3D Copper Foam-Supported CuCo2O4 Nanosheet Arrays as Electrode for Enhanced Non-Enzymatic Glucose Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
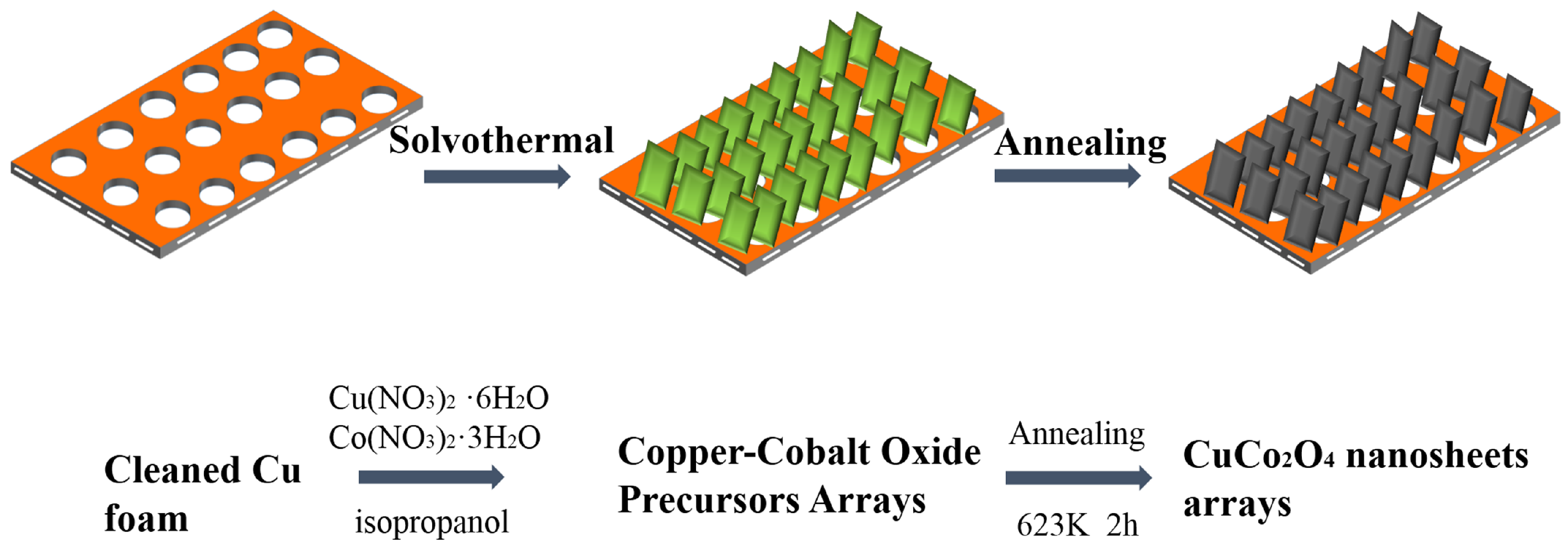
2.2. Fabrication of CuCo2O4 Nanosheets
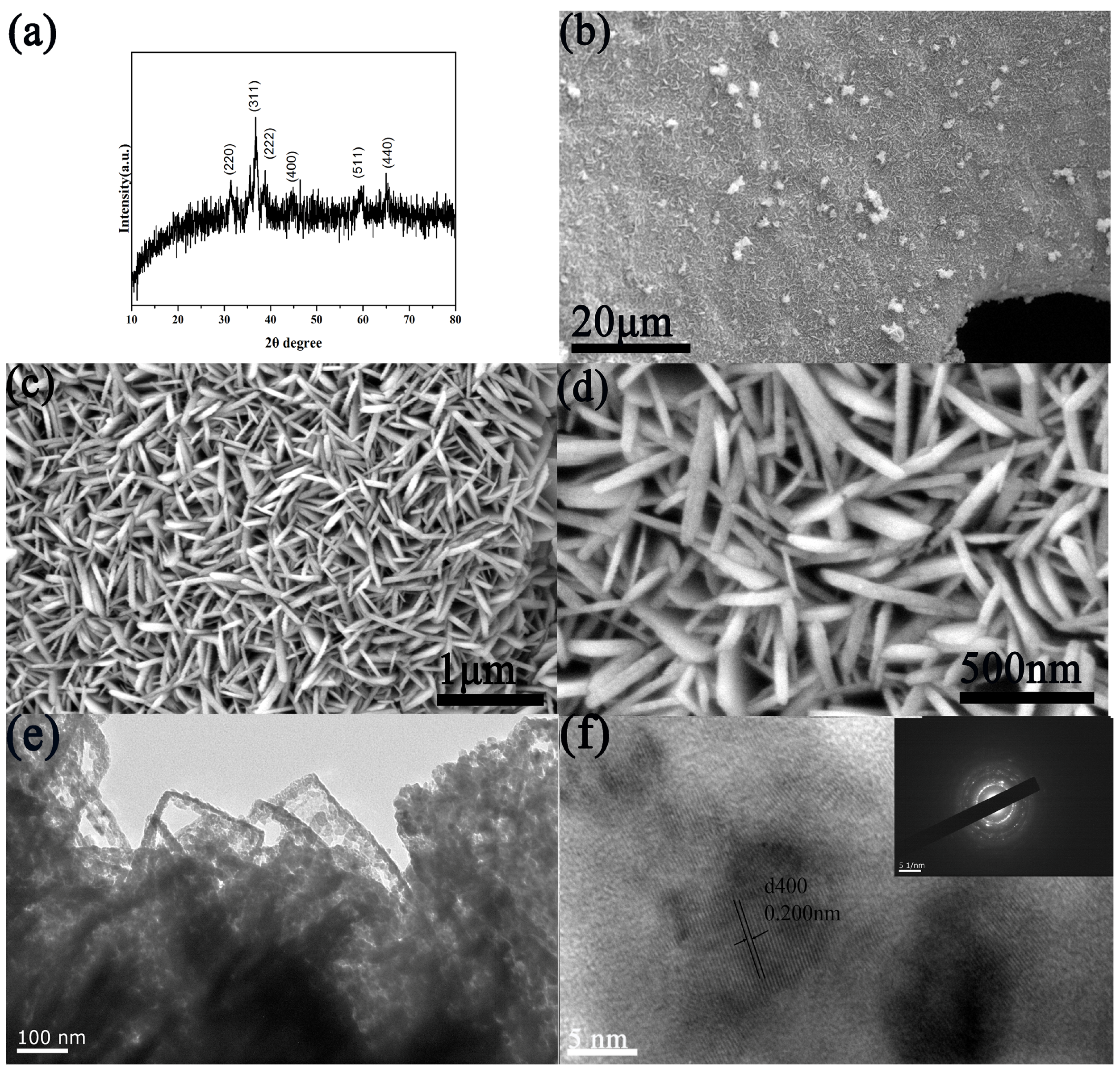
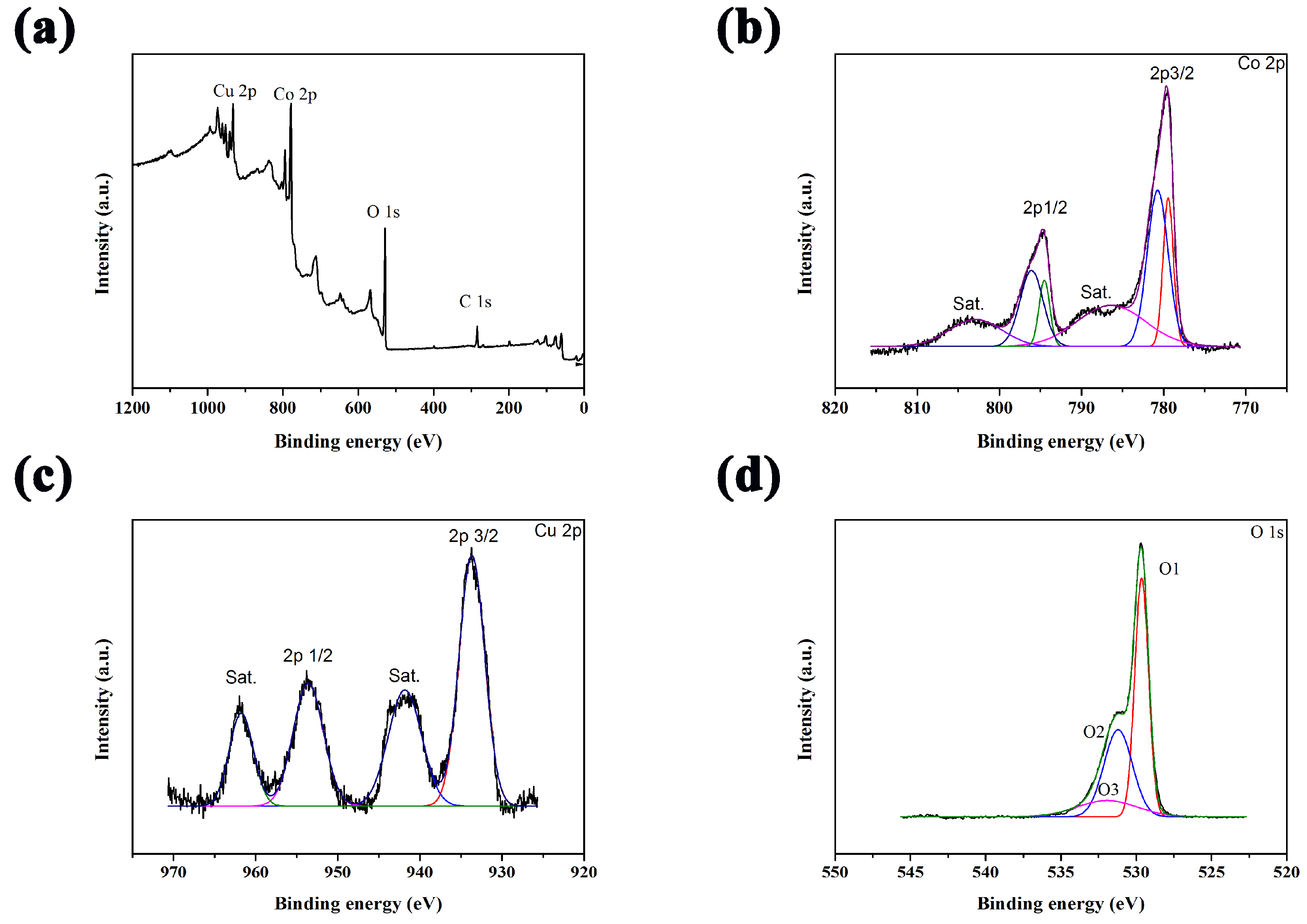
2.3. Characterizations
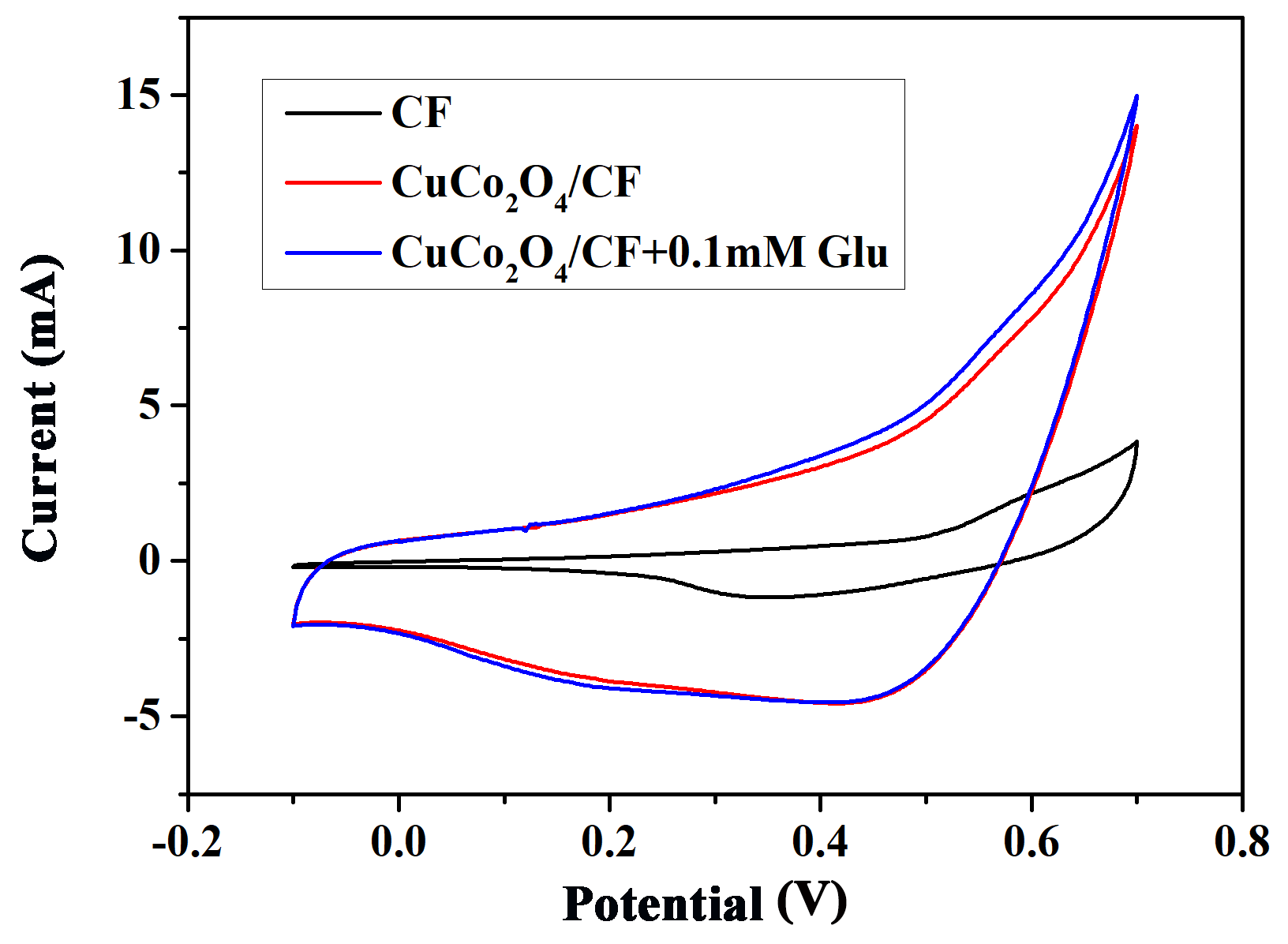
2.4. Electrochemical Measurements
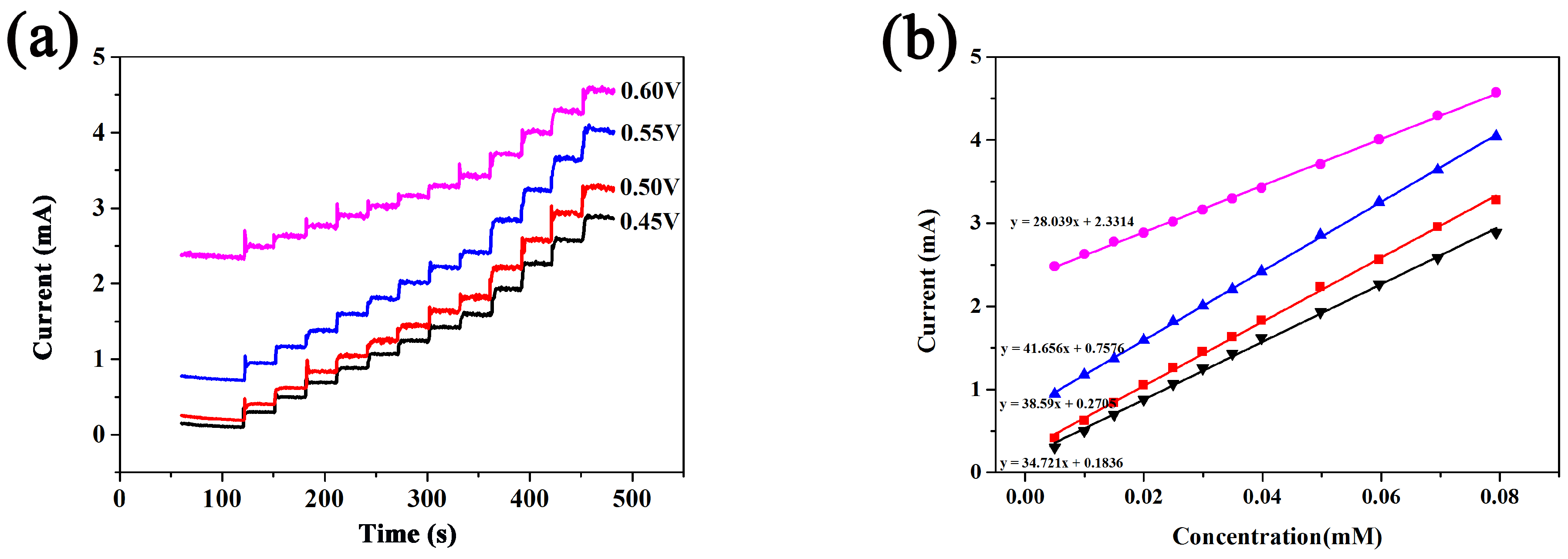
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brouzgou, A.; Tsiakaras, P. Electrocatalysts for glucose electrooxidation reaction: A review. Top. Catal. 2015, 58, 1311–1327. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wei, C.-W.; Liu, Y.-N.; Li, J. Nafion coating the ferrocenylalkanethiol and encapsulated glucose oxidase electrode for amperometric glucose detection. Analyst 2011, 136, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Kamari Kaverlavani, S.; Moosavifard, S.E.; Bakouei, A. Self-templated synthesis of uniform nanoporous CuCo2O4 double-shelled hollow microspheres for high-performance asymmetric supercapacitors. Chem. Commun. 2017, 53, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Liu, X.Y.; Dong, F.; Zhang, Y.X. Tunable design of layered CuCo2O4 nanosheets@MnO2 nanoflakes core-shell arrays on Ni foam for high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 21528–21536. [Google Scholar] [CrossRef]
- Pendashteh, A.; Moosavifard, S.E.; Rahmanifar, M.S.; Wang, Y.; El-Kady, M.F.; Kaner, R.B.; Mousavi, M.F. Highly ordered mesoporous CuCo2O4 nanowires, a promising solution for high-performance supercapacitors. Chem. Mater. 2015, 27, 3919–3926. [Google Scholar] [CrossRef]
- Jia, J.; Li, X.; Chen, G. Stable spinel type cobalt and copper oxide electrodes for O2 and H2 evolutions in alkaline solution. Electrochim. Acta 2010, 55, 8197–8206. [Google Scholar] [CrossRef]
- Niu, F.; Wang, N.; Yue, J.; Chen, L.; Yang, J.; Qian, Y. Hierarchically porous CuCo2O4 microflowers: A superior anode material for li-ion batteries and a stable cathode electrocatalyst for Li-O2 batteries. Electrochim. Acta 2016, 208, 148–155. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. CuCo2O4 based economical electrochemical sensor for the nanomolar detection of hydrazine and metol. J. Electroanal. Chem. 2016, 777, 48–57. [Google Scholar] [CrossRef]
- Yang, J.; Ye, H.; Zhang, Z.; Zhao, F.; Zeng, B. Metal–organic framework derived hollow polyhedron CuCo2O4 functionalized porous graphene for sensitive glucose sensing. Sens. Actuators B Chem. 2017, 242, 728–735. [Google Scholar] [CrossRef]
- Gao, G.; Xiang, Y.; Lu, S.; Dong, B.; Chen, S.; Shi, L.; Wang, Y.; Wu, H.; Li, Z.; Abdelkader, A.; et al. Ctab-assisted growth of self-supported Zn2GeO4 nanosheet network on a conductive foam as a binder-free electrode for long-life lithium-ion batteries. Nanoscale 2018, 10, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hui, K.S.; Hui, K.N. Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS Appl. Mater. Interfaces 2016, 8, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, M.; Bie, L.; He, D.; Zhang, Y.; Jiang, P. CuCo2O4 nanowire arrays supported on carbon cloth as an efficient 3D binder-free electrode for non-enzymatic glucose sensing. RSC Adv. 2017, 7, 23093–23101. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, R.H.; Han, D.W.; Kwon, H.S. Electrochemical performances of Sn anode electrodeposited on porous cu foam for li-ion batteries. Electrochim. Acta 2012, 66, 126–132. [Google Scholar] [CrossRef]
- Gu, S.; Lou, Z.; Ma, X.; Shen, G. CuCo2O4 nanowires grown on a Ni wire for high-performance, flexible fiber supercapacitors. ChemElectroChem 2015, 2, 1042–1047. [Google Scholar] [CrossRef]
- Tang, J.; Ge, Y.; Shen, J.; Ye, M. Facile synthesis of CuCo2S4 as a novel electrode material for ultrahigh supercapacitor performance. Chem. Commun. 2016, 52, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Moosavifard, S.E.; Shamsi, J.; Fani, S.; Kadkhodazade, S. Facile synthesis of hierarchical CuO nanorod arrays on carbon nanofibers for high-performance supercapacitors. Ceram. Int. 2014, 40, 15973–15979. [Google Scholar] [CrossRef]
- Liu, S.; Hui, K.S.; Hui, K.N.; Jadhav, V.V.; Xia, Q.X.; Yun, J.M.; Cho, Y.R.; Mane, R.S.; Kim, K.H. Facile synthesis of microsphere copper cobalt carbonate hydroxides electrode for asymmetric supercapacitor. Electrochim. Acta 2016, 188, 898–908. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Wen, C.; Xie, Y.; Miao, L.; Chen, S.; Li, H.; Li, P.; Song, Y. One-step synthesis of pt-nio nanoplate array/reduced graphene oxide nanocomposites for nonenzymatic glucose sensing. J. Mater. Chem. A 2015, 3, 608–616. [Google Scholar] [CrossRef]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Diao, P. Direct electrochemical detection of pyruvic acid by cobalt oxyhydroxide modified indium tin oxide electrodes. Electrochim. Acta 2011, 56, 10159–10165. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, L.; Zhang, Z.; Ding, Y.; Liu, S.; Deng, D.; Zhao, H.; Chen, Y. Synthesis of MnCo2O4 nanofibers by electrospinning and calcination: Application for a highly sensitive non-enzymatic glucose sensor. J. Mater. Chem. B 2014, 2, 529–535. [Google Scholar] [CrossRef]
- Wu, M.; Meng, S.; Wang, Q.; Si, W.; Huang, W.; Dong, X. Nickel–cobalt oxide decorated three-dimensional graphene as an enzyme mimic for glucose and calcium detection. ACS Appl. Mater. Interfaces 2015, 7, 21089–21094. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Three-dimensional copper foam supported CuO nanowire arrays: An efficient non-enzymatic glucose sensor. Electrochim. Acta 2017, 235, 519–526. [Google Scholar] [CrossRef]
- Naik, K.K.; Sahoo, S.; Rout, C.S. Facile electrochemical growth of spinel copper cobaltite nanosheets for non-enzymatic glucose sensing and supercapacitor applications. Microporous Mesoporous Mater. 2017, 244, 226–234. [Google Scholar] [CrossRef]
- Yuan, R.-M.; Li, H.-J.; Yin, X.-M.; Lu, J.-H.; Zhang, L.-L. 3D CuO nanosheet wrapped nanofilm grown on cu foil for high-performance non-enzymatic glucose biosensor electrode. Talanta 2017, 174, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, C.; Zhao, G.; Mu, J.; Wang, Y. Self-supported porous CoOOH nanosheet arrays as a non-enzymatic glucose sensor with good reproducibility. Sens. Actuators B Chem. 2015, 210, 190–196. [Google Scholar] [CrossRef]
- Niu, X.; Pan, J.; Qiu, F.; Li, X.; Yan, Y.; Shi, L.; Zhao, H.; Lan, M. Anneal-shrinked Cu2O dendrites grown on porous cu foam as a robust interface for high-performance nonenzymatic glucose sensing. Talanta 2016, 161, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Li, Z.; Ma, L.; Wang, J.; Yang, S. One-pot hydrothermal synthesis of CuO with tunable morphologies on ni foam as a hybrid electrode for sensing glucose. RSC Adv. 2014, 4, 23319–23326. [Google Scholar] [CrossRef]



| Electrode Material | Linear Range (mM) | Detection Limit (μM) | Sensitivity (μA·mM−1·cm−2) | Reference |
|---|---|---|---|---|
| CuCo2O4 nanosheet on graphite paper | Up to 0.32 | 0.55 | 3625 | [12] |
| CuCo2O4 nanosheet on ITO | 0.005–0.11 | 5.2 | 8250 | [25] |
| CuCo2O4 NWAs/CC | 0.001–0.93 | 0.5 | 3930 | [13] |
| CuO/Cu nanomaterials | Up to 4 | 0.5 | 4201 | [26] |
| CoOOH nanosheet array | 0.003–1.109 | 1.37 | 526 | [27] |
| Cu2O/Cu foam | 0.001–1.4 | 0.13 | 5040 | [28] |
| CuO/Ni foam | 0.0005–3.5 | 0.16 | 1084 | [29] |
| CuCo2O4/Cu foam | Up to 1.0 | 1 | 20,981 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhuang, Y.; Guo, M.; Chen, Y.; Tu, J.; Ding, L. 3D Copper Foam-Supported CuCo2O4 Nanosheet Arrays as Electrode for Enhanced Non-Enzymatic Glucose Sensing. Sensors 2018, 18, 1131. https://doi.org/10.3390/s18041131
Liu F, Zhuang Y, Guo M, Chen Y, Tu J, Ding L. 3D Copper Foam-Supported CuCo2O4 Nanosheet Arrays as Electrode for Enhanced Non-Enzymatic Glucose Sensing. Sensors. 2018; 18(4):1131. https://doi.org/10.3390/s18041131
Chicago/Turabian StyleLiu, Fangqing, Yi Zhuang, Mingliang Guo, Yongjun Chen, Jinchun Tu, and Lei Ding. 2018. "3D Copper Foam-Supported CuCo2O4 Nanosheet Arrays as Electrode for Enhanced Non-Enzymatic Glucose Sensing" Sensors 18, no. 4: 1131. https://doi.org/10.3390/s18041131
APA StyleLiu, F., Zhuang, Y., Guo, M., Chen, Y., Tu, J., & Ding, L. (2018). 3D Copper Foam-Supported CuCo2O4 Nanosheet Arrays as Electrode for Enhanced Non-Enzymatic Glucose Sensing. Sensors, 18(4), 1131. https://doi.org/10.3390/s18041131




