Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
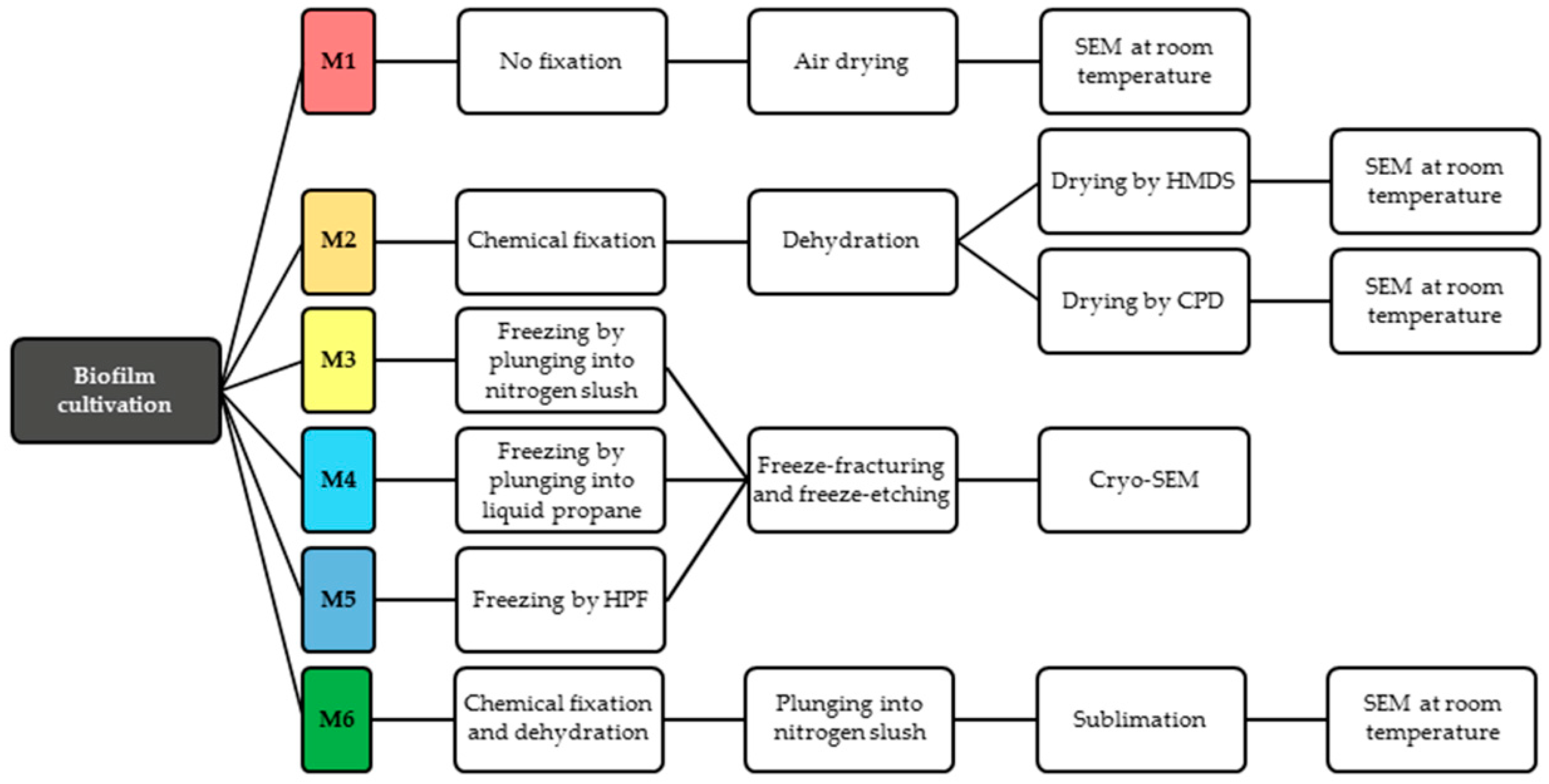
2.1. Biofilm Cultivation
2.2. Conventional SEM
2.2.1. Method 1—Air-Drying (M1)
2.2.2. Method 2—Chemical Preparation (M2)
2.3. Cryo-SEM
2.3.1. Method 3—Plunging into Nitrogen Slush (M3)
2.3.2. Method 4—Plunging into Liquid Ethane (M4)
2.3.3. Method 5—Cryo-Preparation by HPF (M5)
2.4. Method 6—Combined Preparation: Chemical and Cryo-Methods (M6)
2.5. Analysis of the Yeast Biofilm by Raman Spectroscopy
3. Results and Discussion
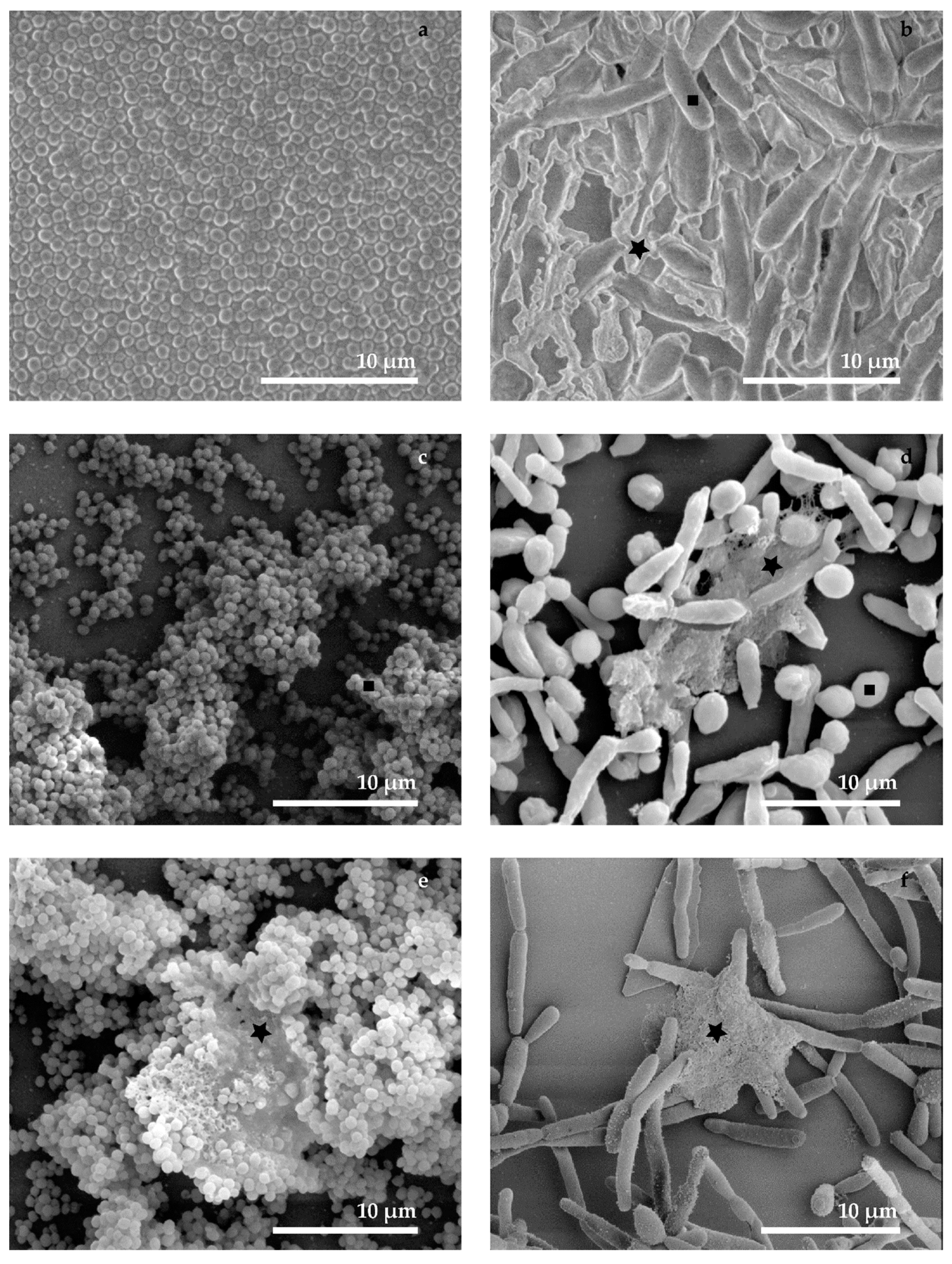
3.1. Conventional SEM (M1 and M2)
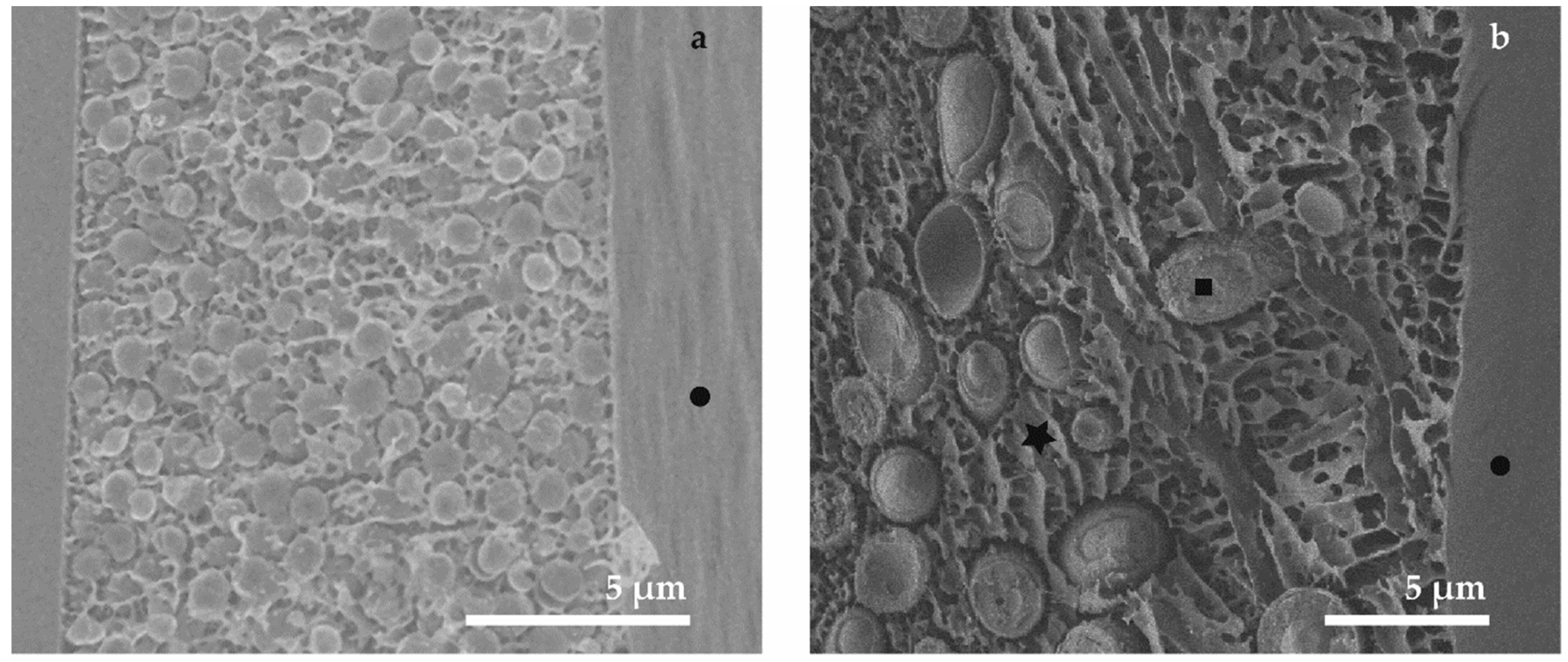
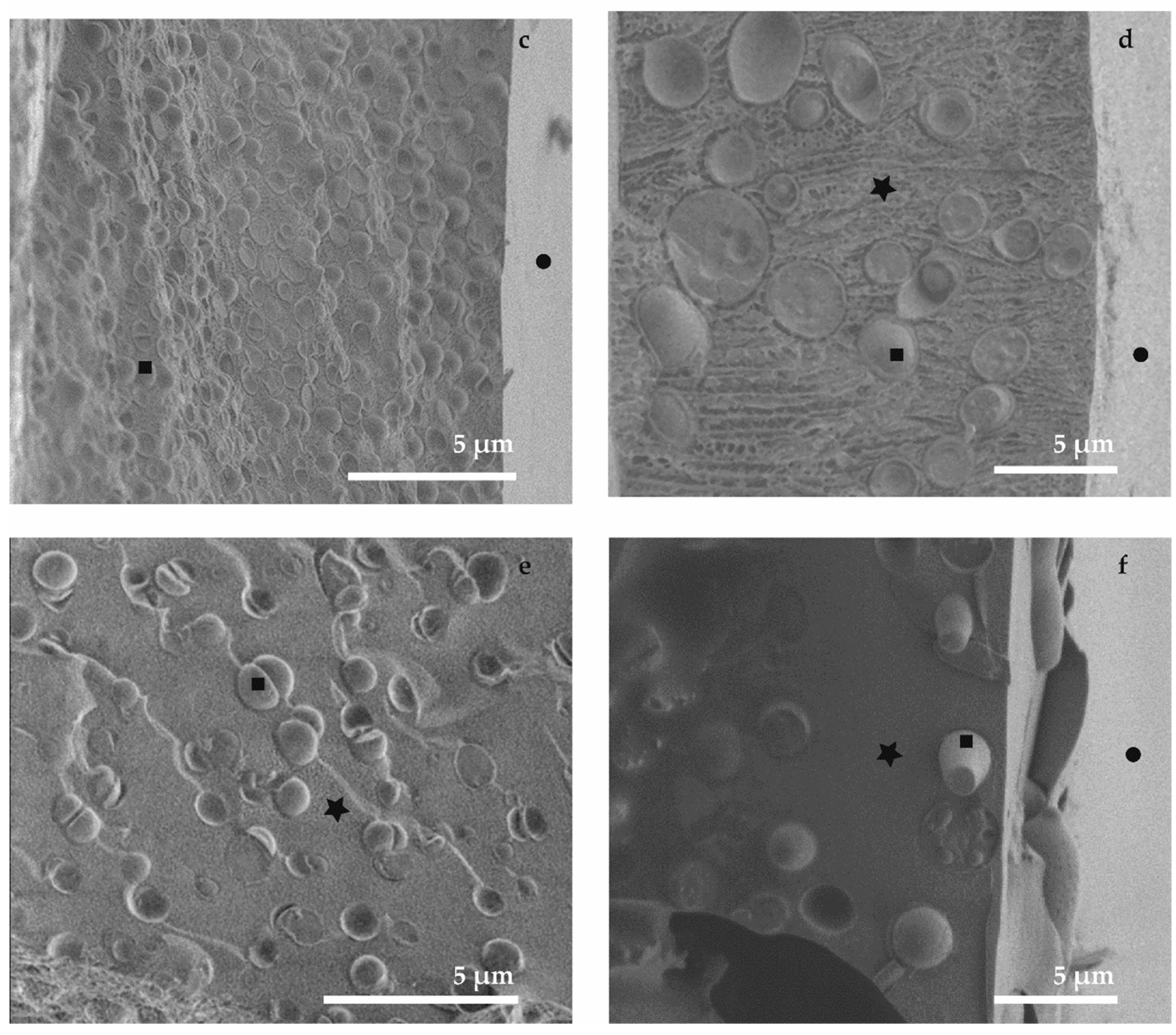
3.2. Cryo-SEM (M3, M4 and M5)
3.3. Combined Preparation—Chemical and Cryo-Methods (M6)
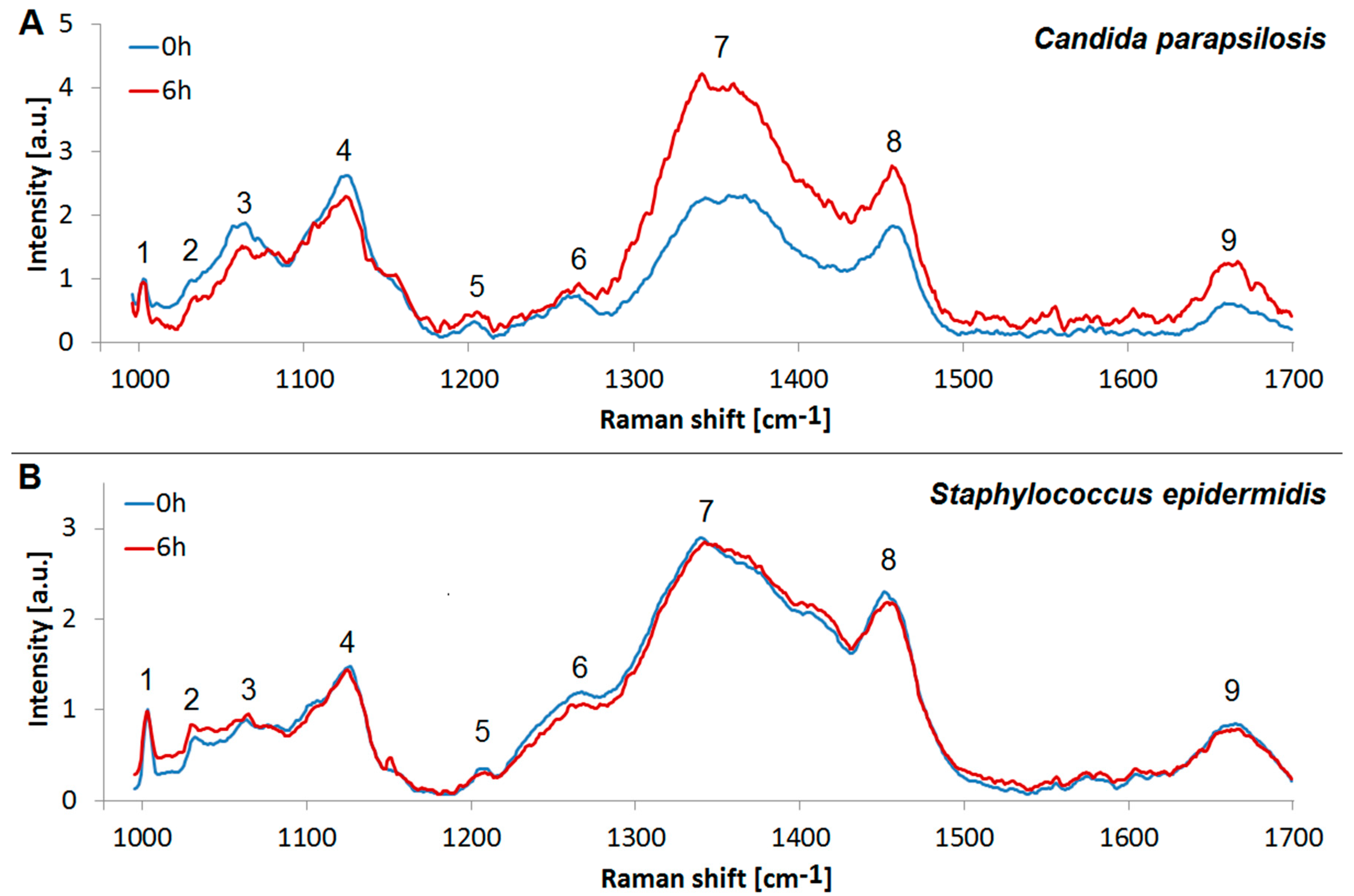
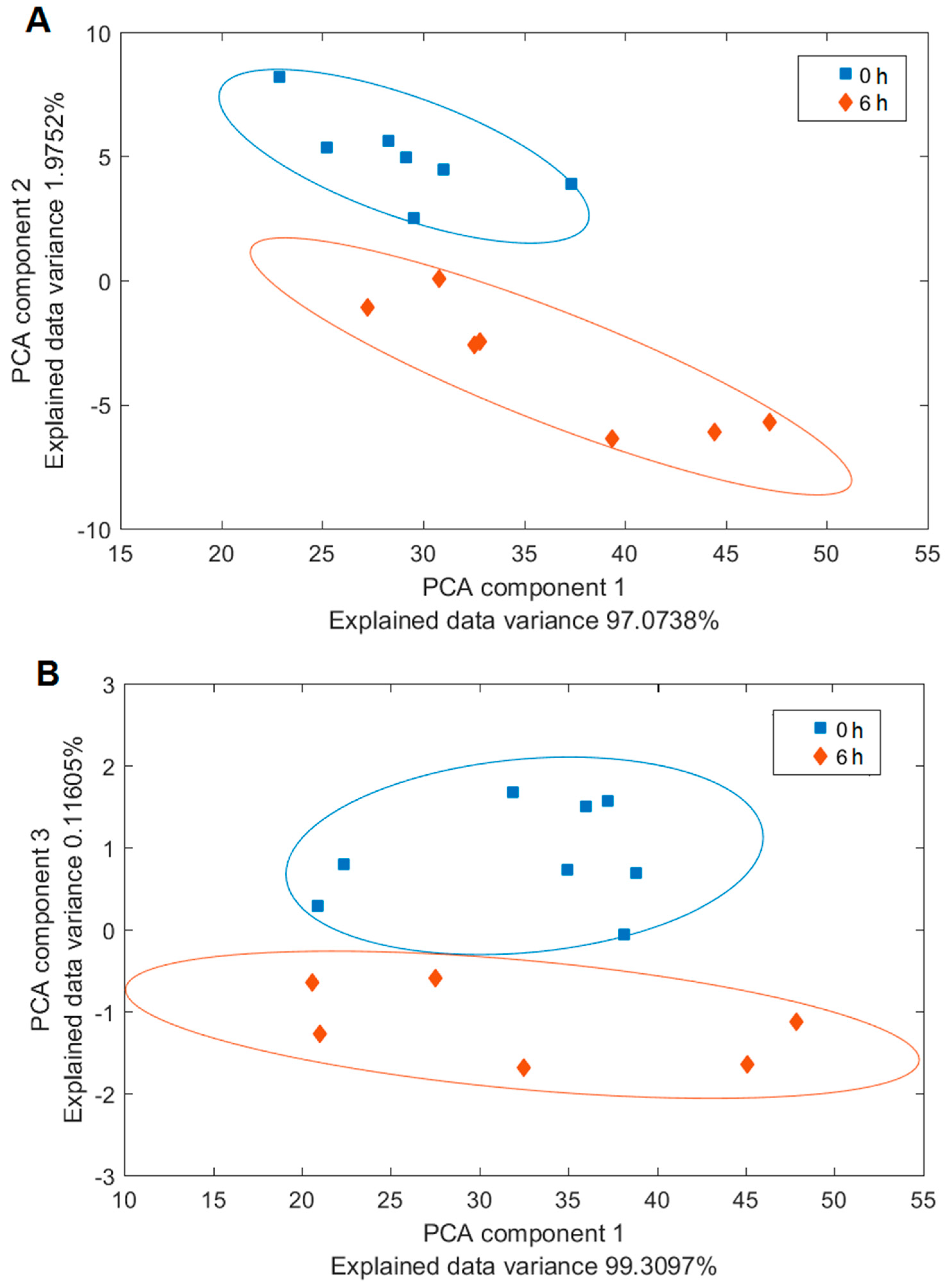
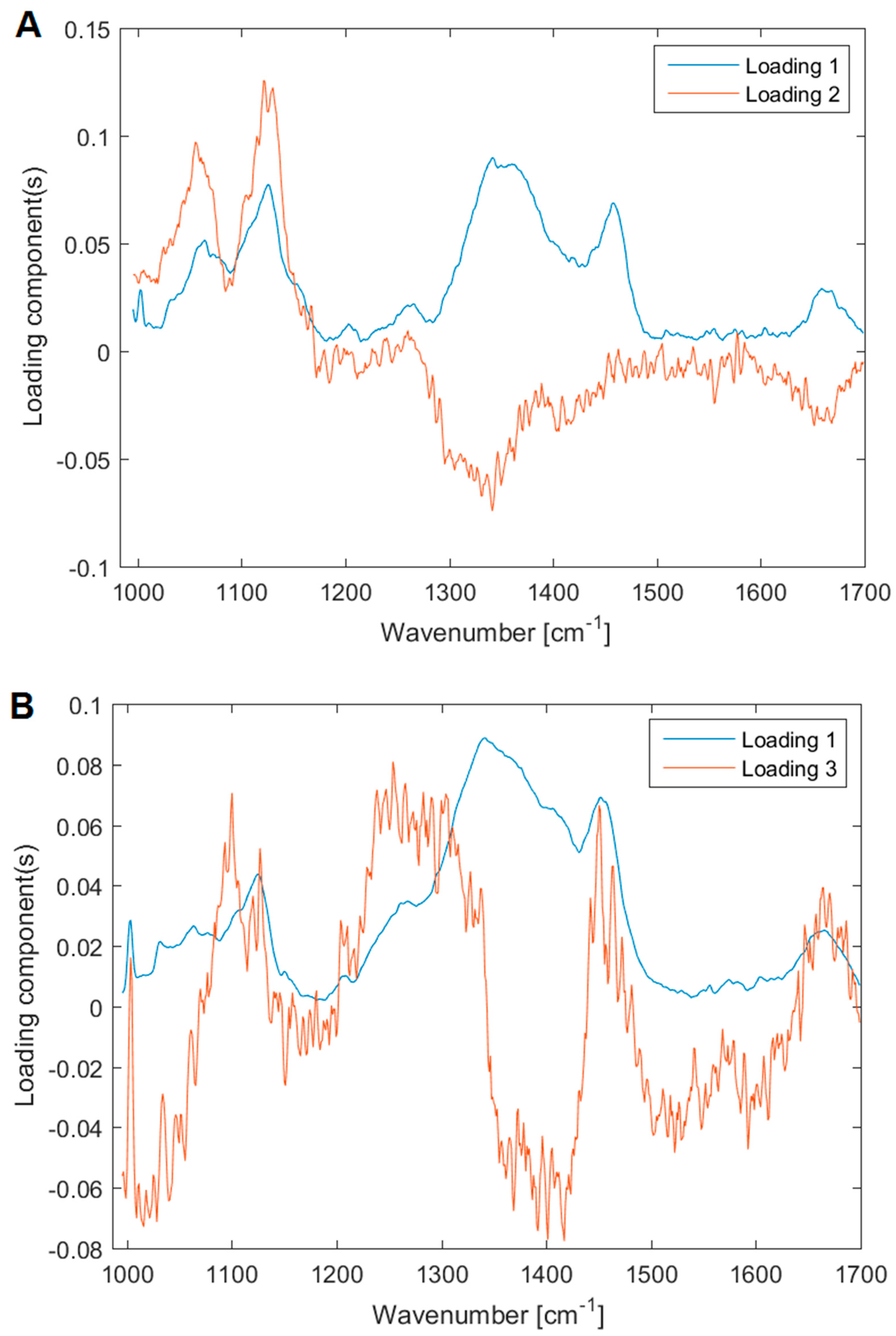
3.4. Analysis of the Yeast and Bacterial Biofilms by Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Alhede, M.; Qvortrup, K.; Liebrechts, R.; Hoiby, N.; Givskov, M.; Bjarnsholt, T. Combination of microscopic techniques reveals a comprehensive visual impression of biofilm structure and composition. FEMS Immunol. Med. Microbiol. 2012, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, F.; Horka, M.; Hola, V. Extracellular Polysaccharides in Microbial Biofilm and Their Influence on the Electrophoretic Properties of Microbial Cells. In Capillary Electrophoresis of Carbohydrates; Volpi, N., Ed.; Humana Press: New York, NY, USA, 2011; pp. 105–126. [Google Scholar]
- Donelli, G. Microbial Biofilms: Methods and Protocols; Humana Press: New York, NY, USA, 2014. [Google Scholar]
- Flemming, H.C.; Meier, M.; Schild, T. Mini-review: Microbial problems in paper production. Biofouling 2013, 29, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ou, Y.; Yang, L.; Zhu, Y.; Tolker-Nielsen, T.; Molin, S.; Qu, D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 2007, 153, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Hola, V.; Ruzicka, F.; Tejkalova, R.; Votava, M. Biofilm formation in nosocomial pathogens of respiratory tract. Int. J. Antimicrob. Agents 2007, 29, S142. [Google Scholar] [CrossRef]
- Wimpenny, J. Microbial Metropolis. Adv. Microb. Physiol. 2009, 56, 29–84. [Google Scholar] [PubMed]
- Marsh, P.D. Plaque as a biofilm: Pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003, 9, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Barghi, A.; Sadati, R.; Larki, R.A. Biological Wastewater Treatment through Biofilm. Iran. J. Public Health 2016, 45, 101. [Google Scholar]
- Voběrková, S.; Hermanová, S.; Hrubanová, K.; Krzyžánek, V. Biofilm formation and extracellular polymeric substances (EPS) production by Bacillus subtilis depending on nutritional conditions in the presence of polyester film. Folia Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cresson, R.; Dabert, P.; Bernet, N. Microbiology and performance of a methanogenic biofilm reactor during the start-up period. J. Appl. Microbiol. 2009, 106, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Liu, N.; Zhu, L.; Xu, Q.; Huang, H.; Jiang, L. Programming a Biofilm-Mediated Multienzyme-Assembly-Cascade System for the Biocatalytic Production of Glucosamine from Chitin. J. Agric. Food Chem. 2018, 66, 8061–8068. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Nunez, C.; Cardoso, M.H.; de Souza Candido, E.; Franco, O.L.; Hancock, R.E. Synthetic antibiofilm peptides. Biochim. Biophys. Acta 2016, 1858, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.; Whitehouse, C.A.; Grim, C.J.; Alam, M.; Colwell, R.R. Biofilms in water, its role and impact in human disease transmission. Curr. Opin. Biotechnol. 2008, 19, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Vik, A.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Adam, B.; Baillie, G.S.; Douglas, L.J. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J. Med. Microbiol. 2002, 51, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Holá, V.; Růžička, F.; Votava, M. The dynamics of Staphylococcus epidermis biofilm formation in relation to nutrition, temperature, and time. Scr. Med. Fac. Med.Univ. Brun. Masaryk. 2006, 79, 169–174. [Google Scholar]
- Liu, H.Y.; Zhao, Y.F.; Zhao, D.; Gong, T.; Wu, Y.C.; Han, H.Y.; Xu, T.; Peschel, A.; Han, S.Q.; Qu, D. Antibacterial and anti-biofilm activities of thiazolidione derivatives against clinical staphylococcus strains. Emerg. Microbes Infect. 2015, 4, e1. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, F.; Horka, M.; Hola, V.; Kubesova, A.; Pavlik, T.; Votava, M. The differences in the isoelectric points of biofilm-positive and biofilm-negative Candida parapsilosis strains. J. Microbiol. Methods 2010, 80, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Deleo, F.; Otto, M.W. Bacterial Pathogenesis: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Paiva, L.C.F.; Vidigal, P.G.; Donatti, L.; Svidzinski, T.I.E.; Consolaro, M.E.L. Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics. Micron 2012, 43, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Lam, O.L.T.; Watt, R.M.; Jin, L.J.; Samaranayake, L.P. Bacterial lipopolysaccharides variably modulate in vitro biofilm formation of Candida species. J. Med. Microbiol. 2010, 59, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Lattif, A.A.; Mukherjee, P.K.; Chandra, J.; Swindell, K.; Lockhart, S.R.; Diekema, D.J.; Pfaller, M.A.; Ghannoum, M.A. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int. J. Med. Microbiol. 2010, 300, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dohnalkova, A.C.; Marshall, M.J.; Arey, B.W.; Williams, K.H.; Buck, E.C.; Fredrickson, J.K. Imaging hydrated microbial extracellular polymers: Comparative analysis by electron microscopy. Appl. Environ. Microb. 2011, 77, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Schaudinn, C.; Stoodley, P.; Hall-Stoodley, L.; Gorur, A.; Remis, J.; Wu, S.; Auer, M.; Hertwig, S.; Guerrero-Given, D.; Hu, F.Z.; et al. Death and Transfiguration in Static Staphylococcus epidermidis Cultures. PLoS ONE 2014, 9, e100002. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Swerhone, G.D.W.; Leppard, G.G.; Araki, T.; Zhang, X.; West, M.M.; Hitchcock, A.P. Scanning transmission X-ray, laser scanning, and transmission electron microscopy mapping of the exopolymeric matrix of microbial biofilms. Appl. Environ. Microb. 2003, 69, 5543–5554. [Google Scholar] [CrossRef]
- Karcz, J.; Bernas, T.; Nowak, A.; Talik, E.; Woznica, A. Application of lyophilization to prepare the nitrifying bacterial biofilm for imaging with scanning electron microscopy. Scanning 2012, 34, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanek, V.; Sporenberg, N.; Keller, U.; Guddorf, J.; Reichelt, R.; Schonhoff, M. Polyelectrolyte multilayer capsules: Nanostructure and visualisation of nanopores in the wall. Soft Matter 2011, 7, 7034–7041. [Google Scholar] [CrossRef]
- Hrubanova, K.; Nebesarova, J.; Ruzicka, F.; Krzyzanek, V. The innovation of cryo-SEM freeze-fracturing methodology demonstrated on high pressure frozen biofilm. Micron 2018, 110, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Biel, S.S.; Wilke, K.; Dunckelmann, K.; Wittern, K.P.; Wepf, R. Light and electron microscopy: Histochemistry on the identical biopsy after high-pressure freezing. J. Histochem. Cytochem. 2004, 52, S62. [Google Scholar]
- Hawser, S.P.; Douglas, L.J. Biofilm Formation by Candida Species on the Surface of Catheter Materials in-Vitro. Infect. Immunity 1994, 62, 915–921. [Google Scholar]
- Kuo, J. Electron Microscopy: Methods and Protocols; Springer Science & Business Media: Berlin, Germany, 2007; Volume 369. [Google Scholar]
- Montesinos, E.; Esteve, I.; Guerrero, R. Comparison between Direct Methods for Determination of Microbial Cell-Volume—Electron-Microscopy and Electronic Particle Sizing. Appl. Environ. Microb. 1983, 45, 1651–1658. [Google Scholar]
- Webster, P.; Wu, S.; Webster, S.; Rich, K.; McDonald, K. Ultrastructural preservation of biofilms formed by non-typeable Hemophilus influenzae. Method Enzymol. 2004, 1, 165–182. [Google Scholar] [CrossRef]
- Graham, L.L.; Beveridge, T.J. Effect of Chemical Fixatives on Accurate Preservation of Escherichia-Coli and Bacillus-Subtilis Structure in Cells Prepared by Freeze-Substitution. J. Bacteriol. 1990, 172, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.A. Principles and Techniques of Scanning Electron Microscopy. Biological Applications, Volume 1; Van Nostrand Reinhold Company: New York, NY, USA, 1974. [Google Scholar]
- Wu, Y.; Liang, J.; Rensing, K.; Chou, T.M.; Libera, M. Extracellular Matrix Reorganization during Cryo Preparation for Scanning Electron Microscope Imaging of Staphylococcus aureus Biofilms. Microsc. Microanal. 2014, 20, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Fassel, T.A.; Edmiston, C.E. Ruthenium red and the bacterial glycocalyx. Biotech. Histochem. 1999, 74, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Reese, S.; Guggenheim, B. A novel TEM contrasting technique for extracellular polysaccharides in in vitro biofilrns. Microsc. Res. Tech. 2007, 70, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Galway, M.E.; Heckman, J.W., Jr.; Hyde, G.J.; Fowke, L.C. Advances in High-Pressure and Plunge-Freeze Fixation. Methods Cell Biol. 1995, 49, 3–19. [Google Scholar] [PubMed]
- Wang, A.B.; Lin, C.H.; Chen, C.C. The critical temperature of dry impact for tiny droplet impinging on a heated surface. Phys. Fluids 2000, 12, 1622–1625. [Google Scholar] [CrossRef]
- Dahl, R.; Staehelin, L.A. High-pressure freezing for the preservation of biological structure: Theory and practice. J. Electron Microsc. Tech. 1989, 13, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Moor, H. Theory and Practice of High Pressure Freezing. In Cryotechniques in Biological Electron Microscopy; Steinbrecht, R., Zierold, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 175–191. [Google Scholar]
- Studer, D.; Michel, M.; Muller, M. High-Pressure Freezing Comes of Age. Scanning Microsc. 1989, 253–269. [Google Scholar]
- Shimoni, E.; Muller, M. On optimizing high-pressure freezing: From heat transfer theory to a new microbiopsy device. J. Microsc. 1998, 192, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Samek, O.; Mlynarikova, K.; Bernatova, S.; Jezek, J.; Krzyzanek, V.; Siler, M.; Zemanek, P.; Ruzicka, F.; Hola, V.; Mahelova, M. Candida parapsilosis Biofilm Identification by Raman Spectroscopy. Int. J. Mol. Sci. 2014, 15, 23924–23935. [Google Scholar] [CrossRef] [PubMed]
- Rebrosova, K.; Siler, M.; Samek, O.; Ruzicka, F.; Bernatova, S.; Jezek, J.; Zemanek, P.; Hola, V. Differentiation between Staphylococcus aureus and Staphylococcus epidermidis strains using Raman spectroscopy. Future Microbiol. 2017, 12, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Hench, L.L. Raman microspectroscopy: A noninvasive tool for studies of individual living cells in vitro. Expert Rev. Med. Devices 2006, 3, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Mc, F.J. The nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 1907, 49, 1176–1178. [Google Scholar]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.P.; Ngo-Thi, N.A.; van Vreeswijk, T.; Stammler, M.; Endtz, H.P.; Bruining, H.A.; Naumann, D.; Puppels, G.J. Prospective study of the performance of vibrational spectroscopies for rapid identification of bacterial and fungal pathogens recovered from blood cultures. J. Clin. Microbiol. 2003, 41, 324–329. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Vancanneyt, M.; De Vos, P.; Moens, L. Methods for extracting biochemical information from bacterial Raman spectra: Focus on a group of structurally similar biomolecules—Fatty acids. Anal. Chim. Acta 2007, 603, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tuma, R. Raman spectroscopy of proteins: From peptides to large assemblies. J. Raman Spectrosc. 2005, 36, 307–319. [Google Scholar] [CrossRef]
- Notingher, I. Raman Spectroscopy cell-based Biosensors. Sensors 2007, 7, 1343–1358. [Google Scholar] [CrossRef]
- Neugebauer, U.; Schmid, U.; Baumann, K.; Ziebuhr, W.; Kozitskaya, S.; Holzgrabe, U.; Schmitt, M.; Popp, J. The influence of fluoroquinolone drugs on the bacterial growth of S-epidermidis utilizing the unique potential of vibrational spectroscopy. J. Phys. Chem. A 2007, 111, 2898–2906. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Perna, G.; Lastella, M.; Lasalvia, M.; Mezzenga, E.; Capozzi, V. Raman spectroscopy and atomic force microscopy study of cellular damage in human keratinocytes treated with HgCl2. J. Mol. Struct. 2007, 834, 182–187. [Google Scholar] [CrossRef]
- Pyrgiotakis, G.; Bhowmick, T.K.; Finton, K.; Suresh, A.K.; Kane, S.G.; Bellare, J.R.; Moudgil, B.M. Cell (A549)-particle (Jasada Bhasma) interactions using Raman spectroscopy. Biopolymers 2008, 89, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Pilat, Z.; Bernatova, S.; Jezek, J.; Kirchhoff, J.; Tannert, A.; Neugebauer, U.; Samek, O.; Zemanek, P. Microfluidic Cultivation and Laser Tweezers Raman Spectroscopy of E. coli under Antibiotic Stress. Sensors 2018, 18, 1623. [Google Scholar] [CrossRef] [PubMed]
- Rebrosova, K.; Siler, M.; Samek, O.; Ruzicka, F.; Bernatova, S.; Hola, V.; Jezek, J.; Zemanek, P.; Sokolova, J.; Petras, P. Rapid identification of staphylococci by Raman spectroscopy. Sci. Rep. 2017, 7, 14846. [Google Scholar] [CrossRef] [PubMed]
- Samek, O.; Obruca, S.; Siler, M.; Sedlacek, P.; Benesova, P.; Kucera, D.; Marova, I.; Jezek, J.; Bernatova, S.; Zemanek, P. Quantitative Raman Spectroscopy Analysis of Polyhydroxyalkanoates Produced by Cupriavidus necator H16. Sensors 2016, 16, 1808. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, F.; Hola, V.; Votava, M.; Tejkalova, R. Importance of biofilm in Candida parapsilosis and evaluation of its susceptibility to antifungal agents by colorimetric method. Folia Microbiol. 2007, 52, 209–214. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hebraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory Effect of Sophorolipid on Candida albicans Biofilm Formation and Hyphal Growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef] [PubMed]
- Ludecke, C.; Jandt, K.D.; Siegismund, D.; Kujau, M.J.; Zang, E.; Rettenmayr, M.; Bossert, J.; Roth, M. Reproducible Biofilm Cultivation of Chemostat-Grown Escherichia coli and Investigation of Bacterial Adhesion on Biomaterials Using a Non-Constant-Depth Film Fermenter. PLoS ONE 2014, 9, e84837. [Google Scholar] [CrossRef] [PubMed]
- Bray, D.F.; Bagu, J.; Koegler, P. Comparison of Hexamethyldisilazane (Hmds), Peldri-Ii, and Critical-Point Drying Methods for Scanning Electron-Microscopy of Biological Specimens. Microsc. Res. Tech. 1993, 26, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hazrin-Chong, N.H.; Manefield, M. An alternative SEM drying method using hexamethyldisilazane (HMDS) for microbial cell attachment studies on sub-bituminous coal. J. Microbiol. Methods 2012, 90, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Osumi, M.; Konomi, M.; Sugawara, T.; Takagi, T.; Baba, M. High-pressure freezing is a powerful tool for visualization of Schizosaccharomyces pombe cells: Ultra-low temperature and low-voltage scanning electron microscopy and immunoelectron microscopy. J. Electron Microsc. 2006, 55, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Psenicka, M.; Tesarova, M.; Tesitel, J.; Nebesarova, J. Size determination of Acipenser ruthenus spermatozoa in different types of electron microscopy. Micron 2010, 41, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Brandt, N.N.; Brovko, O.O.; Chikishev, A.Y.; Paraschuk, O.D. Optimization of the rolling-circle filter for Raman background subtraction. Appl. Spectrosc. 2006, 60, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Kaech, A.; Ziegler, U. High-Pressure Freezing: Current State and Future Prospects. Methods Mol. Biol. 2014, 1117, 151–171. [Google Scholar] [PubMed]
- Kaech, A.; Woelfel, M. Evaluation of Different Freezing Methods for Monolayer Cell Cultures. Microsc. Microanal. 2007, 13, 240–241. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Fungal exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maira-Litran, T.; Kropec, A.; Abeygunawardana, C.; Joyce, J.; Mark, G.; Goldmann, D.A.; Pier, G.B. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immunity 2002, 70, 4433–4440. [Google Scholar] [CrossRef]
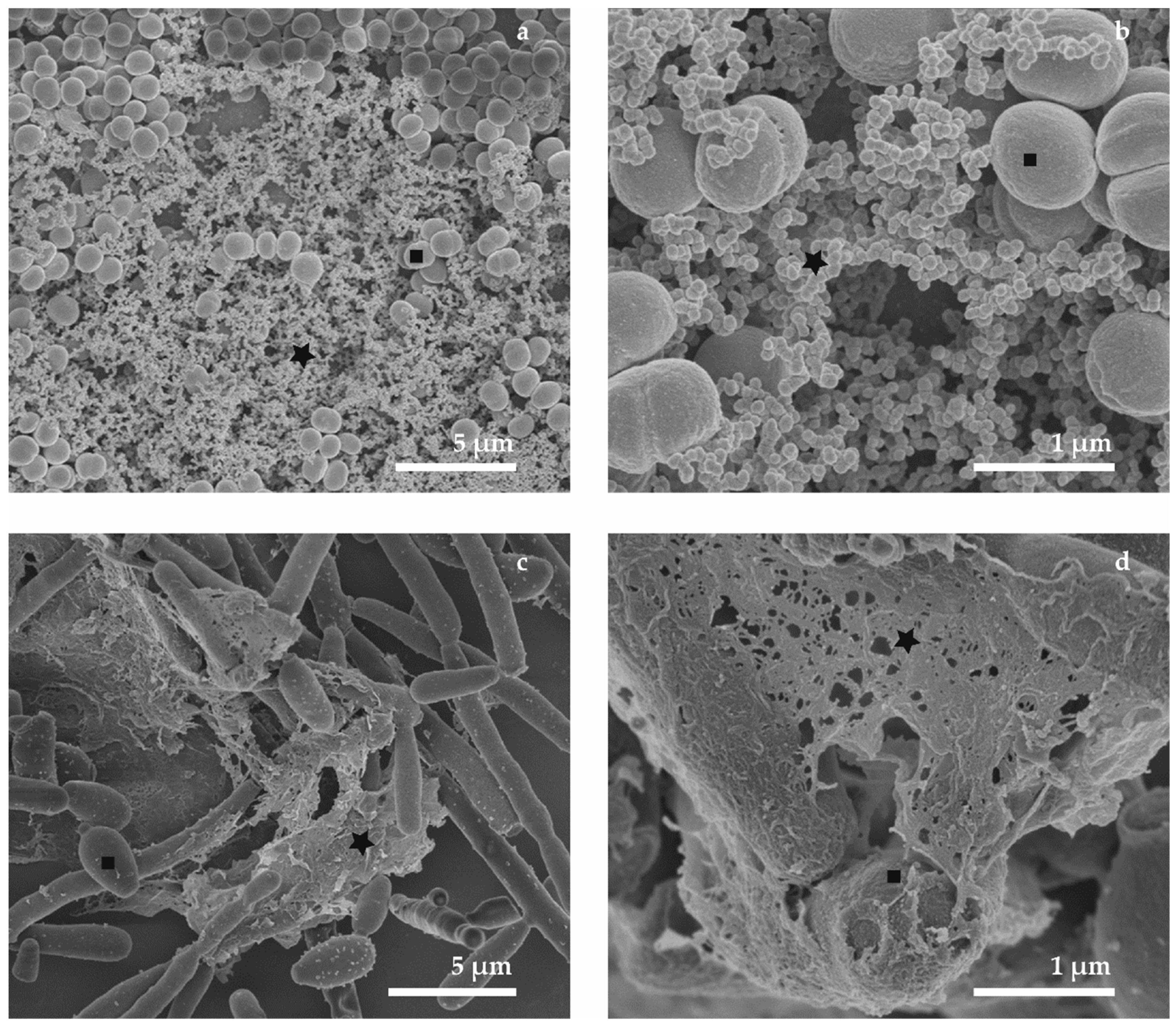
| M | Advantage | Disadvantage | Schema |
|---|---|---|---|
| M1—air-drying | Speed of sample preparation Simplicity Repeatability of measurement in SEM at room temperature Suitable for surface imaging | The loss of the 3D structure Deformation of microbial biofilm Deformation of ECM The possibility of imaging only the sample surface (not interior) | Deformation of biofilm  |
| M2—conventional chemical preparation | Repeatability of measurement in SEM at room temperature The 3D structure is preserved. Suitable for surface imaging | Long-term procedure Damage of soft biofilm sample due to multi-steps washing Artefacts with chemicals treatment (the change of gel-like ECM into fiber structures) The sample surface imaging | Biofilm is washed out 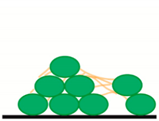 |
| M3—plunging LN2; cryo-SEM | Speed of sample preparation The 3D structure of microbial cells is preserved Possibility of biofilm interior imaging (used freeze-fracturing technique also suitable for M3–M5) | Artefacts with freezing procedure Freezing is sufficient for very thin samples Limitation for surface imaging because of water content in biofilm samples | “Large” ice crystals 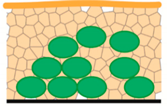 |
| M4—plung. Ethane; cryo-SEM | Speed of sample preparation The 3D structure of microbial cells is preserved Possibility of biofilm interior imaging | Artifacts with freezing procedure (smaller ice crystals inside biofilm than by M3) Freezing of thin samples Limitation in surface imaging because of water content in biofilm samples | “Small” ice crystals 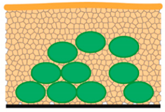 |
| M5—HPF freezing and cryo-SEM | Speed of sample preparation 3D structure of microbial cells/ECM is nicely preserved The best freezing technique for samples with thickness up to 200 µm (exp. tested) Biofilm interior imaging | Limitation in surface imaging (water content in biofilm) Limitations connected with HPF machine–cultivation substrate (sapphire discs for freeze fracturing; Al or Cu-gold discs) | Optimal prep. of biofilm for interior imaging 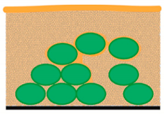 |
| M6—Combined preparation | Speed of sample preparation 3D structure of biofilm Biofilm surface imaging Repeatability of measurement in SEM at room temperature after freeze-drying Less washed out biofilm | Artifacts from chemical fixation (the change of gel-like ECM) The imaging of sample surface | Applicable prep. of chemically fixed biofilm for surface imaging  |
| No. | Wavenumber [cm−1] | Peaks Assignment |
|---|---|---|
| 1 | 1002 | Symmetric-ring breathing of Phe |
| 2 | 1033 | C-H in-plane stretch of Phe |
| 3 | 1065 | C-C stretch of lipids |
| 4 | 1125 | C-N stretch of proteins |
| 5 | 1205 | Proteins |
| 6 | 1267 | Lipids, Amide III |
| 7 | 1340–1360 | Proteins, Carbohydrates |
| 8 | 1456 | CH2 scissoring, Lipids |
| 9 | 1660 | Amide I, Lipids |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Ruzicka, F.; Pilat, Z.; Samek, O. Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors 2018, 18, 4089. https://doi.org/10.3390/s18124089
Hrubanova K, Krzyzanek V, Nebesarova J, Ruzicka F, Pilat Z, Samek O. Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors. 2018; 18(12):4089. https://doi.org/10.3390/s18124089
Chicago/Turabian StyleHrubanova, Kamila, Vladislav Krzyzanek, Jana Nebesarova, Filip Ruzicka, Zdenek Pilat, and Ota Samek. 2018. "Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy" Sensors 18, no. 12: 4089. https://doi.org/10.3390/s18124089
APA StyleHrubanova, K., Krzyzanek, V., Nebesarova, J., Ruzicka, F., Pilat, Z., & Samek, O. (2018). Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors, 18(12), 4089. https://doi.org/10.3390/s18124089





