anal. Chim. Acta 2017, 986, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Li, F.; Li, M.; Chen, L.; Dong, Y.; Wei, Q. Sandwich-type amperometric immunosensor using functionalized magnetic graphene loaded gold and silver core-shell nanocomposites for the detection of Carcinoembryonic antigen. J. Electroanal. Chem. 2017, 795, 1–9. [Google Scholar] [CrossRef]
- Han, J.; Li, Y.; Feng, J.; Li, M.; Wang, P.; Chen, Z.; Dong, Y. A novel sandwich-type immunosensor for detection of carcino-embryonic antigen using silver hybrid multiwalled carbon nanotubes/manganese dioxide. J. Electroanal. Chem. 2017, 786, 112–119. [Google Scholar] [CrossRef]
- Yang, Z.; Lan, Q.; Li, J.; Wu, J.; Tang, Y.; Hu, X. Efficient streptavidin-functionalized nitrogen-doped graphene for the development of highly sensitive electrochemical immunosensor. Biosens. Bioelectron. 2017, 89, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, L.; Du, W.; Liu, S.; Wei, Q.; Li, H. Robust enzyme-free electrochemical immunoassay of CEA enhanced by porous PdCu nanoparticles. Electrochim. Acta 2017, 252, 374–380. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.; Xue, Y.; Liang, J.; Cui, L.; Li, Q.; Zhou, S.; Huang, Y.; Li, G.; Zhao, Y. A Fe3O4@Au-basedpseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal. Biochem. 2017, 534, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, J.; Wang, J.; Zhao, H.; Ren, H.; Li, Z. Dual signal amplification strategy of Au nanopaticles/ZnO nanorods hybridized reduced graphene nanosheet and multienzyme functionalized Au@ZnO composites for ultrasensitive electrochemical detection of tumor biomarker. Biosens. Bioelectron. 2017, 97, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Duangkaew, P.; Wutikhun, T.; Laocharoensuk, R. Triple signal amplification strategy based on size and shape transformation of ultrasmall sub-10 nm gold nanoparticles tag towards sensitivity improvement of electrochemical immunosensors. Sens. Actuators B Chem. 2017, 239, 430–437. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, F.; Feng, J.; Li, M.; Chen, L.; Dong, Y. Ultrasensitive electrochemical immunosensor for quantitative detection of SCCA using Co3O4@CeO2-Au@Pt nanocomposite as enzyme-mimetic labels. Biosens. Bioelectron. 2017, 92, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Lv, H.; Feng, J.; Gao, Z.; Wang, P.; Dong, Y.; Liu, Q.; Zhao, Z. Sandwich-type electrochemical immunosensor based on Au@Ag supported on functionalized phenolic resin microporous carbon spheres for ultrasensitive analysis of α-fetoprotein. Biosens. Bioelectron. 2018, 106, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, W.; Ma, Z. Improved sandwich-format electrochemical immunosensor based on “smart” SiO2@polydopamine nanocarrier. Biosens. Bioelectron. 2018, 109, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, X.; Wang, K.-K.; Xue, Y.; Wang, A.-J.; Wu, L.; Feng, J.-J. A novel enzyme-free sandwich-like electrochemical immunosensor for the detection of carbohydrate antigen 15-3 based on hierarchical AuPd nanochain networks. Sens. Actuators B Chem. 2017, 247, 349–356. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Gao, Z.; Zhang, C.; Zhang, S.; Dong, Y. Enhanced peroxidase-like properties of Au@Pt DNs/NG/Cu2+ and application of sandwich-type electrochemical immunosensor for highly sensitive detection of CEA. Biosens. Bioelectron. 2018, 112, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, Q.; Liu, Q.; Li, Y.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu2O nanoparticles for prostate specific antigen detection. Biosens. Bioelectron. 2018, 99, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Pandey, B.; Neupane, B.; Ligler, F.S. Signal amplification strategies for microfluidic immunoassays. TrAC Trends Anal. Chem. 2016, 79, 326–334. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. Electrochemical nanobiosensing in whole blood: Recent advances. TrAC Trends Anal. Chem. 2016, 80, 167–176. [Google Scholar] [CrossRef]
- Wang, D.; Gan, N.; Zhou, J.; Xiong, P.; Cao, Y.; Li, T.; Pan, D.; Jiang, S. Signal amplification for multianalyte electrochemical immunoassay with bidirectional stripping voltammetry using metal-enriched polymer nanolabels. Sens. Actuators B Chem. 2014, 197, 244–253. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, J.; Chen, G.; Tang, D. Layer-by-layer multienzyme assembly for highly sensitive electrochemical immunoassay based on tyramine signal amplification strategy. Biosens. Bioelectron. 2014, 54, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Tallapragada, S.D.; Layek, K.; Mukherjee, R.; Mistry, K.K.; Ghosh, M. Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry 2017, 118, 25–30. [Google Scholar] [CrossRef] [PubMed]








| Immunosensor | Components | Preparation Conditions | Binding Technique | Target | Ref. |
|---|---|---|---|---|---|
| Ab1/GS/SPCE | GS | Acid treatment of graphite flakes | EDC/NHS (covalent) | CEA, CA125, CA153 | [30] |
| Ab1/N-GS/GCE | N-GS | GO prepared from acid treatment of graphite and then reduced with DMF at 153 °C to get N-GS | glutaraldehyde (covalent) | SCCA | [28] |
| Ab1-ADA/CD-GN/GCE | Ab1-ADA | EDC/NHS chemistry | physical | CEA | [37] |
| CD-GN | GO prepared from acid treatment of graphite and then reduced with hydrazine in presence of ammonia and β-CD at 60 °C to get CD-GN | ||||
| Ab1/MWCNTs/GCE | MWCNT-COOH | Acid treatment of MWCNTs | EDC/NHS (covalent) | AFP | [33] |
| Ab1/CD-GS/GCE | CD-GS | GO prepared from acid treatment of graphite and then reduced with hydrazine in presence of ammonia and β-CD at 180 °C to get CD-GS | physical | AFP | [38] |
| Ab1-GS/GCE | GS | GO prepared from acid treatment of graphite flakes and then reduced with hydrazine at 100 °C to get GS | EDC/NHS (covalent) | CA 15-3 | [31] |
| Ab1-ADA/CD-GS/GCE | Ab1-ADA | EDC/NHS chemistry | EDC/NHS (covalent) | AFP | [39] |
| CD-GS | GO prepared from acid treatment of graphite and then reduced with hydrazine hydrate in presence of ammonia and β-CD at 60 °C to get CD-GS | ||||
| Ab1-PA/AuNP/CSSH-SWCNTs/Au | AuNPs | Sodium citrate based reduction at 100 °C | PA-antibody interaction | AFP | [40] |
| CSSH | EDC/NHS chemistry | ||||
| anti-HER2 Nb/SPE | COOH-SPE | Acid treatment at 1.6 V | EDC/NHS (covalent) | HER2 | [63] |
| Ab1/GS-Thi/GCE | GS-Thi | Thi adsorption on GS | EDC/NHS (covalent) | AFP | [64] |
| Ab1/IL-rGO/GCE | IL-rGO | treating GO with IL-NH2 in KOH at 80 °C | glutaraldehyde (covalent) | CEA, AFP | [29] |
| Ab1/N-GS-CH/GCE | N-GS-CH | GO prepared from acid treatment of graphite and then undergo thermal annealing in ammonia to get N-GS which was then mixed with CH to get N-GS-CH | glutaraldehyde (covalent) | SCC | [65] |
| Ab1/rGO-TEPA/GCE | rGO-TEPA | rGO-TEPA | EDC/NHS (covalent) | CEA, SCCA | [66] |
| Ab1/rGO-TEPA/GCE | rGO-TEPA | rGO-TEPA | EDC/NHS (covalent) | CA72-4 | [67] |
| Ab1-GS/GCE | GS-COOH | GO prepared from acid treatment of graphite and then undergo thermal exfoliation in quartz tube at 1000 °C to get GS which was then treated chloroacetic acid in basic media to generate GS-COOH | EDC/NHS (covalent) | CA15-3 | [32] |
| Ab1/MWCNTs/DAH/GCE | MWCNT-COOH | Nitric acid treatment of MWCNTs | EDC/NHS (covalent) | PSA | [68] |
| DAH monolayer | CV scans in 0.2 and 1.6 V at 20 mV/s | ||||
| Ab1/CH/rGO/SPC/whatman paper | rGO | GO prepared from acid treatment of graphite and reduced electrochemically at −1.0 V CH coating | glutaraldehyde (covalent) | AFP, CEA, CA125, CA153 | [69] |
| Ab1/nafion-AuNP-DN-GR/GCE | GR | GO prepared from acid treatment of graphite and reduced using NaBH4 at 85 °C | physical | CEA | [34] |
| AuNPs | Sodium citrate based reduction at 97 °C | ||||
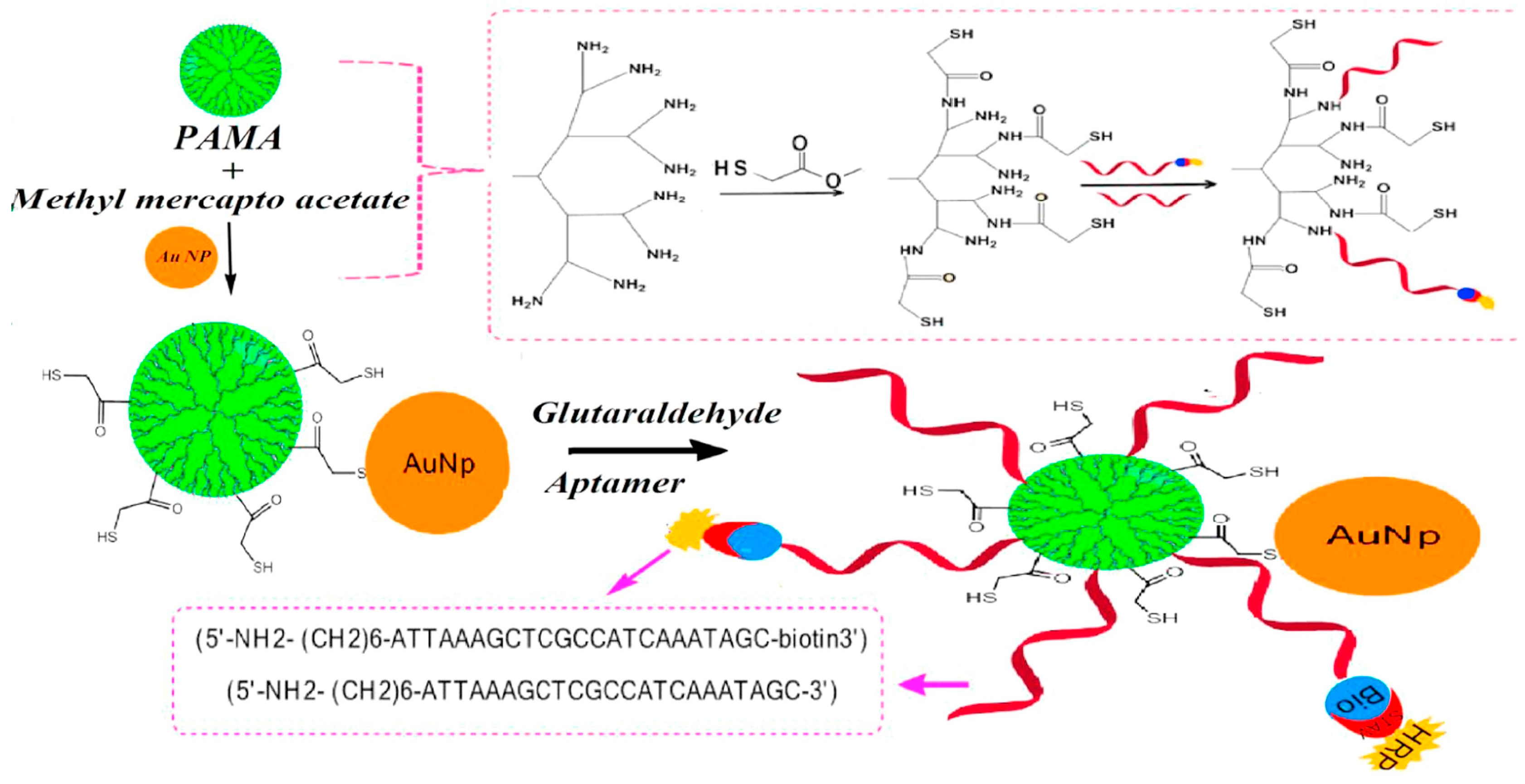
| Ab1/thionine/AuNP-PMMA dendrimer/CH-MWCNTs-IL/GCE | AuNP-PMMA dendrimer | AuNPs prepared via citrate method were mixed and incubated with thiol terminated PAMAM prepared via treating amine-terminated PAMAM dendrimer (G4) with methyl mercaptoacetate at 50 °C | phthaloyl chloride (covalent) | PSA | [42] |
| Ab1-thionine/CH/rGO/GCE | rGO | GO prepared from acid treatment of graphite and reduced electrochemically at −1.0 V CH coating | glutaraldehyde (covalent) | PSA | [70] |
| Ab1/Au@SH-GS/GCE | AuNPs | Sodium citrate based reduction in boiling condition | physical | SCCA | [41] |
| SH-GS | GO prepared from acid treatment of graphite was treated with MPTES at 70 °C followed by treating with hydrazine hydrate at 95 °C to get SH-GS | ||||
| Ab1/AuNPs-IL-rGO/GCE | IL-rGO | GO was mixed with IL-NH2 in KOH and reflux at 80 °C to get IL-rGO | physical | AFP, CEA, PSA | [43] |
| AuNPs-IL-rGO | IL-rGO mixed with HAuCl4 was reduced using ascorbic acid to get AuNPs-IL-rGO nanocomposite | ||||
| Ab1/Au@APTES-GS/GCE | APTES-GS | GO treated with APTES at 70 °C followed by treating with hydrazine hydrate at 95 °C to get APTES-GS | physical | CEA | [45] |
| AuNPs | HAuCl4 reduction using NaBH4 in ice bath | ||||
| Ab1/CH/rGO/GCE | rGO | GO prepared from acid treatment of graphite and reduced electrochemically at −1.0 V after CH coating | glutaraldehyde (covalent) | CEA | [71] |
| Ab1/AuNPs-IL-rGO/GCE | IL-rGO | GO was mixed with IL-NH2 in KOH and reflux at 80 °C to get IL-rGO | physical | CEA, AFP | [44] |
| AuNPs | HAuCl4 reduction using NaBH4 and/or sodium citrate | ||||
| Ab1/Au-GR/GCE | Au-GR | Mix HAuCl4 with GO and perform 5 CV scan in −1.5 V to 0 V at 50 mV/s | physical | AFP, CEA, CA125, PSA | [46] |
| Ab1/Au-PGO/GCE | Au-PGO | Treat GO, HAuCl4 and PEG mixture at 180 °C | physical | CA19-9 | [72] |
| Ab1/Au-Gra/GCE | Au-Gra | Treat GO-AA mixture with HAuCl4 at room temperature | physical | AFP | [73] |
| Ab1/AuNPs/CH-Thi-CNTs/GCE | AuNP | Electrochemical deposition at −200 mV | physical | CEA | [47] |
| Thi-CNT | Modify COOH-CNTs with thionine using EDC/NHS chemistry | ||||
| Ab1/GO-AuNP/GCE | GO AuNP | physical | CEA | [35] | |
| Ab1/HAG/PANI/rGO/GCE | HAG | Electrochemically deposited at −200 mV | physical | CEA, AFP | [36] |
| PANI | Electro-polymerization at 0.75 V | ||||
| Ab1/CH-AuNP/GCE | CH-AuNPs | NaBH4 based reduction of CH-HAuCl4 solution | EDC/NHS (covalent) | CEA, AFP | [52] |
| Ab1/NPG/GCE | NPG | Acid based removal of silver from silver gold alloy | physical | CA72-4 | [55] |
| Ab1/AuNPs/GCE | AuNPs | Electrodeposited at −0.2V | physical | CEA, AFP | [48] |
| Ab1/AuNPs/GCE | AuNPs | Electrodeposited at −0.2V | physical | AFP | [49] |
| Ab1/AuNPs/GCE | AuNPs | Sodium citrate based reduction in boiling condition | physical | AFP | [50] |
| AuNPs/GCE | Electrochemical deposition at 1.5 V | ||||
| Ab1/CH-AuNP/GCE | CH-AuNPs | NaBH4 based reduction of CH-HAuCl4 solution | Physical | CEA, AFP | [53] |
| Ab1/MoS2-Au/GCE | MoS2-Au | Citrate based reduction of HAuCl4-MoS2 nano-sheets solution | Physical | CEA | [56] |
| Ab1-TB/Au@MCM-41/GCE | NH2-MCM-41 | Treating MCM-41 with APTES at 70 °C | physical | AFP | [57] |
| AuNPs | HAuCl4 reduction using NaBH4 in ice bath | ||||
| Ab1/CH-AuNPs/GCE | CH-AuNPs | Refluxing CH-HAuCl4 solution for 1 h | glutaraldehyde (covalent) | CEA, AFP | [54] |
| Ab1-biotin/streptavidin/Au–Fe3O4@SiO2/Au/magnet | Fe3O4 | Treating FeCl2, FeCl3, and PEG 4000 mixture with NaOH at 80 °C | streptavidin-biotin interaction | CA 19-9 | [74] |
| Fe3O4@SiO2 | Treating PDDA-Fe3O4 solution pH 11 (using ammonia) with TEOS at room temperature | ||||
| Au–Fe3O4@SiO2 | Treating PDDA-Fe3O4@SiO2 solution with AuNPs solution | ||||
| Ab1/PSS/IL-rGO/GCE | IL-rGO | GO was mixed with IL-NH2 in KOH and treated at 80 °C | electrostatic | CEA, PSA, AFP | [75] |
| aptamer/AuNP/oPD/Au | oPD/Au | Electropolymerized via CV scans in −0.5 and 0.8 V range at 50 mV/s | physical | MUC 1 | [58] |
| Ab1/AuNPs/GCE | AuNPs | Electrodeposited at −0.2 V | physical | AFP | [51] |
| Ab1/PANI/Au/paper | Au | Seed layer using AuNPs prepared via NaBH4, citrate method; Au layer using growth solution of HAuCl4 cetyltrimethyl ammonium chloride | glutaraldehyde (covalent) | CEA, AFP | [76] |
| PANI | 20 CV scans in −0.1 to 0.8 V range at 50 mV/s | ||||
| Ab1/β-CD/GCE | oxidize GCE | 5 CV scans in H2SO4 solution in 0 to 2 V | physical | CEA | [60] |
| Ab1/PAMAM/GCE | PAMAM/GCE | Using infrared light treatment | EDC/NHS (covalent) | PSA | [61] |
| Ab1/cysteine /Au | cysteine /Au | Self-assembled monolayer | EDC/NHS (covalent) | CEA | [62] |
| PSA aptamer/GDPTS/PDMS | GDPTS/PDMS | Self-assembled monolayer | epoxide chemistry | PSA | [77] |
| Ab1/Au@MWCNTs-SO3H/GCE | MWCNTs-SO3H | Refluxing MWCNTs in H2SO4-HNO3 at 120 °C, 30 min | physical | PSA | [78] |
| AuNPs | Sodium citrate based reduction at 100 °C reflux | ||||
| Ab1/PDA-rGO/GCE | PDA-rGO | Mixing dopamine with GO and stirring for 24 h at 25 °C | physical | CEA | [79] |
| Ab1/AuNPs/GCE | AuNPs | Electrodeposited at −0.2 V | physical | CEA, NSE, CA125, Cyfra21–1, SCCA | [80] |
| Ab1/MPA/Au | MPA/Au | Self-assembled monolayer | EDC/NHS (covalent) | PSA, PSMA, IL-6, PF-4 | [81] |
| Ab1/MUDA-mercapto ethanol/Au | MUDA-mercapto ethanol | Self-assembled monolayer | EDC/NHS (covalent) | PSA | [82] |
| Ab1/PS | physical | PSA | [83] | ||
| Ab1/3D-G-CH/GCE | 3D-G | GO was first prepared from natural graphite powder by Hummer’s method followed by autoclaving at 180 °C to get 3D-G. Dried 3D-G was then mixed in 1% CS | glutaraldehyde (covalent) | CYFRA21-1 | [84] |
| Ab1/polystyrene; AgNP/SPCE | AgNPs | Sodium citrate-based reduction of AgNO3 in boiling condition | physical | AFP | [85] |
| antiHER2/APTMS-Fe3O4/GCE | Fe3O4 | Chemical co-precipitation from FeCl3·6H2O and FeCl2·4H2O mix using ammonia solution | glutaraldehyde (covalent) | HER2 | [86] |
| Anti-CEA/LPA/Au | NHS-LPA/Au | Self-assembly | covalent | CEA | [87] |
| Ab1-AuNPs/CHI/SPE | AuNPs | Electrochemical reduction in 0.5 M H2SO4 via CV scans between −1.5 and 0.5 V at a rate of 30 mV/s | physical | PSA | [88] |
| BSA/anti-CEA/AuNPs/GCE | AuNPs | Electrodeposit deposition by cyclic sweeping in the potential range of −0.5 to 0 V (vs. SCE) at 50 mV/s for 50 segments | physical | CEA | [89] |
| Ab1/Au@Th/GO/GCE | Au@Th/GO | GO synthesized using modified Hummers’ method was mixed with Thi and HAuCl4 solution and stir | physical | PSA | [90] |
| Ab1/Au@MWCNTs-SO3H/GCE | AuNPs | Citrate reduction of HAuCl4 solution; | physical | PSA | [78] |
| Au@MWCNT-SO3H | Physical adsorption of AuNPs on MWCNTs-SO3H | ||||
| Ab1/Au@MPTES-GS/GCE | AuNPs | Citrate reduction of HAuCl4 solution | physical | AFP | [91] |
| MPTES-GS | GO synthesized using modified Hummers’ method was treated with MPTES in ethanol at 70 °C for 2 h followed by treatment with hydrazine solution at 95 °C for 1.5 h | ||||
| Ab1/AuNPs/GCE | AuNPs | Electrochemical reduction at −0.2 V, 30 s | physical | CEA | [92] |
| Ab1/β-CD/MWCNT/GCE | β-CD/MWCNTs | Grind rMWCNTs and β-CD in ethanol | physical | CEA | [93] |
| Ab1/streptavidin-NG-CH/GCE | NG-S | GO synthesized using modified Hummers’ method was refluxed with hydrazine at 100 °C, 24 h. Obtained rGO was then mixed with pyrrole and treated with ammonium peroxydisulphate. Obtained PPY-rGO was heat treated till 600 °C, 2 h | Biotin-streptavidin | [94] | |
| Ab1/AuNPs/GCE | AuNPs | Electrochemical reduction at −0.2V, 30 s | physical | CEA | [95] |
| Fe3O4@AuNPs-Ab1 | Fe3O4 | From ferrous complex via hydrothermal method using H2O2 as oxidizer | physical | AFP | [96] |
| Fe3O4@AuNPs | Mixture of Fe3O4 NPs with PEG 20000 and HAuCl4 was treated with hydroxylamine hydrochloride | ||||
| Ab1/Au/ZnO/RGO/GCE | Au/ZnO/RGO | GO synthesized using modified Hummers’ method was mixed with C12N3. Solution was adjusted to pH 12 and mixed with Zn(NO3)2 and HAuCl4 followed by treatment with hydrazine at 105 °C, 5 h | physical | AFP | [97] |
| Ab1/CH/CNT/SPE | CH/CNT/SPE | Acid treated CNTs were mixed with nafion 117 and drop casted on SPE followed by deposition of CH solution | glutaraldehyde (covalent) | PSA | [98] |
| Ab1/AuNP/GCE | AuNPs | Electrochemical reduction at −0.2 V, 30 s | physical | SCCA | [99] |
| Ab1/AuNP/GCE | AuNPs | Electrochemical reduction at −0.2 V, 30 s | physical | AFP | [100] |
| Ab1-BSA/AuNP/PANI/GCE | PANI/GCE | Phytic acid doped polyaniline via electrochemical co-deposition at 0.8 V, 400 s | physical | PSA | [101] |
| AuNPs | Electrodeposit deposition by cyclic sweeping in the potential range of −1 to 0.2 V at 50 mV/s, 10 cycles | ||||
| Ab1/AuPd NCNs/GCE | AuPd NCNs | Add HAuCl4, H2PdCl4 and PVP sequentially into NaOH solution containing T7AA | physical | CA 15-3 | [102] |
| Ab1/Au@PDA/GCE | Au@PDA | Citrate reduced AuNPs were treated with dopamine in tris buffer | physical | CEA | [103] |
| Ab1/Au@N-GQD/GCE | N-GQD | Dicyandiamide and CA solution was autoclaved at 180°C, 12 h | physical | PSA | [104] |
| Au@N-GQD | HAuCl4 was added to N-GQD, pH adjusted to 10 using NaOH followed by autoclaving at 160 °C, 6 h |
| Detection Probe | Components | Preparation Conditions | Ref. |
|---|---|---|---|
| HRP, Anti-CEA/AuNPs-PAN@CNTs | PAN@CNTs | (NH4)2S2O8 based polymerization of CNTs and aniline monomers solution in HCl at ice bath | [47] |
| AuNPs | Citrate reduction | ||
| AuNPs-PAN@CNTs | Electrostatic assembly of AuNPs | ||
| HRP-PSA aptamer/AuNP-PAMAM | AuNP | Citrate reduction | [70] |
| thiol-PAMAM | Treating amine-terminated PAMAM dendrimer (G4) with methyl mercaptoacetate at 50 °C for 18 h | ||
| AuNP-PAMAM | Incubation for 5 h at RT | ||
| Thi-Anti-AFP/HRP NPs-hollow AuNPs | hollow AuNPs | HAuCl4 reduction in N2 environment using NaBH4, sodium citrate and CoCl2·6H2O mixed solution | [40] |
| HRP NPs-hollow AuNPs | Self-assembly of L-cysteine modified HRP-NPs prepared via desolvation followed by glutaraldehyde chemistry; | ||
| Thi-Anti-AFP | EDC/NHS chemistry | ||
| HRP, anti-AFP/Fe3O4 NPs-MSNs | Fe3O4 NPs-MSNs | Treating APTES modified MSNs with bromine-functionalized Fe3O4 NPs in EtOH | [64] |
| AgNPs-GOx-anti CEA | AgNPs | Ag nanospheres prepared via ethylene glycol (EG) and poly(vinyl pyrrolidone) (PVP) based reduction | [56] |
| HRP-anti CA 19-9/Au@SBA-15 | Au@SBA-15 | incubating PDDA coated SBA-15 particles with AuNPs solution | [74] |
| Anti-CEA/Ag/Au–DN–GR | Ag/Au–DN–GR | (i) HAuCl4, AgNO3, trisodium citrate dihydrate and SDS mixture reduction using NaBH4, (ii) Mix and incubate Ag/Au with DN-graphene | [34] |
| PAMAM-Gr/anti-AFP-HRP | PAMAM-Gr | EDC/NHS chemistry | [50] |
| HRP, GOD, anti-AFP/SWCNHs | Carboxylated SWCNHs | Acid treatment of SWCNHs | [73] |
| AuNPs-MCF | carboxy-MCF | Refluxing MCF in acid | [71] |
| AuNPs-MCF | NaBH4 based reduction of HAuCl4-MCF mixture | ||
| CHIT-PB-AuNP CHIT-FC-AuNP | CHIT-PB | Treating K3Fe(CN)6 and FeCl3 solution (pH 1.5) in CHIT | [52] |
| CHIT-FC | EDC/NHS chemistry; | ||
| AuNP binding | Physical adsorption by mixing | ||
| anti-AFP2,2-AuNPs-Thi@rGO | AuNPs-Thi@rGO | (i) Incubating rGO and Thi for 12 h, (ii) Incubating Thi@rGO with AuNPs for 24 h, (iii) Mixing FeCl3 and K3Fe(CN)6 solution (pH 1.5) to rGO dispersion | [36] |
| anti-CEA2,1-AuNPs-PB@rGO | AuNPs-PB@rGO | (i) Mixing PB@rGO with PDDA, 30 min, (ii) Incubation with AuNPs | |
| Anti-CEA /PB–CS-Au and Anti-CEA/Cd–CS-Au | PB–CS-Au and Cd–CS-Au | (i) PBNPs and CdNPs were prepared using FeCl3 and CdCl2 were first mixed with CS solution in 1% acetic acid, (ii) Incubating PBNPs and CdNPs with AuNPs | [44] |
| PLL-Au-Cd-Apo-Ab2 and PLL-Au-Pb-Apo-Ab2 | PLL-Au | Incubating PLL with citrate reduced AuNPs | [59] |
| Cd-Apo and Pb-Apo | Dropwise adding metal ions (Cd2+, Pb2+) to Apo solution pH 2 and adjusting pH to 8.5 before stirring for 3 h | ||
| anti-CEA/PtPNPs-Cd2+ and anti-AFP /PtPNPs-Cu2+ | PtPNPs-Cd2+ and PtPNPs-Cu2+ | (i) PtPNPs synthesised from chloroplatinic acid treatment with ascorbic acid in KOH, (ii) Mercapto-ethylamine modification of PtPNPs for capture of Cd2+ or Cu2+ ions | [29] |
| CdNCs–Au–anti-CEA and CuNCs– Au–anti-AFP | CdNCs–Au and CuNCs–Au | (i) Treating CdCl2 or CoCl2 in presence of CS with K3Co(CN)6 dispersed PDDA, (ii) Nanocubes incubation with AuNPs prepared via citrate and NaBH4 reduction | [53] |
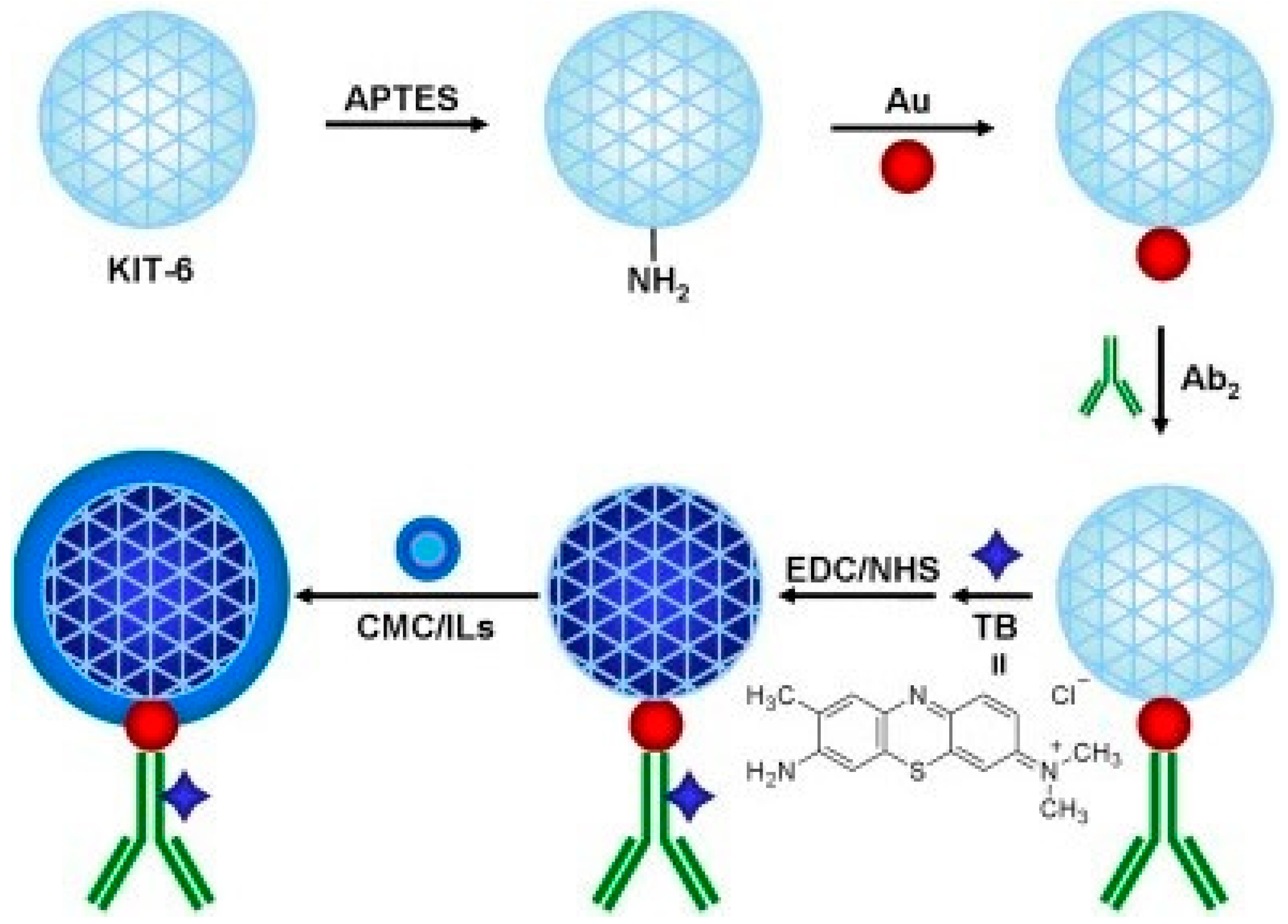
| TB/Au@KIT-6/CMC/ILs-anti-CEA | Au@KIT-6 | Treating APTES silanized KIT-6 with AuNPs prepared via NaBH4 reduction; | [45] |
| TB/Au@KIT-6/CMC/ILs-Ab2 | (i) Physical binding of anti-CEA on Au@KIT-6, (ii) TB binding using EDC NHS, (iii) Mixing and incubating with 1-butyl-pyridine tetrafluoroborate (ILs) dissolved in CMC | ||
| Ab2/M-Alg; (M: Cd, Pb and Cu) | M-Alg | (i) Emulsion A: agitate the mixture of triton x-100, 1-hexyl alcohol, n-octane and sodium for more than 30 min RT, (ii) Emulsion B: agitate mixture of triton x-100, 1-hexyl alcohol, n-octane and Metal salt for more than 30 min RT, (iii) Add emulsion A dropwise to emulsion B and stir for 4 h RT, (iv) Break M-Alg using acetone and ethanol to get M-Alg nanobeads | [43] |
| M/Ab2-Envision copolymer; (M: AuNPs, CdS and PbS) | Ab2-Envision copolymer | Mix and incubate Ab2 with Envision (highly branched polymer) at 4 °C, 24 h | [107] |
| M/Ab2-Envision copolymer | AuNP tagging via physical adsorption, CdS and PbS were bound to HRP modified envision-Ab2 via EDC/NHS chemistry | ||
| Au@MCM-41/TB/Ab2 | Au@MCM-41 | APTES modified MCM-41 was mixed with AuNPs prepared via NaBH4 based reduction of HAuCl4 | [57] |
| Au@CMK-3-anti-CEA-neutral red and Au@CMK-3-anti-SCCA-thionine | Au@CMK-3 | AuNPs were prepared from sodium citrate based reduction of HAuCl4 were mixed and stir with mesoporous carbon CMK-3 for 4 h | [66] |
| AuNPs-Ab2-Cu2+ or Pb2+ | AuNPs | Via sodium citrate based reduction of HAuCl4 | [54] |
| Cu2+ and Pb2+ tagging | Cu(NO3)2 or Pb(NO3)2 incubation with AuNPs-Ab2 | ||
| Redox tag bio-dsDNA/SA/bio-Ab2/Au/SiO2-Fe3O4 | Au/SiO2–Fe3O4 | (i) Nano-sized Fe3O4 via treating FeCl2-FeCl3 mixture with NaOH, (ii) Fe3O4–SiO2 via alkaline hydrolysis of TEOS, (iii) Au/SiO2–Fe3O4 via mixing and incubating PDDA treated SiO2–Fe3O4 with for 20 min | [46] |
| bio-dsDNA/SA/bio-Ab2/Au/SiO2-Fe3O4 | (i) Incubation of biotin-Ab2 Au/SiO2-Fe3O4 12 h, 4 °C, (ii) Treatment with streptavidin (SA), initiator bio-S1, bio-S2 and bio-S3 in sequence to form bio-dsDNA/SA/bio-Ab2/Au/SiO2-Fe3O4 via HCR reaction | ||
| Anti-CA15-3–f-TiO2–Cd2+ | nanoporous TiO2 | (i) Mixing and stirring tetrabutoxytitanium (TBOT) and ethylene glycol for 8 h, RT, (ii) Pouring mixture in acetone-water followed by vigorous stirring 1 h, (iii) Ethanol wash and drying at 50 °C, (iv) Mix with water and reflux for 1 h | [32] |
| f-TiO2–Cd2+ | (i) APTMS treatment to get NH2 functionalized nanoporous TiO2 (f-TiO2), (ii) Mixing f-TiO2 with Cd(NO3)2 and stirring for 24 h at 50 °C | ||
| Anti-PSA/Fc-AuNPs | Fc-AuNPs | Self-assembly of 6-ferrocenyl hexanethiol onto AuNPs | [68] |
| Apt/Thi-AuNPs/SiO2@MWCNTs | Apt/Thi-AuNPs/SiO2@MWCNTs | (i) Treat COOH-MWCNTs (c-MWCNTs) with PDDA, (ii) TEOS modification to get SiO2@MWCNTs, (iii) Treatment with PDDA, (iv) Incubation in AuNPs solution to obtain AuNPs/SiO2@MWCNTs, (v) Mixing and incubating with thionine 1 h, RT, (vi) Incubation with SH-Apt solution | [58] |
| Ab2-PGN | rGO | Mix and refluxing GO with PEI | [48] |
| PGN | Mix H2PtCl6 with rGO and treat with NaBH4 | ||
| Anti-CEA/APTES/3DGS@MB and anti-AFP/APTES/3DGS@Fc-COOH | 3DGS | NaI based reduction of GO prepared from graphite | [76] |
| APTES/3DGS@MB, APTES/3DGS@Fc-COOH | (i) Redox tag (MB for CEA and Fc-COOH for AFP) modification by mixing and stirring; (ii) Treatment with APTES to get amino functionalized composites | ||
| CGN-Thi-anti-CEA, CGN-DAP-anti-PSA and CGN-Cd2+-anti-AFP | CGN | (i) Glucose carbonization in presence of sodium citrate, (ii) AuNPs deposition on carbon particles from HAuCl4 using microwave reaction | [75] |
| Thi or DAP or Cd2+/CGN | Mixing Thi or DAP or Cd(NO3)2 with CGN and stirring for 5 h | ||
| M-Pt-Ab2 | M-Pt | Ascorbic acid based reduction of K2PtCl4 | [30] |
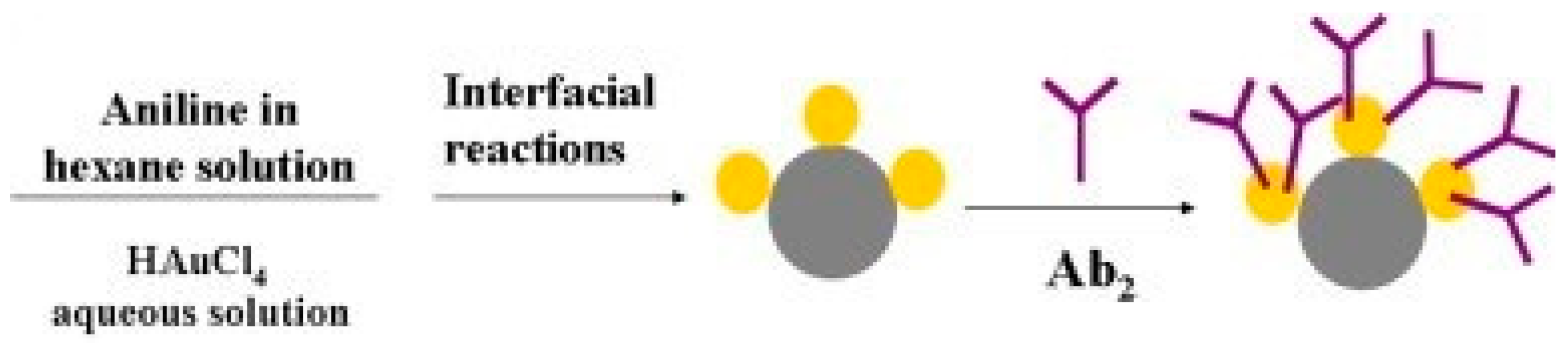
| Anti-CA72-4/PANi–Au AMNPs | PANi–Au AMNPs | Mix and incubate aniline in hexane with HAuCl4 aqueous solution at 45 °C overnight | [55] |
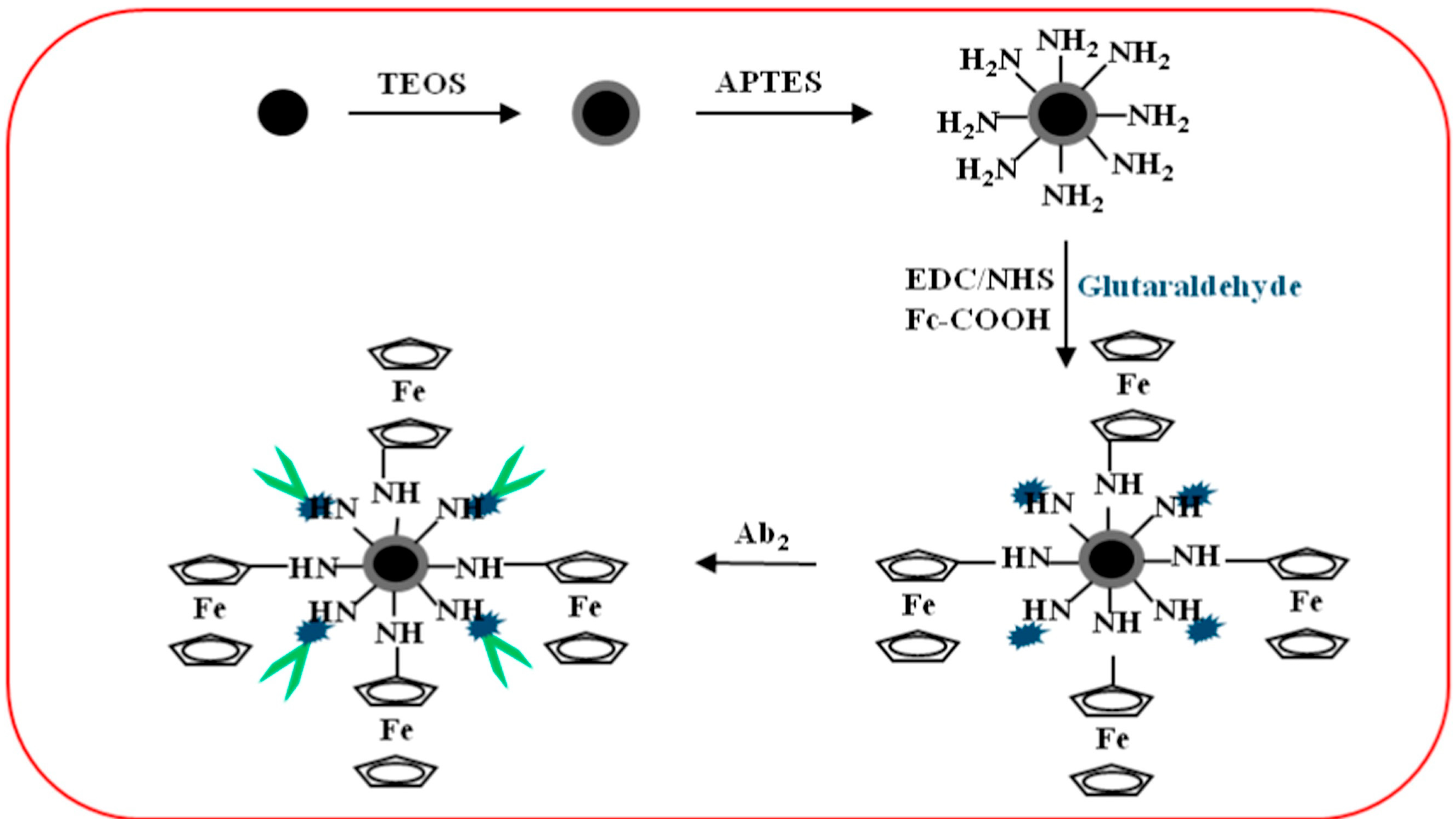
| Fe3O4@SiO2/Fc/GA/anti-CEA | Fe3O4@SiO2/Fc/GA/ | (i) Fe3O4 via solvothermal method; (ii) Treatment with TEOS to obtain Fe3O4@SiO2; (iii) Treatment with APTES to get Fe3O4@SiO2–NH2; (iv) Treatment with EDC/NHS activated Fc-COOH followed by treatment with GA | [35] |
| Anti-SCCA/Pd–Au/C | Pd–Au/C | Mixing activated carbon, PdCl2, HAuCl4 and H2O-tetrahydrofuran via ultra-sonicating and stirring followed by treatment with NaBH4 and Na2CO3 | [28] |
| Cu@Ag-CD/anti-CEA | Cu@Ag-CD | CD-ascorbic acid (pH 11) solution based sequential reduction of CuSO4·5H2O and AgNO3 solution in ammonia, followed by mixing and stirring with HS-β-CD overnight; Obtained Cu@Ag-CD was used for EDC/NHS based binding of Ab2 modified ADA-COOH | [37] |
| Fe3O4@C@Pd/anti-AFP | Fe3O4@C@Pd | (i) Fe3O4@C magnetic nanoparticles via hydrothermal process; (ii) Treatment with PDDA followed by mixing and incubation with PDNPs prepared via citrate and NaBH4 based reduction of Na2PdCl4 | [33] |
| Anti-CA15-3/NP-PtFe | NP-PtFe | By removing Al using NaOH from ternary PtFeAl alloy with 80%Al | [31] |
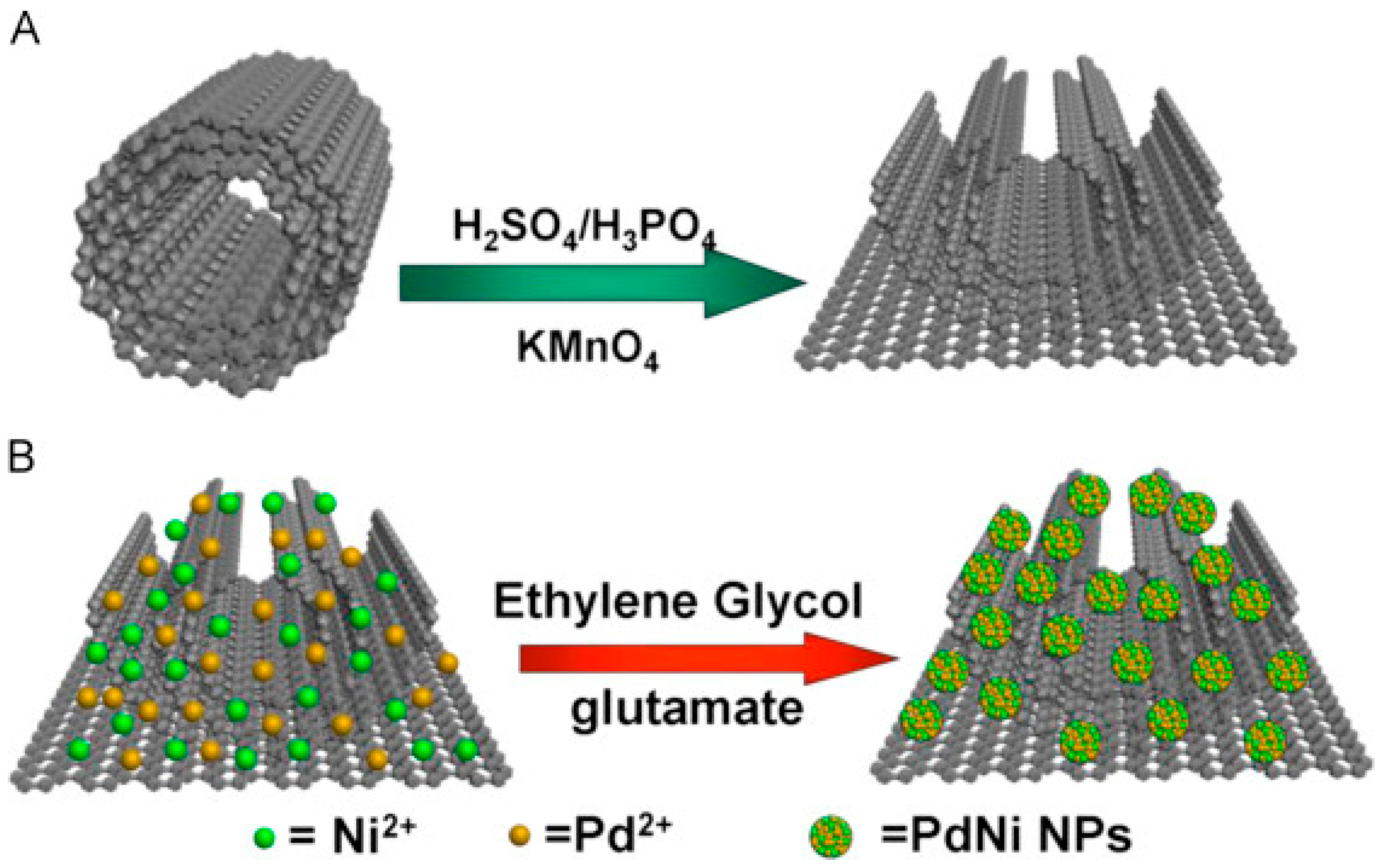
| Anti-AFP/PdNi/N-GNRs | PdNi/N-GNRs | (i) N-GNRs powders via microwave-assisted treatment of N-MWCNTs, (ii) Mix N-GNRs with aqueous solution of Na2PdCl4, NiCl2·6H2O, and glutamate in ethylene glycol (EG), (pH 11) and stirring it for 2h followed by heating at 160 °C for 6 h in autoclave to get PdNi/N-GNRs | [39] |
| Pb2+@Au@MWCNTs-Fe3O4/anti-AFP | Pb2+@Au@MWCNTs-Fe3O4 | (i) MWCNTs-Fe3O4 via autoclaving the mixture of acid treated MWCNTs, FeCl3.6H2O and sodium acetate, (ii) Amino-functionalization via APTES modification, (iii) Mixing and incubation with AuNPs prepared via citrate reduction; (iv) Treatment with lead nitrate solution 24 h to get Pb2+@Au@MWCNTs-Fe3O4 | [49] |
| Au/Ag/Au@anti-SCCA | Au/Ag/Au | (i) Mix AuNPs, ascorbic acid, and AgNO3 in CTAB solution, (ii) Add NaOH dropwise with vigorous stirring to get yellow-golden colored, silver coated Au particles, (iii) Mix with HAuCl4 and ascorbic acid and stirred vigorously to obtain dark-blue Au/Ag/Au NPs solution | [41] |
| Anti-AFP/Pd/APTES-M-CeO2-GS | Pd/APTES-M-CeO2-GS | (i) The Pd octahedral NPs via sonicating followed by heating the mixture of 1-ethenyl-2-pyrrolidinone homopolymer (PVP), citric acid, and Na2PdCl4 dissolved in ethanol and water at 80 °C with stirring and refluxing for 3 h, (ii) M-CeO2-GS prepared by dissolving Ce(NO3)3·6H2O into water followed by adding C2H5COOH, ethylene glycol and GO and then treating at 180 °C for 200 min followed by cooling, centrifuging the ppt and drying at 50 °C for 12 h, (iii) APTES modification of M-CeO2-GS by refluxing, (iv) Pd binding by sonication and stirring to get Pd/APTES-M-CeO2-GS | [51] |
| Anti-SCC-Pt–Fe3O4 | Pt–Fe3O4 | (i) Mix platinum acetylacetonate, oleic acid, oleylamine and octadecane under argon atmosphere followed by heating to 120 °C, (ii) add Fe(CO)5 heat at 280 °C, 20 min, (iii) Precipitation using ethanol addition | [65] |
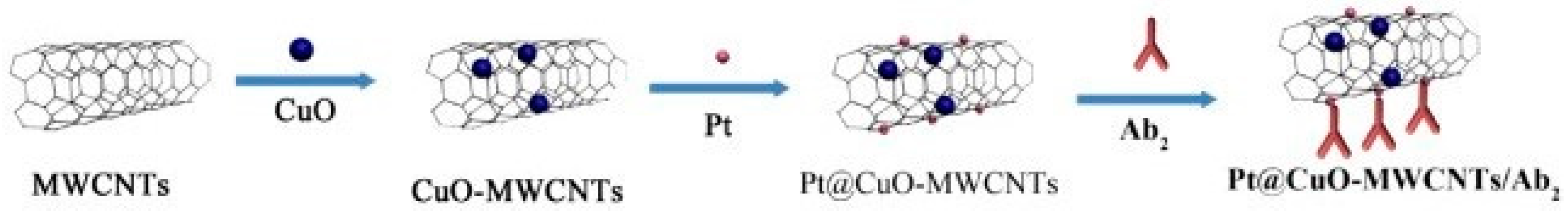
| Anti-AFP/Pt@CuO-MWCNTs | Pt@CuO-MWCNTs | (i) Acid treated MWCNTs mixed with Cu(CH3COO)2·H2O were grounded and calcinated at 350 °C in argon, followed by addition of NH4OH solution, (ii) MWCNTs addition followed by ageing and calcination to get CuO/MWCNTs composite, (iii) Pt loading by adding CuO/MWCNTs nanocomposites to K2PtCl4 solution followed by Pt salt reduction | [38] |
| M-Pd@Pt/NH2-GS/anti-PSA | NH2-GS | (i) Mix GO and ethylene glycol under ultrasonication followed by ammonia water addition, (ii) Autoclave for solvothermal reaction at 180 °C, 10 h | [78] |
| M-Pd@Pt | (i) Mix Pluronic F127 with aqueous solution of K2PtCl4, Na2PdCl4 and hydrochloric, (ii) Reduction using ascorbic acid at 35 °C for 4 h | ||
| M-Pd@Pt/NH2-GS | Mix and sonicate NH2-GS and M-Pd@Pt for 1 h | ||
| Ir NPs-anti-CEA | PVP stabilized Ir NPs | (i) Add aqueous IrCl3 solution dropwise to ethanol solution containing PVP followed by mixing and stirring at 25 °C for 12 h, (ii) Refluxed in air at 100 °C for 6 h followed by evaporation | [79] |
| PBG-Au-anti-CEA; PPP-Au-anti-NSE; PTBO-Auanti-CA125; PMCP-Au-anti-Cyfra21–1; Cd NCs-Auanti-SCCA | PBG-Au | Add and stir TTAB to brilliant green aqueous solution followed by HAuCl4 addition and agitation for 4 h, RT | [80] |
| PPP-Au | Add water with stirring to DMF solution of N-phenyl-p-phenylenediamine followed by HAuCl4 addition and agitation for 4 h, RT | ||
| PTBO-Au | Add HAuCl4 to toluidine blue o aqueous solution and agitate for about 4 h, RT | ||
| PMCP-Au | Add and stir DTAB to m-cresol purple ethanol solution followed by HAuCl4 addition and agitation for 4 h, RT | ||
| Cd NCs-Au | Mix Cd NCs with gold colloid and stirred for 4 h | ||
| HRP-anti-CYFRA21-1/AuNPs/Thi/MWCNT-NH2 | AuNPs | HAuCl4 reduction using NaBH4 in Thi/MWCNT-NH2 solution | [84] |
| MWCNT-NH2 | Acid treatment of MWCNT to get MWCNT-COOH followed by treatment with HMDA in presence of DCC for 96 h at 120 °C | ||
| anti-AFP-Co3O4@MnO2-Thi | Co3O4@MnO2 | Mixture of Co(CH3COO)2·4H2O and MnO2 nanotubes in ammonium hydroxide autoclaved at 150 °C, 5 h followed by calcination at 300 °C, 1 h | [85] |
| Co3O4@MnO2-Thi | Co3O4@MnO2 treatment with APTES at 70 °C, 1.5 h followed by incubation with Thi at 95 °C, 1 h | ||
| anti-AFP-Co3O4@MnO2-Thi | EDC/NHS chemistry | ||
| antiHER2/Hyd@AuNPs-APTMS-Fe3O4 | antiHER2/Hyd@AuNPs-APTMS-Fe3O4 | AuNPs preparation using HAuCl4 reduction via NaBH4, sodium citrate followed by treatment with APTMS-Fe3O4. Resulting AuNPs-APTMS-Fe3O4 were treated with thiolated anti-HER2 followed by treatment with hydrazine | [86] |
| Anti-CEA-AuNPs-Fc | AuNPs | Reduction of chloroauric acid with trisodium citrate | [87] |
| Anti-CEA-AuNPs-Fc | Physical immobilization of anti-CEA on AuNPs followed by chemisorption of Fc-SH | ||
| HRP-anti-CEA-AuNPs-TiO2-graphene | TiO2-graphene | Sonicate graphene with dopamine for 1 h, followed by stirring with TiO2 | [89] |
| HRP-anti-CEA-AuNPs-TiO2-graphene | Treat TiO2-graphene with HAuCl4 under ultraviolet irradiation followed by physical adsorption of HRP-anti-CEA | ||
| PtCu@rGO/g-C3N4/anti-PSA | PtCu@rGO/g-C3N4/anti-PSA | Physical adsorption of anti-PSA on PtCu@rGO/g-C3N4 | [90] |
| M-Pd@Pt/NH2-GS/anti-PSA | NH2-GS | GO prepared via modified Hummer’s method was mixed with ethylene glycol and ammonia followed by autoclaving at 180 °C for 10 h | [78] |
| M-Pd@Pt | Pluronic F127 was mixed with K2PtCl4 and Na2PdCl4 in HCl followed by reducing with ascorbic acid | ||
| Anti-AFP-Pt NPs/Co3O4/graphene | Pt NPs/Co3O4/graphene | Mix GO and Co(NO3)2·6H2O in ethanol and add ammonia solution followed by autoclaving at 190 °C for 24 h. Obtained Co3O4/graphene was mixed with Na2PtCl4 in ethanol aqueous solution and treat with NaBH4 | [91] |
| GS-Fe3O4/Au@Ag/Ni2+-anti-CEA | NH2-GS-Fe3O4 | GO prepared via modified Hummer’s method was mixed with clear solution of FeCl3·6H2O in ethylene glycol along with NaAc and ethanediamine and autoclaved at 200 °C for 8 h. Resulting GS-Fe3O4 was treated with APTES to get NH2-GS-Fe3O4 | [92] |
| Au@Ag | AuNPs prepared via citrate reduction were mixed with ascorbic acid, AgNO3 and CTAB solution and treated with NaOH | ||
| GS-Fe3O4/Au@Ag/Ni2+-anti-CEA | GS-Fe3O4/Au@Ag made by mixing NH2-GS-Fe3O4 and Au@Ag were dispersed in Ni(NO3)2·6H2O solution and stir for 24 h, anti CEA was immobilized via physical adsorption | ||
| Ag NPs-MWCNTs/MnO2-Anti-CEA | Ag NPs-MWCNTs/MnO2 | Acid treated MWCNTs were dispersed in KMnO4 solution and treated with MnSO4. Obtained MWCNTs/MnO2 were mixed with AgNO3 in water followed by reduction NaBH4 | [93] |
| PdCu-anti-CEA | PdCu | Using AA as reducing agent and HDPC as growth inhibitor | [95] |
| anti-AFP-GNPs-HRP | GNP | Citrate reduction | [96] |
| anti-AFP/HRP-Au@ZnO | Au@ZnO | C18N3 was added to mixture of Zn(NO3)2 and HAuCl4 and heated at 145 °C, 5 h | [97] |
| anti-PSA/AuNPs | AuNPs | Citrate reduction in cold for smaller size and in boiling condition for large size | [98] |
| Co3O4@CeO2-Au@Pt-anti-SCCA | Co3O4@CeO2 | Co(NO3)2·6H2O solution was treated with NaOH at 180 °C, 5 h. Obtained Co3O4 cubes were mixed in ethanol aqueous solution followed by addition of Ce(NO3)3 and HMT and refluxing at 70 °C, 2 h | [99] |
| Au@Pt | Citrate reduced AuNPs were mixed with H2PtCl6 under boiling conditions followed by reduction with AA | ||
| Co3O4@CeO2-Au@Pt | APTES treated Co3O4@CeO2 were mixed with Au@Pt and stir for 12 h at room temperature. | ||
| Anti-AFP/Au@Ag/PDA-PR-MCS | PR-MCS | C6H5OH and HCHO were added to solution containing NH4OH and C2H5OH and autoclaved at 100 °C, 24 h. Product was mixed with KOH and treated at 350 °C, 1 h followed by at 700 °C, 2 h | [100] |
| Au@Ag | Citrate reduced AuNPs were mixed with AgNO3 solution and treated with NaBH4 solution | ||
| Au@Ag/PDA-PR-MCS | PR-MCS dispersed in tris buffer was treated with Dopamine hydrochloride 24 h and mixed with Au@Ag solution | ||
| MSN-MB/PDA-anti-PSA | MSN | Mixture of CTAB and pluronics F127 in ethanol, water and ammonia was treated with TEOS | [101] |
| MSN-MB/PDA | MB loaded MSN was treated with dopamine in tris buffer, pH 8.5 | ||
| Au@Pt DNs/NG/Cu2+-anti-CEA | NG | GO prepared via modified Hummer’s method was treated with ammonia solution at 90 °C, 4 h | [103] |
| Au@Pt DNs | NaBH4 and AA reduced HAuCl4 and CTAB solution was mixed with K2PtCl4 and AA and treated at 60 °C, 12 h | ||
| Au@Ag-Cu2O/anti-PSA | Au@Ag-Cu2O | Citrate reduced AuNPs were mixed with CTAC and AgNO3 followed by reduction using AA at 30 °C, 2 h. Obtained Au@Ag solution was mixed with CuCl2 and SDS followed by treatment with NaOH and NH2OH·HCl | [104] |
| Probe | Immunosensor Conditions | Characteristics | Ref. |
|---|---|---|---|
| [DP]: anti-HER2-HRP [Anal]: HER2 [DM]: HQ | [Tran]: amperometry at −280 mV [IC]: (i) anal for 2 min at RT, (ii) [DP] for 20 min [MC]: 2.5 mm H2O2 with HQ in citrate buffer | [L]: 1 and 200 µg/mL, [LgS] [DL]: 1 µg/mL [S]: 18.23 µA/(µg/mL) [SL]: 3 weeks [CR]: 0.9591 | [63] |
| [DP]: anti-CEA-HRP/AuNPs-PAN@CNTs [Anal]: CEA [DM]: H2O2 | [Tran]: DPV in 0.2 to −0.8 V, [PA] 50 mV [IC]: (i) CEA, (ii) [DP] for 55 min at 37 °C, sequentially [MC]: 4 mM H2O2 in 5.0 mL PBS | [L]: (i) 0.02–3.0 ng/mL, (ii) 3.0–80 ng/mL [LS] [DL]: 0.008 ng/mL [S]: (i) 13.9465 µA/(ng/mL), (ii) 0.7342 µA/(ng/mL) [SL]: 30 days [CR]: (i) 0.9875, (ii) 0.9960 | [47] |
| [DP]: AuNP–PAMAM dendrimer/PSA–aptamer-HRP [Anal]: PSA [DM]: thionine | [Tran]: DPV in −0.4 to −0.1 V [IC]: (i) PSA conc. for 15 min, (ii) [DP] for 20 min [MC]: 3 mM H2O2 | [L]: 0.1 pg/mL to 90 ng/mL [LS] [DL]: 10 fg/mL [S]: 0.3635 µA/(pg/mL) [SL]: 3 weeks [CR]: 0.9831 | [70] |
| [DP]: HRP-HRP-NP-hollow Au-NP-Thi@anti-AFP [Anal]: AFP [DM]: thionine | [Tran]: DPV in −0.4 to 0 V [IC]: (i) AFP conc for 16 min, 37 °C, (ii) [DP] for 30 min, RT [MC]: 4.2 mM H2O2 | [L]: 0.025 to 5.0 ng/mL [LgS] [DL]: 8.3 pg/mL [S]: 7.649 µA/(ng/mL) [SL]: 30 days [CR]: 0.9949 | [40] |
| [DP]: anti-AFP, HRP/MSNs-Fe3O4 [Anal]: AFP [DM]: thionine | [Tran]: CV in −0.6 to 0.6 V (vs. SCE) at 100 mV/s in PBS (pH 7.4) [IC]: (i) AFP, (ii) [DP] for 1 h, sequentially [MC]: 5 mmol/L H2O2 in PBS | [L]: 0.01 to 25 ng/mL [LS] [DL]: 4 pg/mL [SL]: 15 days | [64] |
| [DP]: GOx/anti-CEA/AgNPs [Anal]: CEA [DM]: H2O2 | [Tran]: DPV in −0.2 to −0.8 V, [PA]: 50 mV, [PW]: 20 ms [IC]: (i) CEA conc 40 min, RT, (ii) [DP] 1 h, 4 °C [MC]: PBS + 1% glucose | [L]: 1 pg/mL to 50 ng/mL [LgS] [DL]: 0.27 pg/mL [S]: 8.281 µA/(ng/mL) [SL]: 30 days [CR]: 0.9971 | [56] |
| [DP]: HRP-Ag@BSA-anti-CEA [Anal]: CEA [DM]: tyramine | [Tran]: DPV in 0 to -600 mV vs. SCE [PA]: 50mV, [PW]: 50 ms in PBS [IC]: (i) CEA conc 40 min, RT, (ii) [DP] 40 min, RT, (iii) 2 mM H2O2 + HRP-tyramine conjugates 10 min at RT [MC]: 2.5 mM H2O2 in PBS | [L]: 0.005–80 ng/mL [LgS] [DL]: 5.0 pg/mL [S]: 1.617 µA/(ng/mL) [SL]: 28 days [CR]: 0.9867 | [108] |
| [DP]: HRP/HRP-anti-CA 19-9/Au@SBA-15 [Anal]: CA 19-9 [DM]: H2O2 | [Tran]: chronoamperometry PBS pH 6 at −0.2 V [IC]: (i) CA 19-9 conc 1 h, 37 °C; (ii) [DP] 1 h, 37 °C [MC]: 3 mM H2O2 in PBS | [L]: 0.05 to 15.65 U/mL [LgS] [DL]: 0.01 U/mL [S]: 20.51 g/L [SL]: 30 days [CR]: 0.992 | [74] |
| [DP]: anti-CEA –Ag/Au–DN-graphene [Anal]: CEA [DM]: Ag | [Tran]: CV in −0.6 to1.0 V (vs. SCE) at 50 mV/s [IC]: (i) CEA conc 30 min, (ii) [DP]: 40 min [MC]: 0.1M PBS (pH7.0). | [L]: 10 to 1.2 × 105 pg/mL [LgS] [DL]: 8 pg/mL [S]: 0.494 µA/(ng/mL) [CR]: 0.9899 | [34] |
| [DP]: PAMAM-Gr/anti-AFP-HRP [Anal]: AFP [DM]: hydroquinone | [Tran]: (i) amperometric at −0.2 V, (ii) CV −0.5 to +0.5 V, 50 mV/s [IC]: (i) AFP conc 40 min, 37 °C, (ii) [DP]: 40 min, 37 °C [MC]: PBS containing 1 mM hydroquinone + 2 mM H2O2 | [L]: 1.0–100 ng/mL [LS] [DL]: 0.45 ng/mL | [50] |
| [DP]: Au@Pd-Gra/Thi-anti-CA 19-9/HRP [Anal]: CA19-9 [DM]: Thionine | [Tran]: DPV in −0.4–0 V, [PA]: 50 mV, [PW]: 50 ms, [PP]: 0.2 s [IC]: (i) CA 19-9 conc 40 min, 25 °C, (ii) [DP] [MC]: 1.5 mM H2O2 | [L]: 0.015 to 150 U/mL [LgS] [DL]: 0.006 U/mL [S]: 9.8328 µA/(U/mL) [SL]: 30 days [CR]: 0.9982 | [72] |
| [DP]: HRP, GOD, anti-AFP/SWCNHs [Anal]: AFP [DM]: 4-CN | [Tran]: Impedance [IC]: (i) AFP conc 40 min, 37 °C, (ii) [DP] 40 min, 37 °C, (iii) 1.0 mM 4-CN and 10.0 mM glucose in 10mM PBS 15 min, RT [MC]: 0.01M PBS (pH 7.4) containing 5 mM FeCN63−/4− and 0.1M KCl | [L]: 0.001 to 60 ng/mL [LgS] [DL]: 0.33 pg/mL [S]: 230.60 Ω/(ng/mL) [SL]: 30 days [CR]: 0.996 | [73] |
| [DP]: anti-CEA/Au/MCF [Anal]: CEA [DM]: Ag | [Tran]: ASV in −0.08 to 0.2 V, 50 mV/s [IC]: (i) CEA conc 40 min, 37 °C, (ii) [DP] 40 min, 37 °C, (iii) silver-deposition with enhancer solutions 4 min, 37 °C, [MC]: 1.0 M KCl | [L]: 0.05 pg/mL to 1 ng/mL [LgS] [DL]: 0.024 pg/mL [SL]: 15 days [CR]: 0.9997 | [71] |
| [DP]: anti-AFP/CHIT–PB–AuNP; anti-CEA/CHIT–Fc–AuNPs, [Anal]: CEA, AFP [DM]: PB, Fc | [Tran]: DPV in −0.2 to 0.8 V [IC]: 45 min incubation for CEA, AFP concentration [MC]: PBS | [L]: 0.05–100 ng mL−1 for AFP and CEA [LgS] [DL]: 0.03 ng mL−1 and 0.02 ng mL−1 for AFP and CEA [S]: 0.47067 µA/(ng/mL), 0.51106 µA/(ng/mL) for AFP and CEA [CR]: 0.99712 for AFP and 0.99806 for CEA | [52] |
| [DP]: anti-AFP-AuNPs-Thi@rGO and anti-CEA-AuNPs-PB@rGO [Anal]: CEA, AFP [DM]: PB, Thionine | [Tran]: DPV 600 to −600 mV; [PA] 50 mV. [IC]: (i) CEA/AFP conc 50 min, 37 °C, (ii) [DP] 50 min, 37 °C [MC]: PBS pH 6.5 | [L]: 0.6–80 ng/mL for both [LS] [DL]: 0.12 ng/mL and 0.08 ng/mL for CEA and AFP [S]: 0.0188 µA/(ng/mL), 0.0273 µA/(ng/mL) for CEA and AFP [SL]: 30 days [CR]: 0.9908, 0.9936 for CEA and AFP | [36] |
| [DP]: Anti-CEA/PB–CS-Au and anti APF/Cd–CS-Au [Anal]: CEA, AFP [DM]: PB, Cd | [Tran]: DPV in −0.1V to 0.9V (vs. Ag/AgCl), [PA]: 50 mV, [PW]: 50 ms [IC]: (i) CEA/AFP conc 40 min, (ii) [DP] mixture 1:1 [MC]: 0.1 M pH 6.5 phosphate buffered solution (PBS) | [L]: 0.01 to 100 ng/mL range for both [LgS] [DL]: 0.006 ng/mL for AFP and 0.01 ng/mL for CEA [S]: 1.771 µA/(ng/mL), 1.751 µA/(ng/mL) for CEA and AFP [CR]: 0.996 and 0.995 for CEA and AFP | [44] |
| [DP]: PLL-Au-Cd-Apo-anti-AFP and PLL-Au-Pb-Apo-anti-CEA [Anal]: AFP and CEA [DM]: Cd, PB | [Tran]: SWV scan from −1.0 to −0.3 V with frequency of 15 Hz, [PA]: 25 mV, potential step 4 mV, quiet time 2 s to measure AFP and CEA at −0.78 V and −0.53 V [IC]: (i) CEA/AFP conc 20 min, RT, (ii) [DP] 20 min, RT [MC]: (i) immuno-complex in acetate buffer containing 400 µg/L bismuth, (ii) deposition of bismuth film and metal ions in situ at −1.2 V for 120 s | [L]: 0.01–50 ng/mL for both [LgS] [DL]: 4 pg/mL for both [S]: 6.65 µA/(ng/mL), 6.62 µA/(ng/mL), for AFP and CEA [SL]: 25 days [CR]:0.992, 0.994 for AFP and CEA | [59] |
| [DP]: anti-CEA-PtPNP-Cd2+ and anti-AFP-PtPNPs-Cu2+ [Anal]: CEA and AFP [DM]:Cd2+, Cu2+ | [Tran]: DPV in 0.2 to −0.9 V with [PA]: 50 mV, [PW]: 50 ms and quiet time of 2 s were recorded for CEA and AFP at −0.736 V and 0.004 V respectively [IC]: (i) CEA/AFP conc 1 h, 37 °C, (ii) [DP] 1 h, 37 °C [MC]: acetate buffer solution (0.2 M, pH 4.5). | [L]: 0.05 ng/mL to 200 ng/mL range for both CEA and AFP [LgS] [DL]: 0.002 ng/mL and 0.05 ng/mL for CEA and AFP [S]: 2.26 µA/(ng/mL), 1.06 µA/(ng/mL), for CEA and AFP [CR]: 0.997, 0.998 for CEA and AFP | [29] |
| [DP]: CdNCs–Au–anti-CEA and CuNCs–Au– anti-AFP [Anal]: CEA and AFP [DM]: CdNCs and CuNCs | [Tran]: SWV in 0.1 to −0.9 V with [PA]: 25 mV, pulse frequency 15 Hz, were recorded for CEA and AFP at −0.7 V and −0.1 V (vs. Ag/AgCl), [IC]: (i) CEA/AFP conc 50 min, 37 °C, (ii) [DP] 50 min, 37 °C [MC]: acetate buffer solution (0.2 M, pH 6). | [L]: 0.025 to 250 ng/mL range for both [LgS] [DL]: 0.0175 ng/mL and 0.0109 ng/mL for CEA and AFP [S]: 4.31 µA/(ng/mL), 3.858 µA/(ng/mL), for CEA and AFP [CR]: 0.998 for CEA and AFP | [53] |
| [DP]: TB/Au@KIT-6/CMC/ILs-anti-CEA [Anal]: CEA [DM]: TB | [Tran]: DPV in −0.6 V to 0 V [IC]: (i) CEA conc 1 h, RT, (ii) [DP] 1 h [MC]: PBS pH 6.8 | [L]: 10−5 ng/mL to 102 ng/mL [LgS] [DL]: 3.3 fg/mL [S]: 3.32 µA/(ng/mL) [SL]: 2 weeks [CR]: 0.99 | [45] |
| [DP]: Cd-Alg-anti-AFP, Pb-Alg-anti-CEA and Cu-Alg-anti-PSA [Anal]: AFP, CEA and PSA [DM]: Cd, Pb, Cu | [Tran]: DPV in −0.9 to 0.2 V to measure AFP, CEA and PSA at −0.76 V, −0.5 V and 0.12 V (vs. Ag/AgCl) [IC]: (i) CEA/AFP/PSA conc 50 min, 37 °C, (ii) [DP] 50 min, 37 °C [MC]: acetate buffer solution (0.2 M, pH 5). | [L]: 0.01 to 100 ng mL−1 for all [DL]: 0.01, 0.0086 and 0.0075 ng/mL for AFP, CEA and PSA [S]: 5.548 µA/(ng/mL), 3.737 µA/(ng/mL), 4.586 µA/(ng/mL), for AFP, CEA and PSA [SL]: 15 days [CR]: 0.993, 0.994, 0.996 for AFP, CEA and PSA | [43] |
| [DP]: anti-CA19-92/Envision/Au, anti-AFP2/Envision/CdS and anti-CEA2/Envision/PbS [Anal]: CA19-9, CEA and AFP [DM]: Au (via CSV), CdS, PbS (via ASV) | [Tran]: ASV with accumulation at −1.2 V for 120 s, and scanning from −1.0 to −0.3 V, with [PS]: 4 mV, frequency 15 Hz, and [PA]: 25 mV. CSV +1.3 V for 30 s, immediately followed by DPV detection from +0.6 V to 0 V, with [PS]: 4 mV, [PA]: 50 mV, and pulse period of 0.2 s. [IC]: (i) Ca 19-9/CEA/AFP conc 30 min, RT, (ii) [DP] 30 min, RT [MC]: (i) GCE was incubated in pH 2.0 bismuth nitrate solution in acetate and treated at −1.2 V for 120 s, (ii) immune-complex in 0.1 M HCl | [L]: 5 pg/mL–100 ng/mL, 1 pg/mL–50 ng/mL, and 1 pg/mL–50 ng/mL for CA19-9, CEA and AFP [LgS] [DL]: 0.3, 0.05, 0.02 pg/mL for CA19-9, CEA and AFP [S]: 6.65, 7.32, 0.60 µA/(ng/mL) for AFP , CEA and CA19-9 [SL]: 60 days [CR]:0.99, 0.997, 0.993 for AFP , CEA and CA19-9 | [107] |
| [DP]: Au@MCM-41/TB/anti-AFP [Anal]: AFP [DM]: TB | [Tran]: DPV in −0.6 V to 0.2 V. [IC]: (i) AFP conc 1 h, RT, (ii) [DP] 1 h, RT [MC]: PBS pH 6.8 | [L]: 10−4 ng/mL to 103 ng/mL [LgS] [DL]: 0.05 pg/mL [S]: 1.43 µA/(ng/mL), [SL]: 2 weeks [CR]: 0.99 | [57] |
| [DP]: Au@CMK-3-anti-CEA-neutral red and Au@CMK-3-anti-SCCA-thionine [Anal]: CEA and SCCA [DM]: neutral red, thionine | [Tran]: DPV in −0.7 to 1 V for recording −0.62 V (neutral red), and −0.17V (thionine) [IC]: (i) CEA/SCCA conc 1 h, RT, (ii) [DP] 1 h [MC]: PBS pH 7.4 | [L]: 0.05 to 20 ng/mL and 0.03 to 20 ng/mL range for CEA and SCCA [LS] [DL]: 0.013 ng/mL and 0.010 ng/mL for CEA and SCCA [SL]: 10 days | [66] |
| [DP]: AuNPs–anti-CEA–Cu2+ and AuNPs–anti-AFP–Pb2+ [Anal]: CEA and AFP [DM]: Cu2+, Pb2+ | [Tran]: DPV in −0.7 V to 0.3 V (vs. SCE), [PA] 50 mV, [PW] 50 ms [IC]: (i) CEA/AFP conc 35 min, 37 °C, (ii) [DP] 45 min, 37 °C [MC]: HAc/NaAc (0.2 M, pH 3.5) | [L]: 0.01–50 ng/mL for both [LgS] [DL]: 4.6 pg/mL and 3.1 pg/mL for CEA and AFP [S]: 3.3 µA/(ng/mL), 4.86 µA/(ng/mL), for CEA and AFP [CR]:0.9967, 0.9991 for CEA and AFP | [54] |
| [DP]: Aq-SA/bio-dsDNA/SA/bio-anti-AFP/Au/SiO2–Fe3O4, Thi-SA/bio-dsDNA/SA/bio-anti-CEA/Au/SiO2–Fe3O4, Co-SA/bio-dsDNA/SA/bio-anti-CA125/Au/SiO2–Fe3O4, Fc-SA/bio-dsDNA/SA/bio-anti-PSA/Au/SiO2–Fe3O4 [Anal]: AFP, CEA, CA125 and PSA [DM]: Aq, Thi, Co, Fc | [Tran]: DPV in −0.7 to 0.7 V incre: 0.004 V, [PA]: 0.05 V, [PW]: 0.05 s, sampling width: 0.0167 s, pulse period: 0.2 s to record AFP at −0.52 V, CEA at −0.21V, CA125 at 0.0V, PSA at 0.26V [IC]: (i) AFP/CEA/CA125/PSA conc 40 min, 37 °C, (ii) [DP]: 40 min, 37 °C [MC]: PBS 0.1 M, pH: 7.4 | [L]: 0.2 to 800 pg/mL, 0.2 to 600 pg/mL, 0.2 to 1000 pg/mL, and 0.2 to 800 pg/mL for AFP, CEA, CA125 and PSA [LgS] [DL]: 62, 48, 77 and 60 fg/mL for AFP, CEA, CA125 and PSA [S]: 22.71 µA/(pg/mL), 21.91 µA/(pg/mL), 33.69 µA/(pg/mL),21.30 µA/(pg/mL), for for AFP, CEA, CA125 and PSA [SL]: 14 days [CR]: 0.9797, 0.9696, 0.9791, 0.9786 for AFP, CEA, CA125 and PSA | [46] |
| [DP]: anti-CEA-AuNP-So [Anal]: CEA [DM]: MB | [Tran]: DPV 0 to −500 mV vs SCE [IC]: (i) CEA conc + [DP] 40 min, RT, (ii) hybridization mix 80 min, RT, (iii) hemin incubation 50 min RT, (iv) methylene blue incubation 30 min at RT [MC]: PBS (pH 7.0) containing 3.0 mM H2O2 | [L]: 1.0 fg/mL to 20 ng/mL [LgS] [DL]: 0.5 fg/mL [S]: 1.9636 µA/(ng/mL), [SL]: 30 days [CR]: 0.9973 | [60] |
| [DP]: anti-CA15-3–f-TiO2–Cd2+ [Anal]: CA15-3 [DM]: Cd2+ | [Tran]: SWV in −1 to −0.45 V [IC]: (i) CA15-3 conc 1 h, 4 °C, (ii) [DP] 1 h, 4 °C [MC]: PBS (pH 5.4) | [L]: 0.02–60 U/mL [LS] [DL]: 0.008 U/mL [S]: 1.806 µA/(U/mL), [SL]: 4 weeks [CR]: 0.998 | [32] |
| [DP]: PtNP@ICP-anti-PSA [Anal]: PSA [DM]: FcDA | [Tran]: DPV at 0.31 V vs. SCE [IC]: (i) PSA conc 30 min, RT, (ii) [DP] 30 min, RT, [MC]: 5.0 mM H2O2 in the PBS pH 7.0 | [L]: 0.001 to 60 ng/mL [LgS] [DL]: 0.3 pg/mL [S]: 1.85129 µA/(ng/mL), [SL]: 1 week [CR]: 0.988 | [61] |
| [DP]: anti-PSA-Fc-AuNP [Anal]: PSA [DM]: Fc | [Tran]: DPV in 0 to 0.6 V [IC]: (i) PSA conc 75 min, 37 °C, (ii) [DP] 90 min, 37 °C, [MC]: PBS | [L]: 10 pg/mL to 100 ng/mL [LS] [DL]: 5.4 pg/mL [S]: 0.137 µA/(ng/mL), [CR]: 0.9907 | [68] |
| [DP]: Aptamer/Thi-AuNPs/SiO2@MWCNTs [Anal]: MUC 1 [DM]: Thionine | [Tran]: DPV in −0.33 to −0.1 V [IC]: (i) MUC1 conc 40 min, 37 °C, (ii) [DP] 40 min, 37 °C, [MC]: PBS pH 7.4 | [L]: 10−3 to 1 nM, 1–100 nM [LS] [DL]: 1 pM [S]: 1.647 nA/nM [SL]: 30 days [CR]: 0.98 | [58] |
| [DP]: HRP-GOD/Fc-anti-AFP/PGN, HRP-GOD/Thi-anti-CEA/PGN [Anal]: CEA and AFP [DM]: Fc, Thi | [Tran]: SWV in −0.6 V to 0.6 V with a frequency of 15 Hz and a [PA]: of 25 mV (vs. SCE) to record Thi (at −0.15 V) and Fc (at 0.35 V) [IC]: (i) CEA/AFP conc 45 min, 37 °C, (ii) [DP] 45 min, 37 °C, [MC]: PBS (0.1 M pH 6.5) with 4 mM glucose | [L]: 0.01–100 ng/mL for both [LgS] [DL]: 1.64 pg/mL and 1.33 pg/mL for CEA and AFP [SL]: 30 days [CR]: 0.998, 0.994 for CEA and AFP | [48] |
| [DP]: 3DGS@MB-anti-CEA and 3DGS@Fc-anti-AFP [Anal]: CEA and AFP [DM]: MB, Fc | [Tran]: DPV in −0.4 to 0.4 V with [PA]: 50 mV and [PW] 50 ms [IC]: (i) CEA/AFP conc 40 min, RT, (ii) [DP], [MC]: PBS (pH 7.0, containing 0.1 M KCl) | [L]: 0.001 to 100 ng/mL for both [LgS] [DL]: 0.5 and 0.8 pg/mL for CEA and AFP [S]: 11.19, 27.866 µA/(ng/mL), for CEA and AFP [SL]: 10 days [CR]: 0.9985, 0.9957 for CEA and AFP | [76] |
| [DP]: CGN-Thi-anti-CEA, CGN-DAP-anti-PSA and CGN-Cd2+-anti-AFP [Anal]: PSA, CEA, AFP [DM]: DAP, Thi, Cd2+ | [Tran]: SWV −1.2 V to 0.2 V (vs. SCE) [PA] 50 mV [PW] 50 ms to record Thi, DAP and Cd2+ at −0.05 V, −0.35 V and −0.65 V [IC]: (i) PSA/CEA/AFP conc 35 min, 37 °C, (ii) [DP] 45 min, 37 °C, [MC]: PBS (pH 6.5, 0.1 M) | [L]: 0.01–100 ng/mL for all three [LgS] [DL]: 4.8, 2.7 and 3.1 pg/mL for PSA, CEA and AFP [S]: 4.12, 5.84, 5.48 µA/(ng/mL), for PSA, CEA and AFP [SL]: 2 weeks [CR]: 0.997, 0.995, 0.997 for PSA, CEA and AFP | [75] |
| [DP]: M-Pt-anti-CA125, M-Pt-anti-CA153, M-Pt-anti-CEA [Anal]: CEA, CA153, CA125 | [Tran]: DPV in −0.65 to 0.4 V [IC]: (i) CEA, CA153, CA125 conc 1 h, RT, (ii) [DP] 1 h, RT [MC]: PBS (pH 7.4) containing 5 mM H2O2 | [L]: 0.02–20 ng/mL, 0.008–24 U/mL, 0.05–20 U/mL for CEA, CA153 and CA125, [LgS] [DL]: 7.0 pg/mL, 0.001 U/mL and 0.002 U/mL, for CEA, CA153 and CA125 [SL]: one month [CR]: 0.9927, 0.9962, 0.9988 for CEA, CA153 and CA125 | [30] |
| [DP]: anti-CA72-4/PANi–Au AMNPs [Anal]: CA72-4 [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) CA72-4 conc 1 h, RT, (ii) [DP] 1 h, RT [MC]: PBS pH 7.4 with 5.0 mmol/L H2O2 | [L]: 2 to 200 U/mL [LS] [DL]: 0.10 U/mL [S]: 0.814 µA/(U/ml) [SL]: 20 days [CR]: 0.9945 | [55] |
| [DP]: Fe3O4@SiO2–Fc–anti-CEA/HRP [Anal]: CEA [DM]: Fc | [Tran]: DPV in −0.1 to 0.8 V [IC]: (i) CEA conc 40 min, RT, (ii) [DP] [MC]: PBS (pH 7.4) with 4 mM H2O2 | [L]: 0.001 to 80 ng/mL [LS] [DL]: 0.0002 ng/mL [S]: 0.3867 µA/(ng/mL) [SL]: 3 weeks [CR]: 0.99 | [35] |
| [DP]: anti-SCCA/Pd–Au/C [Anal]: SCCA [DM]: H2O2 | [Tran]: amperometric at −0.2 V [IC]: (i) SCCA conc, (ii) [DP] 1 h [MC]: PBS pH 6.8 with H2O2 | [L]: 0.005 to 2 ng/mL [LS] [DL]: 1.7 pg/mL [S]: 4.351 µA/(ng/mL) [SL]: 7 days [CR]: 0.9995 | [28] |
| [DP]: Cu@Ag-CD-ADA-anti-CEA [Anal]: CEA [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) CEA conc, 60 min, RT, (ii) [DP] 60 min, RT [MC]: PBS pH 7.0 with 5mM H2O2 | [L]: 0.0001–20 ng/mL [LS] [DL]: 20 fg/mL [S]: (i) 212.46 µA/(ng/mL) below 0.5 ng/mL, (ii) 5.82 µA/(ng/mL) above 0.5 ng/mL [SL]: 1 week [CR]: (i) 0.9955, (ii) 0.9982 | [37] |
| [DP]: anti-AFP/Fe3O4@C@Pd [Anal]: AFP [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) AFP conc, 1 h, (ii) [DP] [MC]: PBS pH 6.5 with 5 mM H2O2 | [L]: 0.5 pg/mL to 10 ng/mL [LgS] [DL]: 0.16 pg/mL [S]: 45.195 µA/(ng/mL) [SL]: 30 days [CR]: 0.981 | [33] |
| [DP]: anti-CEA/NP-PtFe [Anal]: CA15-3 [DM]: H2O2 | [Tran]: chronoamperometry at −0.4V [IC]: (i) CA15-3 conc, 1 h, RT, (ii) [DP] 1 h, RT [MC]: PBS pH 7.4 with 5 mM H2O2 | [L]: 0.002 to 40 U/mL [LS] [DL]: 3 × 10−4 U/mL [S]: 1.879 µA/(U/mL) [SL]: 10 days [CR]: 0.9988 | [31] |
| [DP]: anti-AFP/PdNi/N-GNRs [Anal]: AFP [DM]: H2O2 | [Tran]: DPV [IC]: (i) AFP conc, 1 h, RT, (ii) [DP] 1 h, RT [MC]: PBS pH 7.0 with 5 mM H2O2 | [L]: 0.0001–16 ng/mL [LS] [DL]: 0.03 pg/mL [S]: (i) 161.86 µA/(ng/mL) below 0.2 ng/mL, (ii) 9.09 µA/(ng/mL) above 0.2 ng/mL [SL]: 20 days [CR]: (i) 0.9946, (ii) 0.9969 | [39] |
| [DP]: Pb2+@Au@MWCNT-Fe3O4/anti-AFP [Anal]: AFP [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) AFP conc, 1 h; RT (ii) [DP] [MC]: PBS pH 7.4 with 5 mM H2O2 | [L]: 10 fg/mL to 100 ng/mL [LgS] [DL]: 3.33 fg/mL [S]: 11.19 µA/(ng/mL) [SL]: 4 weeks [CR]: 0.9984 | [49] |
| [DP]: anti-SCCA/Au/Ag/Au NPs [Anal]: SCCA [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) SCCA conc, 1 h, RT, (ii) [DP] 1 h, RT [MC]: PBS pH 7.17 with 5 mM H2O2 | [L]: 0.5 pg/mL to 40 ng/mL [LgS] [DL]: 0.18 pg/mL [S]: 25.33 µA/(ng/mL) [SL]: 2 weeks [CR]: 0.9880 | [41] |
| [DP]: anti-AFP/Pd/APTES-M-CeO2-GS [Anal]: AFP [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) AFP conc, 1 h, 4°C, (ii) [DP] 1 h, [MC]: PBS pH 7.4 with 5 mM H2O2 | [L]: 0.1 pg/mL to 50 ng/mL [LgS] [DL]: 0.033 pg/mL [S]: 10.1 µA/(ng/mL) [SL]: 4 weeks [CR]: 0.99 | [51] |
| [DP]: anti-SCC/Pt–Fe3O4 NPs [Anal]: SCC [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) SCC conc, 1 h, (ii) [DP] 1 h, [MC]: PBS pH 7.4 with 5 mM H2O2 | [L]: 0.05 to 18 ng/mL [DL]: 15.3 pg/mL [SL]: 20 days | [65] |
| [DP]: anti-CA72-4/PtPd-Fe3O4 NPs [Anal]: CA72-4 [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) SCC conc, 1 h, 4 °C, (ii) [DP] 1 h, [MC]: PBS pH 7.0 with 5 mM H2O2 | [L]: 0.001–10 U/mL [DL]: 0.0003 U/mL [SL]: 10 days | [67] |
| [DP]: CNTs/PDDA/HRP/ConA/HRP-anti-CEA [Anal]: CEA [DM]: hydroquinone | [Tran]: DPV in −0.4 to 0.2 V (vs. SCE) at a scan rate of 50 mV/s [IC]: (i) CEA conc, 40 min, RT, (ii) [DP] 60 min, RT [MC]: PBS (0.02 M, pH 7.5) with 2 mM H2O2 and 3 mM HQ | [L]: (i) 0.05–5 ng/mL and (ii) 5–200 ng/mL, [LS] [DL]: 0.018 ng/mL [S]: (i) 1.29 µA/(ng/mL), (ii) 0.0315 µA/(ng/mL) [SL]: 15 days [CR]: (i) 0.998, (ii) 0.998 | [62] |
| [DP]: M-Pd@Pt/NH2-GS/anti-PSA [Anal]: PSA [DM]: H2O2 | [Tran]: amperometric at −0.4 V [IC]: (i) PSA conc, 1 h, 4 °C, (ii) [DP] 40 min, RT, [MC]: PBS pH 7.38 with 5 mM H2O2 | [L]: 10 fg/mL–50 ng/mL [LgS] [DL]: 3.3 fg/mL [S]: 11.96 µA/(ng/mL), [SL]: 4 weeks [CR]: 0.9988 | [78] |
| [DP]: Ir NPs-anti-CEA [Anal]: CEA [DM]: H2O2 | [Tran]: amperometric at −0.6 V [IC]: (i) CEA conc, (ii) [DP] 1 h, 37 °C, [MC]: PBS pH 7.4 with 5 mM H2O2 | [L]: 0.5 pg/mL–5 ng/mL [LgS] [DL]: 0.23 pg/mL [S]: 0.435 µA/(ng/mL), [SL]: 30 days [CR]: 0.99 | [79] |
| [DP]: PBG-Au-anti-CEA; PPP-Au-anti-NSE; PTBO-Au anti-CA125; PMCP-Au-anti-Cyfra21–1; Cd NCs-Au anti-SCCA [Anal]: CEA, NSE, CA125, Cyfra21–1, SCCA [DM]: PBG-Au, PPP-Au, PTBO-Au, PMCP-Au and Cd NCs at 0.4 V, 0.15 V, −0.14 V, −0.5 V, −0.75 V | [Tran]: SWV in −1.0 V to 0.8 V to record peaks at 0.4 V, 0.15 V, −0.14 V, −0.5 Vand −0.75 V (vs. Ag/AgCl) for simultaneously detection of CEA, NSE, CA125, Cyfra21–1 and SCCA [IC]: (i) CEA, SCCA, CA125, Cyfra21–1 and NSE mix, (ii) PBG-Au-anti-CEA, PPP-Au-anti-NSE, PTBO-Au-anti-CA125, PMCP-Au-anti-Cyfra21–1, Cd NCs-Au-anti-SCCA probes mixture 45 min, 37 °C. [MC]: PB (0.1 M, pH 6.0). | [L]: 0.1 to 100 ng/mL for SCCA, 1 to 150 ng/mL for CEA, NSE and Cyfra21–1, and 1 to 150 U/mL for CA125 [LgS] [DL]: 0.2 ng/mL for CEA, 0.9 ng/mL for NSE, 0.9 U/mL for CA125, 0.4 ng/mL for Cyfra21–1 and 0.03 ng/mL for SCCA [S]: 3.06 µA/(ng/mL), 4.9 µA/(ng/mL), 3.7 µA/(U/mL), 2.3 µA/(ng/mL), 2.57 µA/(ng/mL), for CEA, NSE, CA125, Cyfra21–1, SCCA [SL]: 4 weeks [CR]: 0.984, 0.983, 0.997, 0.995 and 0.971 for CEA, NSE, CA125, Cyfra21–1, SCCA | [80] |
| [DP]: HRP-MNP-anti-PSA, HRP-MNP-anti-PSMA, HRP-MNP-anti-IL-6, HRP-MNP-anti-PF-4 [Anal]: PSA, PSMA, IL-6, PF-4 [DM]: HQ | [Tran]: DPV from 0.0 V to −0.4 V vs. Ag/AgCl at 4 mV step, 25 mV amplitude, and 0.5 s pulse and 15 Hz [IC]: (i) [Anal]: mix with [DP], (ii) incubation with sensor [MC]: PBS with 1 mM HQ and 100 µM H2O2 | [L]: 2 pg/mL to 200 ng/mL for PSA, 0.05 pg/mL to 5 ng/mL for IL-6, 0.1 pg/mL to 10 pg/mL for PF-4, and 0.15 pg/mL to 15 ng/mL for PSMA [LgS] [S]: 0.84 ± 0.06 nA/(pg/mL), for PSA, 0.90 ± 0.09 nA/(pg/mL), for IL-6, 0.98 ± 0.07 nA/(pg/mL), for PF-4, and 1.1 ± 0.1 nA/(pg/mL), for PSMA [SL]: 7 days | [81] |
| [DP]: AuNP-HRP, anti-PSA [Anal]: PSA [DM]: TMB | [Tran]: amperometric at −0.1 V [IC]: (i) PSA conc 250 µL at 30 µL/min flow, (ii) [DP] [MC]: PBS pH 7.4 with TMB-H2O2 | [L]: 0.2–12.5 ng/mL [LS] [DL]: 0.2 ng/mL [S]: 2.24 nA/(ng/mL) [CR]: 0.94 | [82] |
| [DP]: Primer-AuNP-PSA aptamer [Anal]: PSA [DM]: CuNPs | [Tran]: DPSV in −0.2 to 0.6 V with 4 mV step, amplitude 0.05 V, [PW]: 0.05 s, pulse period 0.5 s, deposition potential −0.5 V, deposition time 300 s [IC]: (i) PSA conc, 1 h, 37 °C, (ii) [DP] 1 h, 37 °C, (iii) RCA reaction, (iv) CuNP formation, 30 min, RT [MC]: Cu2+ in 0.5 M HNO3 | [L]: 0.05–500 fg/mL [LgS] [DL]: 0.02 fg/mL [S]: 3.48 µA/(fg/mL) [CR]: 0.995 | [83] |
| [DP]: anti-CYFRA-1-HRP/AuNPs/Thi/MWCNT-NH2 [Anal]: CYFRA-1 [DM]: Thi | [Tran]: DPV in −0.4 to 0 V [IC]: (i) CYFRA-1 conc for 1 h, 35 °C (ii) [DP] for 1 h, 35 °C [MC]: 2 mM H2O2 | [L]: 0.1–150 ng/mL [LS] [DL]: 43 pg/mL [S]: 0.446 µA/(ng/mL) [SL]: 15 days [CR]: 0.9937 | [84] |
| [DP]: anti-AFP-Co3O4@MnO2-Thi [Anal]: AFP [DM]: Thi | [Tran]: DPV in −0.6 to 0.6 V [IC]: (i) AFP conc, (ii) [DP] [MC]: dried 10 µL unbound [DP] on AgNP/SPEC using 50 µL PBS | [L]: 0.001–100 ng/mL [LgS] [DL]: 0.33 pg/mL [S]: 5.24 µA/(ng/mL) [CR]: 0.9977 | [85] |
| [DP]: antiHER2/Hyd@AuNP-APTMS-Fe3O4 [Anal]: HER2 [DM]: AgNPs | [Tran]: DPV in 0 to 0.6 V [IC]: (i) HER2 conc for 30 min, 37 °C, (ii) [DP] 30 min, 37 °C, 0.01 M AgNO3, 25 min [MC]: 0.01 M AgNO3, 25 min | [L]: 5 × 10−4 to 50.0 ng/mL [LgS] [DL]: 2.0 × 10−5 ng/mL [S]: 1.9194 µA/(ng/mL) [CR]: 0.9906 | [86] |
| [DP]: anti-CEA-AuNP-Fc [Anal]: CEA [DM]: Fc | [Tran]: SWV in 0 to 0.6 V [IC]: (i) CEA conc for 45 min, (ii) [DP] for 45 min [MC]: 0.1 M PBS (pH~7.0) | [L]: 0.5 to 10 ng/mL [LS] [DL]: 0.2 ng/mL [S]: 0.4494 µA/(ng/mL) [SL]: 3 weeks [CR]: 0.9968 | [87] |
| [DP]: anti-PSA-HRP [Anal]: PSA [DM]: MB | [Tran]: SWV in −0.4 to 0.15 V [IC]: (i) PSA conc for 25 min, (ii) [DP] for 30 min, [MC]: 1 mM MB + 2.5 mM H2O2 | [L]: 1–18 ng/mL [LS] [DL]: 1 pg/mL [S]: 3.234 µA/(ng/mL) [SL]: 3 weeks [CR]: 0.996 | [88] |
| [DP]: HRP-anti-CEA-AuNP-TiO2-GR [Anal]: CEA [DM]: HQ | [Tran]: DPV in 0.55 to −0.3 V [IC]: (i) CEA conc for 30 min, (ii) [DP] for 1 h, 35 °C [MC]: 2 mM H2O2 + 2.5 mM HQ | [L]: 0.005–200 ng/mL [LgS] [DL]: 3.33 pg/mL [S]: 11.98 µA/(ng/mL) [SL]: 15 days [CR]: 0.994 | [89] |
| [DP]: PtCu@rGO/g-C3N4/anti-PSA [Anal]: PSA [DM]: Thi | [Tran]: amperometric −0.4 V [IC]: (i) PSA conc for 30 min, (ii) [DP] for 50 min, RT [MC]: 5 mM H2O2 | [L]: 50 fg/mL to 40 ng/mL [LgS] [DL]: 16.6 fg/mL [S]: 15.97 µA/(ng/mL) [SL]: 4 weeks [CR]: 0.9913 | [90] |
| [DP]: M-Pd@Pt/NH2-GS/anti-PSA [Anal]: PSA [DM]: H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) PSA conc for 1 h, 4°C, (ii) [DP] for 40 min, RT [MC]: 5 mM H2O2 | [L]: 10 fg/mL to 50 ng/mL [LgS] [DL]: 3.3 fg/mL [S]: 11.96 µA/(ng/mL) [SL]: 4 weeks [CR]: 0.9988 | [78] |
| [DP]: anti-AFP-Pt NPs/Co3O4/graphene [Anal]: AFP [DM]: H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) AFP conc for 1 h, 4 °C, (ii) [DP] for 1 h, 4 °C [MC]: 5 mM H2O2 | [L]: 0.1 pg m/L to 60 ng/mL [LgS] [DL]: 0.029 pg/mL [S]: 9.71 µA/(ng/mL) [SL]: 28 days [CR]: 0.996 | [91] |
| [DP]: GS-Fe3O4/Au@Ag/Ni2+-anti-CEA [Anal]: CEA [DM]: H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) CEA conc for 1 h, RT, (ii) [DP] for 1 h, RT [MC]: 5 mM H2O2 | [L]: 0.1 pg/mL to 100 ng/mL [LgS] [DL]: 0.0697 pg/mL [S]: 6.62 µA/(ng/mL) [SL]: 2 weeks [CR]: 0.998 | [92] |
| [DP]: HRP-anti-CEA [Anal]: CEA [DM]: Thi | [Tran]: DPV in −0.4 to 0.1V [IC]: (i) CEA conc + [DP] for 30 min, RT [MC]: 0.5 mM Thi + 5 mM H2O2in PBS | [L]: 0.02 to 12 ng/mL [LS] [DL]: 0.01 ng/mL [SL]: 4 weeks [CR]: 0.998 | [94] |
| [DP]: PdCu-anti-CEA [Anal]: CEA [DM]: polyaniline | [Tran]: DPV in −0.2 to 0.6 V [IC]: (i) CEA conc for 1 h, (ii) [DP] RT [MC]: aniline polymerization by CV in −1 to 1 V, 200 s, 100 mV/s | [L]: 0.1 pg/mL to 10.0 ng/mL [LgS] [DL]: 0.08 pg/mL [S]: 2.49 µA/(ng/mL) [SL]: 5 weeks [CR]: 0.99 | [95] |
| [DP]: anti-AFP-GNPs-HRP [Anal]: AFP [DM]: HQ | [Tran]: amperometric −0.2 V [IC]: (i) AFP conc + [DP] for 20 min, RT [MC]: 2mM HQ + 2 mM H2O2 | [L]: 20 to 100 ng/mL [LS] [DL]: 0.64 ng/mL [S]: 0.3869 µA/(ng/mL) [SL]: 15 days [CR]: 0.9940 | [96] |
| [DP]: streptavidin-HRP [Anal]: HER2 [DM]: TMB | [Tran]: CV in −0.2 to 0.8 V, 50 mV/s [IC]: (i) HER2 conc for 2 h, RT and dried, (ii) [DP] for 2 h, RT and dried, (iii) streptavidin-HRP 30 min, RT, (iv) TMB-H2O2 20 min | [L]: 5 to 20 ng/mL and 20 to 200 ng/mL [LS] [DL]: 4 ng/mL and 5 ng/mL [S]: 0.087 µA/(ng/mL) and 0.28 µA/(ng/mL) [SL]: 7 days | [109] |
| [DP]: anti-AFP/HRP-Au@ZnO [Anal]: AFP [DM]: TMB | [Tran]: DPV in −0.1 to 0.6 V [IC]: (i) AFP conc for 40 min, 37 °C, (ii) [DP] [MC]: 0.1 mM TMB + 0.2 mM H2O2 | [L]: 0.02 pg/mL to 10ng/mL and 10 to 100 ng/mL [LgS] [DL]: 0.01 pg/mL [S]: 1.48 µA/(10−10 g/mL) and 6.15 µA/(10−10 g/mL) [SL]: 1 week [CR]: 0.9956 and 0.9917 | [97] |
| [DP]: anti-PSA/AuNPs [Anal]: PSA [DM]: Ag (I) ions | [Tran]: Linear sweep anodic striping in −0.2 to 0.5 V, 50 mV/s [IC]: (i) PSA conc for 30 min, RT, (ii) [DP] for 40 min, RT, (iii) 1st Au enhancement, (iv) spiky gold enhancement, (v) silver enhancement [MC]: 1 M KCl | [L]: 1.95 to 125 pg/mL and 0.125 to 10 ng/mL [LS] [DL]: 1.2 pg/mL [S]: 17.51 µA/(ng/mL) and 3.5 µA/(ng/mL) [CR]: 0.99 and 0.9896 | [98] |
| [DP]: Co3O4@CeO2-Au@Pt-anti-SCCA [Anal]: SCCA [DM]: H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) SCCA conc for 1 h, (ii) [DP] for 1 h [MC]: 5 mM H2O2 | [L]: 100 fg/mL to 80 ng/mL [LgS] [DL]: 33 fg/mL [S]: 4.43 µA/(ng/mL) [SL]: 4 weeks [CR]: 0.998 | [99] |
| [DP]: Au@Ag/PDA-PR-MCS [Anal]: AFP [DM]: H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) AFP conc for 30 min, (ii) [DP] for 1 h [MC]: 5 mM H2O2, 30 min | [L]: 20fg/mL to 100 ng/mL [LgS] [DL]: 6.7 fg/mL [S]: 16.07 µA/(ng/mL) [SL]: 1 month [CR]: 0.9987 | [100] |
| [DP]: MSN-MB/PDA-anti-PSA [Anal]: PSA [DM]: MB | [Tran]: SWV −0.7 to 0.3 V [IC]: (i) PSA conc for 50 min, 37 °C, (ii) [DP] for 1 h, 37 °C [MC]: 0.1 M HCl, 40 °C, 15 min | [L]: 10 fg/mL to 100 ng/mL [LgS] [DL]: 1.25 fg/mL [S]: 18.84 µA/(ng/mL) [SL]: 30 days [CR]: 0.992 | [101] |
| [DP]: AuPd NCNs-anti-CA 15-3 [Anal]: CA 15-3 [DM]: H2O2 | [Tran]: amperometric 0.2 V [IC]: (i) CA 15-3 conc. drop and dried 4 °C, (ii) [DP] incubation [MC]: 5 mM H2O2 | [L]: 0.001 pg/mL to 100 ng/mL [LgS] [DL]: 0.35 fg/mL [S]: 14.29 µA/(ng/mL) [SL]: 7 days [CR]: 0.9954 | [102] |
| [DP]: Au@Pt DNa/NG/Cu2+-anti-CEA [Anal]: CEA [DM]: H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) CEA conc, (ii) [DP] for 40 min [MC]: 5 mM H2O2 | [L]: 0.5 pg/mL to 50 ng/mL [LgS] [DL]: 0.167 pg/mL [S]: 17.9 µA/(ng/mL) [SL]: 3 weeks [CR]: 0.9964 | [103] |
| [DP]: Au@Ag-Cu2O/anti-PSA [Anal]: PSA [DM]: 5 mM H2O2 | [Tran]: amperometric −0.4 V [IC]: (i) PSA conc for 1 h, 4 °C, (ii) [DP] [MC]: 5 mM H2O2 | [L]: 0.01 pg/mL to 100 ng/mL [LgS] [DL]: 0.003 pg/mL [S]: 4.98 µA/(ng/mL) [SL]: 16 days [CR]: 0.9998 | [104] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arya, S.K.; Estrela, P. Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors 2018, 18, 2010. https://doi.org/10.3390/s18072010
Arya SK, Estrela P. Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors. 2018; 18(7):2010. https://doi.org/10.3390/s18072010
Chicago/Turabian StyleArya, Sunil K., and Pedro Estrela. 2018. "Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection" Sensors 18, no. 7: 2010. https://doi.org/10.3390/s18072010
APA StyleArya, S. K., & Estrela, P. (2018). Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors, 18(7), 2010. https://doi.org/10.3390/s18072010






