Miniaturized Sample Preparation and Rapid Detection of Arsenite in Contaminated Soil Using a Smartphone
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
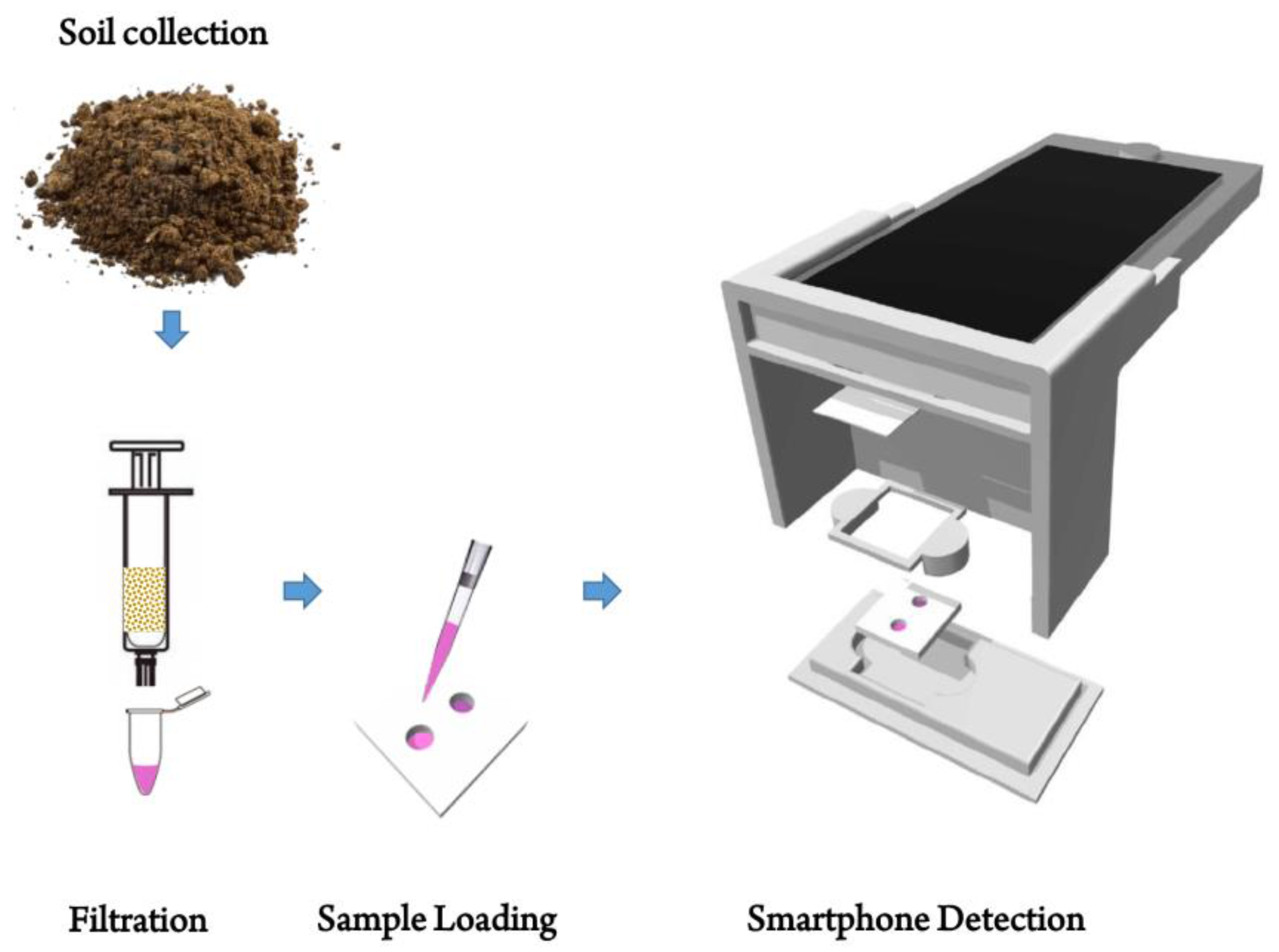
2.2. Extraction Procedure for Arsenic
2.3. Fabrication of PDMS Chip
2.4. Arsenic Detection Standard Protocol
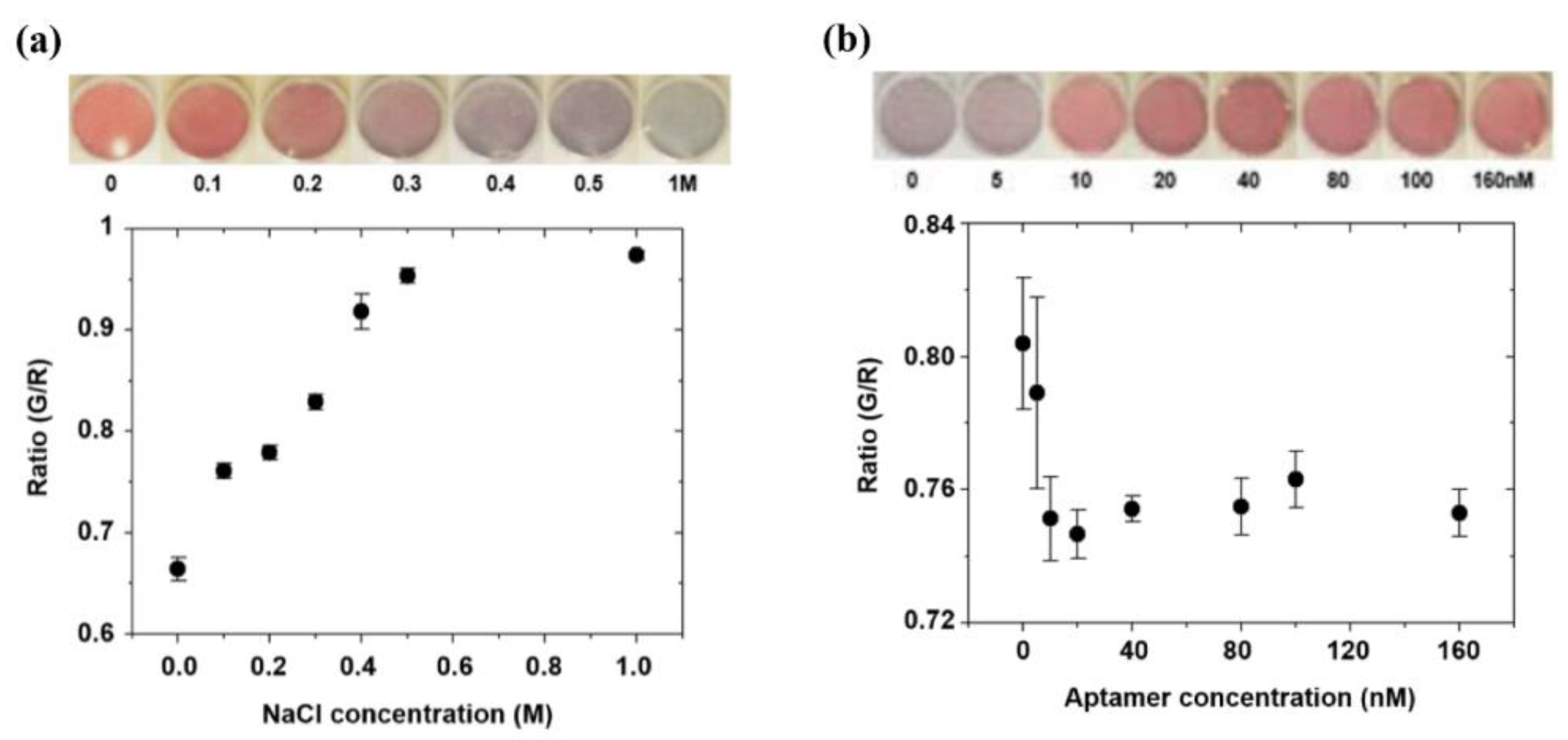
2.4.1. Salt Concentration Optimization
2.4.2. Aptamer Concentration Optimization
2.5. Specificity Test for Arsenic As(III) Determination
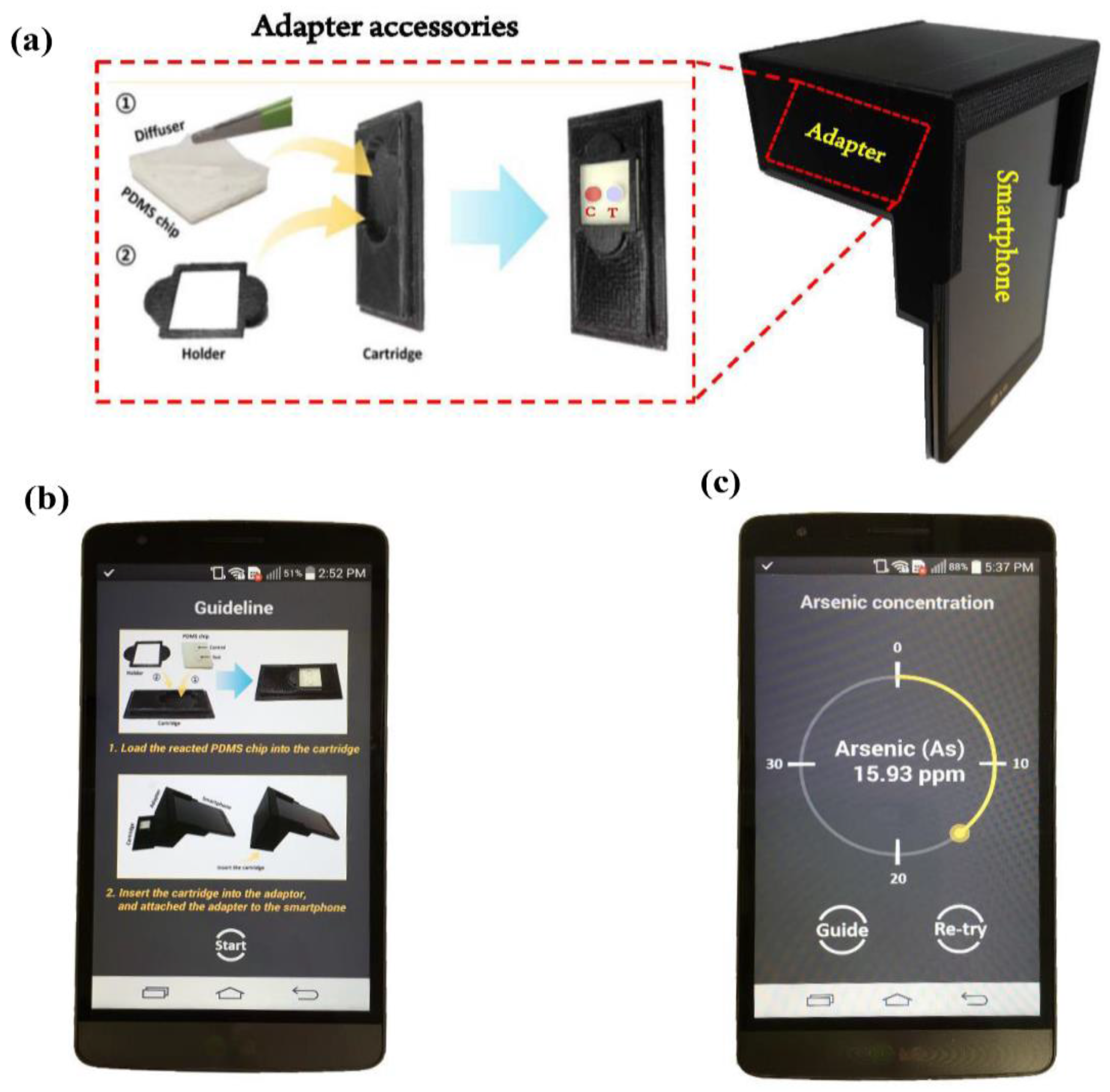
2.6. Image Acquisition & Design of Smartphone-Based Optical Device
3. Results and Discussion
3.1. Android-Based Smartphone Application
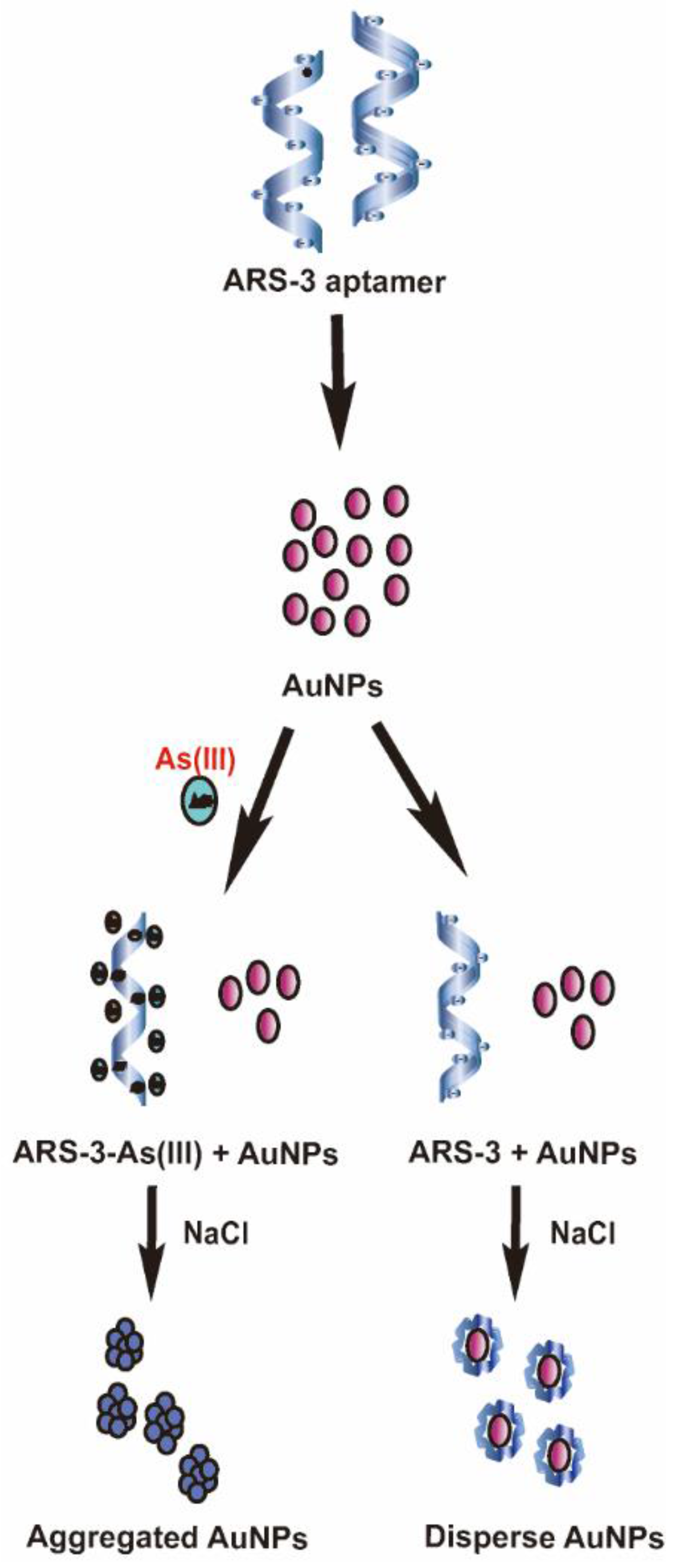
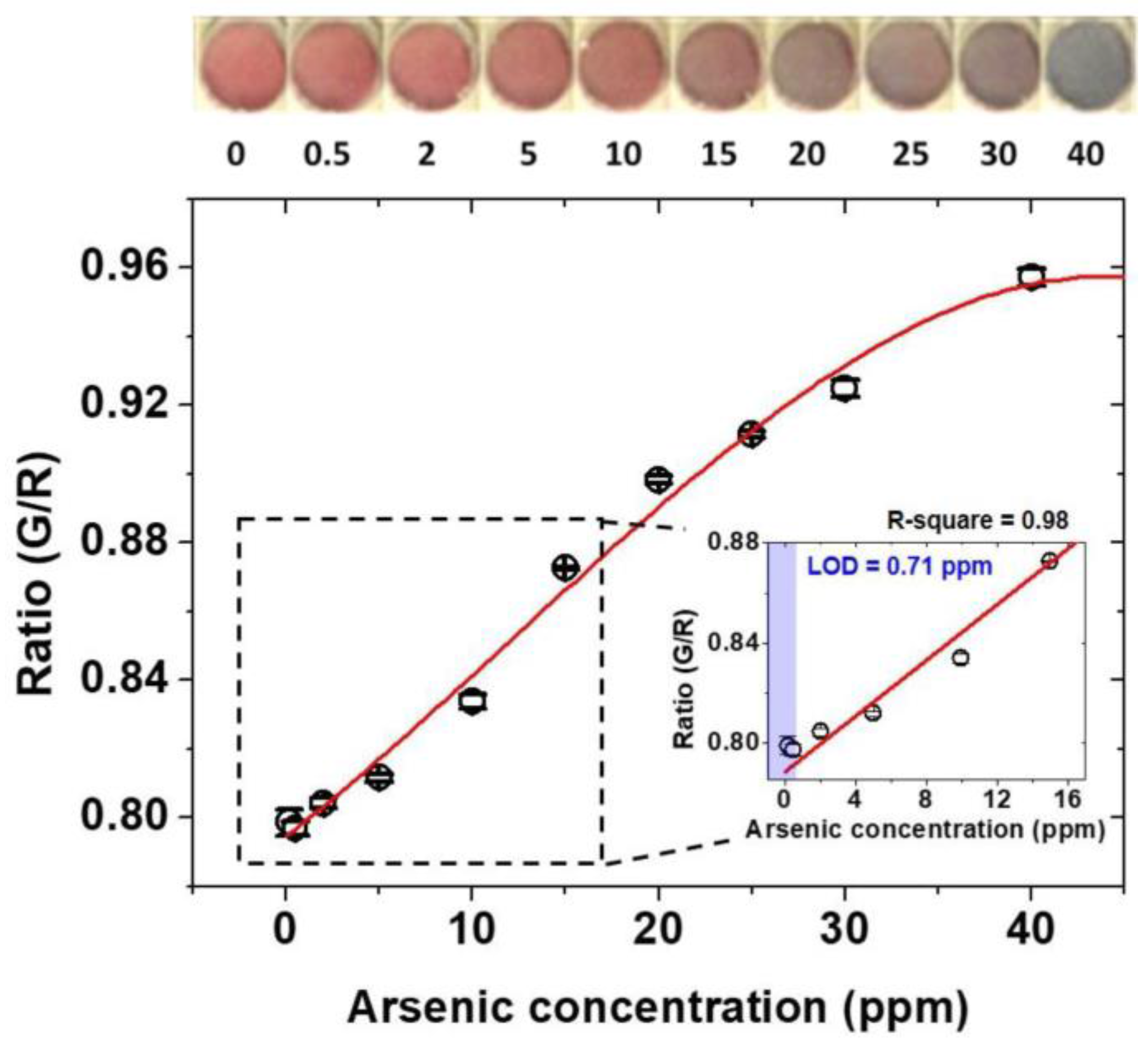
3.2. Colorimetric Detection Mechanism of Arsenic As(III) and Standard Calibration Test for Arsenic As(III) Detection
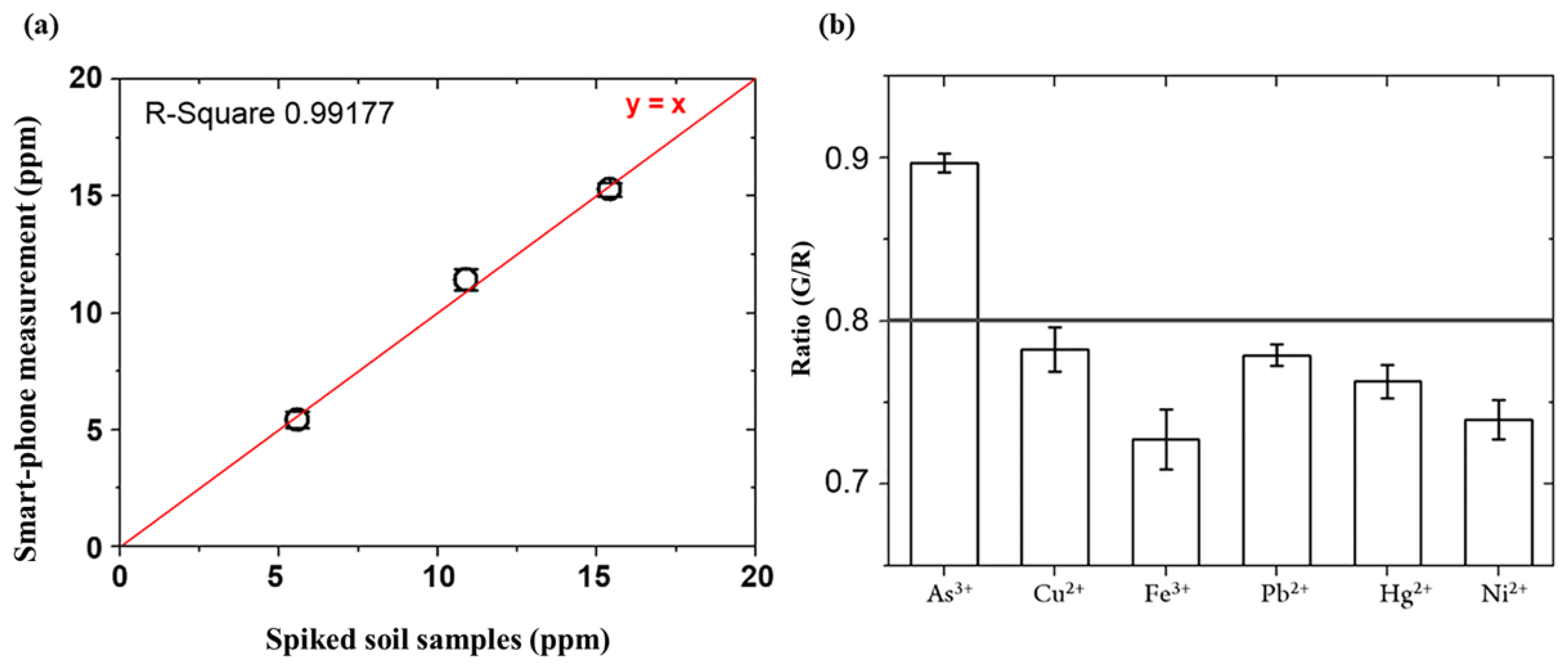
3.3. Detection of Arsenic As(III) in Spiked Soil Sample
3.4. Specificity Test for Arsenic As(III) in the Presence of other Heavy Metal Ions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Chen, G.-H.; Chen, W.-Y.; Yen, Y.-C.; Wang, C.-W.; Chang, H.-T.; Chen, C.-F. Detection of mercury (ii) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Ling, L.; Shuai, X. Ultrasensitive detection of lead (ii) with dnazyme and gold nanoparticles probes by using a dynamic light scattering technique. Chem. Commun. 2011, 47, 4192–4194. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, D.N.G. Chronic arsenic toxicity & human health. Indian J. Med. Res. 2008, 128, 436. [Google Scholar]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.M. Arsenic crisis in Bangladesh. Sci. Am. 2004, 291, 86–91. [Google Scholar] [PubMed]
- Nickson, R.; McArthur, J.; Burgess, W.; Ahmed, K.M.; Ravenscroft, P.; Rahmanñ, M. Arsenic poisoning of Bangladesh groundwater. Nature 1998, 395, 338. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Tran, H.C.; Nguyen, T.C.; Pham, H.V.; Schertenleib, R.; Giger, W. Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environ. Sci. Technol. 2001, 35, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Occurrence of arsenic contamination in Canada: Sources, behavior and distribution. Sci. Total Environ. 2006, 366, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-L.; Dzeng, S.R.; Yang, M.-H.; Chiu, K.-H.; Shieh, G.-M.; Wai, C.M. Arsenic species in groundwaters of the blackfoot disease area, Taiwan. Environ. Sci. Technol. 1994, 28, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Guidelines for Drinking-Water Quality: Recommendations; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Atsdr, U. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry, Division of Toxicology: Atlanta, GA, USA, 2007. [Google Scholar]
- Teaf, C.M.; Covert, D.J.; Teaf, P.A.; Page, E.; Starks, M.J. Arsenic cleanup criteria for soils in the US and abroad: Comparing guidelines and understanding inconsistencies. Proc. Annu. Int. Conf. Soils Sediments Water Energy 2010, 15, 10. [Google Scholar]
- Barwick, M.; Maher, W. Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Mar. Environ. Res. 2003, 56, 471–502. [Google Scholar] [CrossRef]
- Šlejkovec, Z.; Stajnko, A.; Falnoga, I.; Lipej, L.; Mazej, D.; Horvat, M.; Faganeli, J. Bioaccumulation of arsenic species in rays from the northern Adriatic Sea. Int. J. Mol. Sci. 2014, 15, 22073–22091. [Google Scholar] [CrossRef] [PubMed]
- Dovick, M.A.; Kulp, T.R.; Arkle, R.; Pilliod, D. Bioaccumulation trends of arsenic and antimony in a freshwater ecosystem affected by mine drainage. Environ. Chem. 2015, 13, 149–159. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, R.; Babu, J.N.; Mittal, S. Advances in arsenic biosensor development—A comprehensive review. Biosens. Bioelectron. 2015, 63, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A review of arsenic poisoning and its effects on human health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Shen, S.; Li, X.-F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Esteban, E.; Peñalosa, J.M. The fate of arsenic in soil-plant systems. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2012; pp. 1–37. [Google Scholar]
- Rajpert, L.; Kolvenbach, B.A.; Ammann, E.M.; Hockmann, K.; Nachtegaal, M.; Eiche, E.; Schäffer, A.; Corvini, P.F.X.; Skłodowska, A.; Lenz, M. Arsenic mobilization from historically contaminated mining soils in a continuously operated bioreactor: Implications for risk assessment. Environ. Sci. Technol. 2016, 50, 9124–9132. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- Davenport, J.R.; Peryea, F.J. Phosphate fertilizers influence leaching of lead and arsenic in a soil contaminated with lead arsenate. Water Air Soil Pollut. 1991, 57, 101–110. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H., Jr. Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ. Sci. Technol. 1991, 25, 1414–1419. [Google Scholar] [CrossRef]
- Lin, Y.; Gritsenko, D.; Feng, S.; Teh, Y.C.; Lu, X.; Xu, J. Detection of heavy metal by paper-based microfluidics. Biosens. Bioelectron. 2016, 83, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Klaue, B.; Blum, J.D. Trace analyses of arsenic in drinking water by inductively coupled plasma mass spectrometry: High resolution versus hydride generation. Anal. Chem. 1999, 71, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Wahed, M.A.; Chowdhury, D.; Nermell, B.; Khan, S.I.; Ilias, M.; Rahman, M.; Persson, L.Å.; Vahter, M. A modified routine analysis of arsenic content in drinking-water in Bangladesh by hydride generation-atomic absorption spectrophotometry. J. Health Popul. Nutr. 2006, 24, 36–41. [Google Scholar] [PubMed]
- United States Environmental Protection Agency Office of Water (EPA). Analytical Methods Support Document for Arsenic in Drinking Water; EPA: Washington, DC, USA, 1999; Volume 22027.
- Yogarajah, N.; Tsai, S.S.H. Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics. Environ. Sci. Water Res. Technol. 2015, 1, 426–447. [Google Scholar] [CrossRef]
- Bird, F.C.J. The gutzeit test for arsenic. Analyst 1901, 26, 181–188. [Google Scholar] [CrossRef]
- Baghel, A.; Singh, B.; Pandey, P.; Sekhar, K. A rapid field detection method for arsenic in drinking water. Anal. Sci. 2007, 23, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, S.; Karp, M.; Chang, W.; Virta, M. Recombinant luminescent bacteria for measuring bioavailable arsenite and antimonite. Appl. Environ. Microbiol. 1997, 63, 4456–4461. [Google Scholar] [PubMed]
- Wackwitz, A.; Harms, H.; Chatzinotas, A.; Breuer, U.; Vogne, C.; Der Meer, V.; Roelof, J. Internal arsenite bioassay calibration using multiple bioreporter cell lines. Microb. Biotechnol. 2008, 1, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Prabhakar, N.; Pandey, M.K.; Malhotra, B.D. Surface plasmon resonance-based DNA biosensor for arsenic trioxide detection. Int. J. Environ. Anal. Chem. 2009, 89, 49–57. [Google Scholar] [CrossRef]
- Lohar, S.; Sahana, A.; Banerjee, A.; Banik, A.; Mukhopadhyay, S.K.; Matalobos, J.S.; Das, D. Antipyrine based arsenate selective fluorescent probe for living cell imaging. Anal. Chem. 2013, 85, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Sahana, A.; Banerjee, A.; Lohar, S.; Panja, S.; Mukhopadhyay, S.K.; Matalobos, J.S.; Das, D. Fluorescence sensing of arsenate at nanomolar level in a greener way: Naphthalene based probe for living cell imaging. Chem. Commun. 2013, 49, 7231–7233. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Labuda, J.; Bubnicova, K.; Kovalova, L.; Vanickova, M.; Mattusch, J.; Wennrich, R. Voltammetric detection of damage to DNA by arsenic compounds at a DNA biosensor. Sensors 2005, 5, 411–423. [Google Scholar] [CrossRef]
- Date, A.; Pasini, P.; Daunert, S. Integration of spore-based genetically engineered whole-cell sensing systems into portable centrifugal microfluidic platforms. Anal. Bioanal. Chem. 2010, 398, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Buffi, N.; Merulla, D.; Beutier, J.; Barbaud, F.; Beggah, S.; Van Lintel, H.; Renaud, P.; van der Meer, J.R. Development of a microfluidics biosensor for agarose-bead immobilized escherichia coli bioreporter cells for arsenite detection in aqueous samples. Lab Chip 2011, 11, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, S.; Wang, F.; He, L.; Zhi, W.; Zhou, P. Cationic polymers and aptamers mediated aggregation of gold nanoparticles for the colorimetric detection of arsenic (iii) in aqueous solution. Chem. Commun. 2012, 48, 4459–4461. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Um, H.-J.; Bang, S.; Lee, S.-H.; Oh, S.-J.; Han, J.-H.; Kim, K.-W.; Min, J.; Kim, Y.-H. Arsenic removal from Vietnamese groundwater using the arsenic-binding DNA aptamer. Environ. Sci. Technol. 2009, 43, 9335–9340. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Zhang, Y.; Cai, J.; Cai, W.; Gao, T. Aptamer-based fluorescent biosensors. Curr. Med. Chem. 2011, 18, 4175–4184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, L.; Zhan, S.; Wang, F.; Zhou, P. Ultrasensitive aptamer biosensor for arsenic (iii) detection in aqueous solution based on surfactant-induced aggregation of gold nanoparticles. Analyst 2012, 137, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, S.; Xing, H.; He, L.; Xu, L.; Zhou, P. Nanoparticles assembled by aptamers and crystal violet for arsenic (iii) detection in aqueous solution based on a resonance Rayleigh scattering spectral assay. Nanoscale 2012, 4, 6841–6849. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Zhan, S.; Liu, L.; Luo, Y.; Zhou, P. Regulation of hemin peroxidase catalytic activity by arsenic-binding aptamers for the colorimetric detection of arsenic (iii). Rsc Adv. 2013, 3, 25614–25619. [Google Scholar] [CrossRef]
- Stratton, G.; Whitehead, H.C. Colorimetric determination of arsenic in water with silver diethyldithiocarbamate. J. (Am. Water Works Assoc.) 1962, 54, 861–864. [Google Scholar] [CrossRef]
- Alam, G.M.; Tokunaga, S. Chemical extraction of arsenic from contaminated soil. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2006, 41, 631–643. [Google Scholar] [CrossRef]
- Wei, Q.; Nagi, R.; Sadeghi, K.; Feng, S.; Yan, E.; Ki, S.J.; Caire, R.; Tseng, D.; Ozcan, A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 2014, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.F.; Nagi, R.; Sadeghi, K.; Phillips, S.; Ozcan, A. Albumin testing in urine using a smart-phone. Lab Chip 2013, 13, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xiao, W.; Fu, Q.; Wu, Z.; Yao, C.; Shen, H.; Tang, Y. A portable chromium ion detection system based on a smartphone readout device. Anal. Methods 2016, 8, 6877–6882. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-Y.; Oh, M.-A.; Jung, J.-K.; Choi, S.-I.; Lee, J.-Y. Assessment of soil washing efficiency for arsenic contaminated site adjacent to Jang Hang refinery. J. Soil Groundw. Environ. 2011, 16, 71–81. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Ripp, J. Analytical Detection Limit Guidance & Laboratory Guide for Determining Method Detection Limits; Laboratory Certification Program; Wisconsin Department of Natural Resources: Madison, WI, USA, 1996. [Google Scholar]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491. [Google Scholar] [CrossRef]
- Hianik, T.; Ostatna, V.; Sonlajtnerova, M.; Grman, I. Influence of ionic strength, pH and aptamer configuration for binding affinity to thrombin. Bioelectrochemistry 2007, 70, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Schwertfeger, D.M.; Hendershot, W.H. Spike/leach procedure to prepare soil samples for trace metal ecotoxicity testing: Method development using copper. Commun. Soil Sci. Plant Anal. 2013, 44, 1570–1587. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.F.; Kim, S.; Jeon, H.; Kim, T.; Joo, C.; Park, S. Miniaturized Sample Preparation and Rapid Detection of Arsenite in Contaminated Soil Using a Smartphone. Sensors 2018, 18, 777. https://doi.org/10.3390/s18030777
Siddiqui MF, Kim S, Jeon H, Kim T, Joo C, Park S. Miniaturized Sample Preparation and Rapid Detection of Arsenite in Contaminated Soil Using a Smartphone. Sensors. 2018; 18(3):777. https://doi.org/10.3390/s18030777
Chicago/Turabian StyleSiddiqui, Mohd Farhan, Soocheol Kim, Hyoil Jeon, Taeho Kim, Chulmin Joo, and Seungkyung Park. 2018. "Miniaturized Sample Preparation and Rapid Detection of Arsenite in Contaminated Soil Using a Smartphone" Sensors 18, no. 3: 777. https://doi.org/10.3390/s18030777
APA StyleSiddiqui, M. F., Kim, S., Jeon, H., Kim, T., Joo, C., & Park, S. (2018). Miniaturized Sample Preparation and Rapid Detection of Arsenite in Contaminated Soil Using a Smartphone. Sensors, 18(3), 777. https://doi.org/10.3390/s18030777



