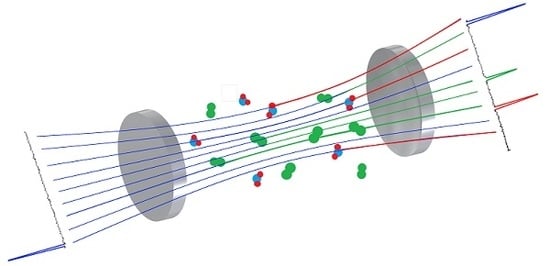
Cavity-Enhanced Raman Spectroscopy for Food Chain Management
Abstract
1. Introduction
2. Methods
2.1. Experimental Setup
2.2. Simulation
3. Results and Discussions
3.1. Cavity Characterization
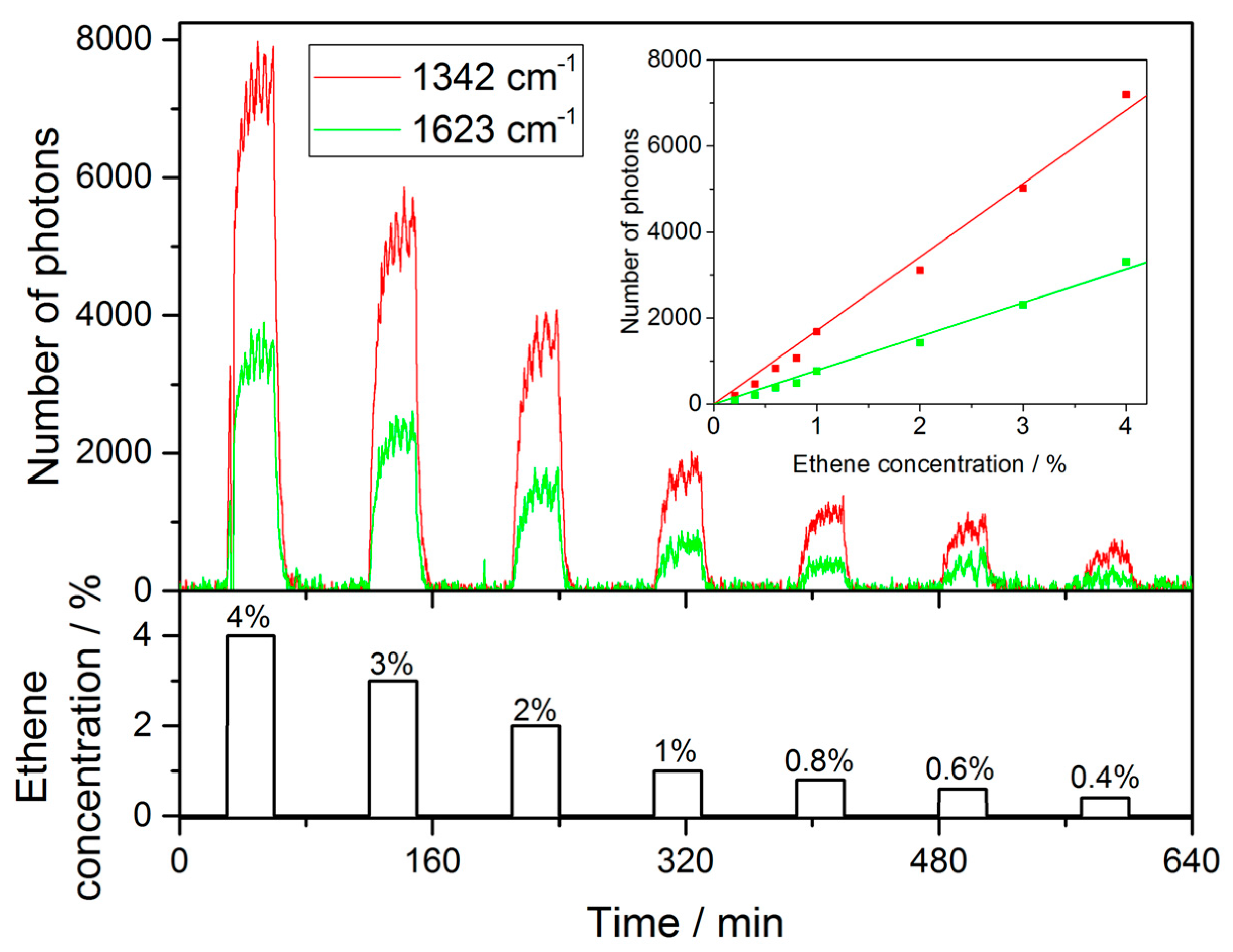
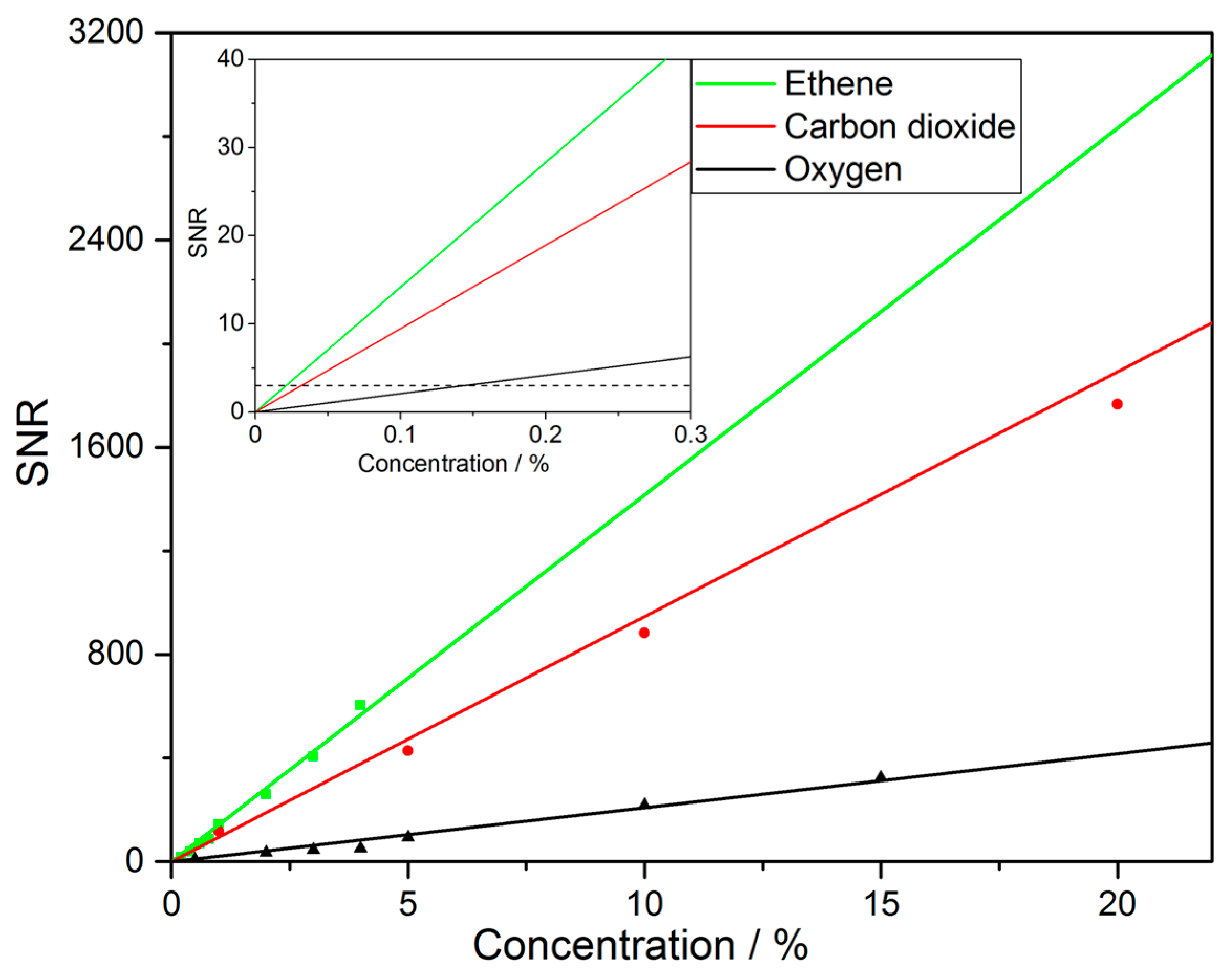
3.2. Gas Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paul, V.; Pandey, R. Role of internal atmosphere on fruit ripening and storability—A review. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Kim, Y.H.; Kim, Y.J.; Yoon, Y.J. Functional antenna integrated with relative humidity sensor using synthesised polyimide for passive RFID sensing. Electron. Lett. 2007, 43, 259. [Google Scholar] [CrossRef]
- Amador, C.; Emond, J.-P.; do Nascimento Nunes, M.C. Application of RFID technologies in the temperature mapping of the pineapple supply chain. Sens. Instrum. Food Qual. Saf. 2009, 3, 26–33. [Google Scholar] [CrossRef]
- Abad, E.; Palacio, F.; Nuin, M.; De Zárate, A.G.; Juarros, A.; Gómez, J.M.; Marco, S. RFID smart tag for traceability and cold chain monitoring of foods: Demonstration in an intercontinental fresh fish logistic chain. J. Food Eng. 2009, 93, 394–399. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Role of Ethylene in Fruit Ripening. Plant Physiol. 1962, 37, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gane, R. Production of Ethylene by Some Ripening Fruits. Nature 1934, 134, 1008. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Ahmad, S.; Thompson, A.K.; Asi, A.A.; Khan, M.; Chatha, G.A.; Shahid, M.A. Effect of Reduced O2 and Increased CO2 (Controlled Atmosphere Storage) on the Ripening and Quality of Ethylene Treated Banana Fruit. Int. J. Agric. Biol. 2001, 3, 486–490. [Google Scholar]
- Jedermann, R.; Geyer, M.; Praeger, U.; Lang, W. Sea transport of bananas in containers—Parameter identification for a temperature model. J. Food Eng. 2013, 115, 330–338. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Knobelspies, S.; Bierer, B.; Ortiz Perez, A.; Wöllenstein, J.; Kneer, J.; Palzer, S. Low-cost gas sensing system for the reliable and precise measurement of methane, carbon dioxide and hydrogen sulfide in natural gas and biomethane. Sens. Actuators B Chem. 2016, 236, 885–892. [Google Scholar] [CrossRef]
- Holm, T. Aspects of the mechanism of the flame ionization detector. J. Chromatogr. A 1999, 842, 221–227. [Google Scholar] [CrossRef]
- Kneer, J.; Wöllenstein, J.; Palzer, S. Specific, trace gas induced phase transition in copper(II)oxide for highly selective gas sensing. Appl. Phys. Lett. 2014, 105, 73509. [Google Scholar] [CrossRef]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Wöllenstein, J.; Palzer, S. New method to selectively determine hydrogen sulfide concentrations using CuO layers. Sens. Actuators B Chem. 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Kneer, J.; Wöllenstein, J.; Palzer, S. Manipulating the gas-surface interaction between copper(II) oxide and mono-nitrogen oxides using temperature. Sens. Actuators B Chem. 2016, 229, 57–62. [Google Scholar] [CrossRef]
- Ortiz Pérez, A.; Kallfaß-de Frenes, V.; Filbert, A.; Kneer, J.; Bierer, B.; Held, P.; Klein, P.; Wöllenstein, J.; Benyoucef, D.; Kallfaß, S.; et al. Odor-Sensing System to Support Social Participation of People Suffering from Incontinence. Sensors 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Fonollosa, J.; Fernández, L.; Huerta, R.; Gutiérrez-Gálvez, A.; Marco, S. Temperature optimization of metal oxide sensor arrays using Mutual Information. Sens. Actuators B Chem. 2013, 187, 331–339. [Google Scholar] [CrossRef]
- Rock, F.; Barsan, N.; Weimar, U.; Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, J.; Smith, R.; Ho, W.O.; Saffell, J.R.; Tatam, R.P. Non-dispersive infra-red (NDIR) measurement of carbon dioxide at 4.2 μm in a compact and optically efficient sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Jiang, B. A One ppm NDIR Methane Gas Sensor with Single Frequency Filter Denoising Algorithm. Sensors 2012, 12, 12729–12740. [Google Scholar] [CrossRef]
- Scholz, L.; Ortiz Perez, A.; Bierer, B.; Eaksen, P.; Wollenstein, J.; Palzer, S. Miniature Low-Cost Carbon Dioxide Sensor for Mobile Devices. IEEE Sens. J. 2017, 17, 2889–2895. [Google Scholar] [CrossRef]
- Fonollosa, J.; Halford, B.; Fonseca, L.; Santander, J.; Udina, S.; Moreno, M.; Hildenbrand, J.; Wöllenstein, J.; Marco, S. Ethylene optical spectrometer for apple ripening monitoring in controlled atmosphere store-houses. Sens. Actuators B Chem. 2009, 136, 546–554. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. Meas. Sci. Technol. 2013, 24, 12004–12059. [Google Scholar] [CrossRef]
- Harris, S.J.; Weiner, A.M. Detection of atomic oxygen by intracavity spectroscopy. Opt. Lett. 1981, 6, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Wartewig, S.; Schorn, C.; Bigler, P. IR and Raman Spectroscopy; Wiley-VCH Verlag GmbH & Co. KGaA: Darmstadt, Germany, 2003; ISBN 352730245X. [Google Scholar]
- Scholz, L.; Palzer, S. Photoacoustic-based detector for infrared laser spectroscopy; Photoacoustic-based detector for infrared laser spectroscopy. Cit. Appl. Phys. Lett. 2016, 109. [Google Scholar] [CrossRef]
- Griffiths, P.R.; de Haseth, J.A. Fourier Transform Infrared Spectrometry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 9780470106310. [Google Scholar]
- Gremlich, H.-U. Infrared and Raman Spectroscopy. In Handbook of Analytical Techniques; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 465–507. ISBN 9783527618323. [Google Scholar]
- Demirgian, J.C. Gas chromatography-Fourier transform infrared spectroscopy-mass spectrometry. A powerful tool for component identification in complex organic mixtures. TrAC Trends Anal. Chem. 1987, 6, 58–64. [Google Scholar] [CrossRef]
- Hammer, S.; Griffith, D.W.T.; Konrad, G.; Vardag, S.; Caldow, C.; Levin, I. Assessment of a multi-species in situ FTIR for precise atmospheric greenhouse gas observations. Atmos. Meas. Tech. 2013, 6, 1153–1170. [Google Scholar] [CrossRef]
- Grob, R.L.; Barry, E.F. Modern Practice of Gas Chromatography, 4th ed.; Grob, R.L., Barry, E.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 0471229830. [Google Scholar]
- Eiceman, G.A. Instrumentation of Gas Chromatography. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–9. ISBN 9780470027318. [Google Scholar]
- Sparkman, O.D.; Penton, Z.; Kitson, F.G. Gas Chromatography and Mass Spectrometry : A Practical Guide; Academic Press: Cambridge, MA, USA, 2011; ISBN 9780080920153. [Google Scholar]
- Repa, P.; Tesar, J.; Gronych, T.; Peksa, L.; Wild, J. Analyses of gas composition in vacuum systems by mass spectrometry. J. Mass Spectrom. 2002, 37, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.E.; Hayes, J.M. Isotope-ratio-monitoring gas chromatography-mass spectrometry. Anal. Chem. 1978, 50, 1465–1473. [Google Scholar] [CrossRef]
- Zoubir, A. Raman Imaging; Zoubir, A., Ed.; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2012; Volume 168, ISBN 978-3-642-28251-5. [Google Scholar]
- Raman, C.V. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Hyatt, H.A.; Cherlow, J.M.; Fenner, W.R.; Porto, S.P.S. Cross section for the Raman effect in molecular nitrogen gas. J. Opt. Soc. Am. 1973, 63, 1604. [Google Scholar] [CrossRef]
- Mooradian, A. Laser Raman Spectroscopy. Science 1970, 169, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Demtröder, W. Laserspektroskopie 2. Experimentelle Techniken, 6th ed.; Springer Spektrum: Berlin, Germany, 2013; ISBN 9783662442166. [Google Scholar]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: San Diego, CA, USA, 2011; ISBN 9780123870186. [Google Scholar]
- Long, D.A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules; John Wiley & Sons Ltd.: Chichester, UK, 2002; ISBN 0471490288. [Google Scholar]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Yamamoto, Y.S.; Ozaki, Y. Plasmon-enhanced spectroscopy of absorption and spontaneous emissions explained using cavity quantum optics. Chem. Soc. Rev. 2017, 46, 3904–3921. [Google Scholar] [CrossRef] [PubMed]
- Bougeard, D.; Buback, M.; Cao, A.; Gerwert, K.; Heise, H.M.; Hoffmann, G.G.; Jordanov, B.; Kiefer, W.; Korte, E.-H.; Kuzmany, H.; et al. Infrared and Raman Spectroscopy : Methods and Applications; Schrader, B., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; ISBN 3527615423. [Google Scholar]
- Matsko, A.B.; Savchenkov, A.A.; Letargat, R.J.; Ilchenko, V.S.; Maleki, L. On cavity modification of stimulated Raman scattering. J. Opt. B Quantum Semiclassical Opt. 2003, 5, 272–278. [Google Scholar] [CrossRef]
- Bloembergen, N. The Stimulated Raman Effect. Am. J. Phys. 1967, 35, 989–1023. [Google Scholar] [CrossRef]
- Regnier, P.R.; Moya, F.; Taran, J.P.E. Gas Concentration Measurement by Coherent Raman Anti-Stokes Scattering. AIAA J. 1974, 12, 826–831. [Google Scholar] [CrossRef]
- Tolles, W.M.; Nibler, J.W.; McDonald, J.R.; Harvey, A.B. A Review of the Theory and Application of Coherent Anti-Stokes Raman Spectroscopy (CARS). Appl. Spectrosc. 1977, 31, 253–271. [Google Scholar] [CrossRef]
- Buric, M.P.; Chen, K.; Falk, J.; Velez, R.; Woodruff, S. Raman Sensing of Fuel Gases Using a Reflective Coating Capillary Optical Fiber. Fiber Opt. Sensors Appl. VI 2009, 7316, 731608. [Google Scholar] [CrossRef]
- Stone, J. cw Raman fiber amplifier. Appl. Phys. Lett. 1975, 26, 163–165. [Google Scholar] [CrossRef]
- Hanf, S.; Keiner, R.; Yan, D.; Popp, J.; Frosch, T. Fiber-Enhanced Raman Multigas Spectroscopy: A Versatile Tool for Environmental Gas Sensing and Breath Analysis. Anal. Chem. 2014, 86, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- Hanf, S.; Bögözi, T.; Keiner, R.; Frosch, T.; Popp, J. Fast and Highly Sensitive Fiber-Enhanced Raman Spectroscopic Monitoring of Molecular H2 and CH4 for Point-of-Care Diagnosis of Malabsorption Disorders in Exhaled Human Breath. Anal. Chem. 2015, 87, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Jochum, T.; Rahal, L.; Suckert, R.J.; Popp, J.; Frosch, T. All-in-one: A versatile gas sensor based on fiber enhanced Raman spectroscopy for monitoring postharvest fruit conservation and ripening. Analyst 2016, 141, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, V.; Trabold, B.; Abdolvand, A.; Bolwien, C.; Russell, P.; Wöllenstein, J.; Palzer, S. Monitoring the Wobbe Index of Natural Gas Using Fiber-Enhanced Raman Spectroscopy. Sensors 2017, 17, 2714. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, A.; Bolwien, C.; Herbst, J.; Kühnemann, F.; Sandfort, V.; Wolf, S. Neue Methoden der laserbasierten Gasanalytik. Chemie Ing. Tech. 2016, 88, 746–755. [Google Scholar] [CrossRef]
- Petrov, D.V. Multipass optical system for a Raman gas spectrometer. Appl. Opt. 2016, 55, 9521. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Mulac, A.J.; Hackett, C.E. Retroreflecting multipass cell for Raman scattering. Appl. Opt. 1977, 16, 2004–2006. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, S.; Krischke, F.; Popovska-Leipertz, N.; Seeger, T.; Breuer, G.; Jeleazcov, C.; Schüttler, J.; Leipertz, A. Demonstration of a signal enhanced fast Raman sensor for multi-species gas analyses at a low pressure range for anesthesia monitoring. J. Raman Spectrosc. 2015, 46, 708–715. [Google Scholar] [CrossRef]
- Hippler, M. Cavity-Enhanced Raman Spectroscopy of Natural Gas with Optical Feedback cw-Diode Lasers. Anal. Chem. 2015, 87, 7803–7809. [Google Scholar] [CrossRef] [PubMed]
- Salter, R.; Chu, J.; Hippler, M. Cavity-enhanced Raman spectroscopy with optical feedback cw diode lasers for gas phase analysis and spectroscopy. Analyst 2012, 137, 4669. [Google Scholar] [CrossRef] [PubMed]
- Hanf, S.; Fischer, S.; Hartmann, H.; Keiner, R.; Trumbore, S.; Popp, J.; Frosch, T. Online investigation of respiratory quotients in Pinus sylvestris and Picea abies during drought and shading by means of cavity-enhanced Raman multi-gas spectrometry. Analyst 2015, 140, 4473–4481. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.J.; Adams, N.I. Laser-Excited Rotation–Vibration Raman Scattering in Ultra-Small Gas Samples. J. Opt. Soc. Am. 1968, 58, 311. [Google Scholar] [CrossRef]
- Neely, G.O.; Nelson, L.Y.; Harvey, A.B. Modification of a Commercial Argon Ion Laser for Enhancement of Gas Phase Raman Scattering. Appl. Spectrosc. 1972, 26, 553–555. [Google Scholar] [CrossRef]
- Hickman, R.S.; Liang, L. Intracavity Laser Raman Spectroscopy Using a Commercial Laser. Appl. Spectrosc. 1973, 27, 425–427. [Google Scholar] [CrossRef]
- Ohara, S.; Yamaguchi, S.; Endo, M.; Nanri, K.; Fujioka, T. Performance Characteristics of Power Build-Up Cavity for Raman Spectroscopic Measurement. Opt. Rev. 2003, 10, 342–345. [Google Scholar] [CrossRef]
- Friss, A.J.; Limbach, C.M.; Yalin, A.P. Cavity-enhanced rotational Raman scattering in gases using a 20 mW near-infrared fiber laser. Opt. Lett. 2016, 41, 3193. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.S.; Repasky, K.S.; Roos, P.A.; Carlsten, J.L. Widely tunable continuous-wave Raman laser in diatomic hydrogen pumped by an external-cavity diode laser. Opt. Lett. 2000, 25, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Kogelnik, H.; Li, T. Laser beams and resonators. Proc. IEEE 1966, 54, 1312–1329. [Google Scholar] [CrossRef]
- Ricci, L.; Weidemüller, M.; Esslinger, T.; Hemmerich, A.; Zimmermann, C.; Vuletic, V.; König, W.; Hänsch, T.W. A compact grating-stabilized diode laser system for atomic physics. Opt. Commun. 1995, 117, 541–549. [Google Scholar] [CrossRef]
- Saleh, B.E.A.; Teich, M.C. Fundamentals of Photonics, 2nd ed.; Saleh, B.E.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 0471358320. [Google Scholar]
- Rouissat, M.; Borsali, A.R.; Chikh-Bled, M.E. Free Space Optical Channel Characterization and Modeling with Focus on Algeria Weather Conditions. Int. J. Comput. Netw. Inf. Secur. 2012, 4, 17–23. [Google Scholar] [CrossRef]
- Drever, R.W.P.; Hall, J.L.; Kowalski, F.V.; Hough, J.; Ford, G.M.; Munley, A.J.; Ward, H. Laser phase and frequency stabilization using an optical resonator. Appl. Phys. B Photophys. Laser Chem. 1983, 31, 97–105. [Google Scholar] [CrossRef]
- Black, E.D. An introduction to Pound–Drever–Hall laser frequency stabilization. Am. J. Phys. 2001, 69, 79–87. [Google Scholar] [CrossRef]
- Black, E. Notes on the Pound-Drever-Hall technique. Technology 1998, 4, 16–98. [Google Scholar]
- Kneer, J.; Eberhardt, A.; Walden, P.; Ortiz Pérez, A.; Wöllenstein, J.; Palzer, S. Apparatus to characterize gas sensor response under real-world conditions in the lab. Rev. Sci. Instrum. 2014, 85, 55006. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Seeger, T.; Steuer, S.; Schorsch, S.; Weikl, M.C.; Leipertz, A. Design and characterization of a Raman-scattering-based sensor system for temporally resolved gas analysis and its application in a gas turbine power plant. Meas. Sci. Technol. 2008, 19, 85408. [Google Scholar] [CrossRef]
- Dieing, T.; Hollricher, O.; Toporski, J. Confocal Raman Microscopy; Dieing, T., Hollricher, O., Toporski, J., Eds.; Springer Series in Optical Sciences; Springer: Berlin/Heidelberg, Germany, 2011; Volume 158, ISBN 978-3-642-12521-8. [Google Scholar]
- Demtröder, W. Experimentalphysik 2; Springer-Lehrbuch; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-68210-3. [Google Scholar]
- Steck, D.A. Rubidium 87 D Line Data. Available online: http://steck.us/alkalidata (accessed on 4 December 2017).
- Steck, D.A. Rubidium 85 D Line Data. Available online: http://steck.us/alkalidata (accessed on 4 December 2017).
- McCreery, R.L. Raman Spectroscopy for Chemical Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2000; ISBN 0471252875. [Google Scholar]



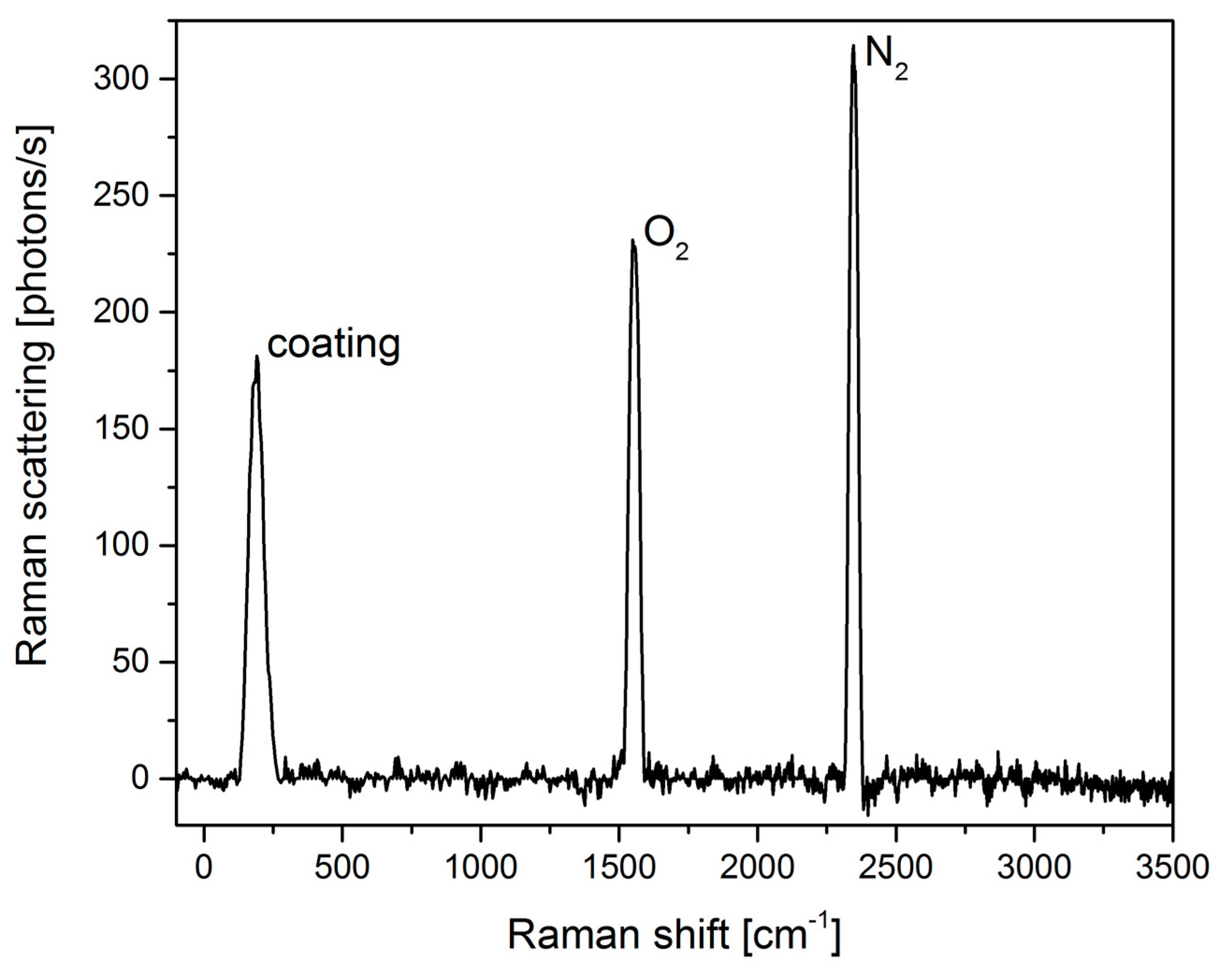

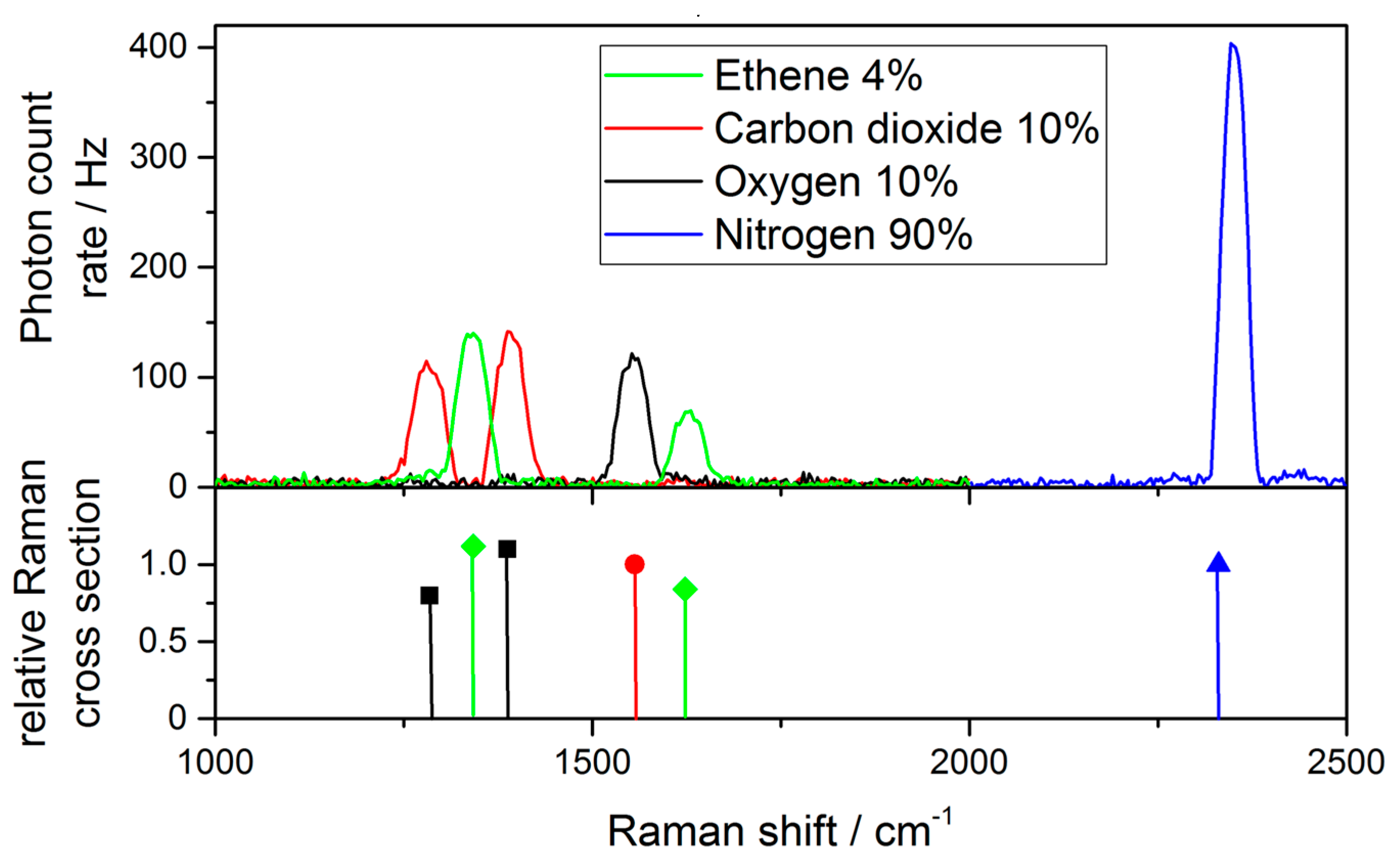
| Gas | Raman Shift [cm−1] | Relative Cross Section |
|---|---|---|
| Oxygen | 1555 | 1 |
| Carbon dioxide | 1285 | 0.8 |
| 1388 | 1.1 | |
| Ethene | 1342 | 2.8 |
| 1623 | 2.1 | |
| Nitrogen | 2331 | 1 |
| Gas | A [ppm−1] | LOD@tint = 30 s [ppm] |
|---|---|---|
| Oxygen | ( | 1412 28 |
| Carbon dioxide | 317 8 | |
| Ethene | 261 9 | |
| Nitrogen | (8.46 0.64) | 3540 267 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandfort, V.; Goldschmidt, J.; Wöllenstein, J.; Palzer, S. Cavity-Enhanced Raman Spectroscopy for Food Chain Management. Sensors 2018, 18, 709. https://doi.org/10.3390/s18030709
Sandfort V, Goldschmidt J, Wöllenstein J, Palzer S. Cavity-Enhanced Raman Spectroscopy for Food Chain Management. Sensors. 2018; 18(3):709. https://doi.org/10.3390/s18030709
Chicago/Turabian StyleSandfort, Vincenz, Jens Goldschmidt, Jürgen Wöllenstein, and Stefan Palzer. 2018. "Cavity-Enhanced Raman Spectroscopy for Food Chain Management" Sensors 18, no. 3: 709. https://doi.org/10.3390/s18030709
APA StyleSandfort, V., Goldschmidt, J., Wöllenstein, J., & Palzer, S. (2018). Cavity-Enhanced Raman Spectroscopy for Food Chain Management. Sensors, 18(3), 709. https://doi.org/10.3390/s18030709