Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion
Abstract
:1. Introduction
- (1)
- Electrode mass: We hypothesize that increasing the mass of the electrode can cause greater electrode displacement relative to the scalp during motion and, therefore, increase motion artifacts.
- (2)
- Electrode surface area: We hypothesize that increasing the electrode surface area can lead to better signal quality during motion, because of the decrease in impedance of the electrode-electrolyte junction.
- (3)
- Cable sway: We hypothesize that cable sway contributes the most to motion artifacts. Therefore, isolating cable motion from electrode motion will give us a better insight into motion artifact generation.
2. Materials and Methods
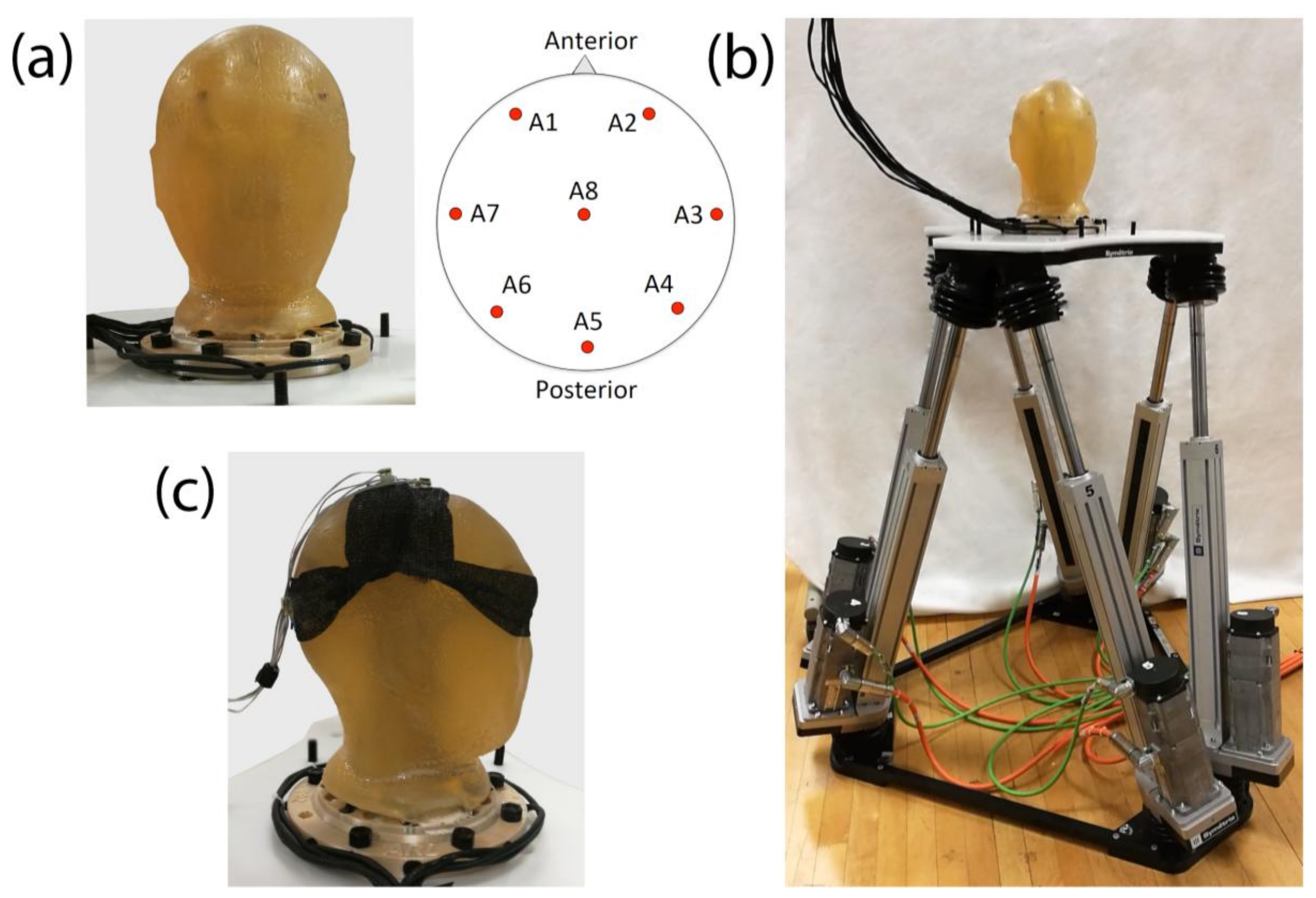
2.1. The Phantom Head
2.2. The Motion Hexapod
2.3. EEG Acquisition
2.4. Electrode Mass
2.5. Electrode Surface Area
2.6. Cable Sway
2.7. EEG Analysis
2.8. Statistical Analysis
3. Results
3.1. Electrode Mass
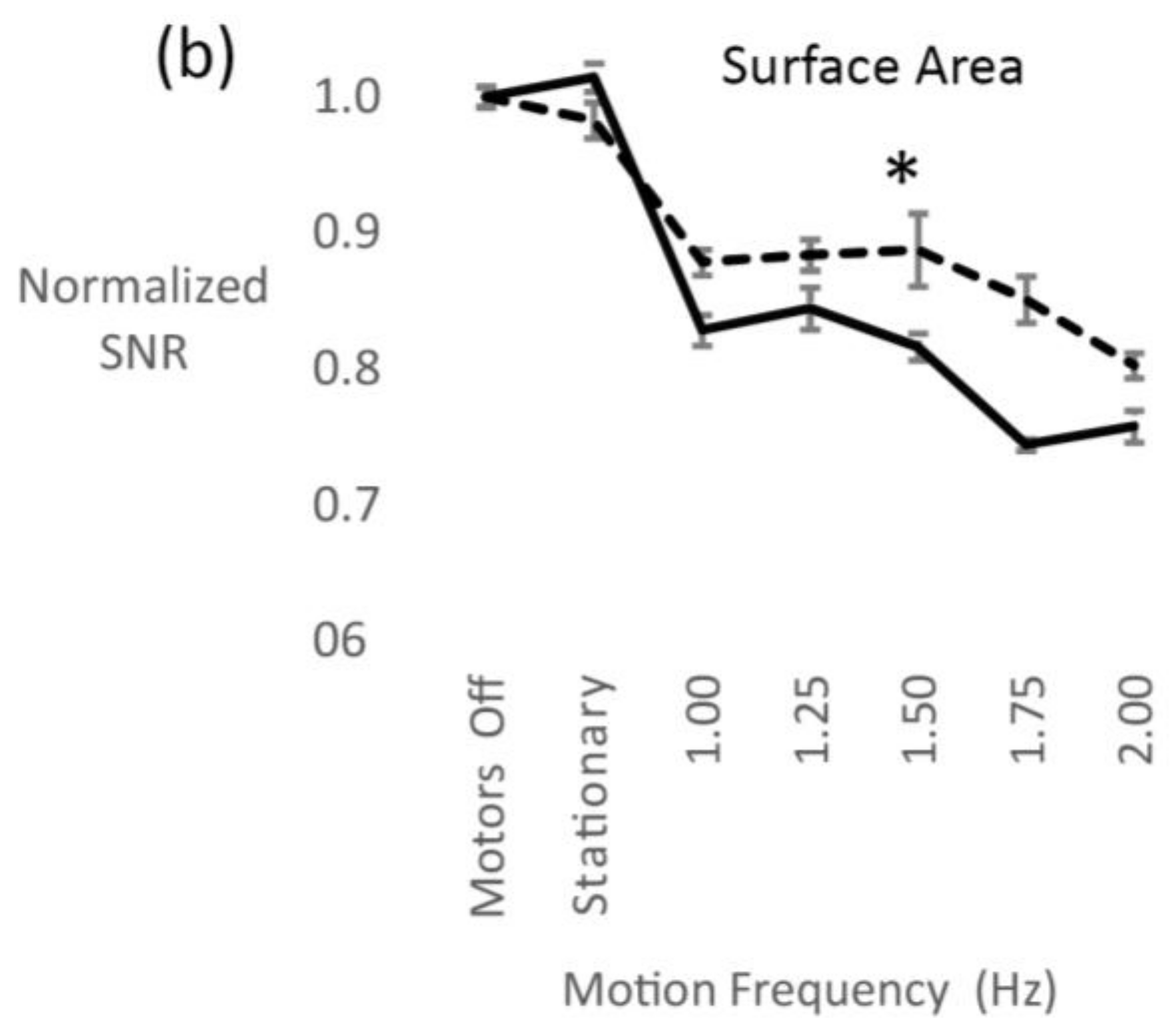
3.2. Electrode Surface Area
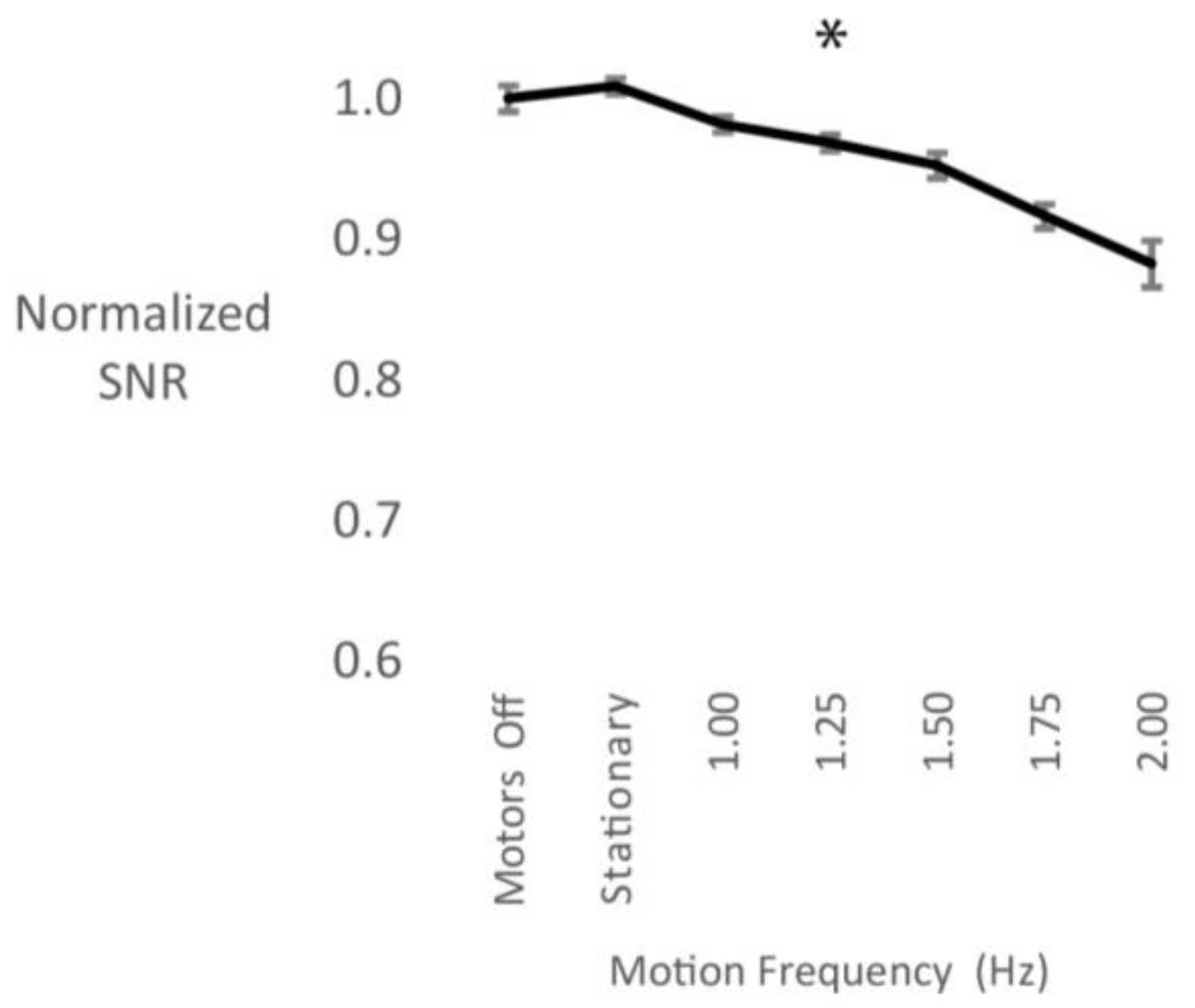
3.3. Cable Sway
4. Discussion
4.1. Electrode Mass
4.2. Electrode Surface Area
4.3. Cable Sway
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2002, 2, 1–11. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Salinsky, M.; Kanter, R.; Dasheiff, R.M. Effectiveness of Multiple EEGs in Supporting the Diagnosis of Epilepsy: An Operational Curve. Epilepsia 1987, 28, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, F. EEG and MEG: Relevance to neuroscience. Neuron 2013, 80, 1112–1128. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C. Brain-Computer Interfaces. In The Frontiers Collection; Graimann, B., Pfurtscheller, G., Allison, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 47–64. ISBN 978-3-642-02090-2. [Google Scholar]
- Tallgren, P.; Vanhatalo, S.; Kaila, K.; Voipio, J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clin. Neurophysiol. 2005, 116, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.G. Reducing Motion Artifacts and Interference in Biopotential Recording. IEEE Trans. Biomed. Eng. 1984, BME-31, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Petruzzello, S.J.; Landers, D.M.; Hatfield, B.D.; Kubitz, K.A.; Salazar, W. A Meta-Analysis on the Anxiety-Reducing Effects of Acute and Chronic Exercise: Outcomes and Mechanisms. Sport. Med. 1991, 11, 143–182. [Google Scholar] [CrossRef]
- Daly, J.J.; Wolpaw, J.R. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage 2011, 54, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Removal of Movement Artifact From High-Density EEG Recorded During Walking and Running. J. Neurophysiol. 2010, 103, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Metting van Rijn, A.C.; Peper, A.; Grimbergen, C.A. High-quality recording of bioelectric events—Part 1 Interference reduction, theory and practice. Med. Biol. Eng. Comput. 1990, 28, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Huigen, E.; Peper, A.; Grimbergen, C.A. Investigation into the origin of the noise of surface electrodes. Med. Biol. Eng. Comput. 2002, 40, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.J.; Konopacki, R.A.; Abbs, J.H. A miniature electrode for surface electromyography during speech. J. Acoust. Soc. Am. 1983, 74, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Induction and separation of motion artifacts in EEG data using a mobile phantom head device. J. Neural Eng. 2016, 13, 36014. [Google Scholar] [CrossRef] [PubMed]
- Simakov, A.B.; Webster, J.G. Motion artifact from electrodes and cables. Iran. J. Electr. Comput. Eng. 2010, 9, 139–143. [Google Scholar]
- MettingVanRijn, A.C.; Kuiper, A.P.; Dankers, T.E.; Grimbergen, C.A. Low-cost active electrode improves the resolution in biopotential recordings. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; pp. 101–102. [Google Scholar] [CrossRef]
- Lee, S.M.; Sim, K.S.; Kim, K.K.; Lim, Y.G.; Park, K.S. Thin and flexible active electrodes with shield for capacitive electrocardiogram measurement. Med. Biol. Eng. Comput. 2010, 48, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Geddes, L.A.; Baker, L.E. The relationship between input impedance and electrode area in recording the ECG. Med. Biol. Eng. 1966, 4, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Geddes, L. A Historical evolution of circuit models for the electrode-electrolyte interface. Ann. Biomed. Eng. 1997, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mihajlović, V.; Li, H.; Grundlehner, B.; Penders, J.; Schouten, A.C. Investigating the impact of force and movements on impedance magnitude and EEG. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar] [CrossRef]
- Mihajlovic, V.; Patki, S.; Grundlehner, B. The impact of head movements on EEG and contact impedance: An adaptive filtering solution for motion artifact reduction. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 5064–5067. [Google Scholar] [CrossRef]
- Mihajlovic, V.; Grundlehner, B. The effect of force and electrode material on electrode-to-skin impedance. In Proceedings of the 2012 IEEE Biomedical Circuits and Systems Conference (BioCAS), Hsinchu, Taiwan, 28–30 November 2012; pp. 57–60. [Google Scholar] [CrossRef]
- Hairston, W.; Slipher, G.; Yu, A. Ballistic gelatin as a putative substrate for EEG phantom devices. arXiv, 2016; arXiv:1609.07691. [Google Scholar]
- Nathan, K.; Contreras-Vidal, J.L. Negligible Motion Artifacts in Scalp Electroencephalography (EEG) During Treadmill Walking. Front. Hum. Neurosci. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Barrett, R.S.; Morrison, S. Upper body accelerations during walking in healthy young and elderly men. Gait Posture 2004, 20, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.J.; Morrison, S.; Barrett, R.S. Coordination of head and trunk accelerations during walking. Eur. J. Appl. Physiol. 2005, 94, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.A.D.; Beijer, T.R.; Canning, C.G.; Lord, S.R. Head and pelvis stride-to-stride oscillations in gait: Validation and interpretation of measurements from wearable accelerometers. Physiol. Meas. 2015, 36, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Hirasaki, E.; Moore, S.T.; Raphan, T. Effects of walking velocity on vertical head and body movements during locomotion. Exp. Brain Res. 1999, 127, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.E.; Huang, H.J.; Snyder, K.L.; Ferris, D.P. Isolating gait-related movement artifacts in electroencephalography during human walking. J. Neural Eng. 2015, 12, 46022. [Google Scholar] [CrossRef] [PubMed]
- Castermans, T.; Duvinage, M.; Cheron, G.; Dutoit, T. About the cortical origin of the low-delta and high-gamma rhythms observed in EEG signals during treadmill walking. Neurosci. Lett. 2014, 561, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, W.; Hof, A.L. Displacement of the pelvis during human walking: Experimental data and model predictions. Gait Posture 1997, 6, 249–262. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Schlink, B.R.; Hairston, W.D.; König, P.; Ferris, D.P. Restricted vision increases sensorimotor cortex involvement in human walking. J. Neurophysiol. 2017, 118, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Bradford, J.C.; Lukos, J.R.; Ferris, D.P. Electrocortical activity distinguishes between uphill and level walking in humans. J. Neurophysiol. 2016, 115, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.L.; Kline, J.E.; Huang, H.J.; Ferris, D.P. Independent Component Analysis of Gait-Related Movement Artifact Recorded using EEG Electrodes during Treadmill Walking. Front. Hum. Neurosci. 2015, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.E.; Poggensee, K.; Ferris, D.P. Your brain on speed: Cognitive performance of a spatial working memory task is not affected by walking speed. Front. Hum. Neurosci. 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.M.; Gwin, J.T.; Ferris, D.P. Walking reduces sensorimotor network connectivity compared to standing. J. Neuroeng. Rehabil. 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.M.; Gwin, J.T.; McDowell, K.G.; Ferris, D.P. Weighted phase lag index stability as an artifact resistant measure to detect cognitive EEG activity during locomotion. J. Neuroeng. Rehabil. 2012, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Gramann, K.; Gwin, J.T.; Bigdely-Shamlo, N.; Ferris, D.P.; Makeig, S. Visual Evoked Responses During Standing and Walking. Front. Hum. Neurosci. 2010, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Biosemi EEG ECG EMG BSPM NEURO Amplifiers Systems. Available online: https://www.biosemi.com/faq/cms&drl.htm (accessed on 30 January 2018).
- Onton, J.; Makeig, S. Information-based modeling of event-related brain dynamics. Prog. Brain Res. 2006, 159, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Ödman, S.; Åke Öberg, P. Movement-induced potentials in surface electrodes. Med. Biol. Eng. Comput. 1982, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kruse, J. Biopotential Electrode Sensors in ECG/EEG/EMG Systems. Motorcontrol. Analog. Commun. 2008, 1–2. Available online: https://s3.amazonaws.com/academia.edu.documents/36298011/ECG-EEG-EMG_FINAL.pdf?AWSAccessKeyId=AKIAIWOWYYGZ2Y53UL3A&Expires=1522728460&Signature=RNGguL2pIjVTBoI2pjEpyFRI%2Ffs%3D&response-content-disposition=inline%3B%20filename%3DECG-EEG-EMG_FINAL.pdf (accessed on 3 April 2018).
- Lee, J.S.; Heo, J.; Lee, W.K.; Lim, Y.G.; Kim, Y.H.; Park, K.S. Flexible capacitive electrodes for minimizing motion artifacts in ambulatory electrocardiograms. Sensors 2014, 14, 14732–14743. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, V.; Grundlehner, B.; Vullers, R.; Penders, J. Wearable, wireless EEG solutions in daily life applications: What are we missing? IEEE J. Biomed. Heal. Inform. 2015, 19, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Melnik, A.; Legkov, P.; Izdebski, K.; Kärcher, S.M.; Hairston, W.D.; Ferris, D.P.; König, P. Systems, Subjects, Sessions: To What Extent Do These Factors Influence EEG Data? Front. Hum. Neurosci. 2017, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- David Hairston, W.; Whitaker, K.W.; Ries, A.J.; Vettel, J.M.; Cortney Bradford, J.; Kerick, S.E.; McDowell, K. Usability of four commercially-oriented EEG systems. J. Neural Eng. 2014, 11, 46018. [Google Scholar] [CrossRef] [PubMed]
- Berka, C.; Levendowski, D.J.; Lumicao, M.N.; Yau, A.; Davis, G.; Zivkovic, V.T.; Olmstead, R.E.; Tremoulet, P.D.; Craven, P.L. EEG correlates of task engagement and mental workload in vigilance, learning, and memory tasks. Aviat. Space. Environ. Med. 2007, 78, B231–B244. [Google Scholar] [PubMed]
- Debener, S.; Minow, F.; Emkes, R.; Gandras, K.; de Vos, M. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology 2012, 49, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Izdebski, K.; Oliveira, A.S.; Schlink, B.R.; Legkov, P.; Kärcher, S.; Hairston, W.D.; Ferris, D.P.; König, P. Usability of EEG Systems. In Proceedings of the 9th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Island, Greece, 29 June–1 July 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Cheron, G.; Duvinage, M.; De Saedeleer, C.; Castermans, T.; Bengoetxea, A.; Petieau, M.; Seetharaman, K.; Hoellinger, T.; Dan, B.; Dutoit, T.; et al. From spinal central pattern generators to cortical network: Integrated BCI for walking rehabilitation. Neural Plast. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Vidal, J.L.; Grossman, R.G. NeuroRex: A clinical neural interface roadmap for EEG-based brain machine interfaces to a lower body robotic exoskeleton. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1579–1582. [Google Scholar] [CrossRef]
- Cheron, G.; Petit, G.; Cheron, J.; Leroy, A.; Cebolla, A.; Cevallos, C.; Petieau, M.; Hoellinger, T.; Zarka, D.; Clarinval, A.M.; et al. Brain oscillations in sport: Toward EEG biomarkers of performance. Front. Psychol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symeonidou, E.-R.; Nordin, A.D.; Hairston, W.D.; Ferris, D.P. Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion. Sensors 2018, 18, 1073. https://doi.org/10.3390/s18041073
Symeonidou E-R, Nordin AD, Hairston WD, Ferris DP. Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion. Sensors. 2018; 18(4):1073. https://doi.org/10.3390/s18041073
Chicago/Turabian StyleSymeonidou, Evangelia-Regkina, Andrew D. Nordin, W. David Hairston, and Daniel P. Ferris. 2018. "Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion" Sensors 18, no. 4: 1073. https://doi.org/10.3390/s18041073
APA StyleSymeonidou, E.-R., Nordin, A. D., Hairston, W. D., & Ferris, D. P. (2018). Effects of Cable Sway, Electrode Surface Area, and Electrode Mass on Electroencephalography Signal Quality during Motion. Sensors, 18(4), 1073. https://doi.org/10.3390/s18041073




