Sensitive and Reproducible Gold SERS Sensor Based on Interference Lithography and Electrophoretic Deposition
Abstract
:1. Introduction
2. Materials and Methods
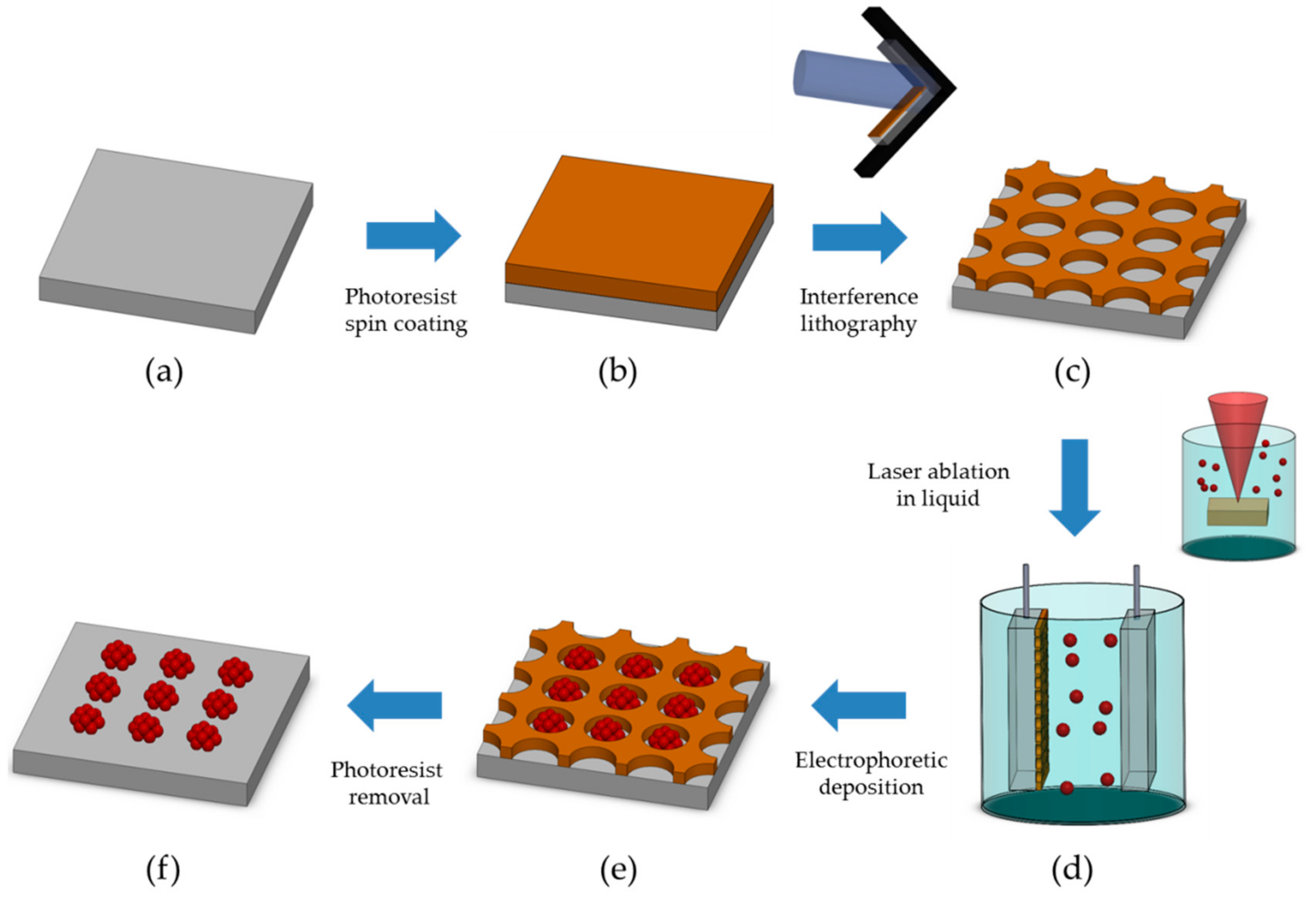
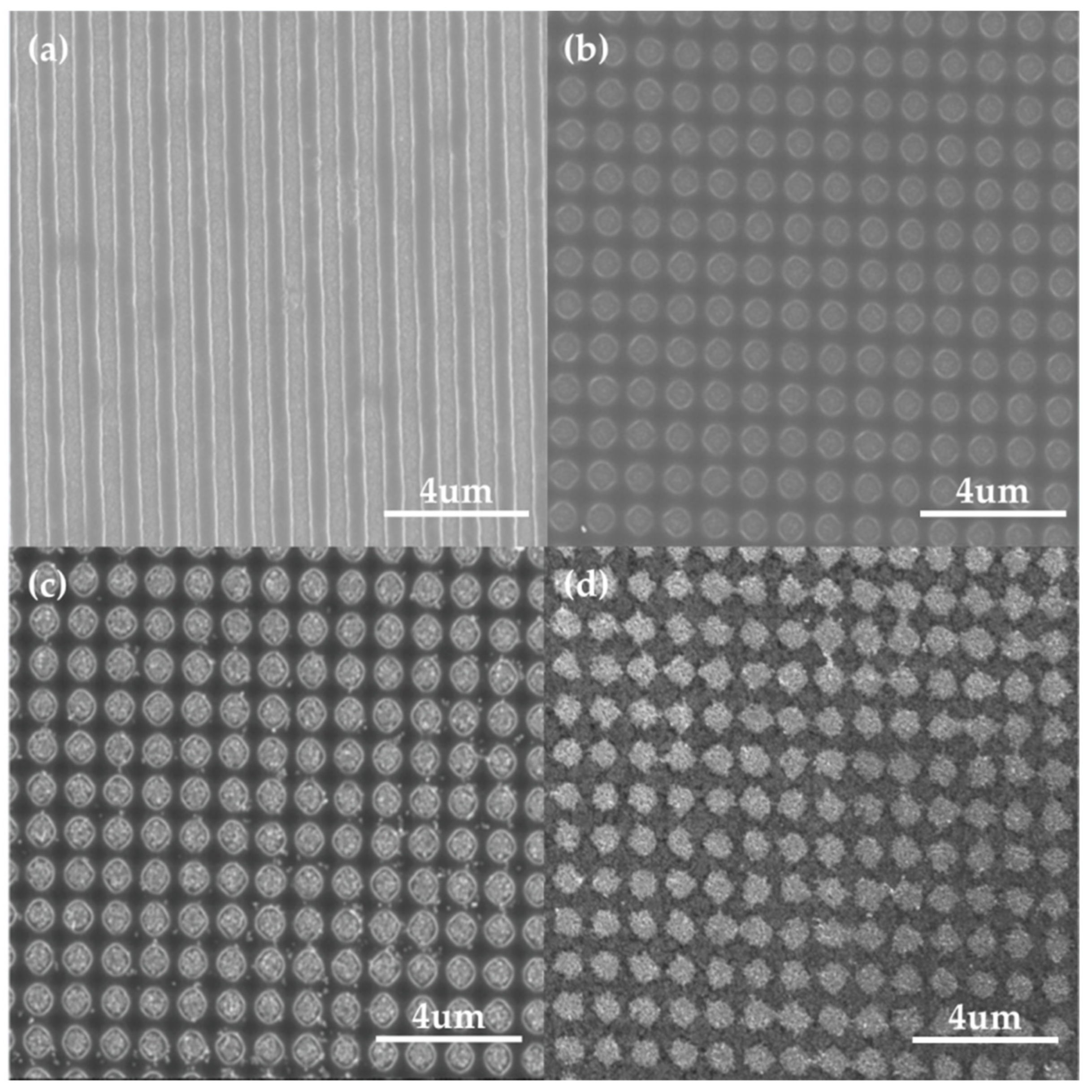
2.1. PR Nanohole Patterning on Electrodes through LIL
2.2. Preparation of Au Colloidal Solution
2.3. EPD of Au NP
2.4. Characterization
3. Results and Discussion
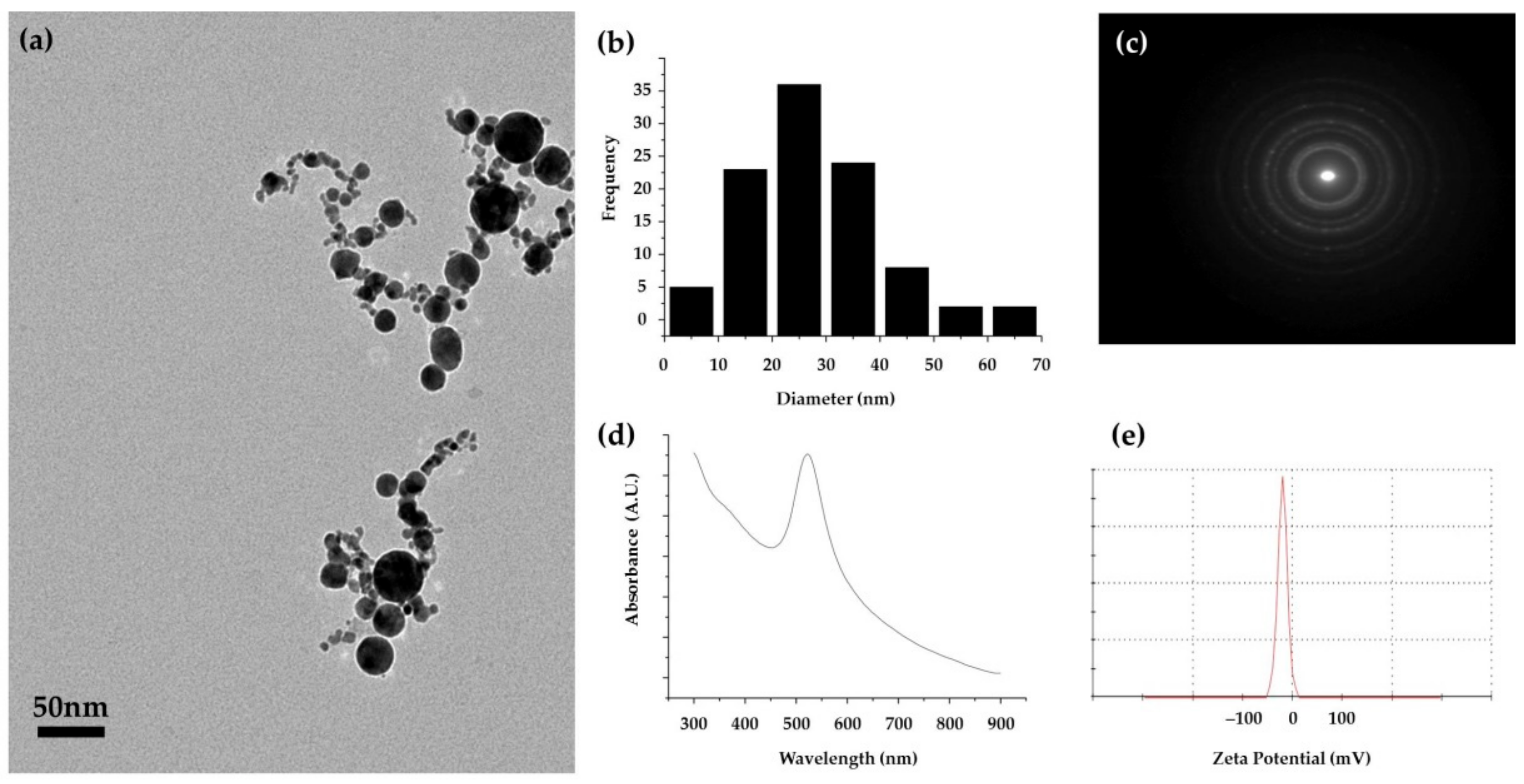
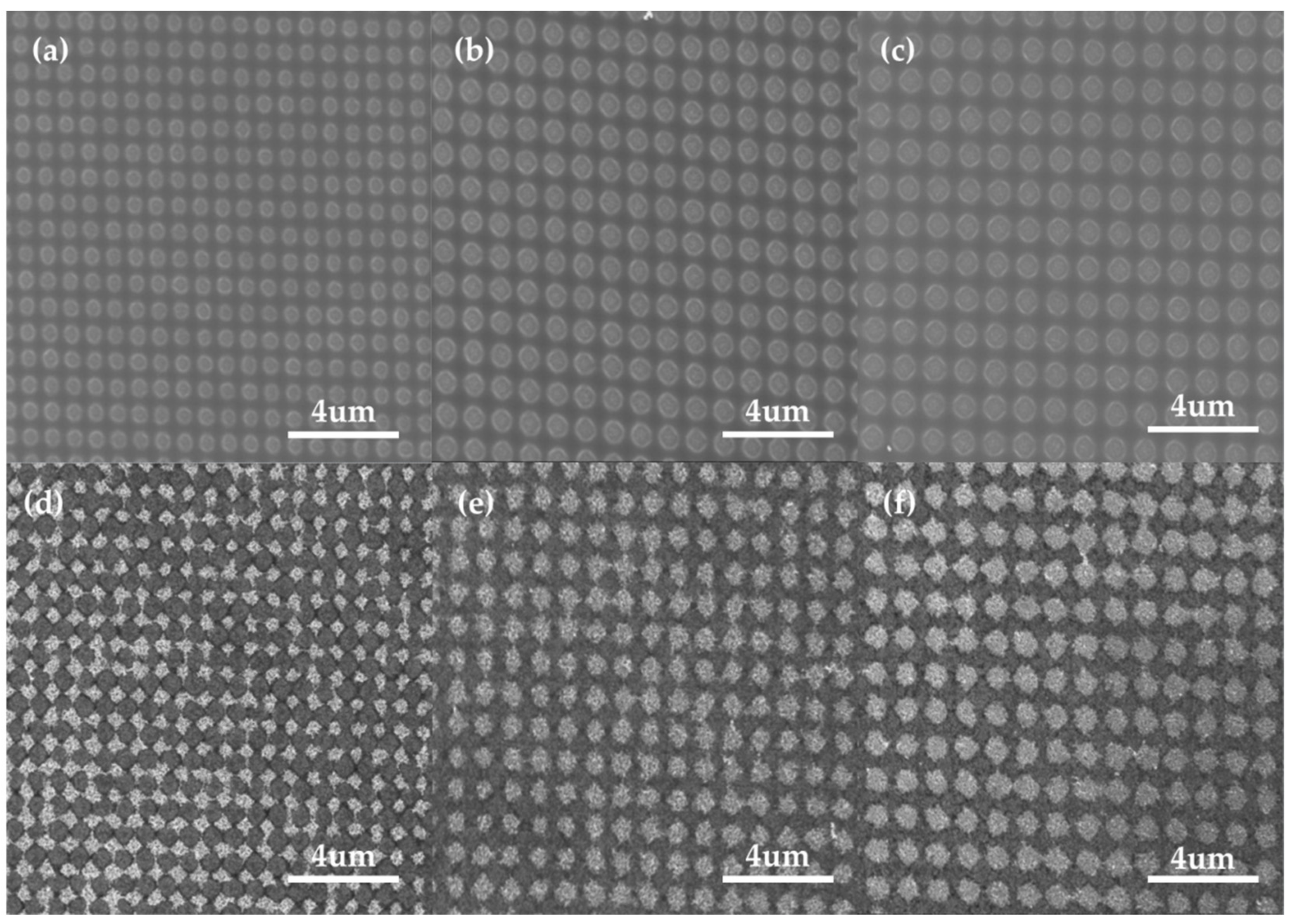
3.1. Characterization of the Au NP Produced by PLAL
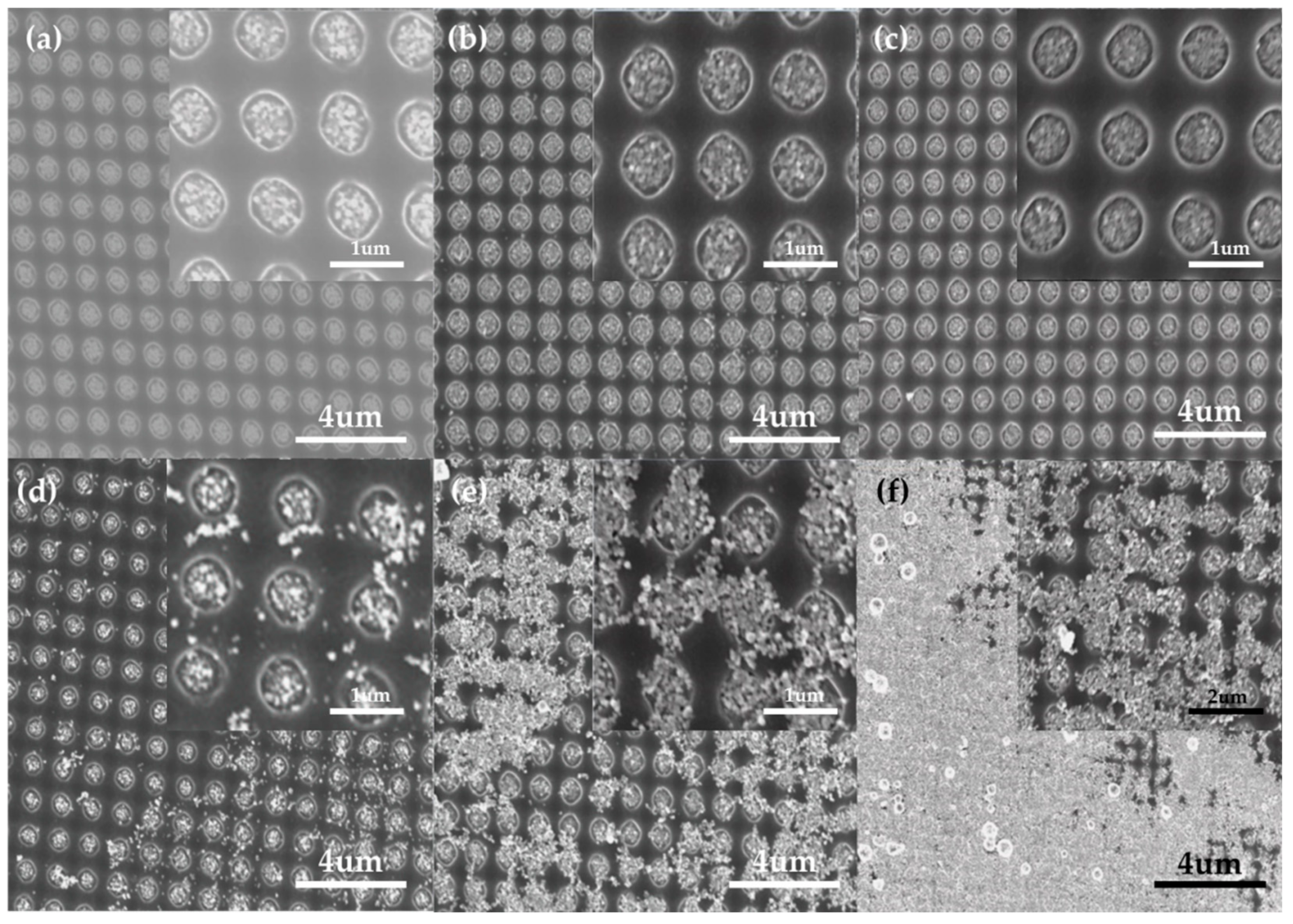
3.2. EPD of Au NP
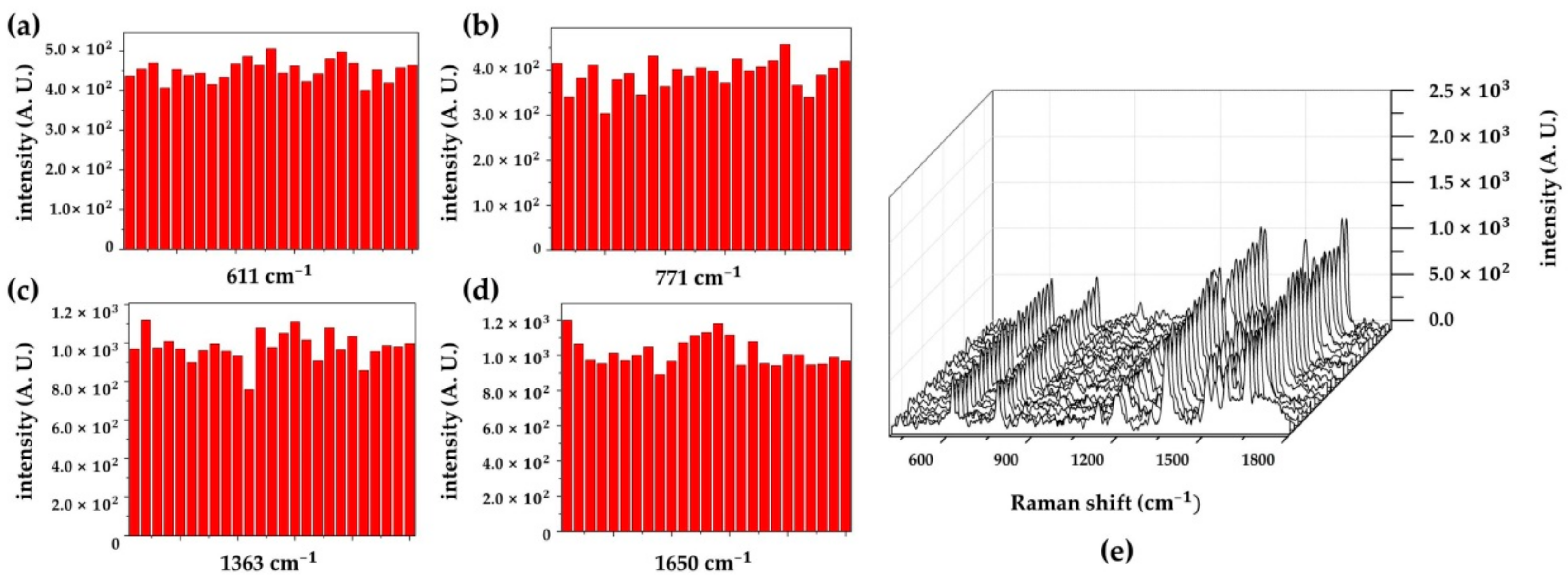
3.3. Raman Measurement
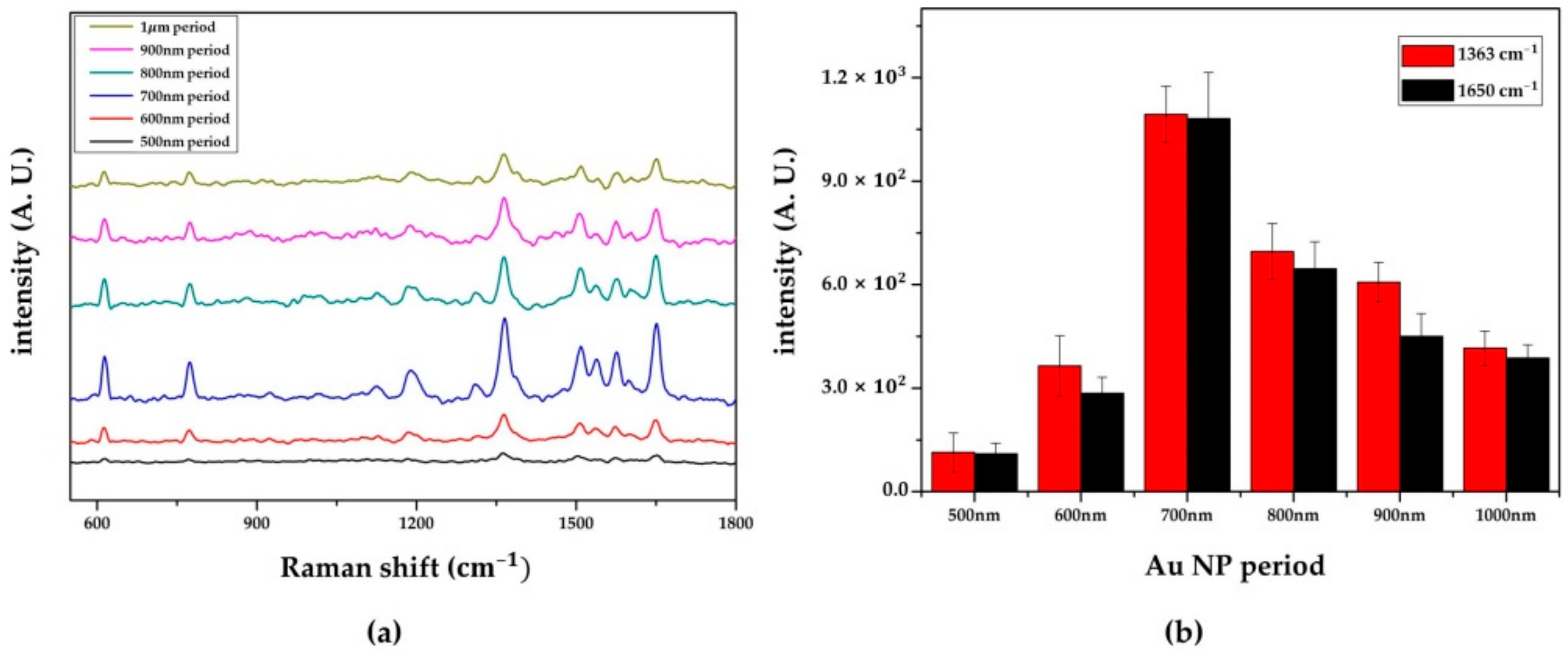
3.3.1. SERS Activity Depending on the Different Periods
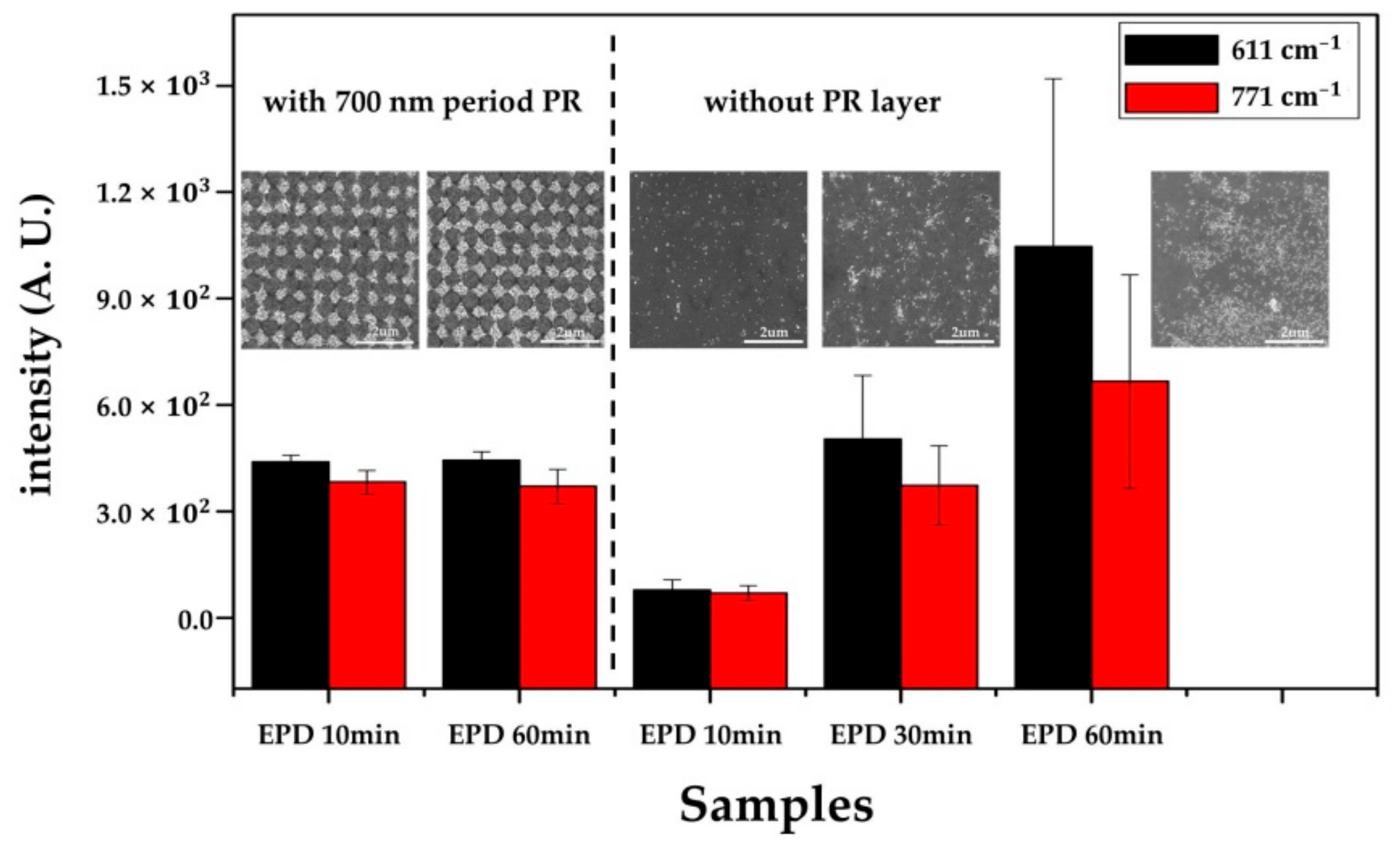
3.3.2. SERS Substrate With/Without the Patterned PR Layer
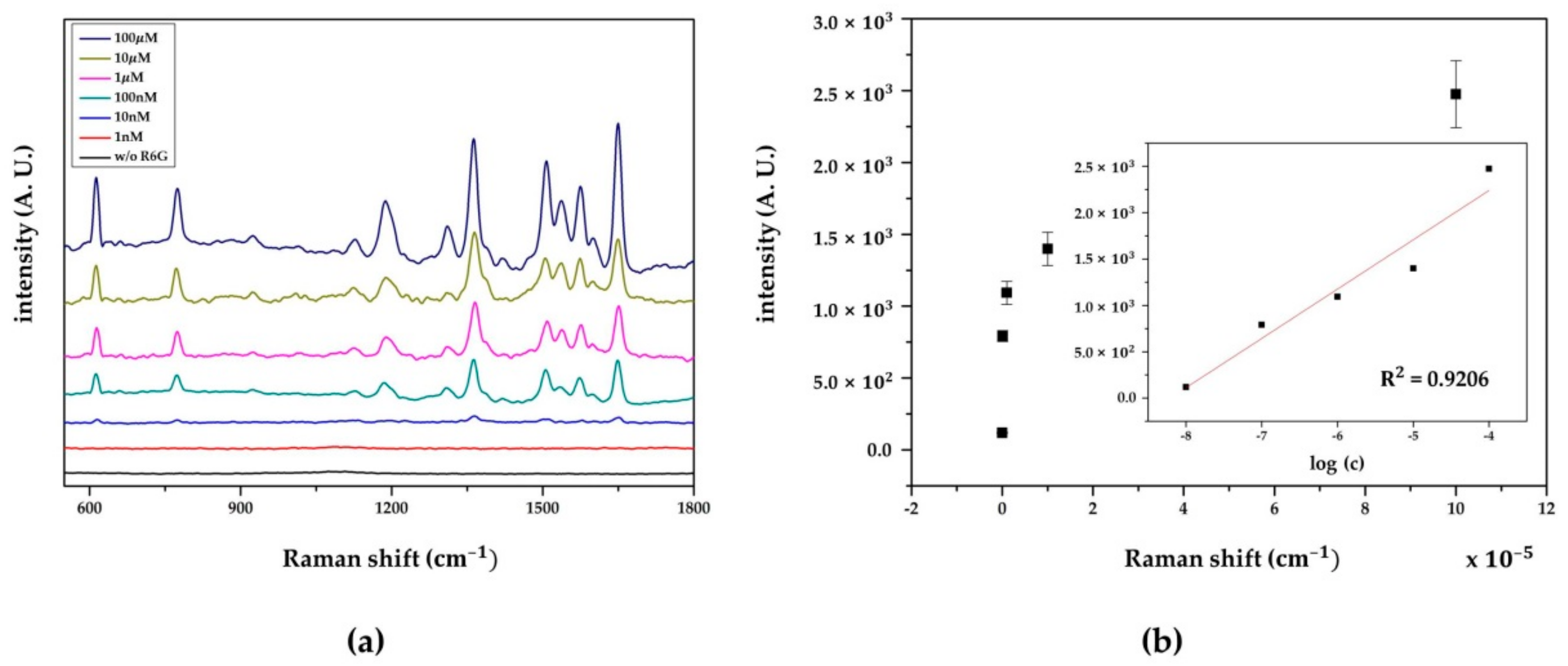
3.3.3. SERS Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mosier-Boss, P.; Lieberman, S. Detection of volatile organic compounds using surface enhanced Raman spectroscopy substrates mounted on a thermoelectric cooler. Anal. Chim. Acta 2003, 488, 15–23. [Google Scholar] [CrossRef]
- Qian, C.; Guo, Q.; Xu, M.; Yuan, Y.; Yao, J. Improving the SERS detection sensitivity of aromatic molecules by a PDMS-coated Au nanoparticle monolayer film. RSC Adv. 2015, 5, 53306–53312. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, J.; Qing, Z.; Zheng, M.; Yang, J.; Yang, S.; Ying, L.; Yang, R. Detection of Circulating Tumor DNA in Human Blood via DNA-Mediated Surface-Enhanced Raman Spectroscopy of Single-Walled Carbon Nanotubes. Anal. Chem. 2016, 88, 4759–4765. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Liao, J.-D.; Yang, M.-L.; Wu, C.-L. Target-size embracing dimension for sensitive detection of viruses with various sizes and influenza virus strains. Biosens. Bioelectron. 2012, 35, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, B.; Premasiri, W.R.; Ziegler, L.D.; Negro, L.D.; Reinhard, B.M. Engineering Nanoparticle Cluster Arrays for Bacterial Biosensing: The Role of the Building Block in Multiscale SERS Substrates. Adv. Funct. Mater. 2010, 20, 2619–2628. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; Mcquillan, A.J. Raman spectra of pyridzne adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 4. [Google Scholar] [CrossRef]
- Quester, K.; Avalos-Borja, M.; Vilchis-Nestor, A.R.; Camacho-López, M.A.; Castro-Longoria, E. SERS Properties of Different Sized and Shaped Gold Nanoparticles Biosynthesized under Different Environmental Conditions by Neurospora crassa Extract. PLoS ONE 2013, 8, e77486. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-K.; Jeon, K.-S.; Hwang, J.-H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.-M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars For Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C Nanomater. Interfaces 2008, 2008, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Q.; Yi, J.; Hu, S.; Liu, B.-J.; Liu, J.-Y.; Wang, X.; Ren, B. Size Effect on SERS of Gold Nanorods Demonstrated via Single Nanoparticle Spectroscopy. J. Phys. Chem. C 2016, 120, 20806–20813. [Google Scholar] [CrossRef]
- Yap, F.L.; Thoniyot, P.; Krishnan, S.; Krishnamoorthy, S. Nanoparticle Cluster Arrays for High-Performance SERS through Directed Self-Assembly on Flat Substrates and on Optical Fibers. ACS Nano 2012, 6, 2056–2070. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Teng, F.; Wang, Z.; Lu, N. Droplet-Confined Electroless Deposition of Silver Nanoparticles on Ordered Superhydrophobic Structures for High Uniform SERS Measurements. ACS Appl. Mater. Interfaces 2017, 9, 21548–21553. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, C.; Zhou, F.; Zhang, T.; Zhang, H.; Li, X.; Duan, G.; Cai, W.; Li, Y. Rapid Synthesis of Monodisperse Au Nanospheres through a Laser Irradiation -Induced Shape Conversion, Self-Assembly and Their Electromagnetic Coupling SERS Enhancement. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Smyth, C.A.; Mirza, I.; Lunney, J.G.; McCabe, E.M. Surface-enhanced Raman spectroscopy (SERS) using Ag nanoparticle films produced by pulsed laser deposition. Appl. Surf. Sci. 2013, 264, 31–35. [Google Scholar] [CrossRef]
- Yang, S.; Dai, X.; Stogin, B.B.; Wong, T.-S. Ultrasensitive surface-enhanced Raman scattering detection in common fluids. Proc. Natl. Acad. Sci. USA 2016, 113, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, S.; Wang, L.; Zhao, J.; Zhu, Z.; Liu, B.; Zhong, J.; Sun, X. Large-scale uniform Au nanodisk arrays fabricated via x-ray interference lithography for reproducible and sensitive SERS substrate. Nanotechnology 2014, 25, 245301. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.M.; Retterer, S.D.; Oran, J.M.; Sepaniak, M.J. Controllable Nanofabrication of Aggregate-like Nanoparticle Substrates and Evaluation for Surface-Enhanced Raman Spectroscopy. ACS Nano 2009, 3, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-Y.; Liao, J.-D.; Ju, Y.-H.; Chang, C.-W.; Shiau, A.-L. Focused ion beam-fabricated Au micro/nanostructures used as a surface enhanced Raman scattering-active substrate for trace detection of molecules and influenza virus. Nanotechnology 2011, 22, 185308. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Zito, G.; Managò, S.; Galeotti, F.; Pisco, M.; De Luca, A.; Cusano, A.; Quero, G.; Zito, G.; Managò, S.; et al. Nanosphere Lithography on Fiber: Towards Engineered Lab-On-Fiber SERS Optrodes. Sensors 2018, 18, 680. [Google Scholar] [CrossRef] [PubMed]
- Cottat, M.; Lidgi-Guigui, N.; Tijunelyte, I.; Barbillon, G.; Hamouda, F.; Gogol, P.; Aassime, A.; Lourtioz, J.-M.; Bartenlian, B.; de la Chapelle, M.L. Soft UV nanoimprint lithography-designed highly sensitive substrates for SERS detection. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Quilis, N.; Lequeux, M.; Venugopalan, P.; Khan, I.; Knoll, W.; Boujday, S.; Lamy de la Chapelle, M.; Dostalek, J. Tunable laser interference lithography preparation of plasmonic nanoparticle arrays tailored for SERS. Nanoscale 2018, 10, 10268–10276. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, Y.; Kong, W.; Liu, K.; Du, W.; Zhao, C.; Gao, P.; Zhao, Z.; Wang, C.; Pu, M.; et al. Large area deep subwavelength interference lithography with a 35 nm half-period based on bulk plasmon polaritons. Opt. Mater. Express 2018, 8, 199. [Google Scholar] [CrossRef]
- Park, J.-M.; Nalwa, K.S.; Leung, W.; Constant, K.; Chaudhary, S.; Ho, K.-M. Fabrication of metallic nanowires and nanoribbons using laser interference lithography and shadow lithography. Nanotechnology 2010, 21, 215301. [Google Scholar] [CrossRef] [PubMed]
- Päivänranta, B.; Langner, A.; Kirk, E.; David, C.; Ekinci, Y. Sub-10 nm patterning using EUV interference lithography. Nanotechnology 2011, 22, 375302. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Chen, Y.-C.; Braun, P.V.; Wiltzius, P. Fabrication of Three-Dimensional Photonic Crystals Using Multibeam Interference Lithography and Electrodeposition. Adv. Mater. 2009, 21, 3012–3015. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, B.; Guo, H.; Tetreault, N.; Giessen, H.; Friend, R.H. Large-area two-dimensional photonic crystals of metallic nanocylinders based on colloidal gold nanoparticles. Appl. Phys. Lett. 2007, 90, 133114. [Google Scholar] [CrossRef]
- Liberman, V.; Yilmaz, C.; Bloomstein, T.M.; Somu, S.; Echegoyen, Y.; Busnaina, A.; Cann, S.G.; Krohn, K.E.; Marchant, M.F.; Rothschild, M. A Nanoparticle Convective Directed Assembly Process for the Fabrication of Periodic Surface Enhanced Raman Spectroscopy Substrates. Adv. Mater. 2010, 22, 4298–4302. [Google Scholar] [CrossRef] [PubMed]
- Simão, T.; Chevallier, P.; Lagueux, J.; Côté, M.-F.; Rehbock, C.; Barcikowski, S.; Fortin, M.-A.; Guay, D. Laser-synthesized ligand-free Au nanoparticles for contrast agent applications in computed tomography and magnetic resonance imaging. J. Mater. Chem. B 2016, 4, 6413–6427. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cai, W.; Lin, Y.; Chen, B. Au nanochain-built 3D netlike porous films based on laser ablation in water and electrophoretic deposition. Chem. Commun. 2010, 46, 7223–7225. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lin, Y.; Liu, G.; Cai, W. Au nanoparticle-built mesoporous films based on co-electrophoresis deposition and selective etching. Electrochem. Commun. 2014, 46, 71–74. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Kong, L.; Lei, Y. Surface Nanometer-Scale Patterning in Realizing Large-Scale Ordered Arrays of Metallic Nanoshells with Well-Defined Structures and Controllable Properties. Adv. Funct. Mater. 2010, 20, 2527–2533. [Google Scholar] [CrossRef]
- Semaltianos, N.G.; Hendry, E.; Chang, H.; Wears, M.L. Electrophoretic deposition on graphene of Au nanoparticles generated by laser ablation of a bulk Au target in water. Laser Phys. Lett. 2015, 12, 046201. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Liu, G.; Zeng, H. From Nanoparticles to Nanoplates: Preferential Oriented Connection of Ag Colloids during Electrophoretic Deposition. J. Phys. Chem. C 2009, 113, 7692–7696. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chrisey, D.B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Sarkar, P.; Nicholson, P.S. Electrophoretic Deposition (EPD): Mechanisms, Kinetics, and Application to Ceramics. J. Am. Ceram. Soc. 1996, 79, 1987–2002. [Google Scholar] [CrossRef]
- He, L.; Liu, C.; Hu, J.; Gu, W.; Zhang, Y.; Dong, L.; Fu, X.; Tang, J. Hydrophobic ligand-mediated hierarchical Cu nanoparticles on reduced graphene oxides for SERS platform. CrystEngComm 2016, 18, 7764–7771. [Google Scholar] [CrossRef]
- Guillot, N.; Shen, H.; Frémaux, B.; Péron, O.; Rinnert, E.; Toury, T.; Lamy de la Chapelle, M. Surface enhanced Raman scattering optimization of gold nanocylinder arrays: Influence of the localized surface plasmon resonance and excitation wavelength. Appl. Phys. Lett. 2010, 97, 023113. [Google Scholar] [CrossRef]
- Yu, Q.; Guan, P.; Qin, D.; Golden, G.; Wallace, P.M. Inverted Size-Dependence of Surface-Enhanced Raman Scattering on Gold Nanohole and Nanodisk Arrays. Nano Lett. 2008, 8, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Wang, Z.; Yang, Y.; Chen, L.; Syed, A.; Wong, K.; Wang, X. Electron-beam lithography of gold nanostructures for surface-enhanced Raman scattering. J. Micromech. Microeng. 2012, 22, 125007. [Google Scholar] [CrossRef]
- Kavitha, C.; Bramhaiah, K.; John, N.S.; Ramachandran, B.E. Low cost, ultra-thin films of reduced graphene oxide–Ag nanoparticle hybrids as SERS based excellent dye sensors. Chem. Phys. Lett. 2015, 629, 81–86. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.S.; Yang, M. Sensitive and Reproducible Gold SERS Sensor Based on Interference Lithography and Electrophoretic Deposition. Sensors 2018, 18, 4076. https://doi.org/10.3390/s18114076
Hwang JS, Yang M. Sensitive and Reproducible Gold SERS Sensor Based on Interference Lithography and Electrophoretic Deposition. Sensors. 2018; 18(11):4076. https://doi.org/10.3390/s18114076
Chicago/Turabian StyleHwang, June Sik, and Minyang Yang. 2018. "Sensitive and Reproducible Gold SERS Sensor Based on Interference Lithography and Electrophoretic Deposition" Sensors 18, no. 11: 4076. https://doi.org/10.3390/s18114076
APA StyleHwang, J. S., & Yang, M. (2018). Sensitive and Reproducible Gold SERS Sensor Based on Interference Lithography and Electrophoretic Deposition. Sensors, 18(11), 4076. https://doi.org/10.3390/s18114076



