Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics
Abstract
1. Introduction
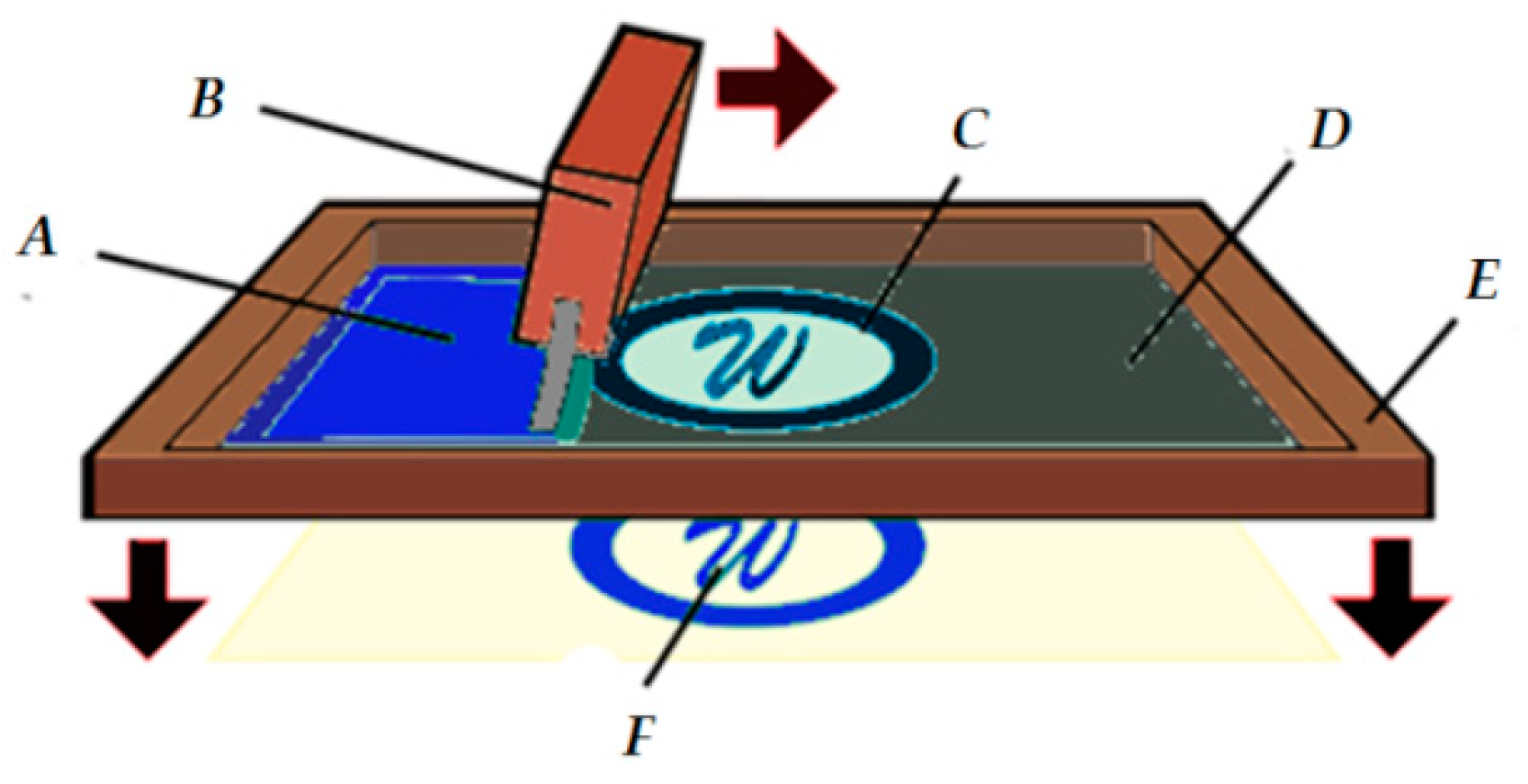
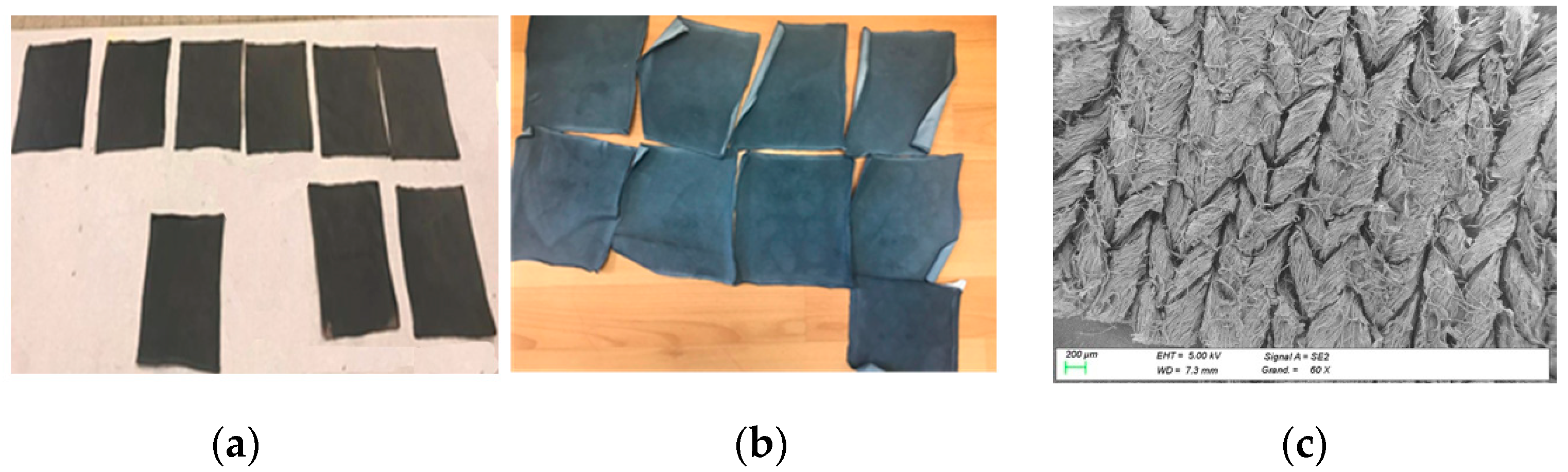
2. Materials and Methods
3. Results and Discussion
3.1. PEDOT:PSS Absorption
3.2. Electrical Characterization
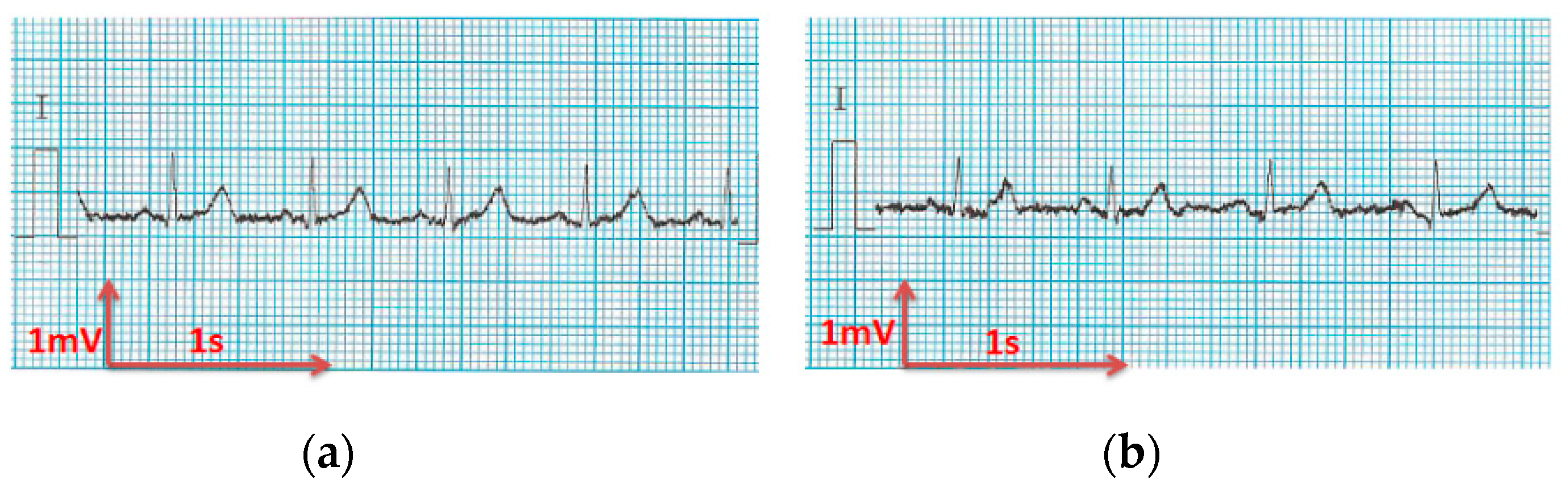
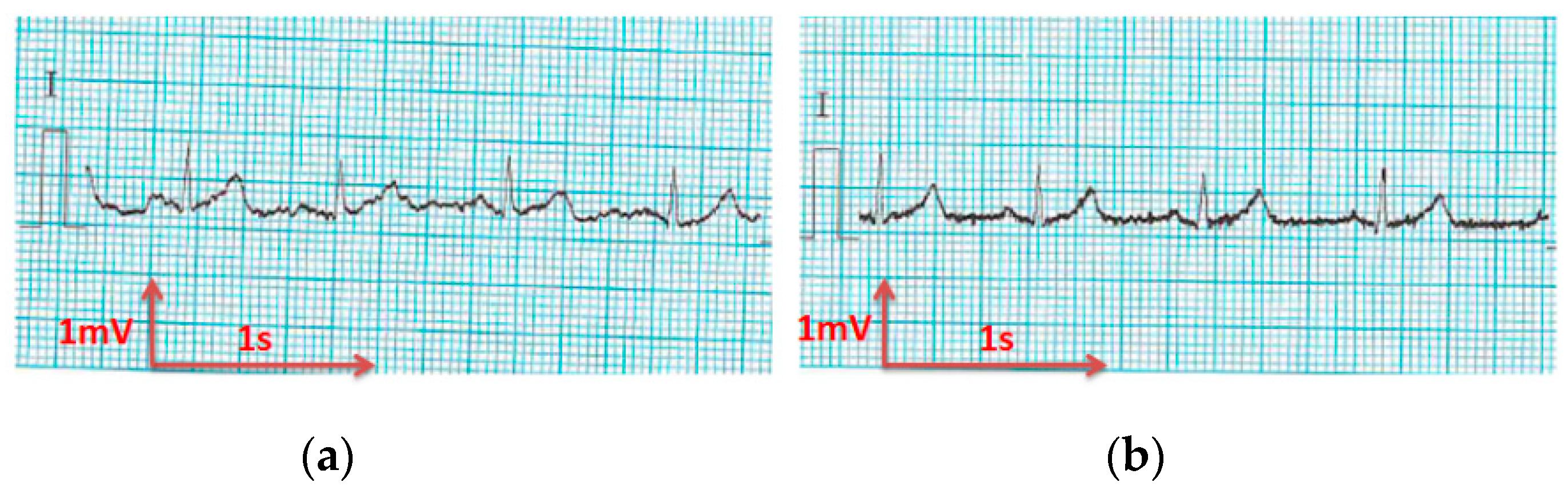
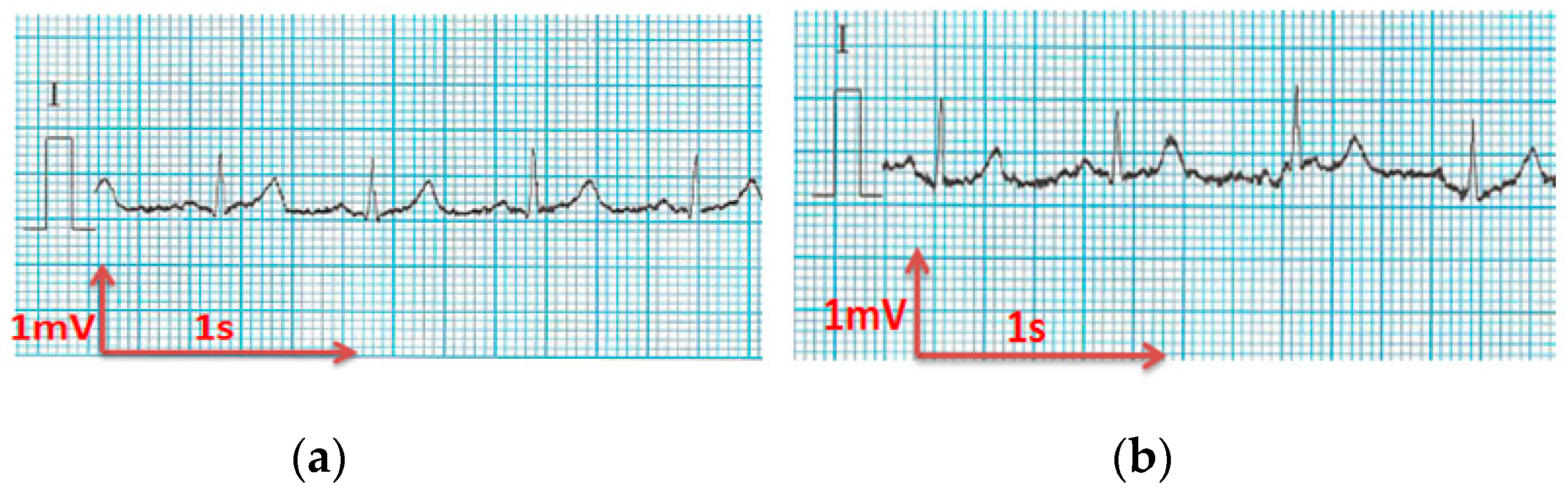
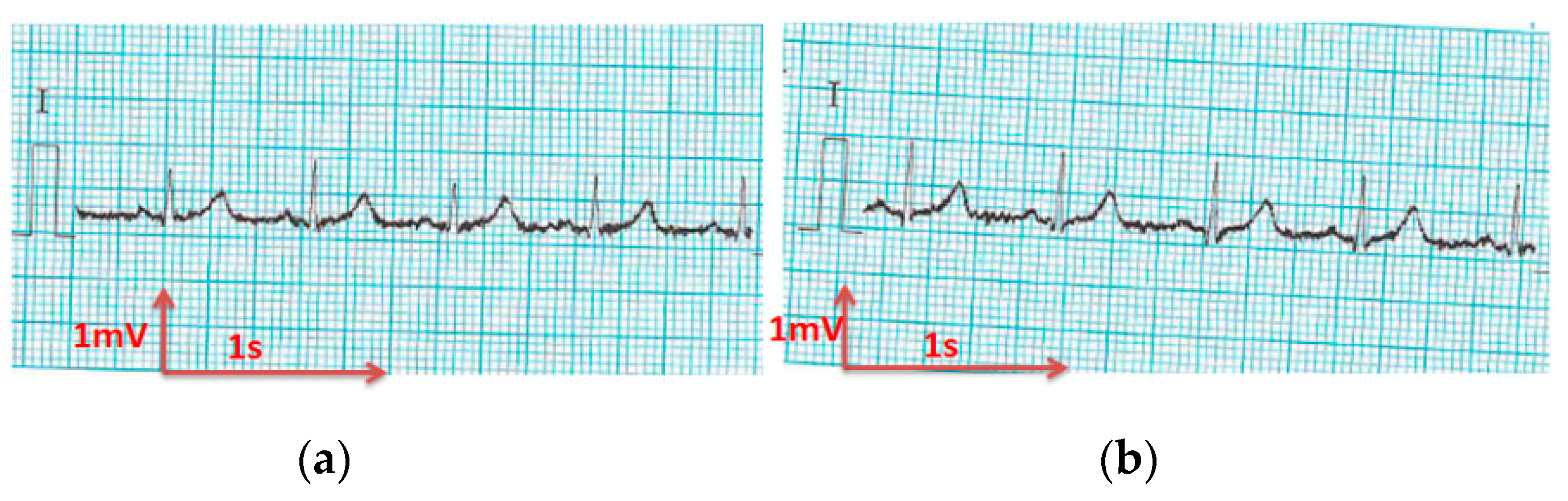
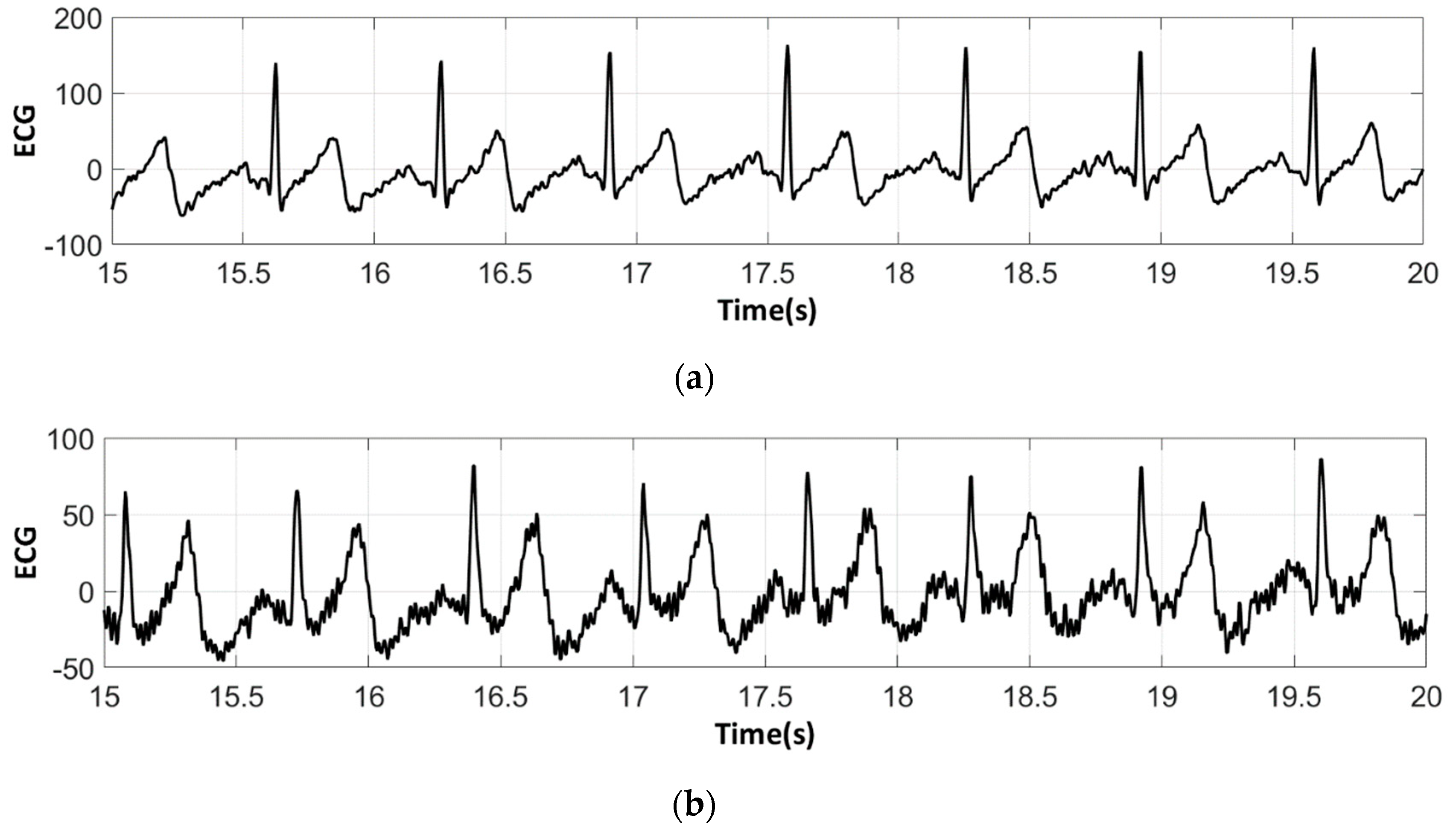
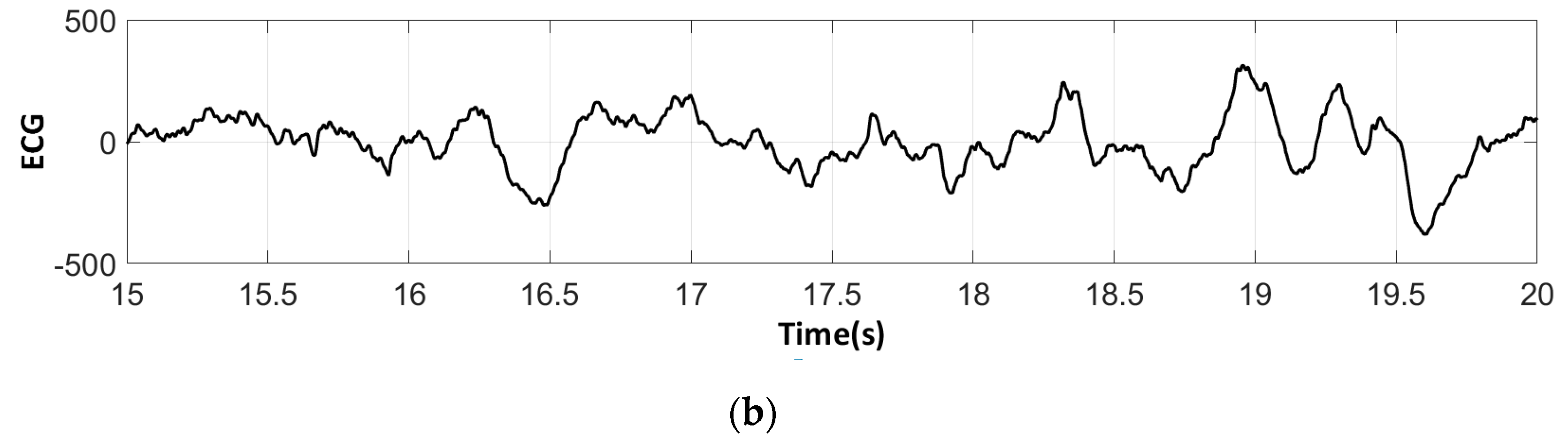
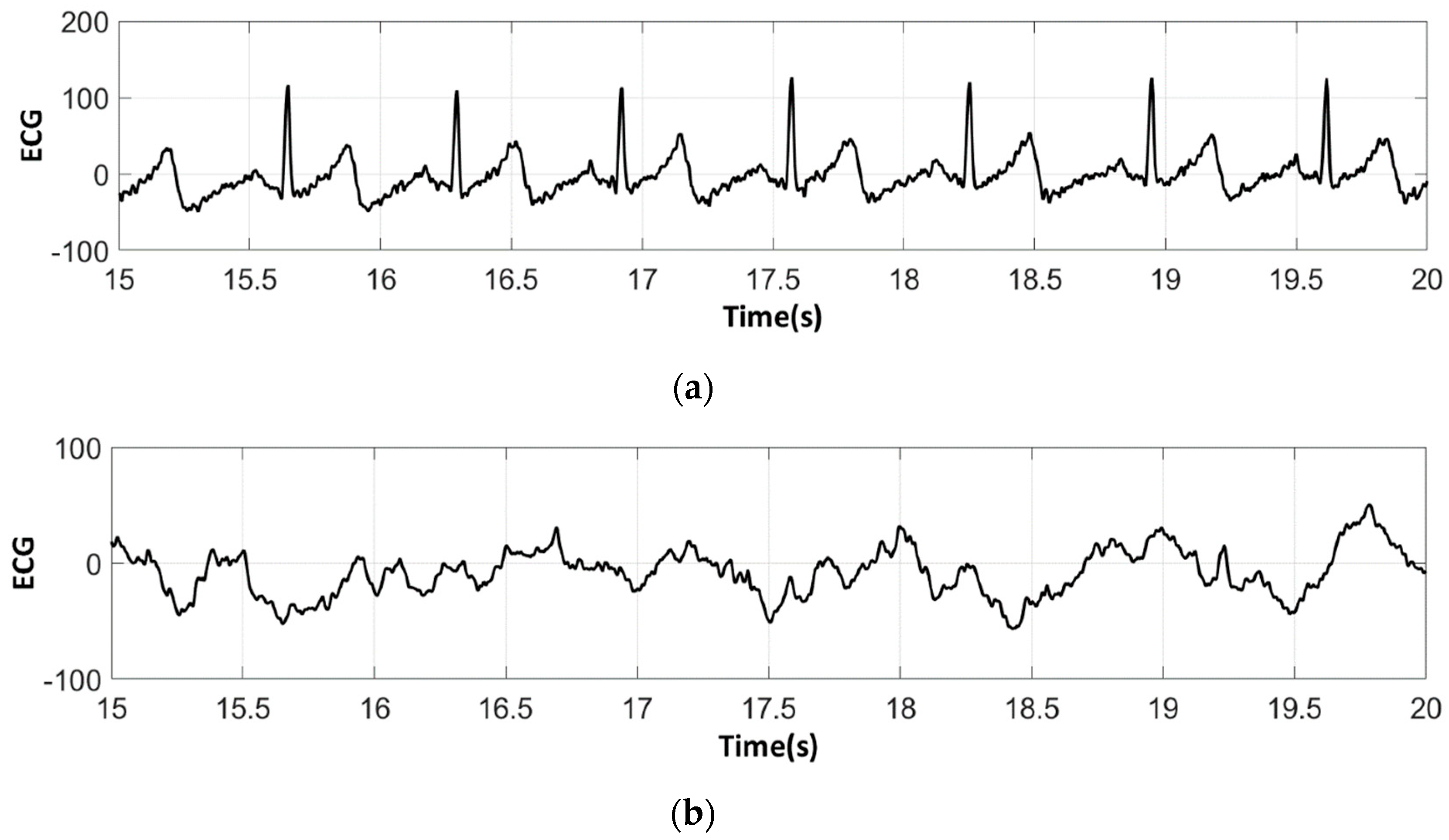
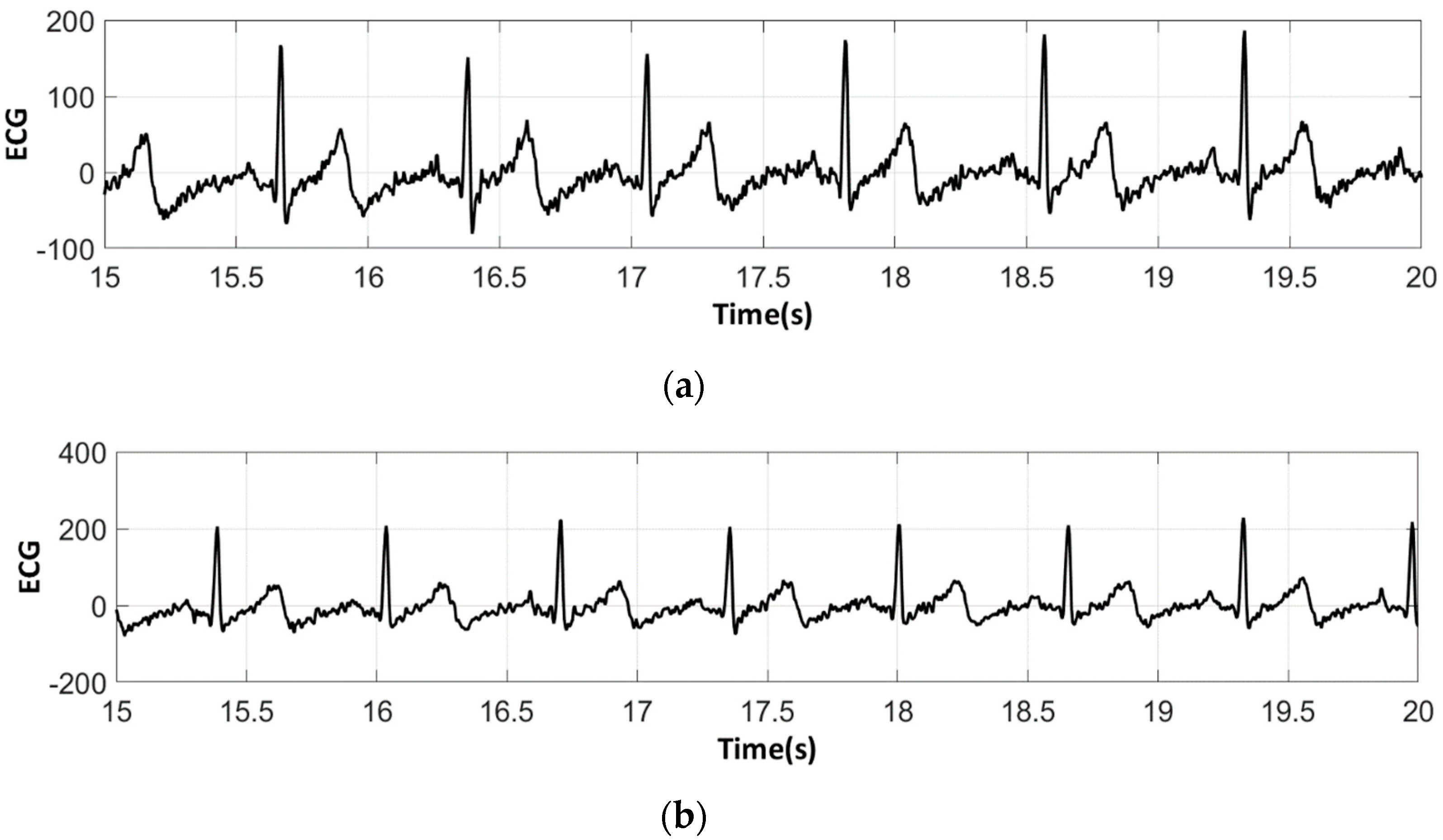
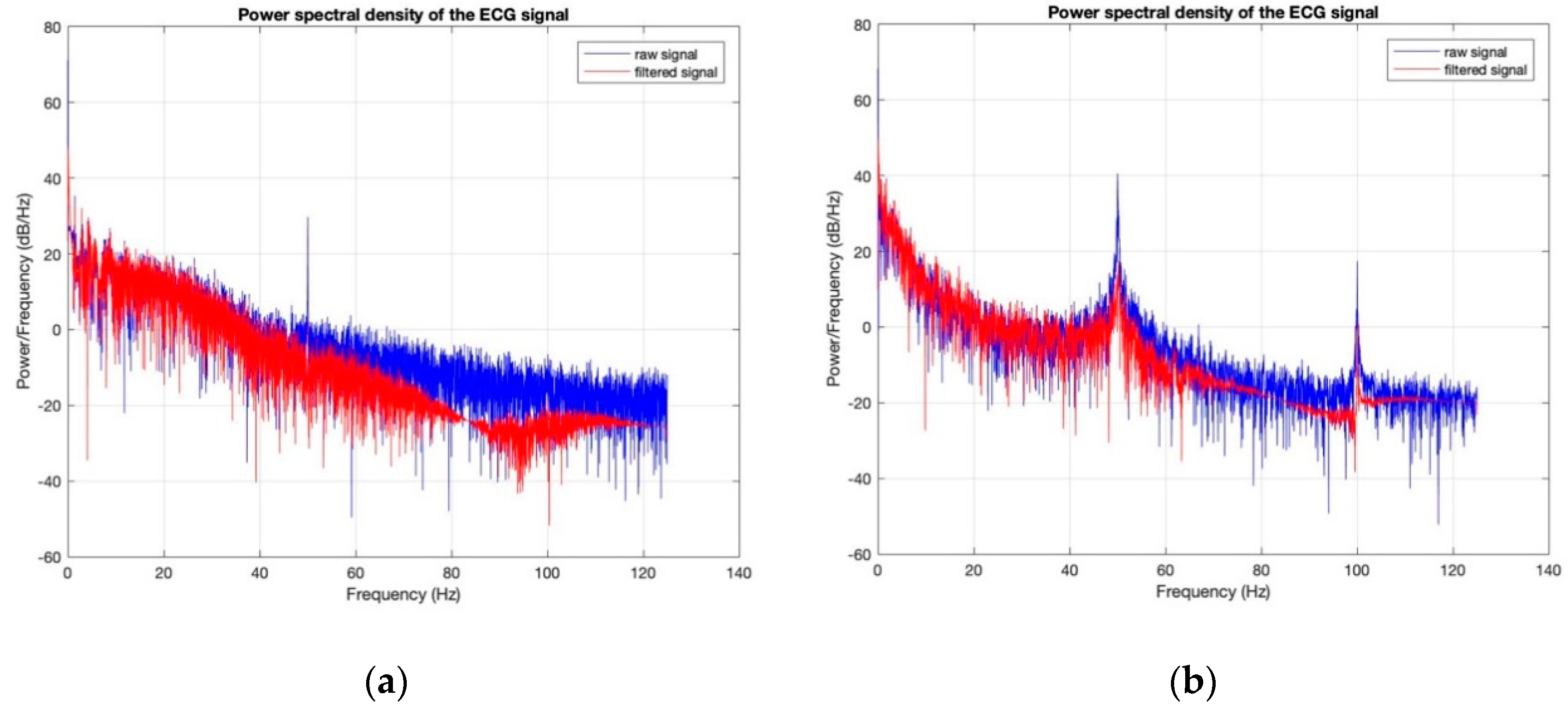
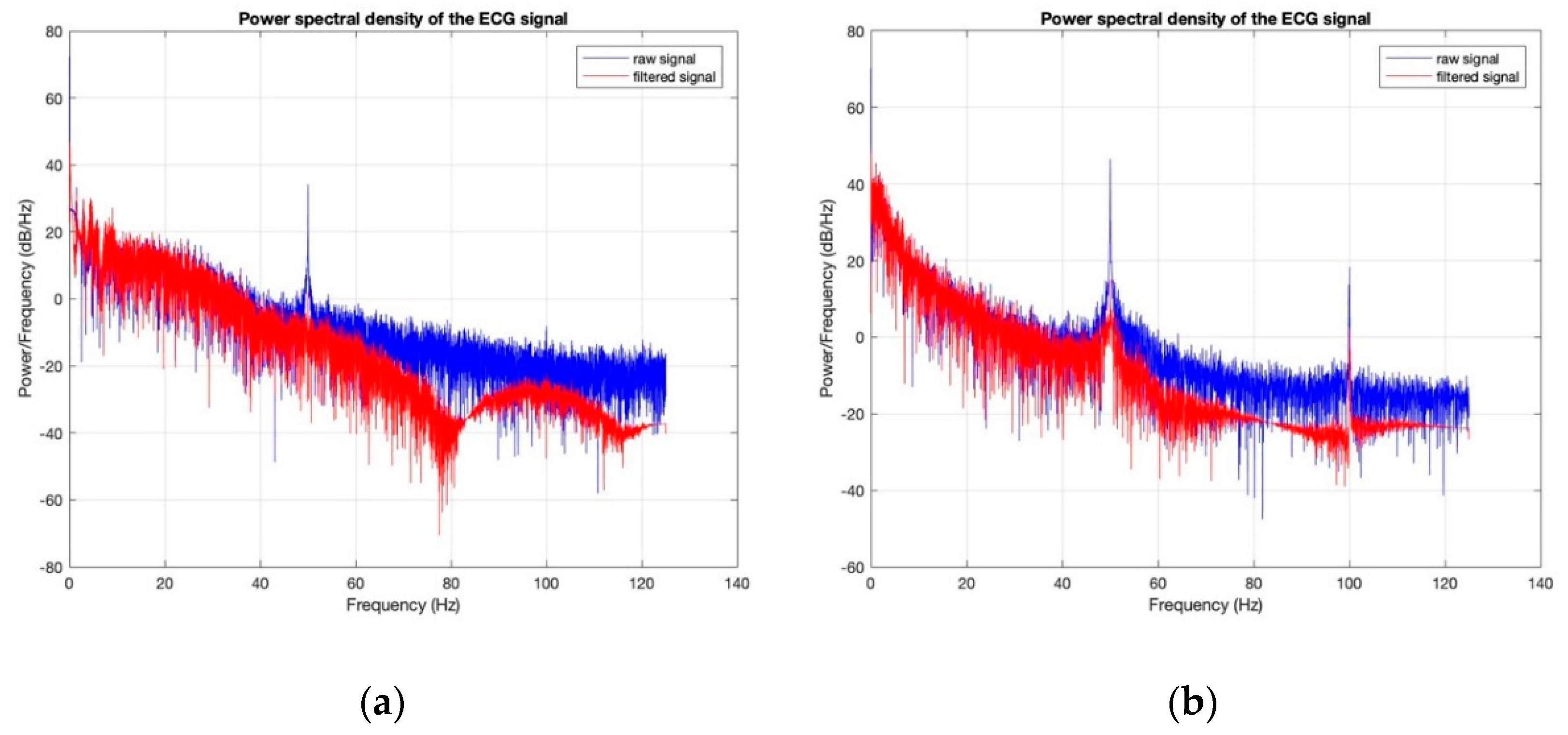
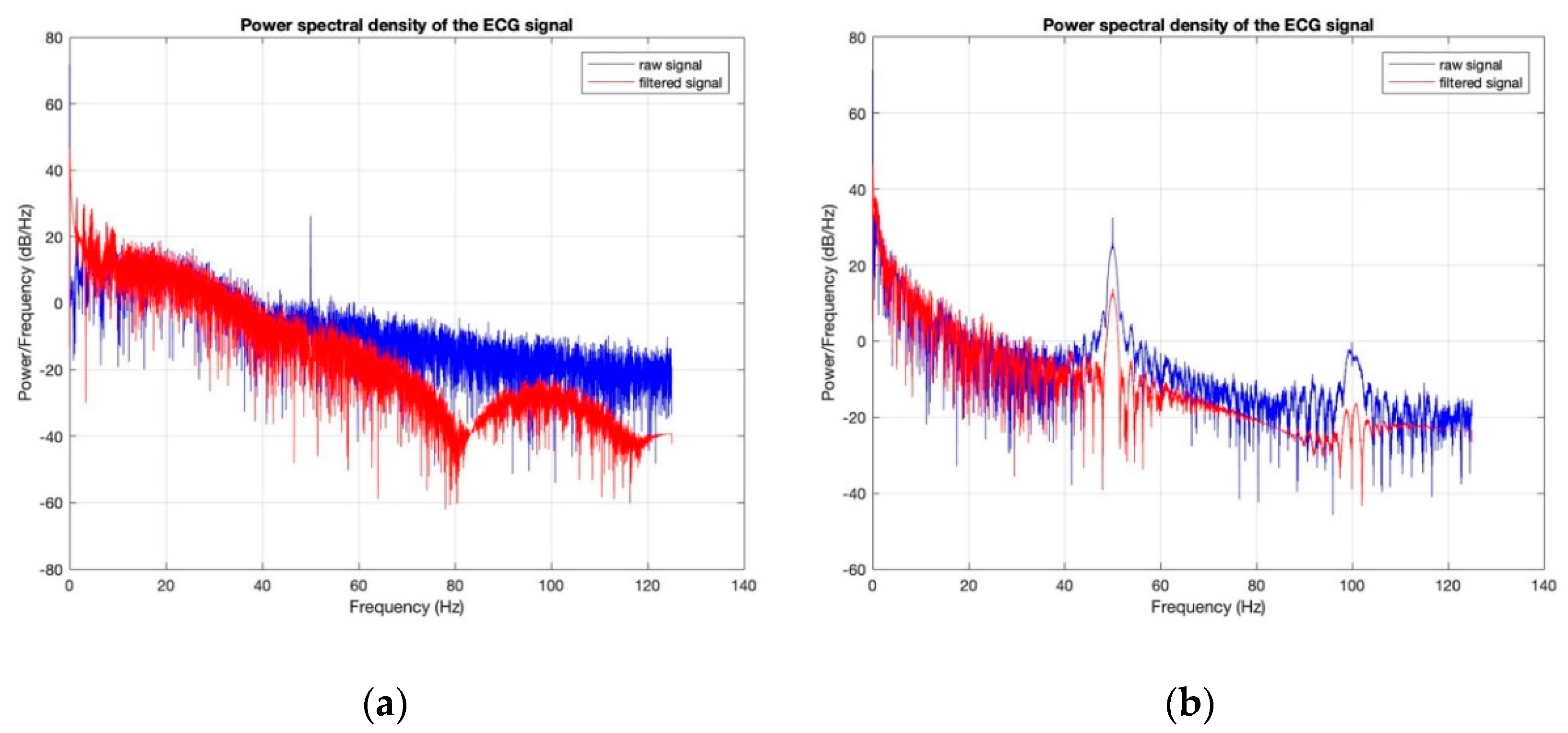
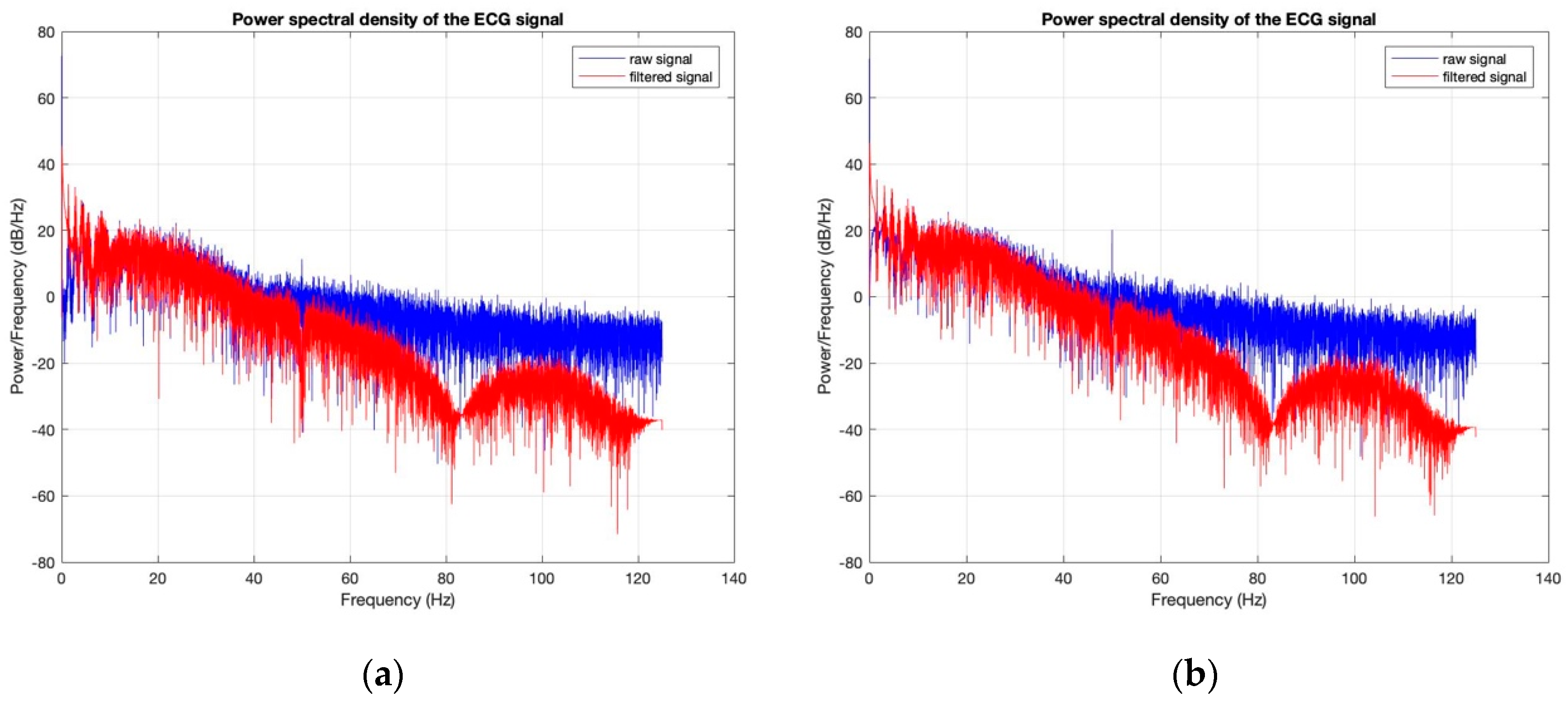
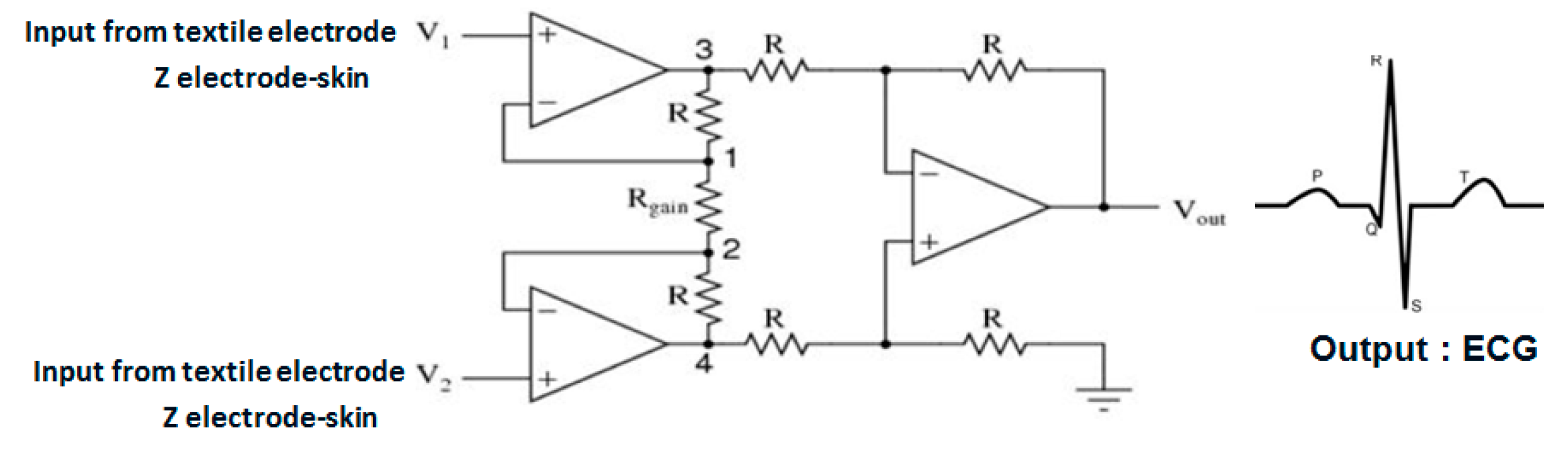
3.3. Electrocardiography Analysis
3.3.1. ECG Recorded by Portable Medical Device
3.3.2. ECG Recorded by Low-Cost Arduino Based Open Source
4. Conclusions
- The thickness of the fabric plays a dominant role in increasing the surface resistivity while using small amount of PEDOT:PSS. The thinner the fabric, the better the distribution of PEDOT:PSS coating will be.
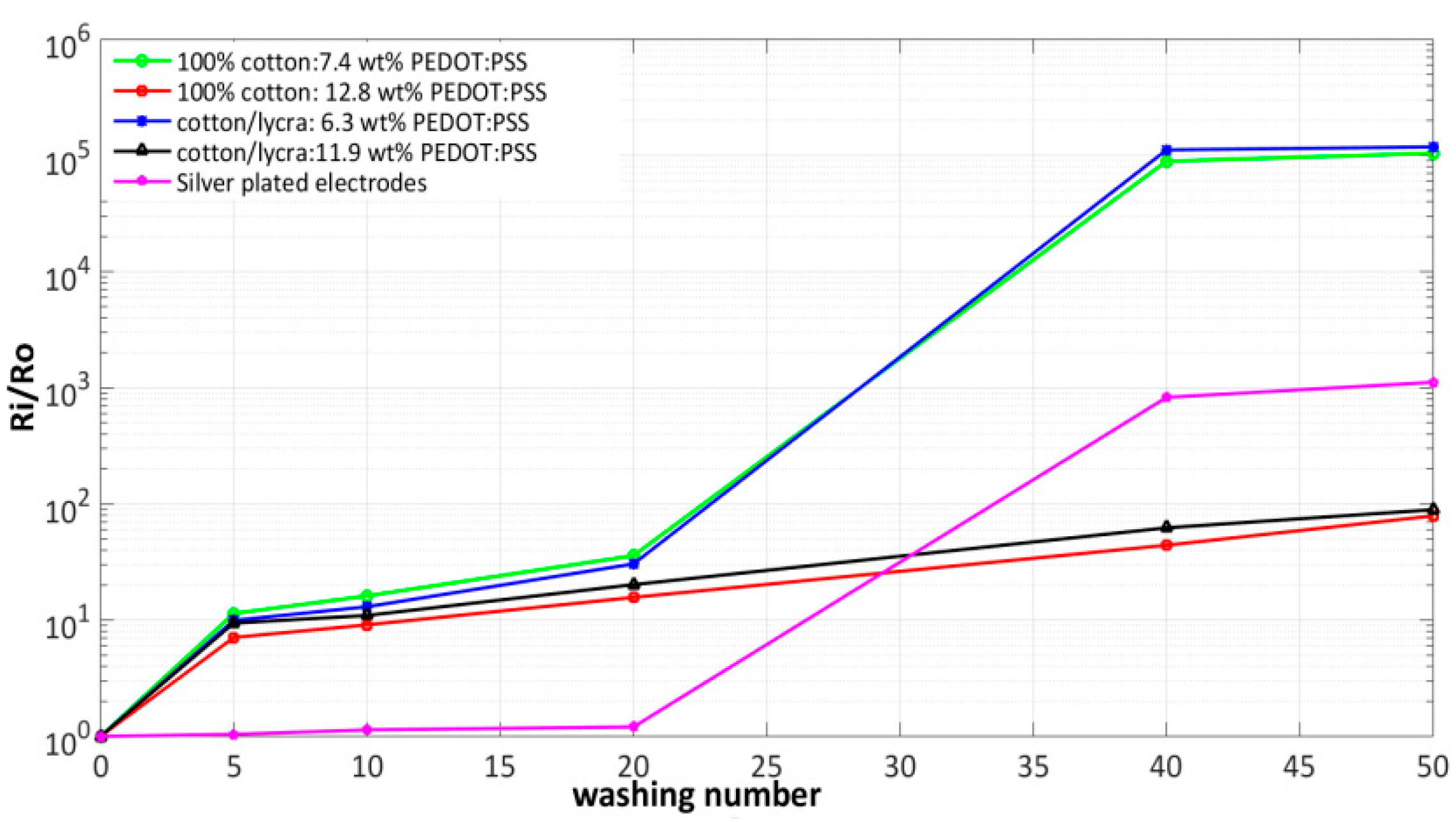
- In this work, silver-plated electrodes were also used to compare their performance with our developed PEDOT:PSS textile electrodes. The conductivity of cotton textile electrodes was enhanced by increasing the amount of PEDOT:PSS coating, which decrease the surface resistivity until obtaining closed value to that of silver electrodes. Subsequently, the effects of repetitive washing process on electrical properties, morphology and ECG quality of textile electrodes were also investigated. After 50 washing cycles, the ratio Ri/Ro increased by 5 orders magnitude for 6.3 wt.% PEDOT:PSS pure cotton electrode and 7.4 wt.% PEDOT:PSS cotton/Lycra electrode, 3 orders magnitude for silver-plated electrodes and by 1 order magnitude for electrodes with high amounts of PEDOT:PSS (11.9 wt.% on cotton/Lycra electrodes and 12.8 wt.% on pure cotton). Results reveal that adding 5.6 wt.% PEDOT:PSS to cotton/Lycra and 5.4 wt.% PEDOT:PSS to pure cotton decreases the resistance ratio Ri/Ro by 4 orders magnitude after 50 washing cycles. Consequently, pure cotton electrodes with 12.8 wt.% PEDOT:PSS are the best choice from the viewpoint of the electrical resistance.
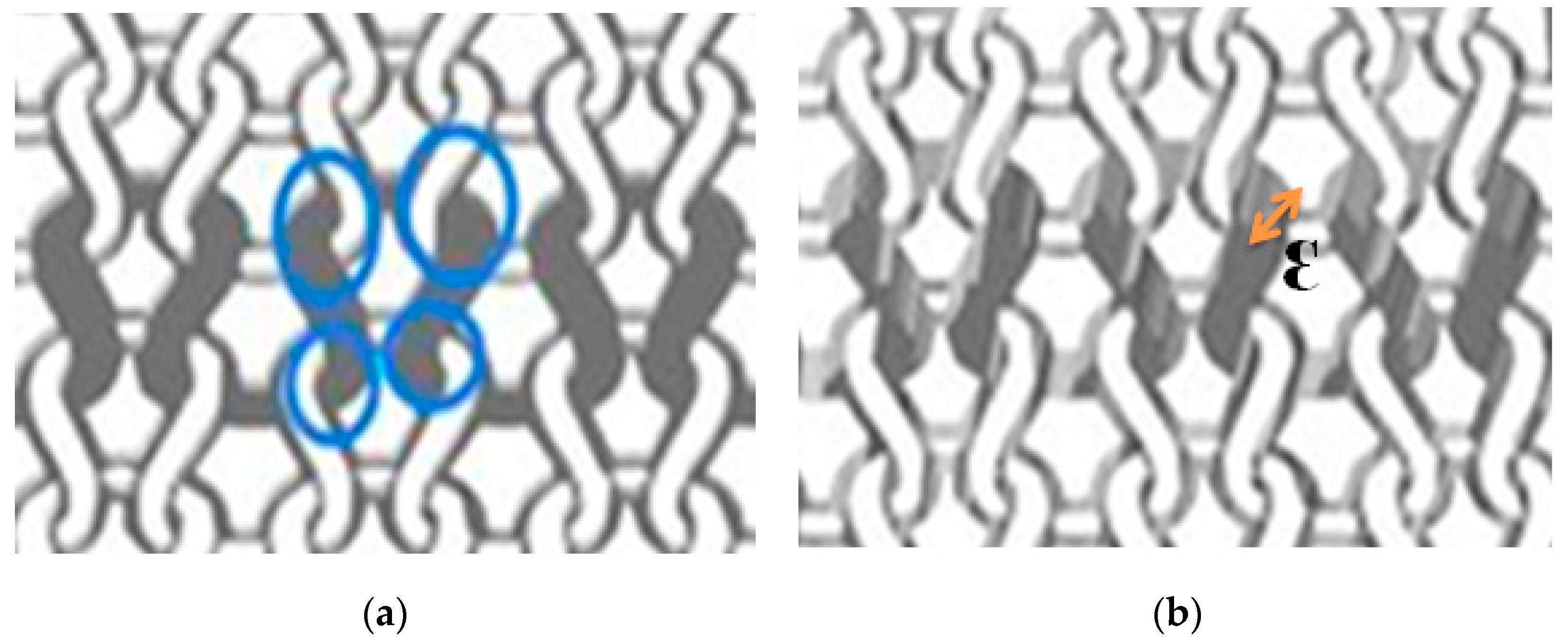
- Mechanical stresses during the washing process affect the surface morphology of textile electrodes. They lead to the entanglements of loose fibers that form roughly spherical accumulation of fibers called “pills”, that are distinct from the electrode surface.
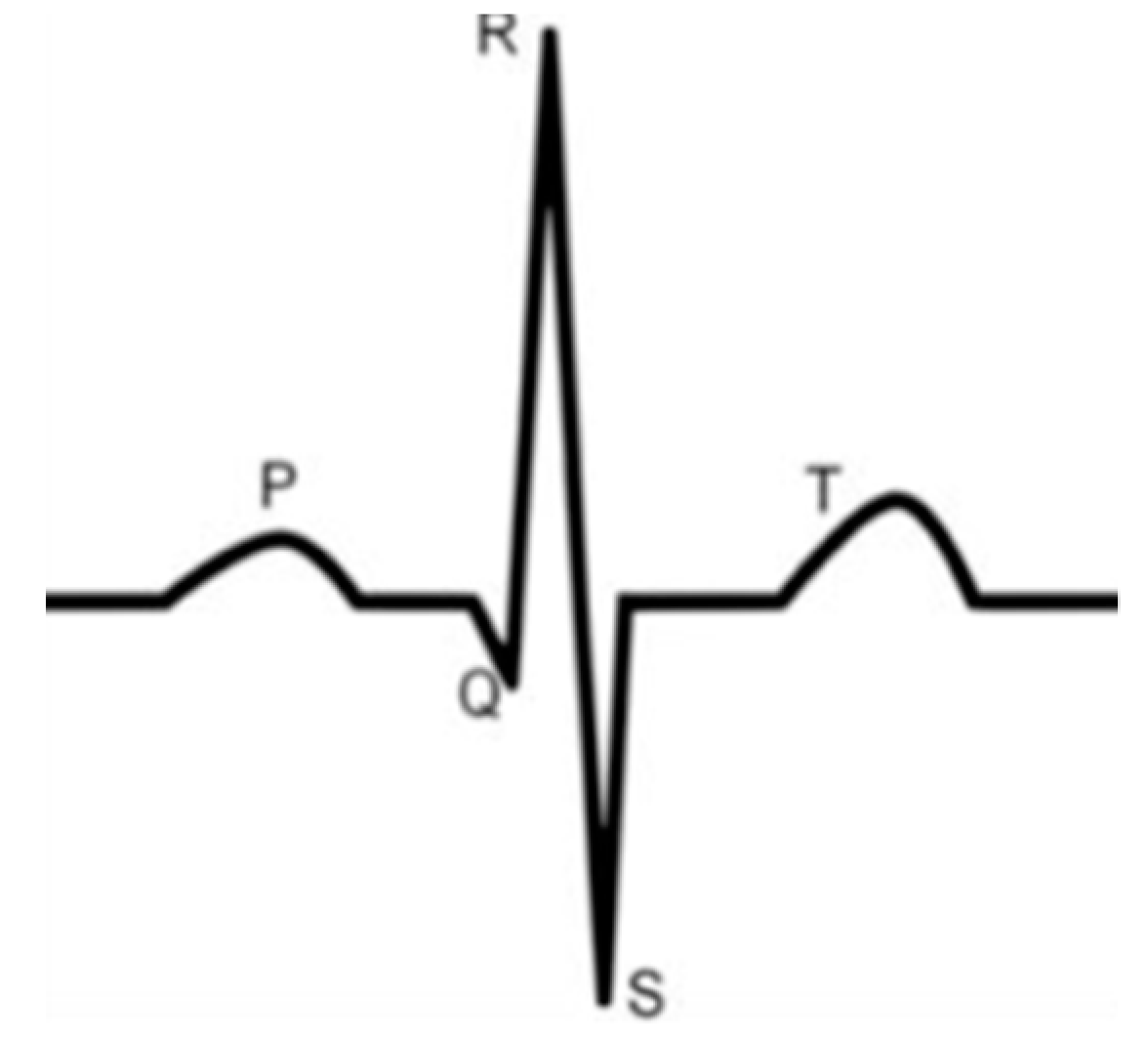
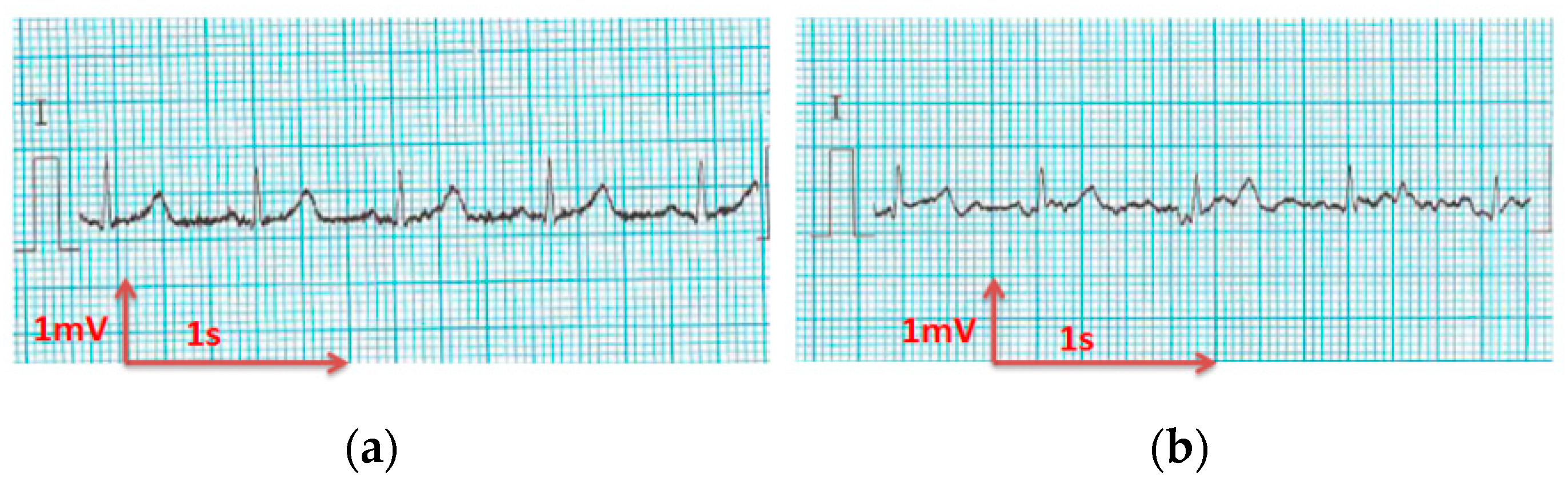
- For all textile electrodes, ECG signals were recorded by a portable medical device as well as with a low-cost, Arduino-based open source device. Using portable medical devices, all electrocardiographic P, T waves, and QRS complexes were identifiable, despite higher contact impedance of PEDOT:PSS textile electrodes. Signal quality analysis by cardiologist showed that these textile electrodes, used in ambulatory conditions for heart monitoring, allow the detection of rhythmic disorders (Atrial Fibrillation, ventricular tachycardia, etc.) and conduction troubles (sinus dysfunction, atrioventricular block etc.), and can detect myocardial ischemia (ST segment underlining) in some cases. Such a system, integrated into garments, can be used in real-time and in continuous mode to monitor the user’s heart in a comfortable way, and to detect possible issues that, with additional analysis if needed, could be avoided, such as strokes. Regarding low-cost devices, assuming that high contact impedance between the skin and textile electrodes is the cause of no detectability or noise and severe distortion of cardiac waveforms, it was concluded that the amplifiers of the low-cost device are not appropriate to record ECG of our developed PEDOT:PSS textile electrodes, and also, that of commercial silver-plated electrodes. Textile electrodes inherently provide high contact skin-electrode impedance because the ECG recording is based only on natural skin moisture and perspiration and without any added gel. Therefore, compatible recording amplifiers with high input impedance are required to compensate for the high contact impedance skin-electrode, which is related to the low mobility of ions across the highly-resistant stratum corneum layer of the skin that causes weak conductivity between the electrodes and the skin.
- The authors consider that the pure cotton electrodes could be embedded into garments for long-term ECG monitoring.
Author Contributions
Funding
Conflicts of Interest
References
- Walraven, G. Basic Arrhythmias; Pearson: London, UK, 2011; pp. 1–11. [Google Scholar]
- Braunwald, E. Heart Disease: A Textbook of Cardiovascular Medicine, 5th ed.; W.B. Saunders Company: Philadelphia, PA, USA; p. 108.
- Lopez-Gordo, M.; Sanchez-Morillo, D.; Valle, F. Dry EEG Electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef] [PubMed]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef] [PubMed]
- Kazani, I.; Hertleer, C.; De Mey, G.; Schwarz, A.; Guxho, G.; Van Langenhove, L. Electrical conductive textiles obtained by screen printing. Fibres Text. East. Eur. 2012, 20, 57–63. [Google Scholar]
- Schwarz, A.; Hakuzimana, J.; Kaczynska, A.; Banaszczyk, J.; Westbroek, P.; McAdams, E.; Moody, G.; Chronis, Y.; Priniotakis, G.; De Mey, G.; Tseles, D.; Van Langenhove, L. Gold coated para-aramid yarns through electroless deposition. Surf. Coat. Technol. 2010, 204, 1412–1418. [Google Scholar] [CrossRef]
- Schwarz, A.; Hakuzimana, J.; Gasana, E.; Westbroek, P.; Van Langenhove, L. Gold Coated Polyester Yarn. Adv. Sci. Technol. 2008, 60, 47–51. [Google Scholar] [CrossRef]
- Banaszczyk, J.; De Mey, G.; Schwarz, A.; Van Langenhove, L. Current distribution modelling in electroconductive fabrics. Fibres Text. East. Eur. 2009, 17, 28–33. [Google Scholar]
- Wallace, G.G. Campbell TE and Innis PC. Putting function into fashion: Organic conducting polymer fibres and textiles. Fibers Polym. 2007, 8, 135–142. [Google Scholar] [CrossRef]
- Shu, L.; Hua, T.; Wang, Y.; Li, Q.; Feng, D.D.; Tao, X. In-shoe plantar pressure measurement and analysis system based on fabric pressure sensing array. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 767–775. [Google Scholar] [PubMed]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Dalle Mura, G.; Lorussi, F.; Tognetti, A.; Anania, G.; Carbonaro, N.; Pacelli, M.; Paradiso, R.; de Rossi, D. Piezoresistive goniometer network for sensing gloves. In Proceedings of the XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013, Seville, Spain, 25–28 September 2013; Springer: Cham, Switzerland, 2014; pp. 1547–1550. [Google Scholar]
- Shorter, K.A.; Kogler, G.F.; Loth, E.; Durfee, W.K.; Hsiao-Wecksler, E.T. A portable powered ankle-foot orthosis for rehabilitation. J. Rehabil. Res. Dev. 2011, 48, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Mattana, G. Realisation and Characterisation of Organic Electronic Devices for E–Textiles Applications. Ph.D. Thesis, University of Cagliari, Cagliari, Italy, March 2011. [Google Scholar]
- Tamburri, E.; Orlanducci, S.; Toschi, F.; Terranova, M.L.; Passeri, D. Growth mechanisms, morphology, and electroactivity of PEDOT layers produced by electrochemical routes in aqueous medium. Synth. Met. 2009, 159, 406–414. [Google Scholar] [CrossRef]
- Kaynak, A.; Wang, L.; Hurren, C.; Wang, X. Characterization of conductive polypyrrole coated woolyarns. Fibers Polym. 2002, 3, 24. [Google Scholar] [CrossRef]
- Lin, J.; Lin, Z.; Pan, Y.; Hsieh, C.; Lee, M.; Lou, C. Manufacturing techniques and property evaluations of conductive composite yarns coated with polypropylene and multi-walled carbon nanotubes. Compos. Part A 2016, 84, 354. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Skrifvars, M.; Persson, N. Synthesis of high performance, conductive PEDOT-coated polyester yarns by OCVD technique. Polym. Adv. Technol. 2012, 23, 611–617. [Google Scholar] [CrossRef]
- Merilampi, S.; Laine-Ma, T.; Ruuskanen, P. The characterization of electrically conductive silver ink patterns of flexible substrates. Microelectron. Reliab. 2009, 49, 782. [Google Scholar] [CrossRef]
- Pudas, M.; Halonen, N.; Granat, P.; Vähäkangas, J. Gravure printing of conductive prticulate polymer inks on flexible substrates. Prog. Org. Coat. 2005, 54, 310. [Google Scholar] [CrossRef]
- Yang, K.; Torah, R.; Wei, Y.; Beeby, S. Tudor, J. Waterproof and durable screen printed silver conductive tracks on textiles. Text. Res. J. 2013, 83, 2023. [Google Scholar] [CrossRef]
- Kwak, M.K.; Shin, K.H.; Yoon, E.Y.; Suh, K.Y. Fabrication of conductive metal lines by plate-to-roll pattern transfer utilizing edge dewetting and flexographic printing. J. Colloid Interface Sci. 2010, 343, 301. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, I.M.; Martin, G.D. Inkjet Technology for Digital Fabrication; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 1–20. [Google Scholar]
- Moeinzadeh, S.; Jabbari, E. Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 285–297. [Google Scholar]
- Blayo, A.; Pineaux, B. Printing processes and their potential for RFID printing. In Proceedings of the Joint sOc-UESAI Conference, Grenoble, France, 7–10 October 2005; pp. 27–30. [Google Scholar]
- Fong, E.M.; Chung, W.Y. A Hygroscopic Sensor Electrode for Fast Stabilized Non-Contact ECG Signal Acquisition. Sensors 2015, 15, 19237–19250. [Google Scholar] [CrossRef] [PubMed]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.F.; Abächerli, R.; Meyer, V.R.; Rossi, R.M. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 5, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Yapici, M.K.; Alkhidir, T.E. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors 2017, 17, 875. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Ankhili, A.; Tao, X.; Cochrane, C.; Coulon, D.; Koncar, V. Washable and reliable textile electrodes embedded into underwear fabric for electrocardiography (ECG) monitoring. Materials 2018, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- ISO 5084:1996—Textiles—Determination of Thickness of Textiles and Textile Products. Available online: https://www.iso.org/fr/standard/23348.html (accessed on 2 April 2018).
- Standard D 257-99. Standard Test Methods for D-C Resistance or Conductance of Insulating Materials; ASTM International: West Conshohocken, PA, USA, 1999.
- IEC. 61340-5-1 Standard. Electrostatics—Part 5-1: Protection of Electronic Devices from Electrostatic Phenomena-General Requirements; The International Electrotechnical Commission: Geneva, Switzerland, 1998. [Google Scholar]
- Li, Y.; Dai, X.Q. Biomechanical Engineering of Textiles and Clothing; Woodhead Publishing: Sawston/Cambridge, UK, 2006; p. 66. [Google Scholar]
- Spencer, D.J. Knitting Technology: A Comprehensive Handbook and Practical Guide, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]

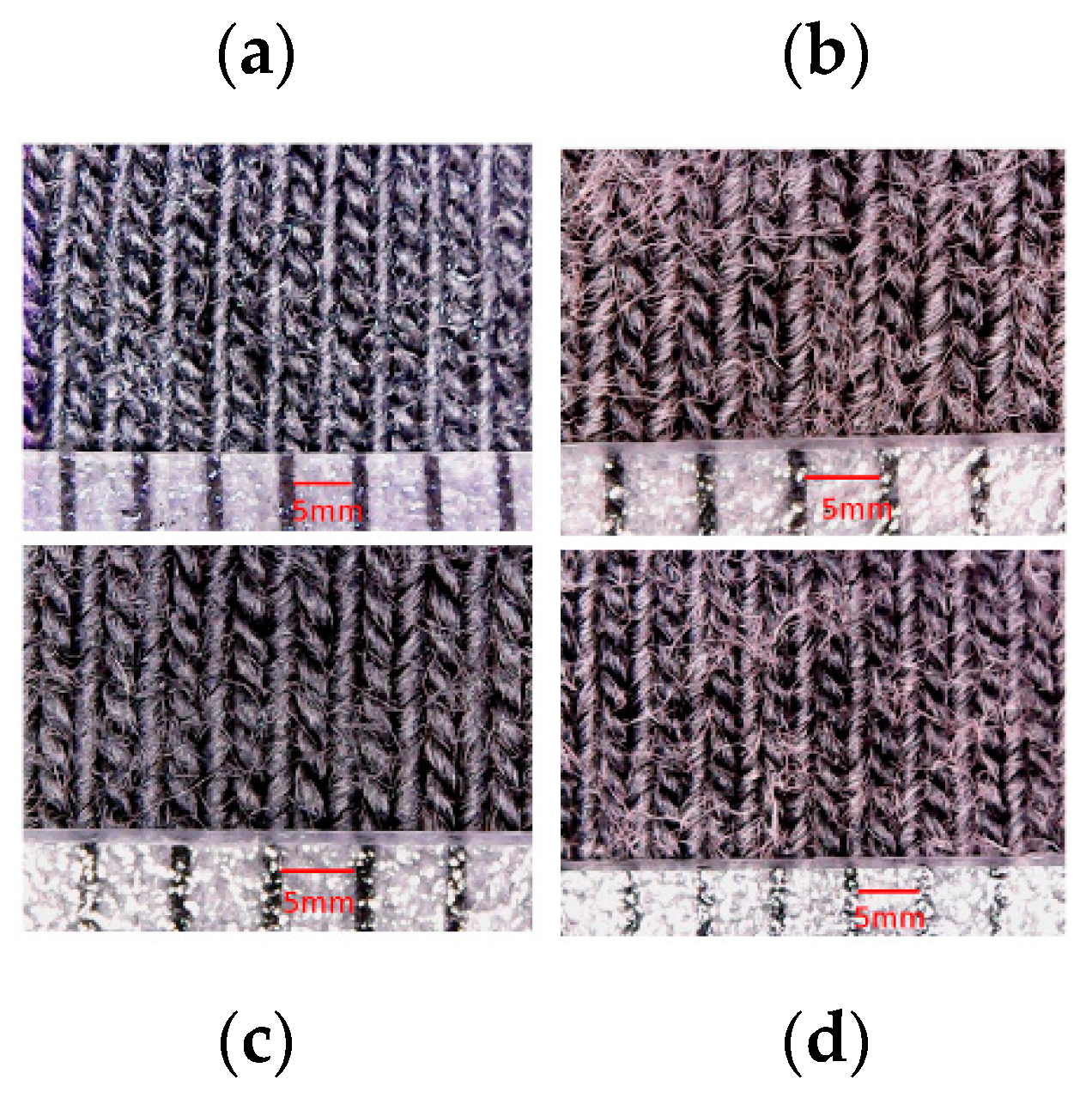

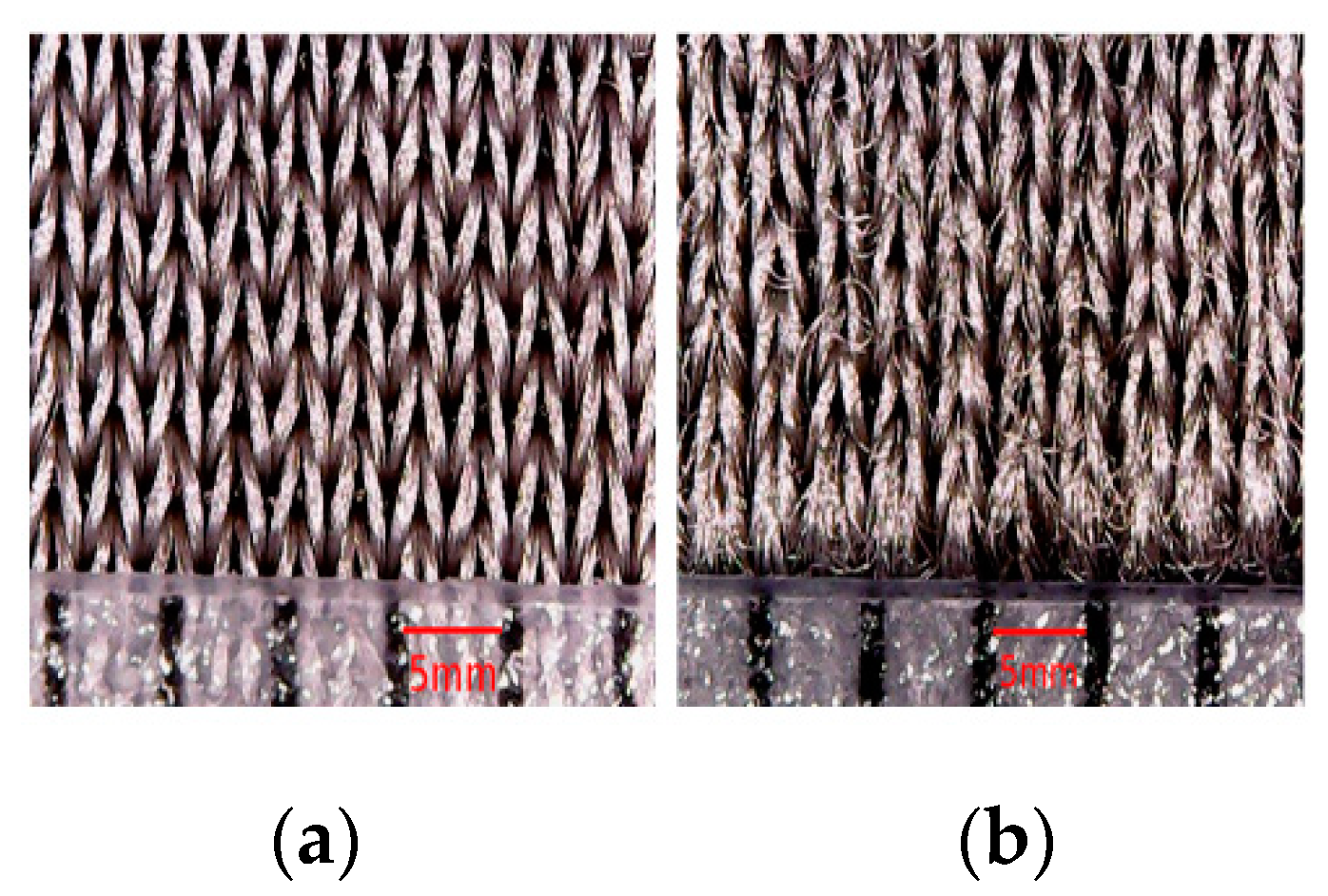



| Thickness of Textile Substrate before Coating (µm) | wt.% of PEDOT:PSS Absorbed after Drying | |||
|---|---|---|---|---|
| Textile electrodes | Avg | St Dev | Avg | ST Dev |
| 100% cotton | 831 | 15.6 | 7.4 | 0.1 |
| 95% Cotton 5% Lycra | 725 | 4.9 | 6.3 | 0.2 |
| Textiles Electrodes | Surface Resistivity before Washing (kΩ) | Standard Deviation (kΩ) |
|---|---|---|
| 100% cotton | 117.3 | 0.217 |
| 95% Cotton 5% Lycra | 90.5 | 0.275 |
| Silver-plated electrode | 21.4 | 0.035 |
| Surface Resistivity (kΩ) | Standard Deviation (kΩ) | |
|---|---|---|
| 100% cotton: 7.4 wt.% PEDOT:PSS | 117.30 | 0.21 |
| 100% cotton: 12.8 wt.% PEDOT:PSS | 22.70 | 0.04 |
| Cotton/Lycra: 6.3 wt.% PEDOT:PSS | 90.50 | 0.27 |
| Cotton/Lycra: 11.9 wt.% PEDOT:PSS | 38.30 | 0.04 |
| Silver-plated electrodes | 21.40 | 0.03 |
| SNR (dB) before Washing | SNR (dB) after Washing | |
|---|---|---|
| 100% cotton: 7.4 wt.% PEDOT:PSS | 24.6311 | 11.8333 |
| 100% cotton: 12.8 wt.% PEDOT:PSS | 33.0505 | 7.6069 |
| Cotton/Lycra: 6.3 wt.% PEDOT:PSS | 17.8022 | 11.5040 |
| Cotton/Lycra: 11.9 wt.% PEDOT:PSS | 27.7690 | 15.6060 |
| Silver-plated electrodes | 34.7203 | 33.1449 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankhili, A.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D.; Tarlet, J.-M. Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics. Sensors 2018, 18, 3890. https://doi.org/10.3390/s18113890
Ankhili A, Tao X, Cochrane C, Koncar V, Coulon D, Tarlet J-M. Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics. Sensors. 2018; 18(11):3890. https://doi.org/10.3390/s18113890
Chicago/Turabian StyleAnkhili, Amale, Xuyuan Tao, Cédric Cochrane, Vladan Koncar, David Coulon, and Jean-Michel Tarlet. 2018. "Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics" Sensors 18, no. 11: 3890. https://doi.org/10.3390/s18113890
APA StyleAnkhili, A., Tao, X., Cochrane, C., Koncar, V., Coulon, D., & Tarlet, J.-M. (2018). Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics. Sensors, 18(11), 3890. https://doi.org/10.3390/s18113890








