Detection of Thrombin Based on Fluorescence Energy Transfer between Semiconducting Polymer Dots and BHQ-Labelled Aptamers
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Preparation of Semiconducting Polymer Quantum Dots (Pdots)
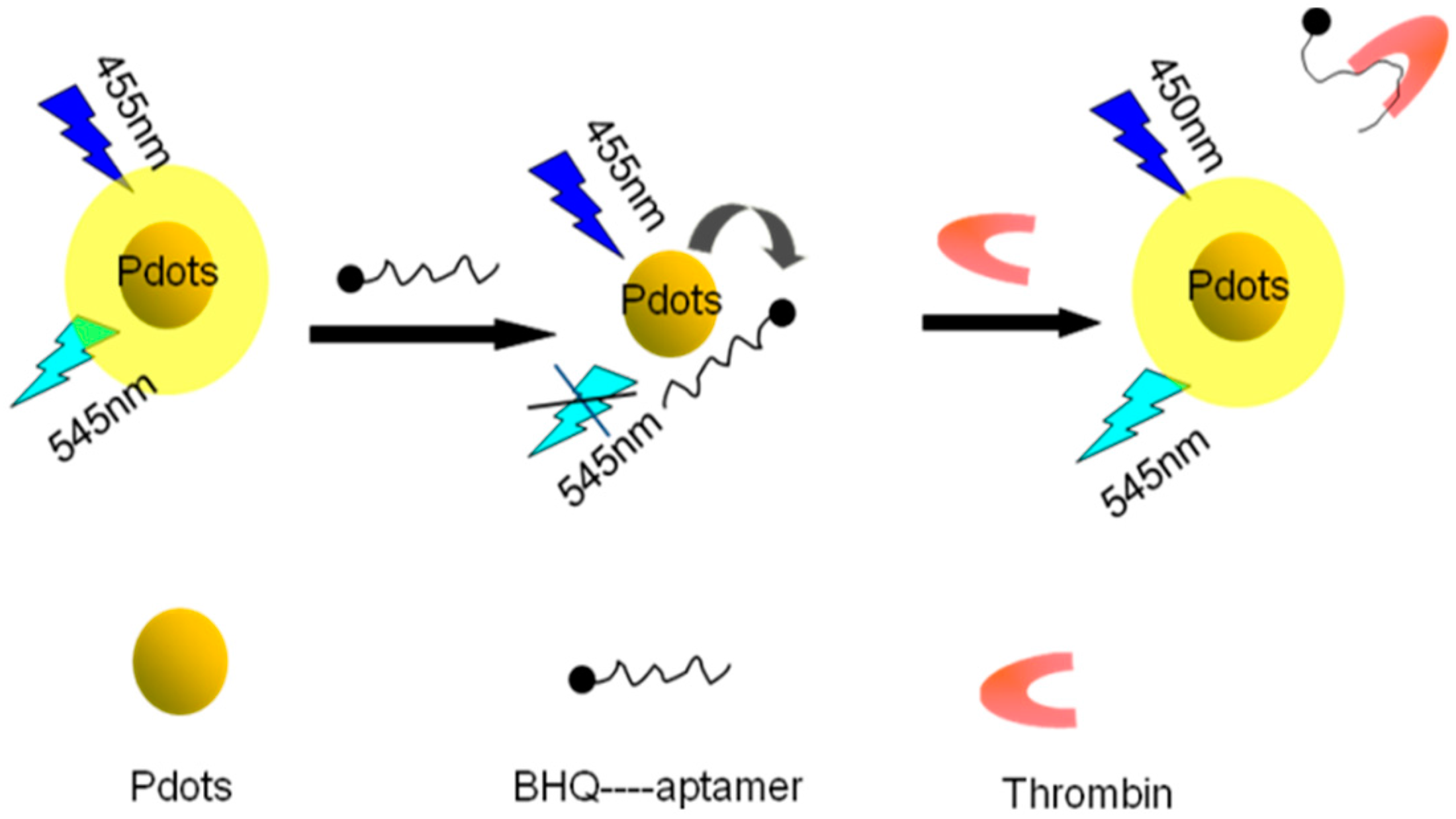
2.3. Interaction between Carboxyl-Functionalized Pdots and BHQ-TBA
2.4. Detection of Thrombin
2.5. Determination of Serum Thrombin
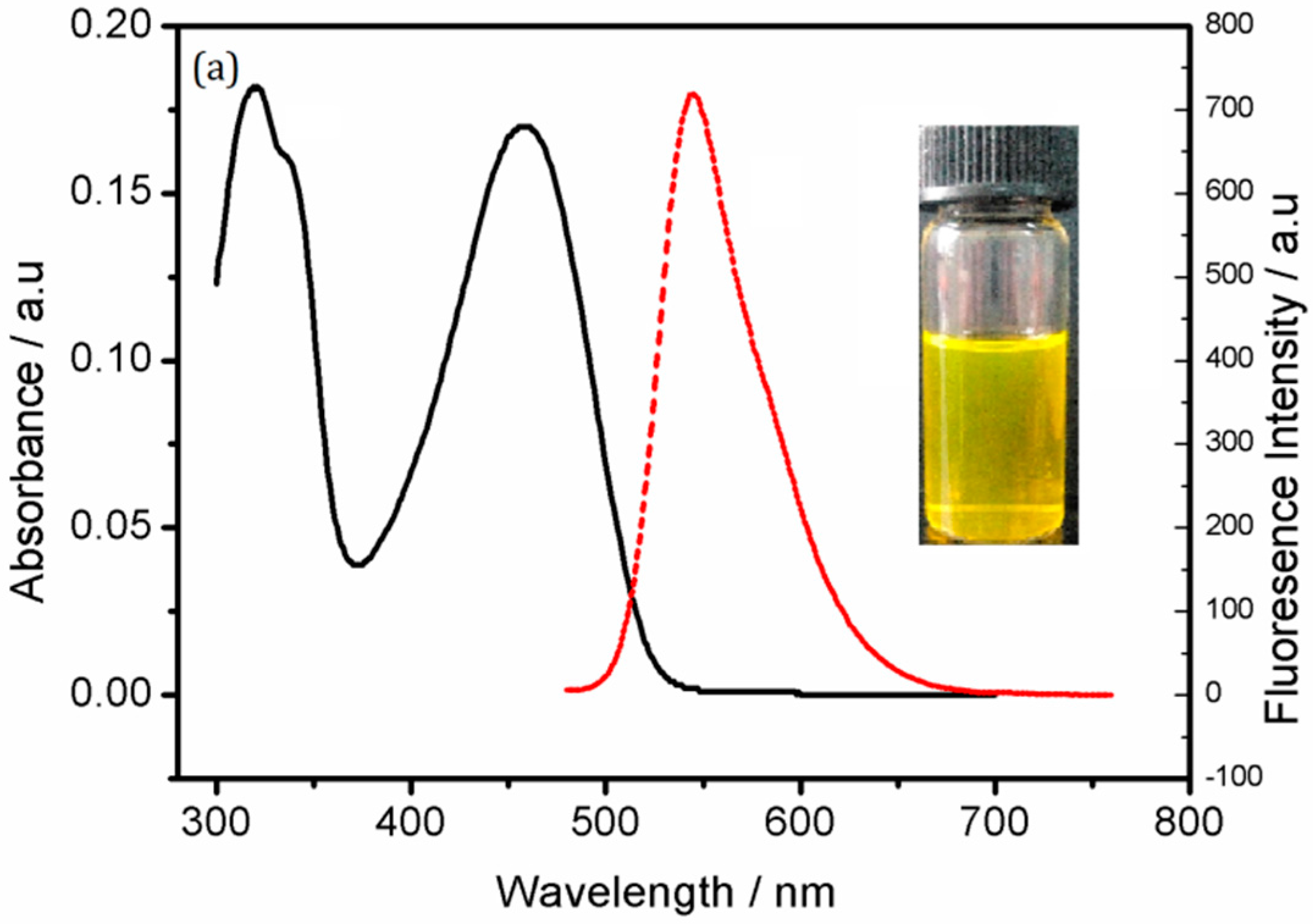
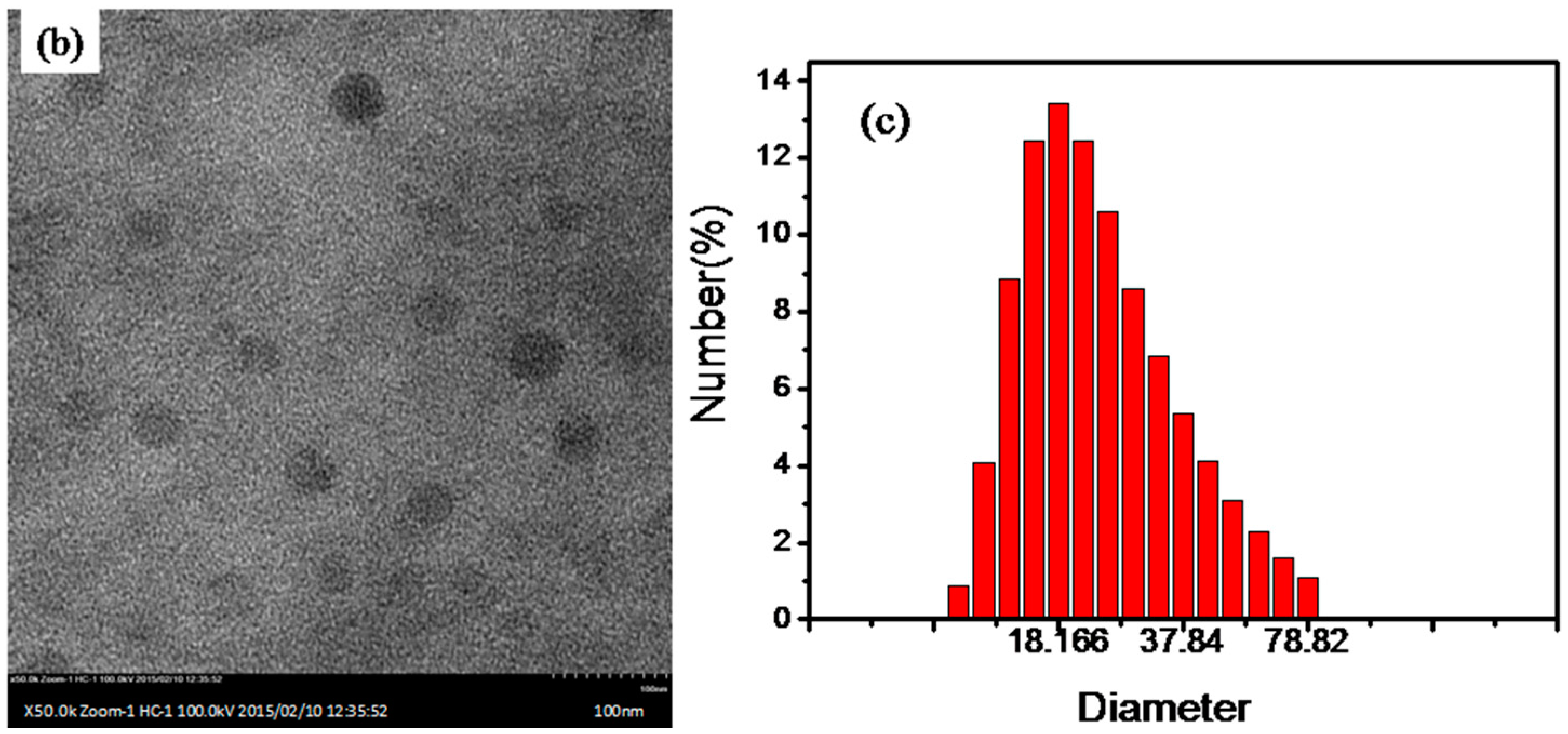
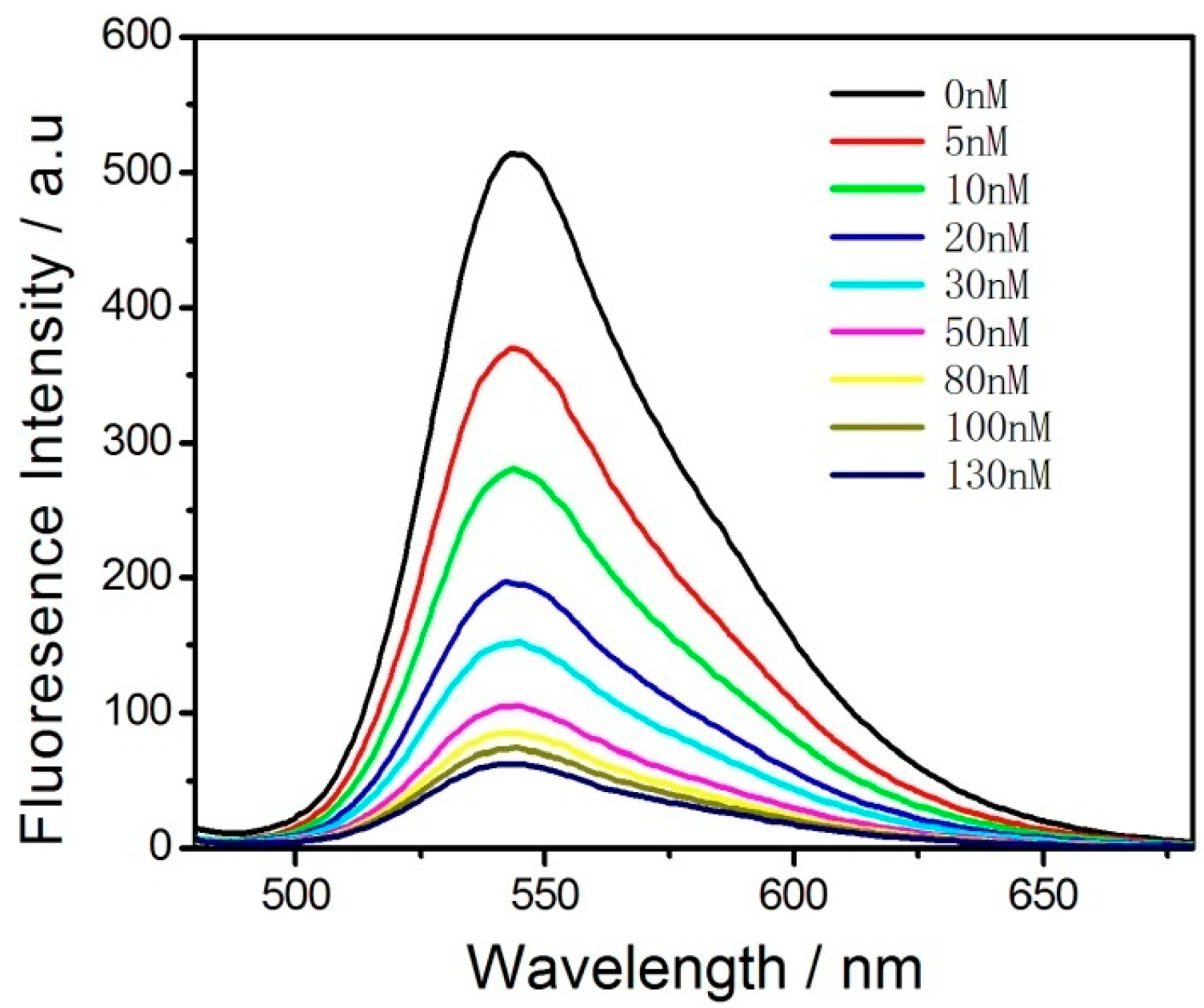
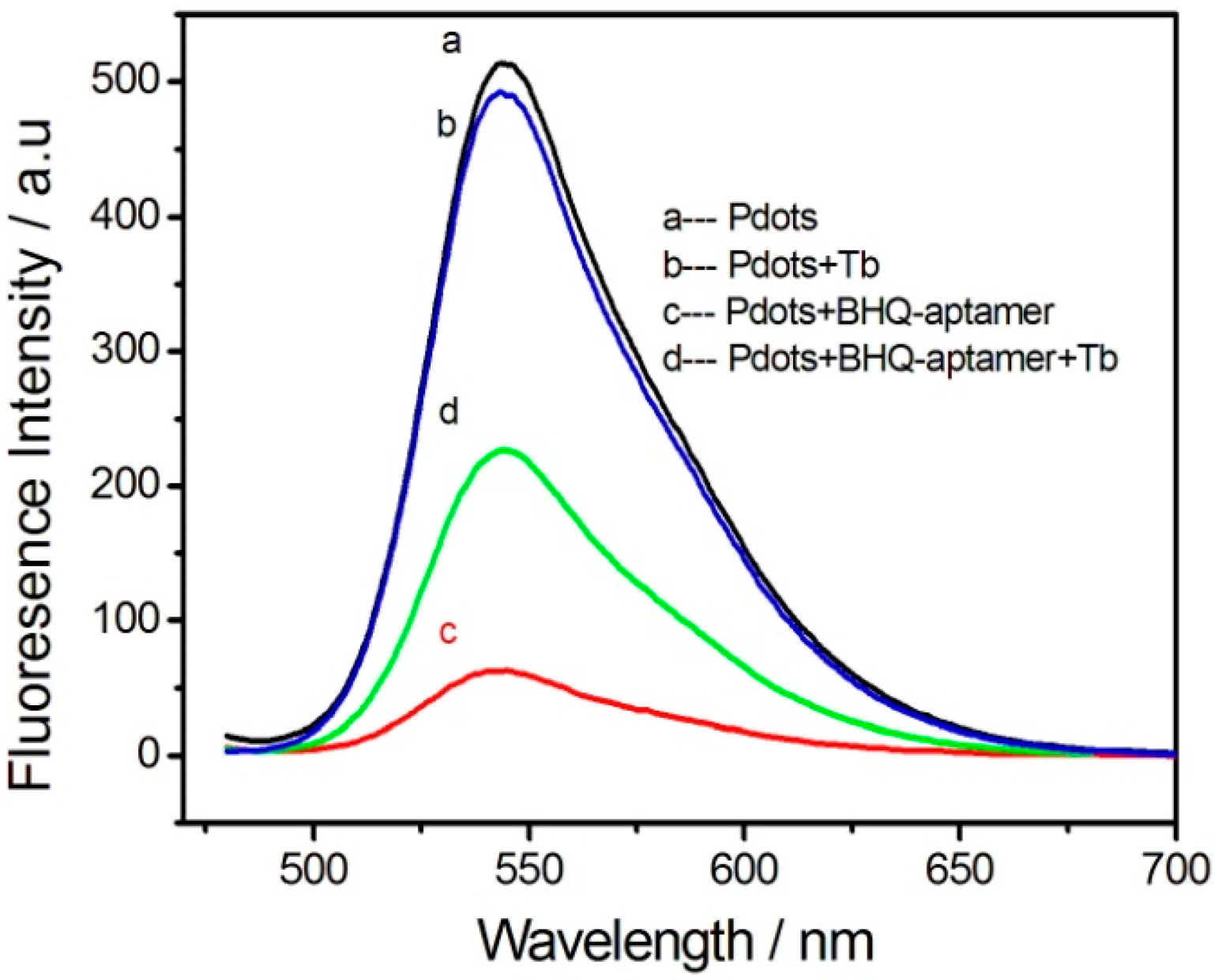
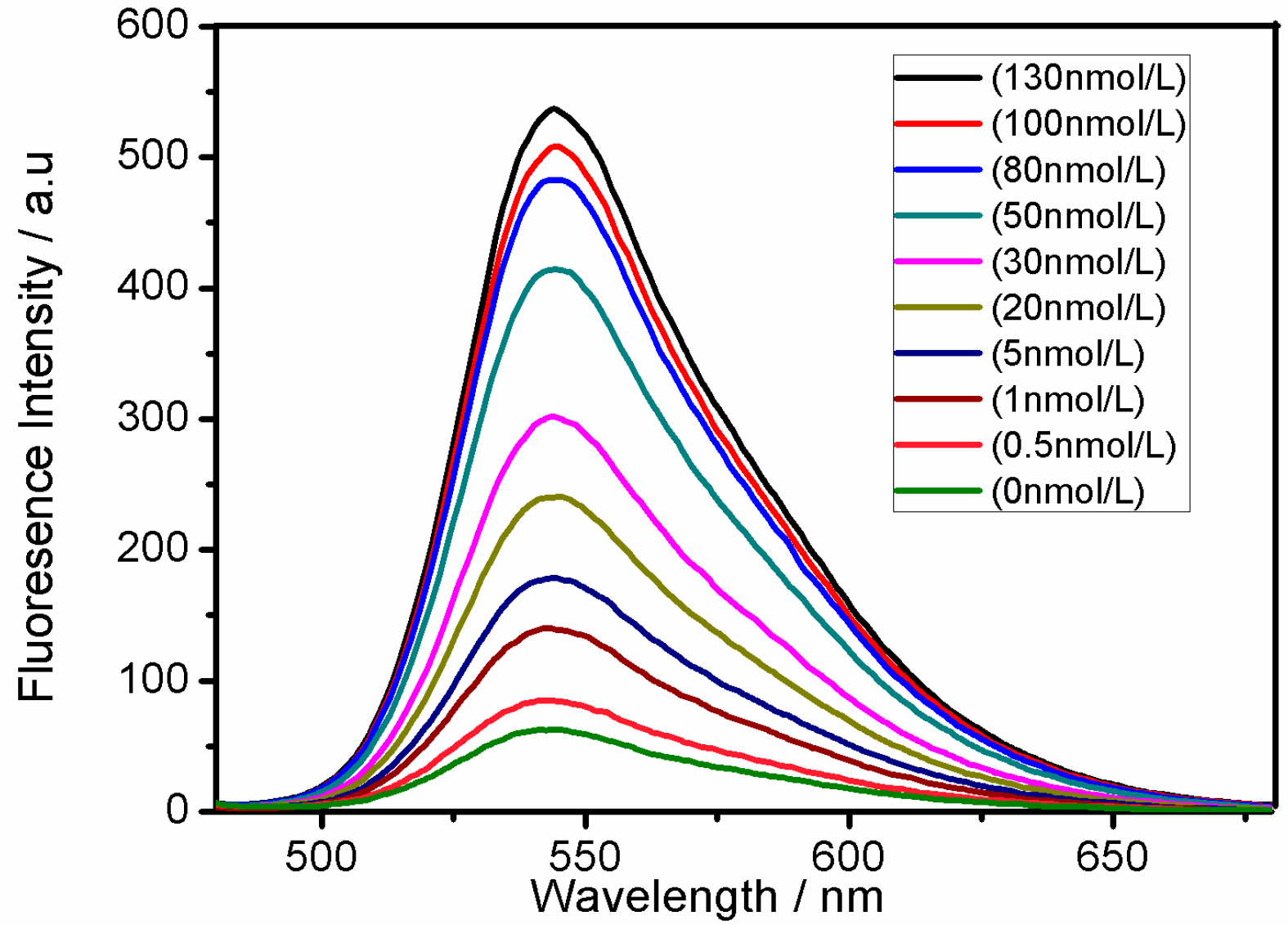
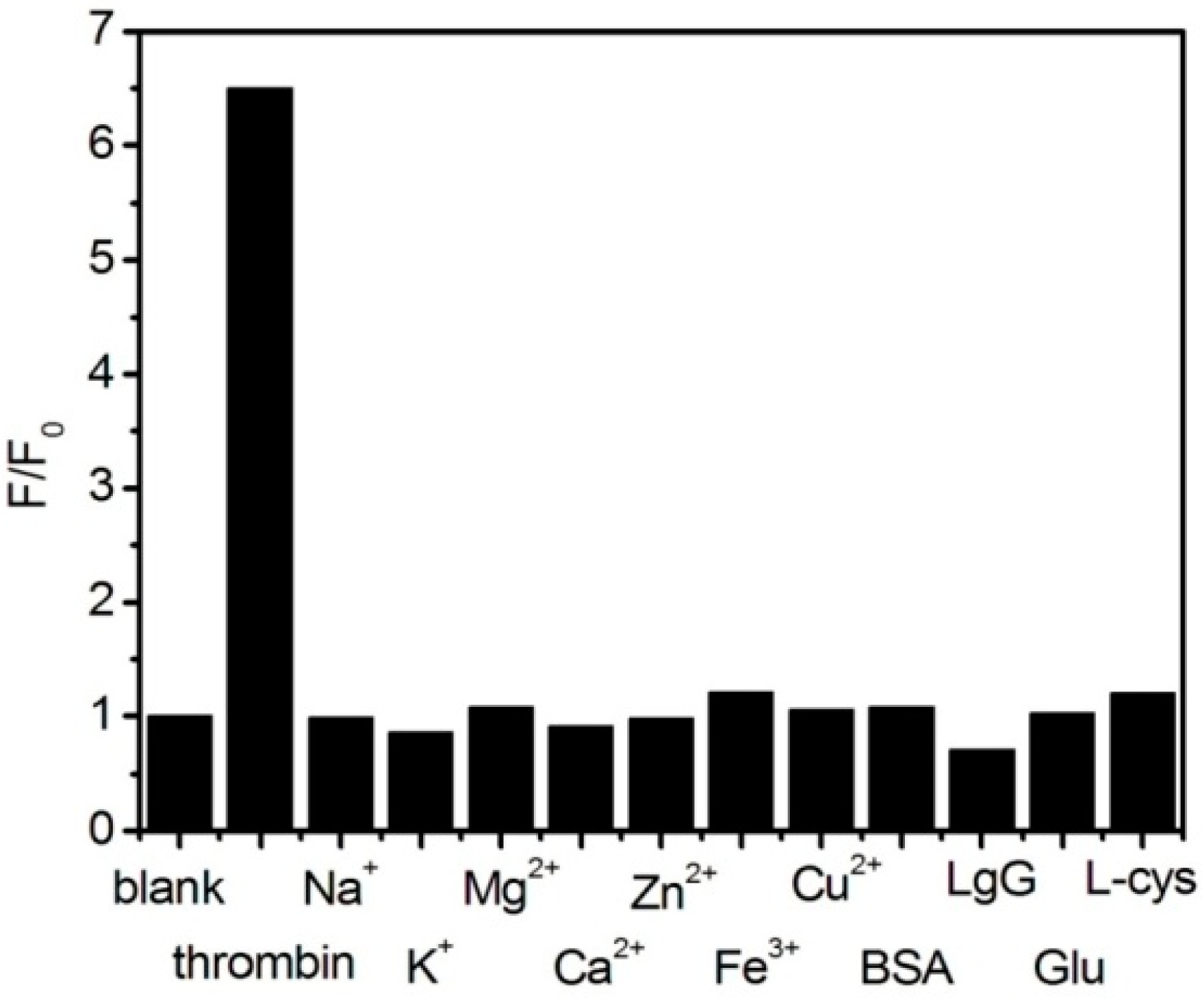
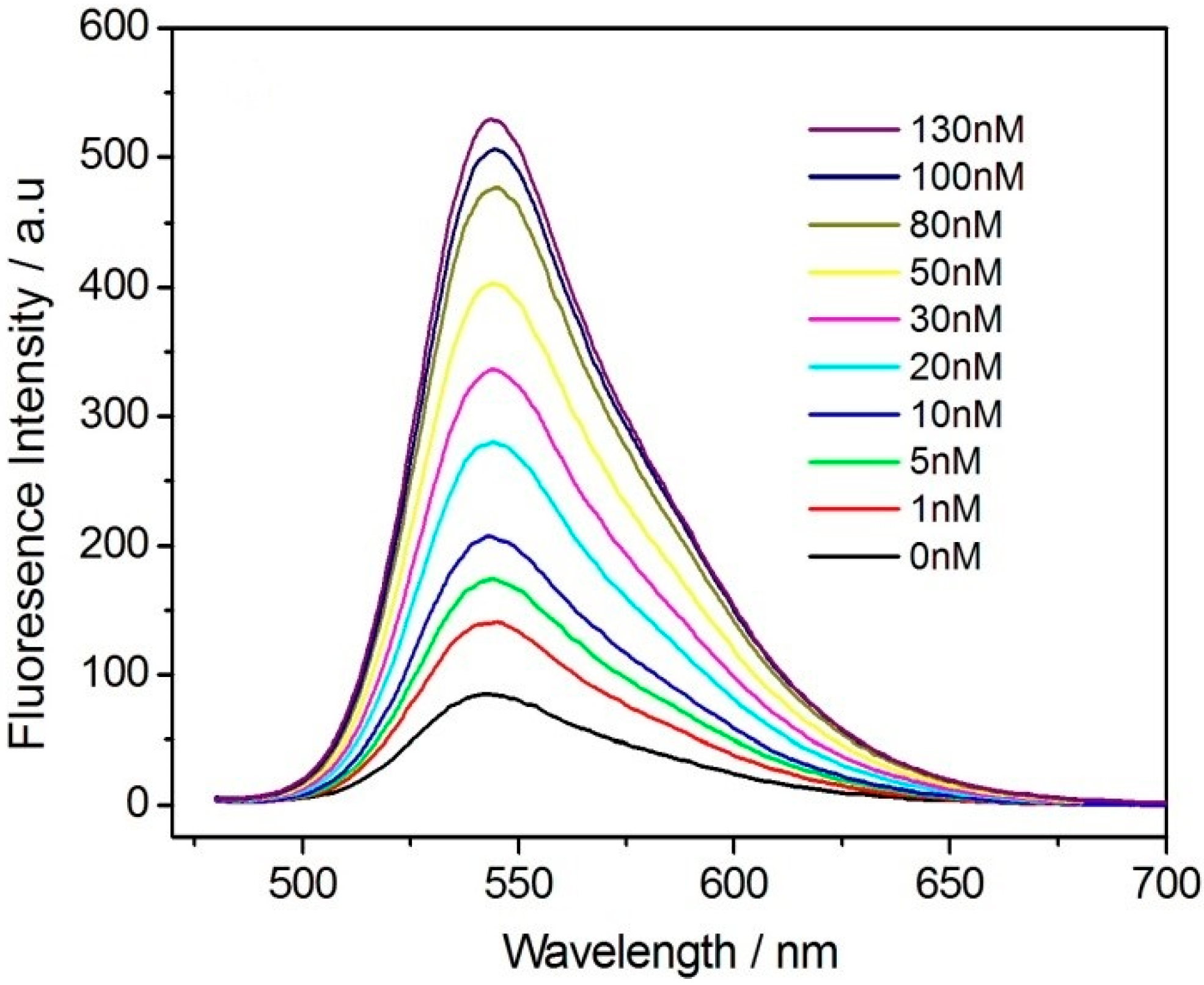
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, R.; Chen, X.; Li, Q.; Yao, C. An electrochemical aptasensor electrocatalyst for detection of thrombin. Anal. Biochem. 2016, 500, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shuman, M.A.; Majerus, P.W. The measurement of thrombin in clotting blood by radioimmunoassay. J. Clin. Investig. 1976, 58, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Bao, Y.G.; Denstedt, J.; Zhang, J. Nanostructured bioluminescent sensor for rapidly detecting thrombin. Biosens. Bioelectron. 2016, 77, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.R.; Betz, A.; Hofsteenge, J. Mechanistic studies on thrombin catalysis. Biochemistry 1991, 30, 9841–9848. [Google Scholar] [CrossRef] [PubMed]
- Lamy, F.; David, F.W. Transformation of prothrombin into thrombin. Phys. Rev. 1954, 34, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.F.; Chen, K.; Wang, X.C.; Lu, Z.K. Dual-stabilizer-capped CdSe quantum dots for “Off–On” electrochemiluminescence biosensing of thrombin by target-triggered multiple amplification. RSC Adv. 2016, 6, 2065–2071. [Google Scholar] [CrossRef]
- German, I.; Buchanan, D.D.; Kennedy, R.T. Aptamers as ligands in affinity probe capillary electrophoresis. Anal. Chem. 1998, 70, 4540–4545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, W.; Luo, H.Q.; Li, N.B. Label-free cascade amplification strategy for sensitive visual detection of thrombin based on target-triggered hybridization chain reaction-mediated in situ generation of DNA zymes and Pt nanochains. Biosens. Bioelectron. 2016, 80, 463–470. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C. Aptasensors-the future of biosensing. Anal. Bioanal. Chem. 2002, 372, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Trapaidze, A.; Hérault, J.P.; Herbert, J.M.; Bancaud, A.; Gué, A.M. Investigation of the selectivity of thrombin-binding aptamers for thrombin titration in murine plasma. Biosens. Bioelectron. 2016, 78, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical applications of aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Hu, X.L.; Wu, X.M.; Dong, Y.M.; Li, Z.J. Fluorescent aptamer-based assay for thrombin with large signal amplification using peroxidase mimetics. Microchim. Acta 2016, 183, 765–771. [Google Scholar] [CrossRef]
- McCauley, T.G.; Hamaguchi, N.; Stanton, M. Aptamer-based biosensor arrays for detection and quantification of biological macromolecules. Anal. Biochem. 2003, 319, 244–250. [Google Scholar] [CrossRef]
- Zhang, L.P.; Li, L. Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim. Acta 2016, 183, 485–490. [Google Scholar] [CrossRef]
- So, H.M.; Won, K.; Kim, Y.H.; Kim, B.K.; Ryu, B.H.; Na, P.S.; Kim, H.; Lee, J.O. Single-walled carbon nanotube biosensors using aptamers as molecular recognition elements. J. Am. Chem. Soc. 2005, 127, 11906–11907. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zheng, P.C.; Jiang, J.H.; Shen, G.L.; Yu, R.Q.; Liu, G.K. Electrostatic interaction based approach to thrombin detection by surface-enhanced Raman spectroscopy. Anal. Chem. 2008, 81, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lubin, A.A.; Heeger, A.J.; Plaxco, K.W. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew. Chem. 2005, 44, 5456–5459. [Google Scholar] [CrossRef] [PubMed]
- Derkus, B.; Arslan, Y.E.; Bayrac, A.T.; Kantarcioglu, I.; Emregul, K.C.; Emregul, E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sens. Actuators B 2016, 228, 725–736. [Google Scholar] [CrossRef]
- Numnuam, A.; Chumbimuni-Torres, K.Y.; Xiang, Y.; Bash, R.; Thavarungkul, P.; Kanatharana, P.; Pretsch, E.; Wang, J.; Bakker, E. Aptamer-Based Potentiometric Measurements of Proteins Using Ion-Selective Microelectrodes. Anal. Chem. 2008, 80, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zhang, Y.; Yan, T.; Fan, D.W.; Du, B.; Ma, H.M.; Wei, Q. Ultrasensitive electrochemical aptasensor for the detection of thrombin based on dual signal amplification strategy of Au@GS and DNA-CoPd NPs conjugates. Biosens. Bioelectron. 2016, 80, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.E.; Sanchez, J.L.A.; Baldrich, E.; Sullivan, C.K.O. Reagentless, reusable, ultrasensitive electrochemical molecular beacon aptasensor. J. Am. Chem. Soc. 2006, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Congur, G.; Mayer, G. Aptasensor platform based on carbon nanofibers enriched screen printed electrodes for impedimetric detection of thrombin. J. Electroanal. Chem. 2015, 758, 12–19. [Google Scholar] [CrossRef]
- Rahman, A.; Song, J.I.; Won, M.S.; Shim, Y.B. Gold nanoparticles doped conducting polymer nanorod electrodes: ferrocene catalyzed aptamer-based thrombin immunosensor. Anal. Chem. 2009, 81, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.Q.; Lu, P.; Yang, P.H. A glassy carbon electrode modified with in-situ generated chromium-loaded CdS nanoprobes and heparin for ultrasensitive electro chemiluminescent determination of thrombin. Microchim. Acta 2016, 183, 123–132. [Google Scholar] [CrossRef]
- Hecht, A.; Kumar, A.A.; Kopelman, R. Label-acquired magnetorotation as a signal transduction method for protein detection: aptamer-based detection of thrombin. Anal. Chem. 2011, 83, 7123–7128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, P.; Zhang, B.C.; Gao, F. Aptamer-based turn-on detection of thrombin in biological fluids based on efficient phosphorescence energy transfer from Mn-doped ZnS quantum dots to carbon nanodots. Chem. Eur. J. 2013, 19, 9242–9250. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Chang, K.W.; Sun, K.; Tang, Y.; Cui, N.; Wang, Y.; Qin, W.P.; Xu, H.; Wu, C.F. Amplified Singlet Oxygen Generation in Semiconductor Polymer Dots for Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, M.; Zeng, Y.Y.; Liao, N.S.; Cai, Z.X.; Liu, G. Lipid-micelles packaged with semiconducting polymer dots as simultaneous MRI /photoacoustic imaging and photodynamic /photo-thermal dual-modal therapeutic agents for liver cancer. J. Mater. Chem. B 2016, 4, 589–599. [Google Scholar] [CrossRef]
- Wu, C.; Chiu, D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. 2013, 52, 3086–3109. [Google Scholar] [CrossRef] [PubMed]
- Massey, M.; Wu, M.; Conroy, E.M.; Algar, W.R. Mind your P’s and Q’s: the coming of age of semiconducting polymer dots and semiconductor quantum dots in biological applications. Curr. Opin. Biotechnol. 2015, 34, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, B.; Li, J.; Wang, E.; Dong, S. Simple and sensitive aptamer-based colorimetric sensing of protein using unmodified gold nanoparticle probes. Chem. Commun. 2007, 36, 3735–3737. [Google Scholar] [CrossRef] [PubMed]
- Che, W.I.; Bo, Y.J.; Mao, Y.F.; Yu, R.; Elena, G.M.; Bryant, F.J.; Yong, S.Z.; Chan, M.H.; Wei, S.; Zhou, X.H.; et al. Squaraine-Based Polymer Dots with Narrow, Bright Near-Infrared Fluorescence for Biological Applications. J. Am. Chem. Soc. 2015, 137, 173–178. [Google Scholar]
- Chan, Y.H.; Wu, P.J. Semiconducting Polymer Nanoparticles as Fluorescent Probes for Biological Imaging and Sensing. Part. Part. Syst. Charact. 2015, 32, 11–28. [Google Scholar] [CrossRef]
- Wan, Y.; Zheng, L.B.; Suna, Y.; Zhang, D. Multifunctional semiconducting polymer dots for imaging, detection, and photo-killing of bacteria. J. Mater. Chem. B 2014, 2, 4818–4825. [Google Scholar] [CrossRef]
- Wu, C.F.; Jin, Y.H.; Schneider, T.; Burnham, D.R.; Smith, P.B.; Chiu, D.T. Ultra bright and bioorthogonal labeling of cellular targets using semiconducting polymer dots and click chemistry. Angew. Chem. Int. Ed. 2010, 49, 9436–9440. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Schneider, T.; Zeigler, M.; Yu, J.B.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, G.Y.; Weng, W.; Qiu, B.; Guo, L.H.; Lin, Z.H.; Chen, G.N. Signal on fluorescence biosensor for MMP-2 based on FRET between semiconducting polymer dots and a metal organic framework. RSC Adv. 2014, 4, 58852–58857. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Z.; Hu, L.; Yuan, Y.; Xu, G. Turn-On Fluorescence Sensor Based on Single-Walled-Carbon-Nanohorn–Peptide Complex for the Detection of Thrombin. Chem. Eur. J. 2012, 18, 16556–16561. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Yuan, J. Novel Magnetic Fe3O4@CdSe Composite Quantum Dot-Based Electrochemiluminescence Detection of Thrombin by a Multiple DNA Cycle Amplification Strategy. Anal. Chem. 2012, 84, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Centi, S.; Tombelli, S.; Minunni, M.; Mascini, M. Aptamer-Based Detection of Plasma Proteins by an Electrochemical Assay Coupled to Magnetic Beads. Anal. Chem. 2007, 79, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Jiang, Y.; Yang, S.; Tan, W.; Yang, R. Combination of π-π stacking and electrostatic repulsion between carboxylic carbon nanoparticles and fluorescent oligonucleotides for rapid and sensitive detection of thrombin. Chem. Commun. 2011, 47, 11321–11323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, L.; Liu, Z.; Pang, D. Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal. Chem. 2011, 83, 8130–8137. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Tang, L.; Wang, Y.; Jiang, J.; Li, J. Graphene Fluorescence Resonance Energy Transfer Aptasensor for the Thrombin Detection. Anal. Chem. 2010, 82, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, F.; Chen, Z. Aptamer-based electrochemical biosensor for label-free voltammetric detection of thrombin and adenosine. Sens. Actuators B 2011, 160, 1380–1385. [Google Scholar] [CrossRef]
- Kim, D.K.; Kerman, K.; Hiep, H.M.; Saito, M.; Yamamura, S.; Takamura, Y.; Kwon, Y.S.; Tamiya, E. Label-free optical detection of aptamer-protein interactions using gold-capped oxide nanostructures. Anal. Biochem. 2008, 379, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Huang, R.; Zhou, Y.; Zhang, M.; Deng, M.; Wang, X.; Weng, X.; Zhou, X. Aptamer-based turn-on fluorescent four-branched quaternary ammonium pyrazine probe for selective thrombin detection. Chem. Commun. 2011, 47, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
| Methods | Signal Output/Reporter | Linear Range (nmol/L) | Detection Limit (nmol/L) | Ref. |
|---|---|---|---|---|
| a DNA cycle amplified method based on ECL quenching of Fe3O4@CdSe QDs by gold NPs | ECL/Fe3O4@CdSe | 0.001–5.0 | 0.00012 | [39] |
| turn-On Fluorescence Sensor Based on single walled carbon nanohorn-Peptide complex | fluorescence/fluorescein | - | 0.1 | [40] |
| a fluorescence detection based on simultaneous electrostatic repulsion and π–π stacking interactions of carboxylic carbon nanoparticles with single-stranded DNA | fluorescence/fluorescein | 0–120 | 5 | [41] |
| a aptamer biosensor based on FRET from upconverting fluorophors to carbon nanoparticles | fluorescence/Yb,Er,NaYF4 | 0.5–20 | 0.18 | [42] |
| FRET aptasensor based on the dye labeled aptamer assembled graphene | fluorescence/fluorescein amidite | 0.0625–0.1875 | 0.0313 | [43] |
| an electrochemical biosensor based on switching structure of aptamers from DNA/DNA duplex to DNA/target complex | current/methylene blue | 6–60 | 3 | [44] |
| the combination of LSPR with RRI spectrum of the gold-capped oxide nanostructure | RRI/gold-capped oxide nanostructure | 0.001–100,000 | 1 | [45] |
| aptamer-based turn-on fluorescent detection | fluorescence/TASPI | 0–2500 | 50 | [46] |
| PET-based aptamer sensor for thrombin | Phosphorescence/Mn-ZnS QDs | 0–40 | 0.013 | [26] |
| This study | Flurorescence/PFBT Pdots | 0–50 | 0.33 |
| Sample | Spiked (nmol/L) | Found (nmol/L ± SD) | Recovery (%) |
|---|---|---|---|
| 1 | 5.0 | 4.81 ± 0.02 | 96.2 ± 0.4 |
| 2 | 10.0 | 10.41 ± 0.03 | 104.1 ± 0.3 |
| 3 | 20.0 | 20.33 ± 0.04 | 101.7± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, X.; Cao, W.; Sun, J.; Gao, F. Detection of Thrombin Based on Fluorescence Energy Transfer between Semiconducting Polymer Dots and BHQ-Labelled Aptamers. Sensors 2018, 18, 589. https://doi.org/10.3390/s18020589
Liu Y, Jiang X, Cao W, Sun J, Gao F. Detection of Thrombin Based on Fluorescence Energy Transfer between Semiconducting Polymer Dots and BHQ-Labelled Aptamers. Sensors. 2018; 18(2):589. https://doi.org/10.3390/s18020589
Chicago/Turabian StyleLiu, Yizhang, Xuekai Jiang, Wenfeng Cao, Junyong Sun, and Feng Gao. 2018. "Detection of Thrombin Based on Fluorescence Energy Transfer between Semiconducting Polymer Dots and BHQ-Labelled Aptamers" Sensors 18, no. 2: 589. https://doi.org/10.3390/s18020589
APA StyleLiu, Y., Jiang, X., Cao, W., Sun, J., & Gao, F. (2018). Detection of Thrombin Based on Fluorescence Energy Transfer between Semiconducting Polymer Dots and BHQ-Labelled Aptamers. Sensors, 18(2), 589. https://doi.org/10.3390/s18020589




