A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of MIP for PFOA and NIP
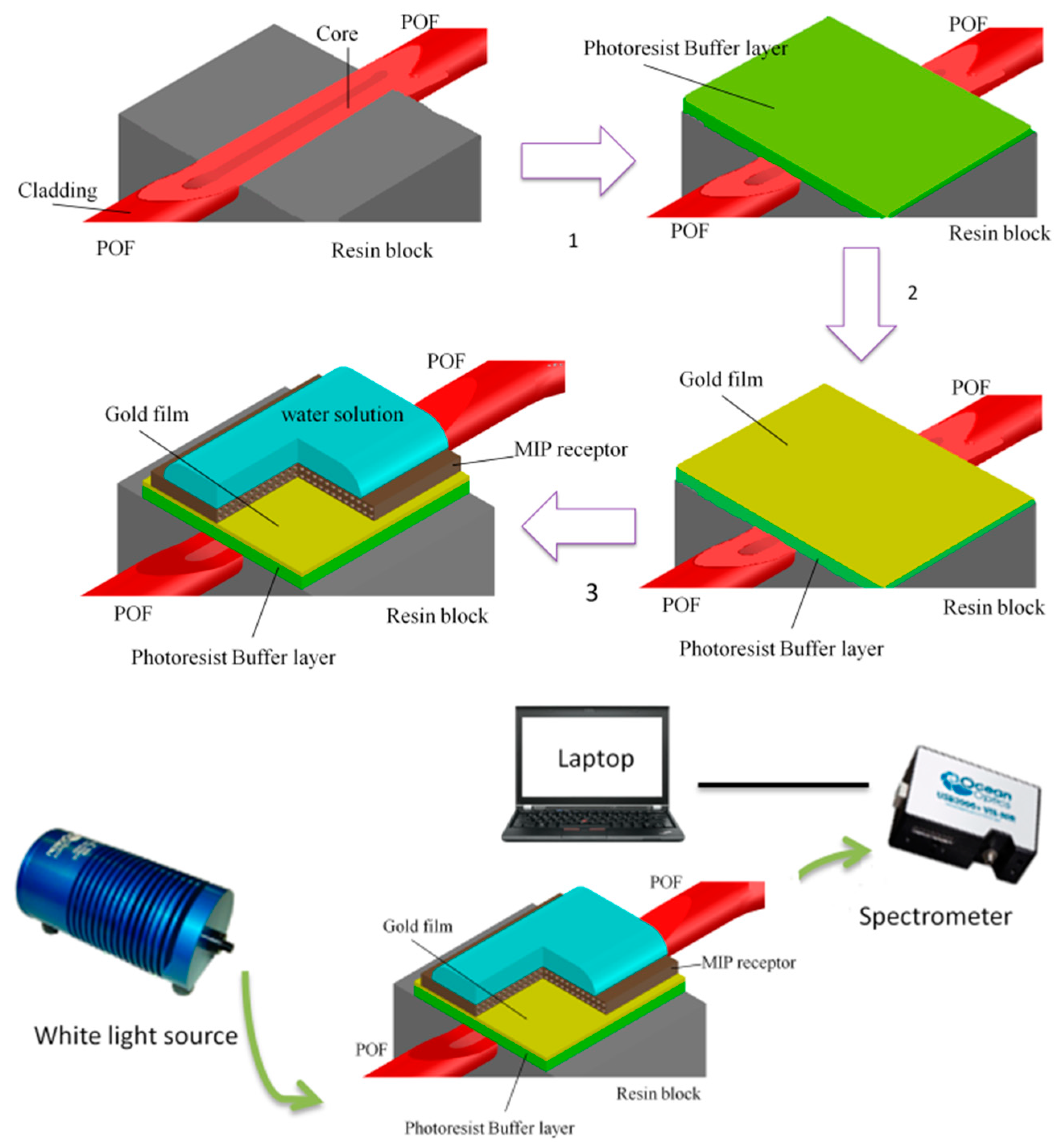
2.3. Optical Sensor Platform
2.4. The Experimental Equipment
2.5. Deposition of the MIP and NIP Layer
2.6. Binding Experiments
3. Results
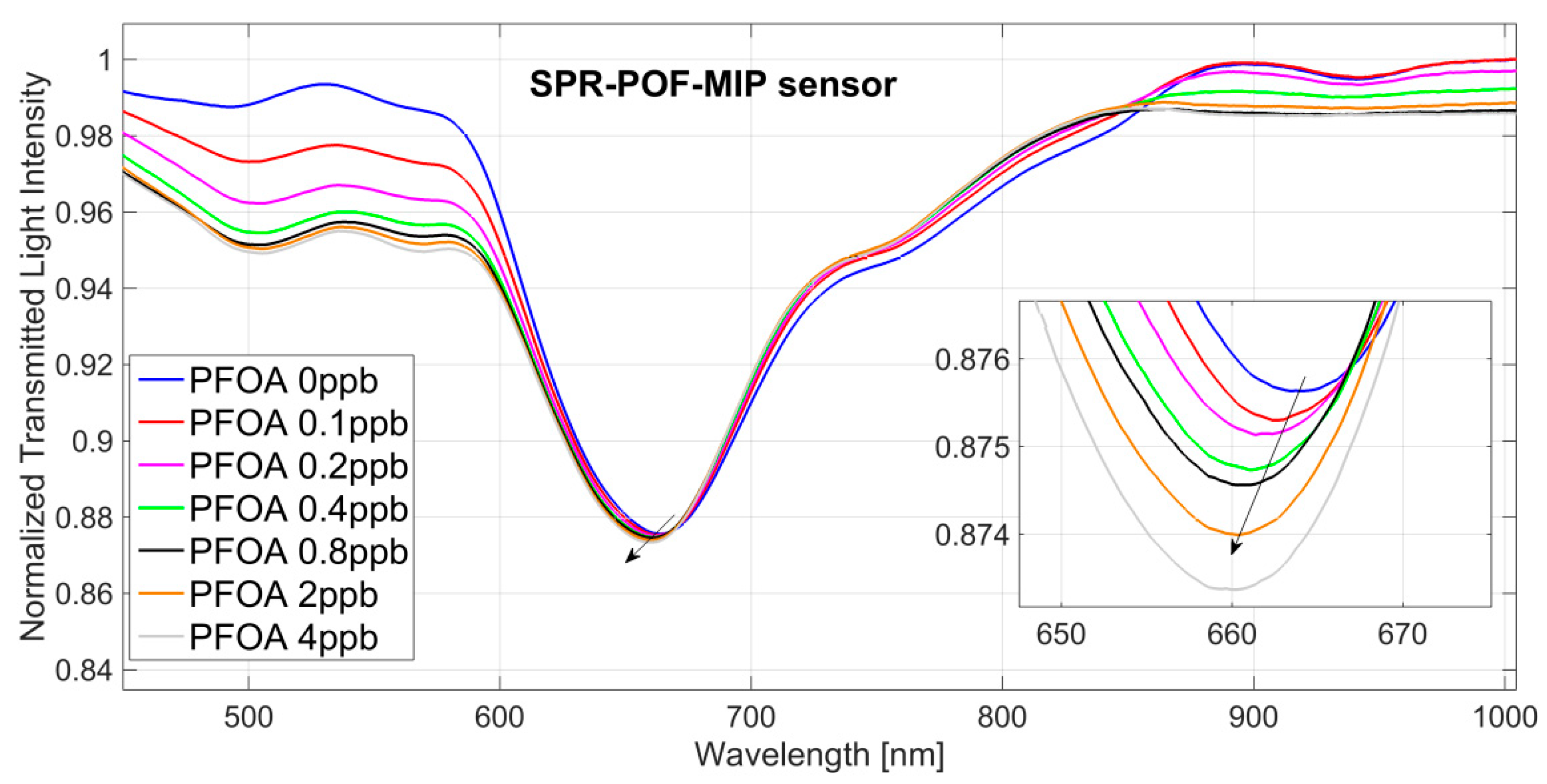
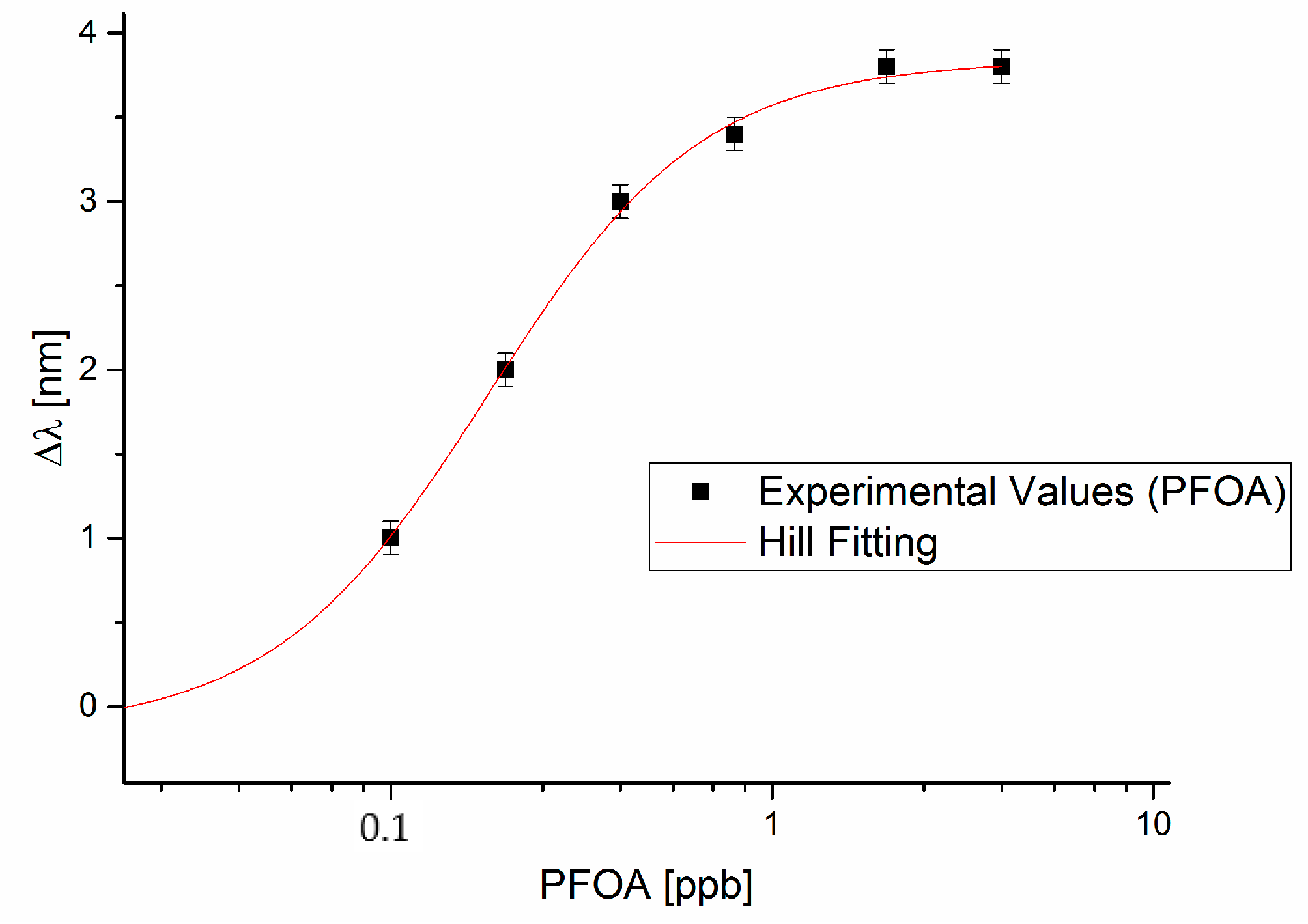
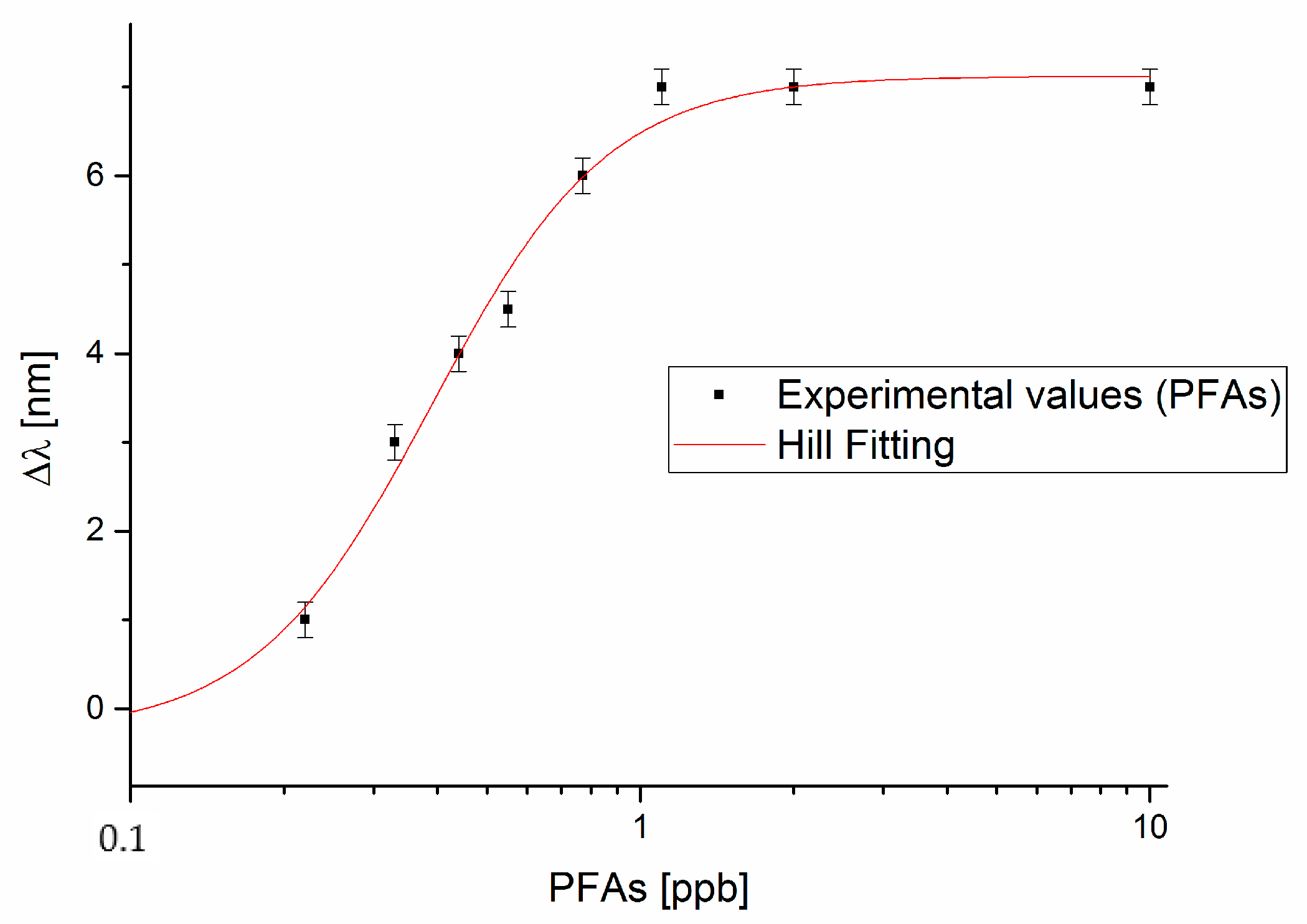
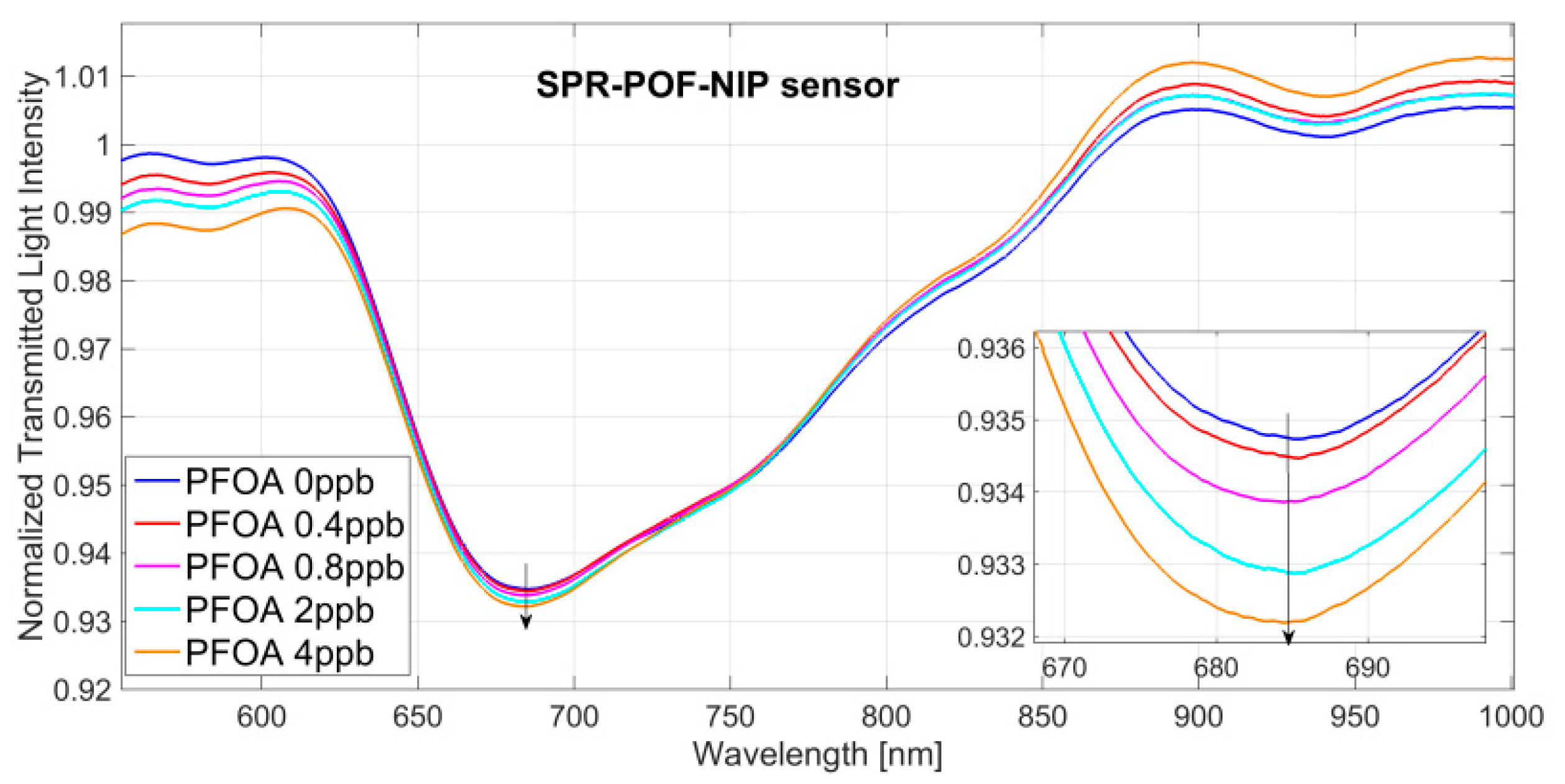
3.1. PFAs Detection
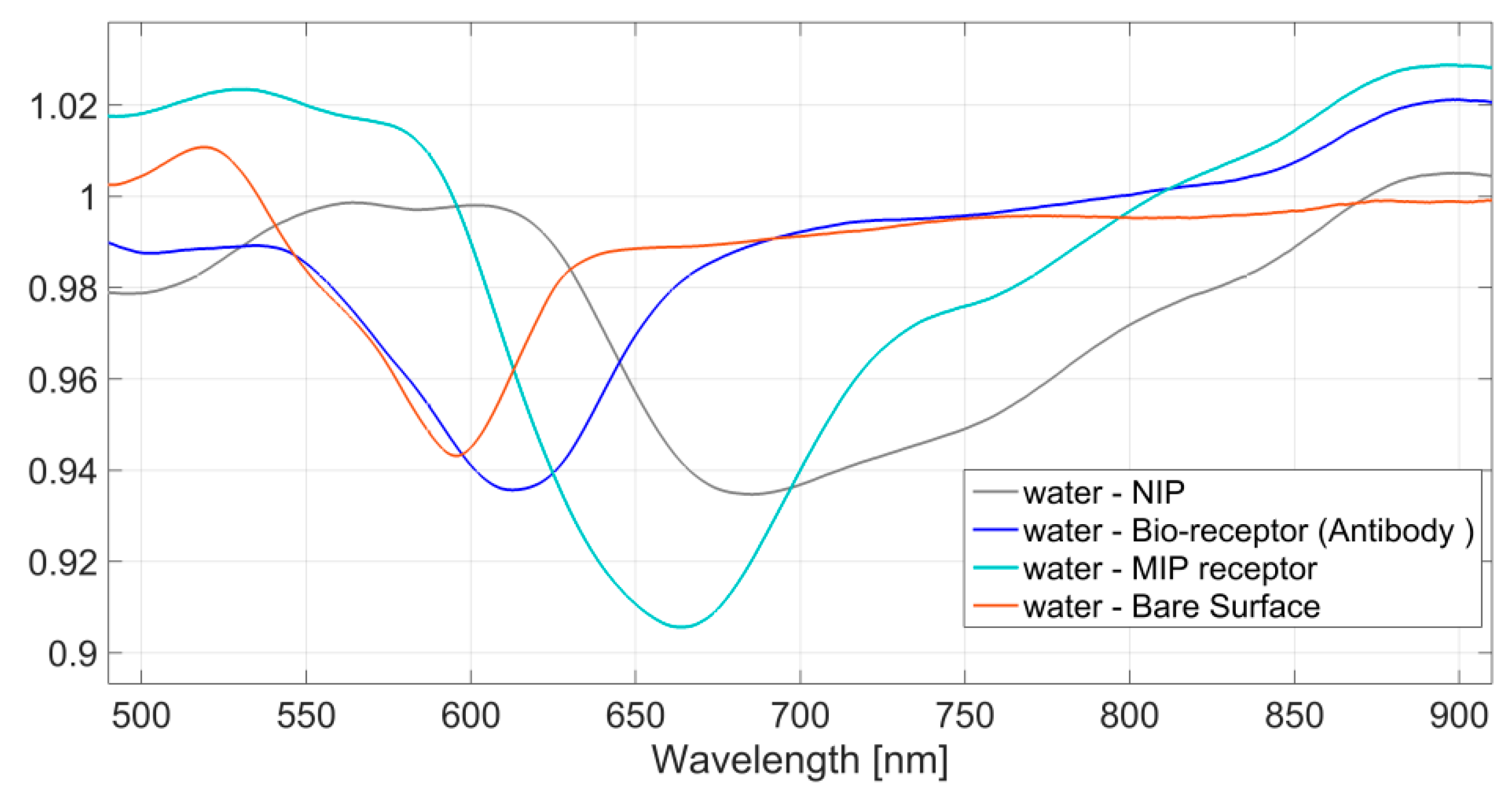
3.2. No Binding Detection
4. Discussion
4.1. Analysis of the Dose-Response Curve
4.2. Surface Characterization by SPR Approach
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- DeWitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immuno-toxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Sangiovanni, E.; Avogadro, A.; Galbiati, V.; Viviani, B.; Marinovich, M.; Galli, C.L.; Dell’Agli, M.; Germolec, D.R. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol. 2012, 258, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Avogadro, A.; Galbiati, V.; Dell’Agli, M.; Marinovich, M.; Galli, C.L.; Germolec, D.R. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol. 2011, 15, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Uemura, E.; Ishizaki, A.; Kataoka, H. Determination of perfluorooctanoic acid and perfluorooctane sulfonate by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. Anal. Chim. Acta 2010, 658, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Young, W.M.; South, P.; Begley, T.H.; Noonan, G.O. Determination of perfluorochemicals in fish and shellfish using liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 11166–11172. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Determinants of plasma PFOA and PFOS levels among 652 Danish men. Environ. Sci. Technol. 2011, 45, 8137–8143. [Google Scholar] [CrossRef] [PubMed]
- Huset, C.A.; Chiaia, A.C.; Barofsky, D.F.; Jonkers, N.; Kohler, H.P.E.; Ort, C.; Giger, W.; Field, J.A. Occurrence and mass flows of fluorochemicals in the Glatt Valley watershed, Switzerland. Environ. Sci. Technol. 2008, 42, 6369–6377. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.F.; Moody, C.A.; Spencer, C.; Small, J.M.; Muir, D.C.G.; Mabury, S.A. Analysis for perfluorocarboxylic acids/anions in surface waters and precipitation using GC-MS and analysis of PFOA from large-volume samples. Environ. Sci. Technol. 2006, 40, 6405–6410. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Lai, C.Z.; Granda, L.P.; Fierke, M.A.; Mandal, D.; Stein, A.; Gladysz, J.A.; Bühlmann, P. Fluorous membrane ion-selective electrodes for perfluorinated surfactants: Trace-level detection and in situ monitoring of adsorption. Anal Chem. 2013, 85, 7471–7477. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Shankar, P.M.; Mutharasan, R. A review of fiber-optic biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
- Bosch, M.E.; Sánchez, A.J.R.; Rojas, F.S.; Ojeda, C.B. Recent development in optical fiber biosensors. Sensors 2007, 7, 797–859. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2013–2015). Anal. Chem. 2016, 88, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Monk, D.J.; Walt, D.R. Optical fiber-based biosensors. Anal. Bioanal. Chem. 2004, 379, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.D.; Kant, R. Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Anuj, K.; Sharma, R.J.; Gupta, B.D. Fiber-optic sensors based on surface Plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Review of plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.C.; Otte, M.A.; Sepulveda, B.; Lechuga, L.M. Trends and challenges of refractometric nanoplasmonic biosensors: A review. Anal. Chim. Acta 2014, 806, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Klantsataya, E.; Jia, P.; Ebendorff-Heidepriem, H.; Monro, T.M.; François, A. Plasmonic Fiber Optic Refractometric Sensors: From Conventional Architectures to Recent Design Trends. Sensors 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low cost sensors based on SPR in a plastic optical fiber for biosensor implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; D’Agostino, G.; Galatus, R.; Bibbò, L.; Pesavento, M.; Zeni, L. Sensors based on surface plasmon resonance in a plastic optical fiber for the detection of trinitrotoluene. Sens. Actuators B 2013, 118, 221–226. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Lunelli, L.; Vanzetti, L.; Pederzolli, C.; Zeni, L.; Pasquardini, L. An easy way to realize SPR aptasensor: A multimode plastic optical fiber platform for cancer biomarkers detection. Talanta 2015, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; De Maria, L.; D’Agostino, G.; Zeni, L.; Pesavento, M. Monitoring of Low Levels of Furfural in Power Transformer Oil with a Sensor System Based on a POF-MIP Platform. Sensors 2015, 15, 8499–8511. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Zeni, L.; Tortora, P.; Regonesi, M.E.; Giusti, A.; Staiano, M.; D’Auria, S.; Varriale, A. A High Sensitivity Biosensor to detect the presence of perfluorinated compounds in environment. Talanta 2018, 178, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Oughena, M.; Moliner-Martinez, Y.; Pico, Y.; Campins-Falco, P.; Barcelo, D. Analysis of 18 perfluorinated compounds in river waters: Comparison of high performance liquid chromatography-tandem mass spectrometry, ultra-high-performance liquid chromatographytandem mass spectrometry and capillary liquid chromatography-mass spectrometry. J. Chromatogr. A 2012, 1244, 88–97. [Google Scholar]
- Trojanowicz, M.; Koc, M. Recent developments in methods for analysis of perfluorinated persistent pollutants. Microchim. Acta 2013, 180, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Di Giovanni, S.; Varriale, A.; Staiano, M.; Di Pietrantonio, F.; Notargiacomo, A.; Zeni, L.; D’Auria, S. Easy to Use Plastic Optical Fiber-Based Biosensor for Detection of Butanal. PLoS ONE 2015, 19, e0116770. [Google Scholar] [CrossRef] [PubMed]
- Burganov, B.I.; Lobanov, A.V.; Borisov, I.A.; Reshetilov, A.N. Criterion for Hill equation validity for description of biosensor calibration curves. Anal. Chim. Acta 2001, 427, 11–19. [Google Scholar] [CrossRef]
| λ0 [nm] | ∆λmax [nm] | K | n | Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Value | Standard Error | Value | Standard Error | Value | Standard Error | Value | Standard Error | Reduced Chi-Sqr | Adj. R-Square |
| PFOA (Figure 3) | −0.138 | 0.941 | 3.833 | 0.108 | 0.179 | 0.060 | 1.537 | 0.411 | 1.075 | 0.995 |
| PFAs (Figure 4) | −0.277 | 0.922 | 7.120 | 0.264 | 0.389 | 0.069 | 2.506 | 0.707 | 11.238 | 0.984 |
| Receptor | Parameters | Value |
|---|---|---|
| MIP Receptor | Sensitivity at low c of PFOA [nm/ppb] | 22.14 |
| Sensitivity at low c of PFAs [nm/ppb] | 18,99 | |
| LOD [ppb] (3 × standard deviation of blank/ sensitivity at low c of PFOA) | 0.13 | |
| LOD [ppb] (3 × standard deviation of blank/ sensitivity at low c of PFAs) | 0.15 | |
| Antibody [24] | Sensitivity at low c of PFOA [nm/ppb] | 29.82 |
| LOD [ppb] (3 × standard deviation of blank/sensitivity at low c of PFOA) | 0.24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, N.; D’Agostino, G.; Porto, G.; Biasiolo, A.; Perri, C.; Arcadio, F.; Zeni, L. A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water. Sensors 2018, 18, 1836. https://doi.org/10.3390/s18061836
Cennamo N, D’Agostino G, Porto G, Biasiolo A, Perri C, Arcadio F, Zeni L. A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water. Sensors. 2018; 18(6):1836. https://doi.org/10.3390/s18061836
Chicago/Turabian StyleCennamo, Nunzio, Girolamo D’Agostino, Gianni Porto, Adriano Biasiolo, Chiara Perri, Francesco Arcadio, and Luigi Zeni. 2018. "A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water" Sensors 18, no. 6: 1836. https://doi.org/10.3390/s18061836
APA StyleCennamo, N., D’Agostino, G., Porto, G., Biasiolo, A., Perri, C., Arcadio, F., & Zeni, L. (2018). A Molecularly Imprinted Polymer on a Plasmonic Plastic Optical Fiber to Detect Perfluorinated Compounds in Water. Sensors, 18(6), 1836. https://doi.org/10.3390/s18061836






