Study and Development of a Fluorescence Based Sensor System for Monitoring Oxygen in Wine Production: The WOW Project
Abstract
:1. Introduction
2. State of the Art
2.1. Methodologies for Wine Monitoring
- optical sensors, based on absorbance, reflectance, luminescence, fluorescence, refractive index, optothermal effect and light scattering;
- electrochemical sensors, including voltammetric and potentiometric devices, amperometric devices and potentiometric solid electrolyte gas sensors;
- electrical sensors including metal oxide and organic semiconductors as well as electrolytic conductivity sensors [18];
- mass sensitive sensors, i.e., piezoelectric devices and those based on surface acoustic waves;
- magnetic sensors (mainly for oxygen) based on paramagnetic gas properties [19];
- thermometric sensors based on the measurement of the heat effect of a specific chemical reaction or adsorption involving the analyte.
2.2. Fluorescence LED-Based Sensors
- intensity: luminescent intensity is clearly the most straightforward approach to achieve the measurement of the parameter. Intensity measurement actually has some important drawbacks: (i) both the incident light and luminescence have to be measured, (ii) the luminescence signal needs to be filtered to eliminate the superimposed excitation spectrum, (iii) thermal quenching strongly affects the luminescent intensity, (iv) each luminophor substrate needs a precise calibration to account for thickness, density and transparency variation; (v) calibrated intensity is sensitive to mechanical alterations of the system and optical material degradation [23,24];
- phase shift: phase shift measurement is more complex with respect to the intensity measurement; it generally requires a lock in amplifier and a precise modulation system for the LED light source. Phase shift presents many advantages being an effective approach to reject noise, making the measurement insensitive to physical properties of the luminophor substrate and limiting the effects of other intensity related system issues. The drawbacks are mainly related to (i) complexity, (ii) sensitivity/bandwidth of the detector and (iii) the luminophor needs to be continuously shined with the sinusoidal signal causing photo-bleaching in particular on sensitive organic compounds [25,26].
- lifetime decay: lifetime transient can be directly sampled and measured to calculate the recombination exponential mean lifetime, thus directly achieving the value of interest. This approach allows not only to measure the lifetime, but also the intensity and if necessary multi-exponential transients. The drawbacks of the transient measurement are: (i) a higher noise sensitivity, (ii) the necessity to achieve a high speed of the reading system/high sensitivity photodiode feedback topology [27].
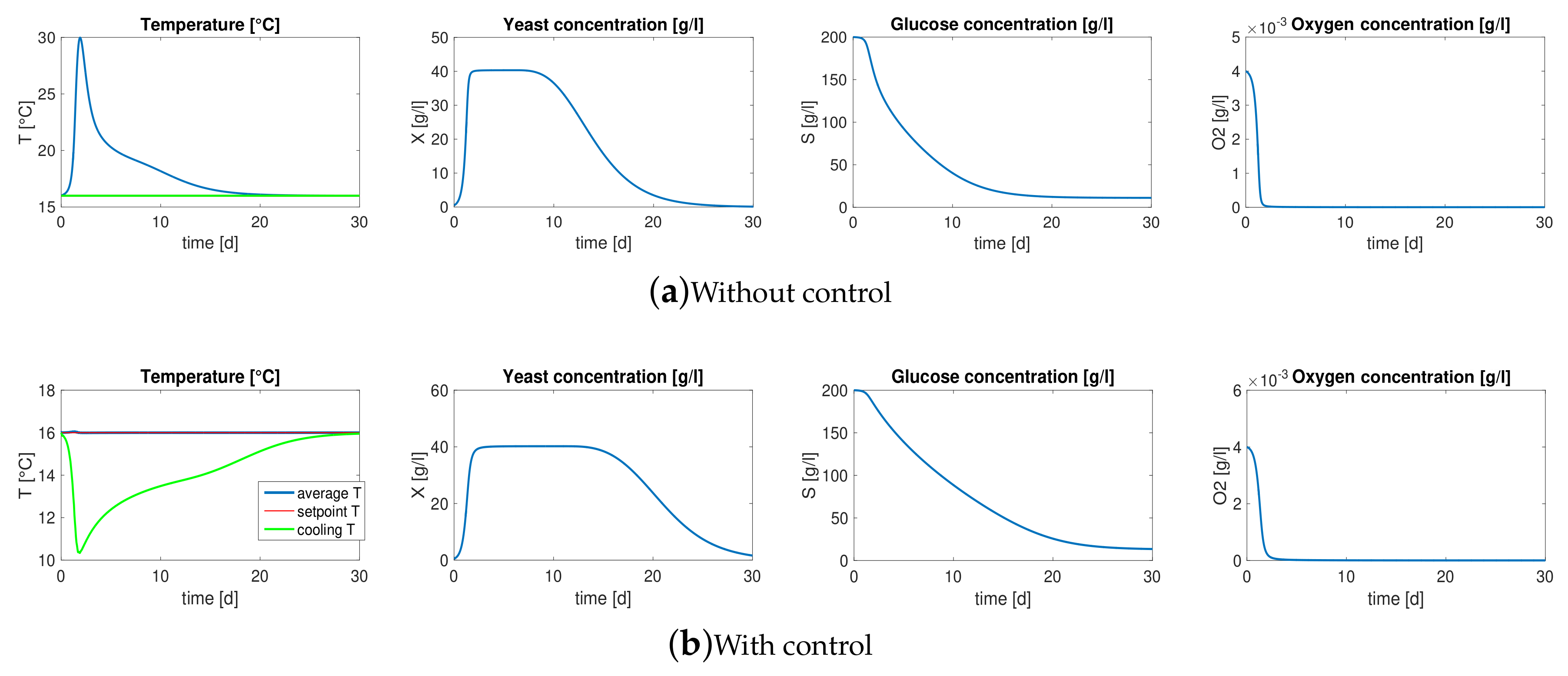
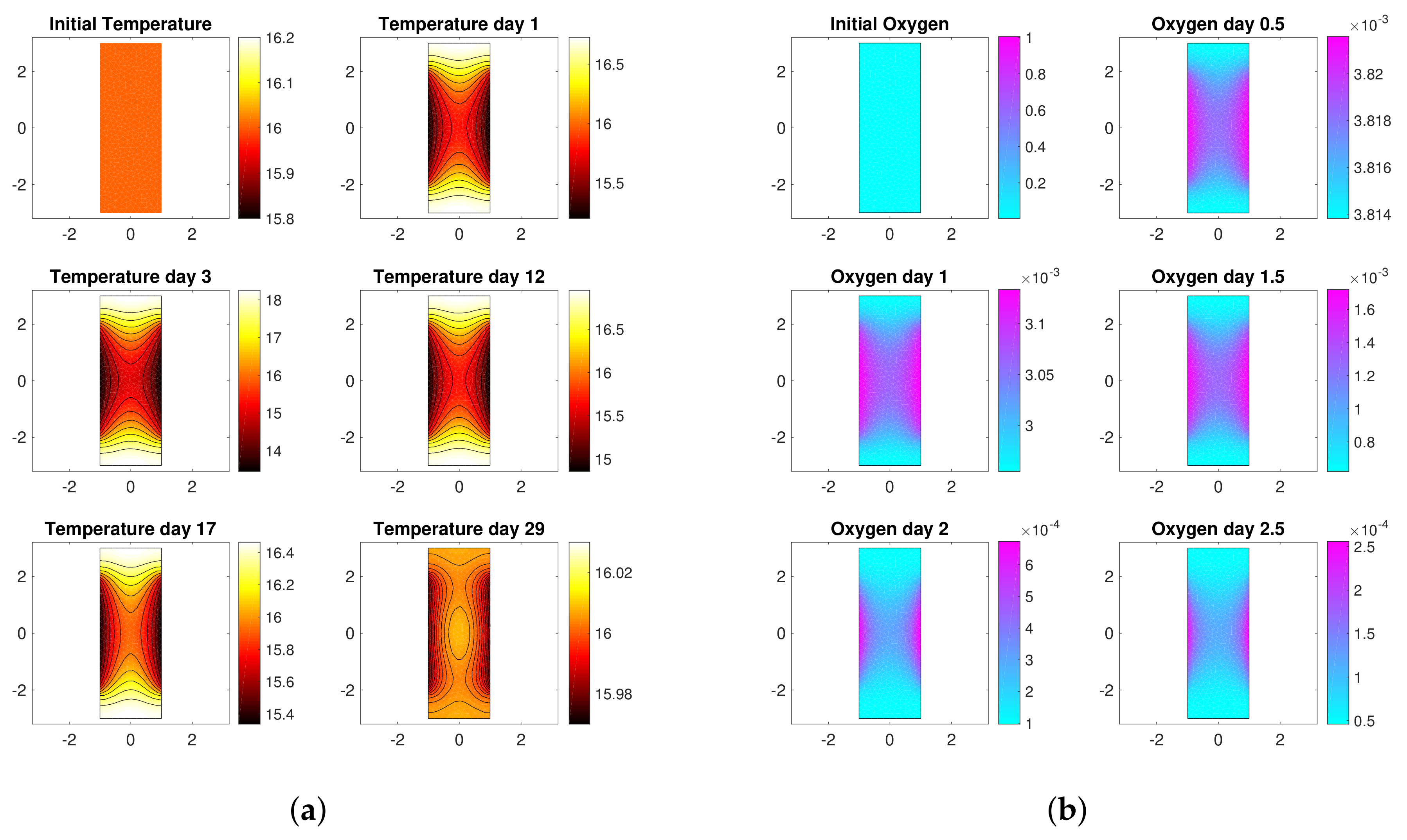
3. Motivation: Model-Based Control
3.1. Reaction-Diffusion PDEs Model
3.2. Boundary Temperature Control
- the values of model parameters, i.e., yield coefficients , Michaelis-Menten constants , diffusivity coefficients and thermal conductivities ,
- the initial conditions of the unknown variable, i.e , , , , , .
4. Monitoring System Design
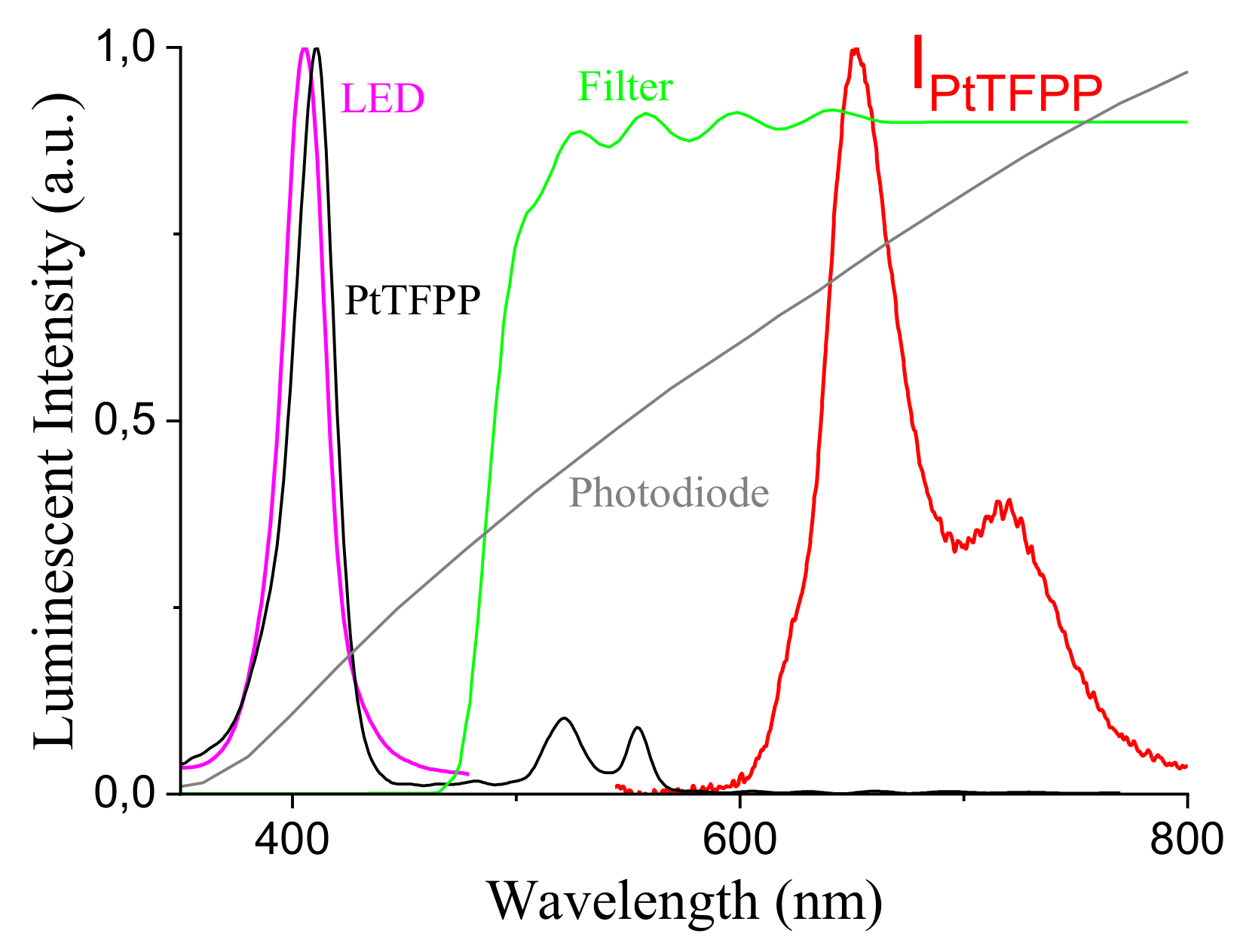
4.1. Luminophor Choice
- ruthenium tris-(4,7-diphenyl-1,10-phenanthroline) bis(octylsulphate) (Ru(dpp)OS, s)
- 5,10,15,20-Tetrakisphenyl-21H,23H-porphine platinum(II) (PtTPP, s)
- 5,10,15,20-Tetrakis(pentafluorophenyl)-21H,23H-porphine platinum(II) (PtTFPP, s)
- 5,10,15,20-Tetrakis(pentafluorophenyl)-21H,23H-porphine palladium(II) (PdTFPP, s)
- 2,3,7,8,12,13,17,18-Octaethyl-21H,23H-porphine palladium(II) (PdOEP, s)
4.2. Sensor Structure and Description
5. Experimental Validation
5.1. Laboratory Experimental Details and Results
5.1.1. Reagents
5.1.2. Membrane Preparation
5.1.3. Sensor Performances
5.1.4. Digitalization of the Luminescence Signal
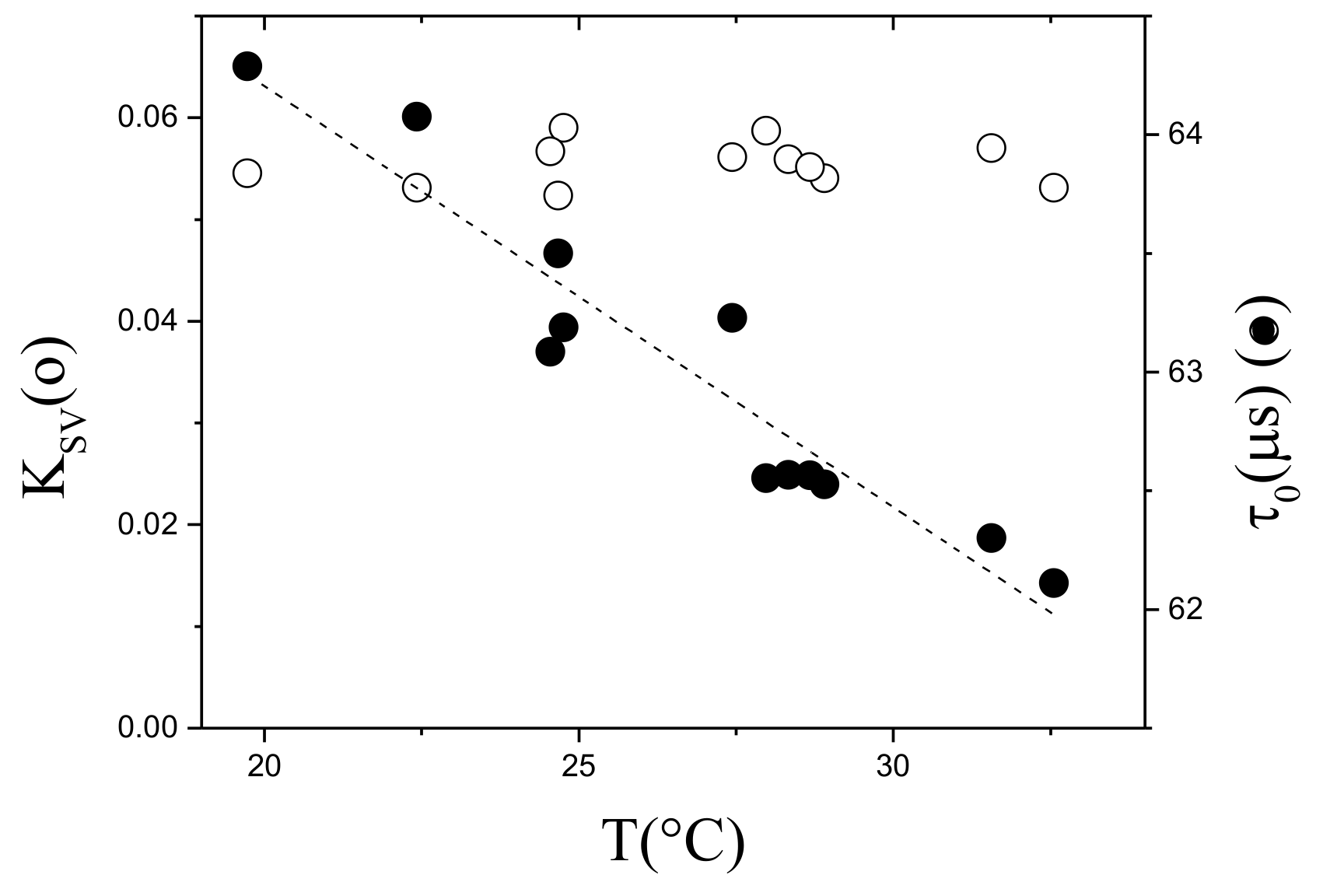
5.1.5. Sensor Characterization in Controlled Environment
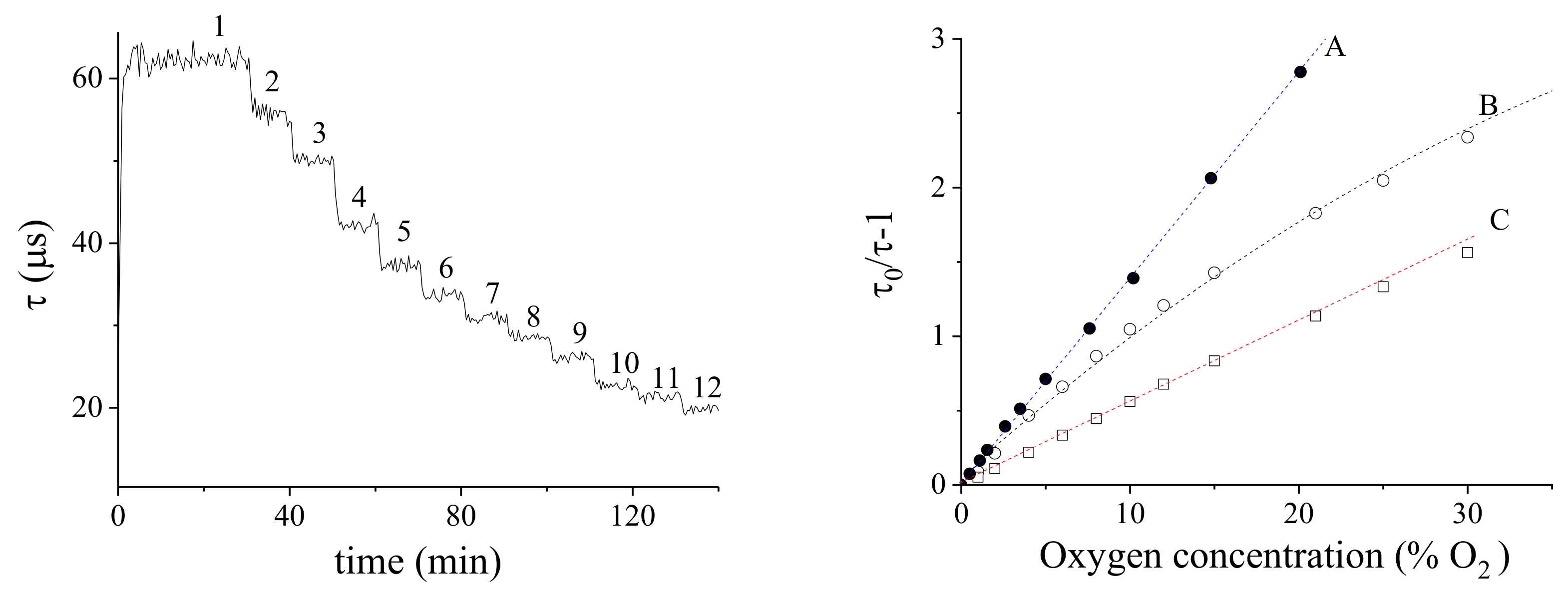
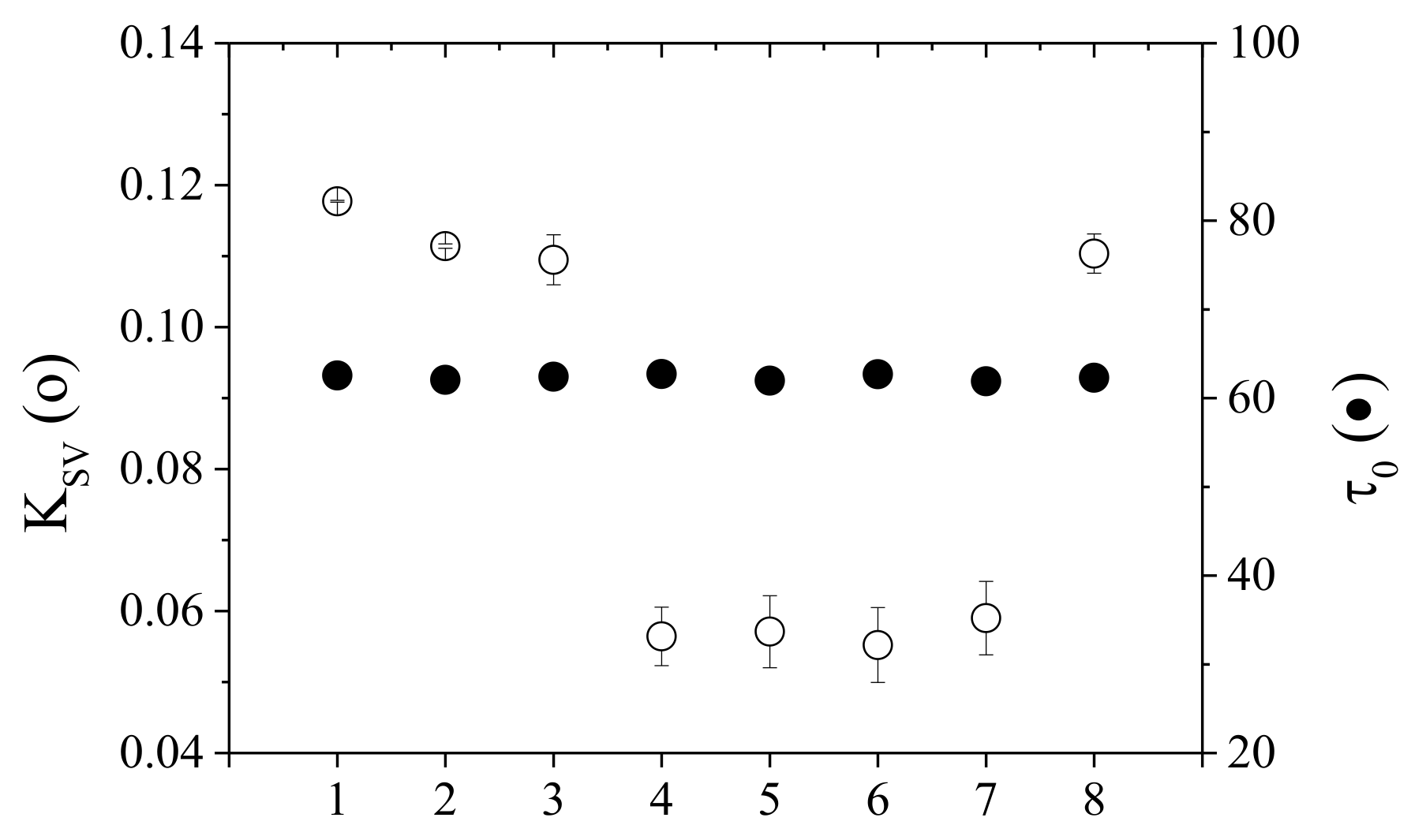
5.1.6. Sensor Calibration
- A:
- in air (membrane supported on glass):
- B:
- in air (membrane supported on Mylar®):
- C:
- in ethanol solution (membrane supported on Mylar®):
5.1.7. Algorithm for Oxygen Determination
5.2. In Situ Experimental Details and Results
5.2.1. Experimental Details
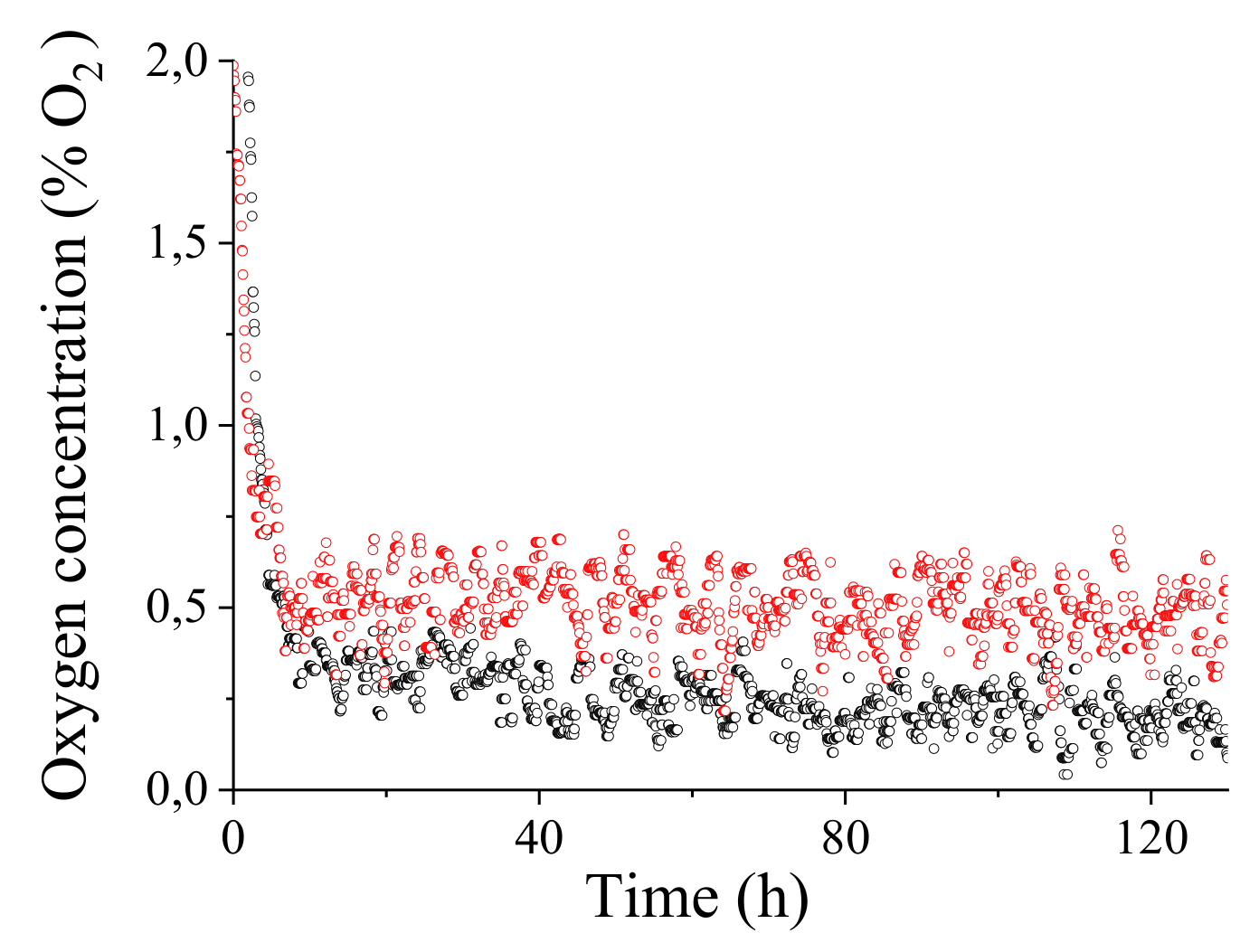
5.2.2. Results
6. Discussion and Conclusions
- the possibility of obtaining a complete and correct fermentation process;
- the reduction of micro-organisms growth risk, since wine naturally contains a microbial load that can proliferate with oxygen;
- the production of a wine with optimized sensory characteristics, namely stabilized color and structure of red wines and stabilization of the aromatic and organoleptic profile;
- the reduction of the perception of dryness and astringency due to the tannic structure;
- less need for antioxidants, such as ascorbic acid and sulfur dioxide, generally added for preservation;
- the increase of the “shelf life” of wine.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moenne, M.I.; Saa, P.; Laurie, V.F.; Pérez-Correa, J.R.; Agosin, E. Oxygen Incorporation and Dissolution During Industrial-Scale Red Wine Fermentations. Food Bioprocess Technol. 2014, 7, 2627–2636. [Google Scholar] [CrossRef]
- Du Toit, W.; Marais, J.; Pretorius, I.; du Toit, M. Oxygen in Must and Wine: A review. S. Afr. J. Enol. Vitic. 2006, 27, 76–94. [Google Scholar] [CrossRef]
- Anli, R.E.; Cavuldak, O.A. A review of microoxygenation application in wine. J. Inst. Brew. 2012, 118, 368–385. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Cano-López, M.; López-Roca, J.; Pardo-Minguez, F.; Plaza, E.G. Oak barrel maturation vs. micro-oxygenation: Effect on the formation of anthocyanin-derived pigments and wine colour. Food Chem. 2010, 119, 191–195. [Google Scholar] [CrossRef]
- The WOW Project Website. Available online: http://automatica.dei.unipd.it/people/cenedese/research/wow.html (accessed on 22 February 2018).
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Sablayrolles, J. Control of alcoholic fermentation in winemaking: Current situation and prospect. Food Res. Int. 2009, 42, 418–424. [Google Scholar] [CrossRef]
- Cywinski, P.J.; Moro, A.J.; Stanca, S.E.; Biskup, C.; Mohr, G.J. Ratiometric porphyrin-based layers and nanoparticles for measuring oxygen in biosamples. Sens. Actuators B Chem. 2009, 135, 472–477. [Google Scholar] [CrossRef]
- O’Mahony, F.C.; O’Riordan, T.C.; Papkovskaia, N.; Kerry, J.P.; Papkovsky, D.B. Non-destructive assessment of oxygen levels in industrial modified atmosphere packaged cheddar cheese. Food Control 2006, 17, 286–292. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, S.; Song, Y.; Zhong, W.; Jiang, J.; Chen, S.; Bai, C. Fluorescence optical fibre sensor provides accurate continuous oxygen detection in rabbit model with acute lung injury. Respirology 2010, 15, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Baleizão, C.; Nagl, S.; Schäferling, M.; Berberan-Santos, M.N.; Wolfbeis, O.S. Dual Fluorescence Sensor for Trace Oxygen and Temperature with Unmatched Range and Sensitivity. Anal. Chem. 2008, 80, 6449–6457. [Google Scholar] [CrossRef] [PubMed]
- Scherer, B.; Woellenstein, J. Optical sensor for online-monitoring of oxygen traces in hydrogen electrolysis. Sens. Actuators B Chem. 2009, 138, 96–99. [Google Scholar] [CrossRef]
- Caillé, S.; Samson, A.; Wirth, J.; Diéval, J.B.; Vidal, S.; Cheynier, V. Sensory characteristics changes of red Grenache wines submitted to different oxygen exposures pre and post bottling. Anal. Chim. Acta 2010, 660, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Liu, J.; Ishii, M.; Igarashi, Y.; Cui, Z. Effect of Oxygen Concentration on the Composting Process and Maturity. Compost Sci. Util. 2007, 15, 184–190. [Google Scholar] [CrossRef]
- Topal, S.Z.; Ertekin, K.; Topkaya, D.; Alp, S.; Yenigul, B. Emission based oxygen sensing approach with tris(2,2′-bipyridyl)ruthenium(II)chloride in green chemistry reagents: room temperature ionic liquids. Microchim. Acta 2008, 161, 209–216. [Google Scholar] [CrossRef]
- Grundler, P. Chemical Sensors—An Introduction for Scientists and Engineers; Springer-Verlag: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mitterdorfer, A.; Gauckler, L. Identification of the reaction mechanism of the Pt, O2(g)|yttria-stabilized zirconia system: Part I: General framework, modelling, and structural investigation. Solid State Ionics 1999, 117, 187–202. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen Sensors: Materials, methods, designs and applications. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Luminescent Sensing and Imaging of Oxygen: Fierce Competition to the Clark Electrode. Bioessays 2015, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Badocco, D.; Mondin, A.; Pastore, P. Signal drift of oxygen optical sensors. Part I: Rationalization of the drift nature and its experimental check with a light intensity detection based sensor. Sens. Actuators B Chem. 2013, 181, 943–948. [Google Scholar] [CrossRef]
- Badocco, D.; Mondin, A.; Pastore, P. Signal drift of oxygen optical sensors. Part II: “Smart” drift correction algorithm and its experimental check with a light intensity detection based sensor. Sens. Actuators B Chem. 2013, 181, 949–954. [Google Scholar] [CrossRef]
- McDonagh, C.; Kolle, C.; McEvoy, A.; Dowling, D.; Cafolla, A.; Cullen, S.; MacCraith, B. Phase fluorometric dissolved oxygen sensor. Sens. Actuators B Chem. 2001, 74, 124–130. [Google Scholar] [CrossRef]
- Valledor, M.; Campo, J.C.; Sánchez-Barragán, I.; Viera, J.C.; Costa-Fernández, J.M.; Sanz-Medel, A. Luminescent ratiometric method in the frequency domain with dual phase-shift measurements: Application to oxygen sensing. Sens. Actuators B Chem. 2006, 117, 266–273. [Google Scholar] [CrossRef]
- Trettnak, W.; Kolle, C.; Reininger, F.; Dolezal, C.; O’Leary, P.; Binot, R. Optical oxygen sensor instrumentation based on the detection of luminescence lifetime. Adv. Space Res. 1998, 22, 1465–1474. [Google Scholar] [CrossRef]
- Nevares, I.; del Alamo, M.; Gonzalez-Muñoz, C. Dissolved oxygen distribution during micro-oxygenation. Determination of representative measurement points in hydroalcoholic solution and wines. Anal. Chim. Acta 2010, 660, 232–239. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Dochain, D.; Mouret, J.R.; Wouwer, A.V.; Sablayrolles, J.M. Dynamical modeling of alcoholic fermentation and its link with nitrogen consumption. IFAC Proc. Vol. 2010, 43, 496–501. [Google Scholar] [CrossRef]
- David, R.; Dochain, D.; Mouret, J.R.; Wouwer, A.V.; Sablayrolles, J.M. Modeling of the aromatic profile in wine-making fermentation: the backbone equations. IFAC Proc. Vol. 2011, 44, 10597–10602. [Google Scholar] [CrossRef]
- Borzì, A.; Merger, J.; Müller, J.; Rosch, A.; Schenk, C.; Schmidt, D.; Schmidt, S.; Schulz, V.; Velten, K.; von Wallbrunn, C.; et al. Novel model for wine fermentation including the yeast dying phase. arXiv, 2014; arXiv:1412.6068. [Google Scholar]
- Merger, J.; Borzì, A.; Herzog, R. Optimal control of a system of reaction-diffusion equations modeling the wine fermentation process. Optim. Control Appl. Methods 2016, 38, 112–132. [Google Scholar] [CrossRef]
- Astrom, K.J.; Murray, R.M. Feedback Systems: An Introduction for Scientists and Engineers; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Adoua, R.; Mietton-Peuchot, M.; Milisic, V. Modelling of oxygen transfer in wines. Chem. Eng. Sci. 2010, 65, 5455–5463. [Google Scholar] [CrossRef]
- Berni, E.; Gosse, I.; Badocco, D.; Pastore, P.; Sojic, N.; Pinet, S. Differential Photoluminescent and Electrochemiluminescent Detection of Anions with a Modified Ruthenium(II)-Bipyridyl Complex. Chem. A Eur. J. 2009, 15, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Badocco, D.; Mondin, A.; Fusar, A.; Pastore, P. Calibration Models under Dynamic Conditions for Determining Molecular Oxygen with Optical Sensors on the Basis of Luminescence Quenching of Transition-Metal Complexes Embedded in Polymeric Matrixes. J. Phys. Chem. C 2009, 113, 20467–20475. [Google Scholar] [CrossRef]
- Badocco, D.; Mondin, A.; Fusar, A.; Favaro, G.; Pastore, P. Influence of the Real Background Signal on the Linearity of the Stern-Volmer Calibration for the Determination of Molecular Oxygen with Optical Sensors. J. Phys. Chem. C 2009, 113, 15742–15750. [Google Scholar] [CrossRef]
- Badocco, D.; Pastore, P. Definition and Use of the Experimental Sensible Parameters To Characterize Sensitivity and Precision of a Generic Oxygen Optical Sensor. Anal. Chem. 2008, 80, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Badocco, D.; Mondin, A.; Pastore, P.; Voltolina, S.; Gross, S. Dependence of calibration sensitivity of a polysulfone/Ru(II)-Tris(4,7-diphenyl-1,10-phenanthroline)-based oxygen optical sensor on its structural parameters. Anal. Chim. Acta 2008, 627, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Craith, B.D.M.; O’Keeffe, G.; McDonagh, C.; McEvoy, A.K. LED-based fibre optic oxygen sensor using sol-gel coating. Electron. Lett. 1994, 30, 888–889. [Google Scholar] [CrossRef]
- Meneghini, M.; Vaccari, S.; Garbujo, A.; Trivellin, N.; Zhu, D.; Humphreys, C.J.; Calciati, M.; Goano, M.; Bertazzi, F.; Ghione, G.; et al. Electroluminescence Analysis and Simulation of the Effects of Injection and Temperature on Carrier Distribution in InGaN-Based Light-Emitting Diodes with Color-Coded Quantum Wells. Jpn. J. Appl. Phys. 2013, 52, 08JG09. [Google Scholar] [CrossRef]
- Graeme, J. Photodiode Amplifiers: OP AMP Solutions; McGraw-Hill Inc.: New York, NY, USA, 1996. [Google Scholar]
- Best, J.A.V.; Mathis, P. Low-noise photodiode amplifier for absorption changes measurements in the microsecond range. Photochem. Photobiol. 1980, 31, 89–92. [Google Scholar] [CrossRef]
- Badocco, D.; Lavagnini, I.; Mondin, A.; Tapparo, A.; Pastore, P. Limit of detection in the presence of instrumental and non-instrumental errors: Study of the possible sources of error and application to the analysis of 41 elements at trace levels by inductively coupled plasma-mass spectrometry technique. Spectrochim. Acta 2015, 107, 178–184. [Google Scholar] [CrossRef]
- Badocco, D.; Lavagnini, I.; Mondin, A.; Pastore, P. Effect of multiple error sources on the calibration uncertainty. Food Chem. 2015, 177, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Azienda Agricola Monteci. Available online: http://www.monteci.it/ (accessed on 22 February 2018).




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivellin, N.; Barbisan, D.; Badocco, D.; Pastore, P.; Meneghesso, G.; Meneghini, M.; Zanoni, E.; Belgioioso, G.; Cenedese, A. Study and Development of a Fluorescence Based Sensor System for Monitoring Oxygen in Wine Production: The WOW Project. Sensors 2018, 18, 1130. https://doi.org/10.3390/s18041130
Trivellin N, Barbisan D, Badocco D, Pastore P, Meneghesso G, Meneghini M, Zanoni E, Belgioioso G, Cenedese A. Study and Development of a Fluorescence Based Sensor System for Monitoring Oxygen in Wine Production: The WOW Project. Sensors. 2018; 18(4):1130. https://doi.org/10.3390/s18041130
Chicago/Turabian StyleTrivellin, Nicola, Diego Barbisan, Denis Badocco, Paolo Pastore, Gaudenzio Meneghesso, Matteo Meneghini, Enrico Zanoni, Giuseppe Belgioioso, and Angelo Cenedese. 2018. "Study and Development of a Fluorescence Based Sensor System for Monitoring Oxygen in Wine Production: The WOW Project" Sensors 18, no. 4: 1130. https://doi.org/10.3390/s18041130
APA StyleTrivellin, N., Barbisan, D., Badocco, D., Pastore, P., Meneghesso, G., Meneghini, M., Zanoni, E., Belgioioso, G., & Cenedese, A. (2018). Study and Development of a Fluorescence Based Sensor System for Monitoring Oxygen in Wine Production: The WOW Project. Sensors, 18(4), 1130. https://doi.org/10.3390/s18041130






