Aerosol Jet Printed 3D Electrochemical Sensors for Protein Detection
Abstract
1. Introduction
2. Materials and Methods
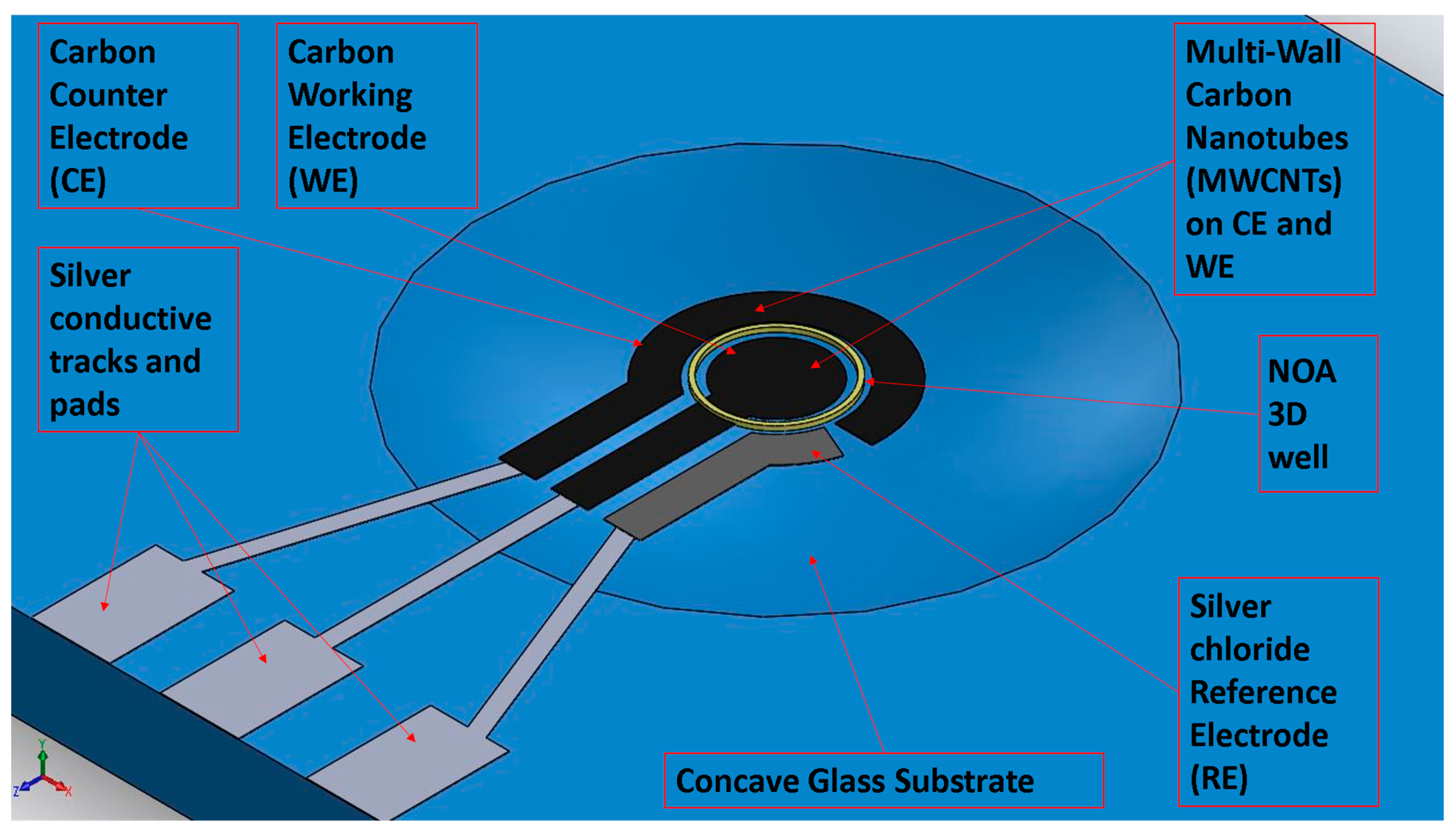
2.1. Sensors Design and Material Choice
2.2. Sensor Fabrication
2.3. Geometrical Analysis and Electrical Resistances
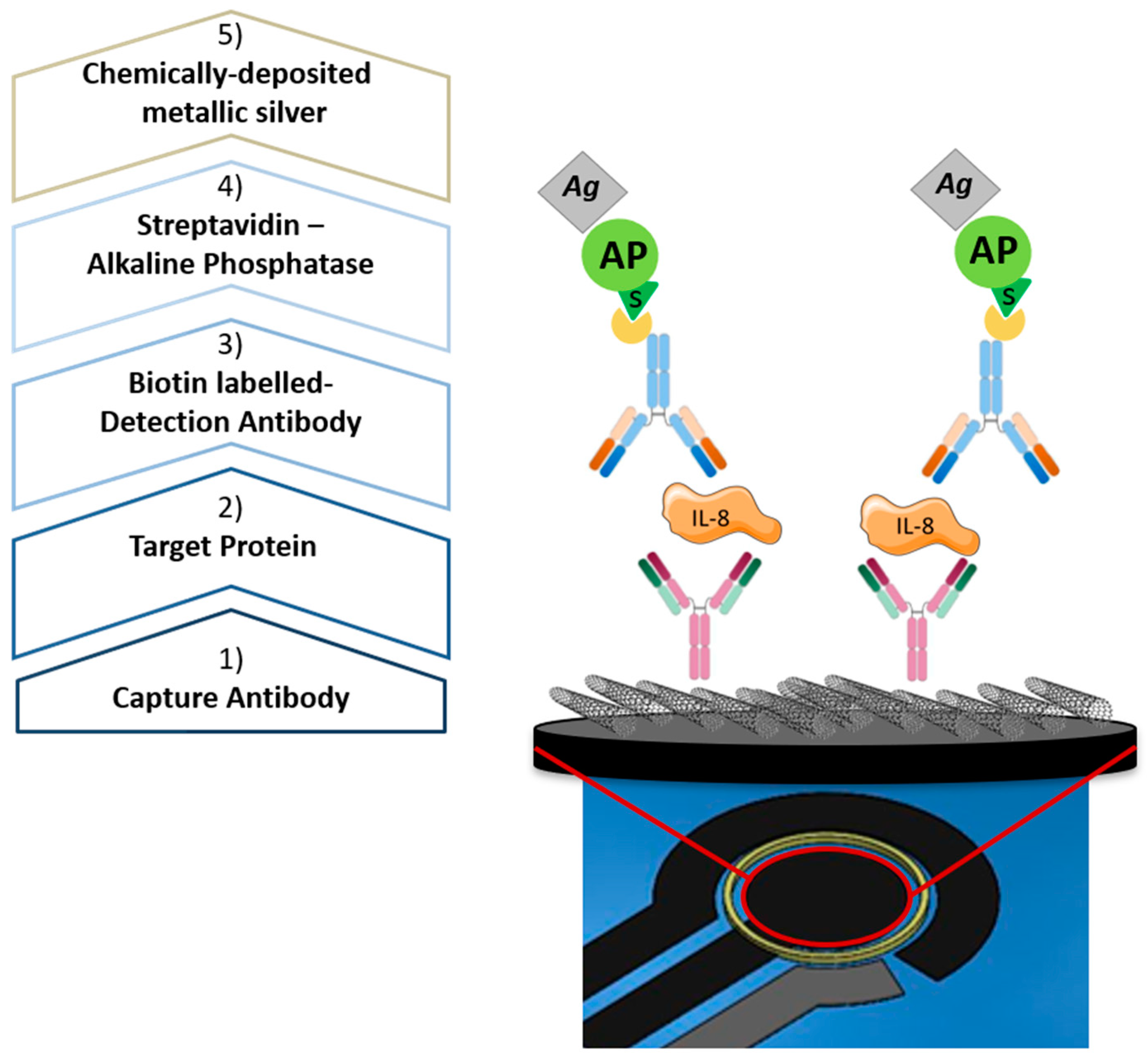
2.4. Voltammetry-Based Protein Quantification
3. Results and Discussion
3.1. Sensors Testing
3.2. Voltammetry-Based Protein Quantification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spanu, A.; Lai, S.; Cosseddu, P.; Tedesco, M.; Martinoia, S.; Bonfiglio, A. An organic transistor-based system for reference-less electrophysiological monitoring of excitable cells. Sci. Rep. 2015, 5, 8807. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rossignol, F.; Macdonald, J. Inkjet printing for biosensor fabrication: Combining chemistry and technology for advanced manufacturing. Lab Chip 2015, 15, 2538–2558. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lorenzelli, L.; Dahiya, R.S. Technologies for printing sensors and electronics over large flexible substrates: A review. IEEE Sens. J. 2015, 15, 3164–3185. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Townsend-Nicholson, A. Bio-electrosprays: The next generation of electrified jets. Biotechnol. J. 2006, 1, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, G.E.; Radspinner, R.; Peyghambarian, N. Screen printing for the fabrication of organic light-emitting devices. IEEE J. Sel. Top. Quantum Electron. 2001, 7, 769–773. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing—Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Lee, C.K.; Oh, J.H. All-inkjet-printed electrical components and circuit fabrication on a plastic substrate. Microelectron. Eng. 2012, 97, 251–254. [Google Scholar] [CrossRef]
- Ragones, H.; Schreiber, D.; Inberg, A.; Berkh, O.; Kósa, G.; Freeman, A.; Shacham-Diamand, Y. Disposable electrochemical sensor prepared using 3D printing for cell and tissue diagnostics. Sens. Actuators B Chem. 2015, 216, 434–442. [Google Scholar] [CrossRef]
- Yang, H.; Rahman, T.; Du, D.; Panat, R.; Lin, Y. 3-D Printed Adjustable Microelectrode Arrays for Electrochemical Sensing and Biosensing. Sens. Actuators. B Chem. 2016, 230, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.B. Principles of aerosol jet printing. Flex. Print. Electron. 2018, 3, 35002. [Google Scholar] [CrossRef]
- Hoey, J.M.; Lutfurakhmanov, A.; Schulz, D.L.; Akhatov, I.S. A review on aerosol-based direct-write and its applications for microelectronics. J. Nanotechnol. 2012, 2012, 324380. [Google Scholar] [CrossRef]
- Binder, S.; Glatthaar, M.; Rädlein, E. Analytical investigation of aerosol jet printing. Aerosol Sci. Technol. 2014, 48, 924–929. [Google Scholar] [CrossRef]
- Clifford, B.; Beynon, D.; Phillips, C.; Deganello, D. Printed-Sensor-on-Chip devices—Aerosol jet deposition of thin film relative humidity sensors onto packaged integrated circuits. Sens. Actuators B Chem. 2018, 255, 1031–1038. [Google Scholar] [CrossRef]
- OPTOMEC. Aerosol Jet ® Printed Electronics Overview; OPTOMEC: Albuquerque, NM, USA, 2018; p. 6. [Google Scholar]
- Seifert, T.; Sowade, E.; Roscher, F.; Wiemer, M.; Gessner, T.; Baumann, R.R. Additive manufacturing technologies compared: Morphology of deposits of silver ink using inkjet and aerosol jet printing. Ind. Eng. Chem. Res. 2015, 54, 769–779. [Google Scholar] [CrossRef]
- Tan, H.W.; Tran, T.; Chua, C.K. A review of printed passive electronic components through fully additive manufacturing methods. Virtual Phys. Prototyp. 2016, 11, 271–288. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Yeong, W.Y. Optimizing aerosol jet printing process of silver ink for printed electronics. IOP Conf. Ser. Mater. Sci. Eng. 2017, 191, 12027. [Google Scholar] [CrossRef]
- Paulsen, J.; Renn, M.; Christenson, K.; Plourde, R. Printing conformal electronics on 3D structures with Aerosol Jet technology. In Proceedings of the FIIW 2012—2012 Future of Instrumentation International Workshop Proceedings, Gatlinburg, TN, USA, 8–9 October 2012; pp. 1–4. [Google Scholar]
- Grunwald, I.; Groth, E.; Wirth, I.; Schumacher, J.; Maiwald, M.; Zoellmer, V.; Busse, M. Surface biofunctionalization and production of miniaturized sensor structures using aerosol printing technologies. Biofabrication 2010, 2, 14106. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R. Rapid Prototyping of Biomaterials: Principles and Applications; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Smith, M.; Choi, Y.S. Optimizing aerosol jet printing process of silver ink for printed electronics Optimizing aerosol jet printing process of silver ink for printed electronics. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Beijing, China, 24–27 October 2017. [Google Scholar]
- Da Silva Neves, M.M.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Future trends in the market for electrochemical biosensing. Curr. Opin. Electrochem. 2018, 10, 107–111. [Google Scholar] [CrossRef]
- Agarwal, K.; Hwang, S.; Bartnik, A.; Buchele, N.; Mishra, A.; Cho, J.-H. Small-Scale Biological and Artificial Multidimensional Sensors for 3D Sensing. Small 2018, 14, 1801145. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.L.; Agarwala, S.; Tan, Y.J.; Yeong, W.Y. A low cost and flexible carbon nanotube pH sensor fabricated using aerosol jet technology for live cell applications. Sens. Actuators B Chem. 2018, 260, 227–235. [Google Scholar] [CrossRef]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, X.-L.; Zeng, Q.; Wang, H.-S.; Wang, L.-S. A multi-walled carbon nanotubes based molecularly imprinted polymers electrochemical sensor for the sensitive determination of HIV-p24. Talanta 2017, 164, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kuberský, P.; Altšmíd, J.; Hamáček, A.; Nešpůrek, S.; Zmeškal, O. An Electrochemical NO2 Sensor Based on Ionic Liquid: Influence of the Morphology of the Polymer Electrolyte on Sensor Sensitivity. Sensors 2015, 15, 28421–28434. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.S.A.; Syu, W.J.; Lei, K.F.; Hwang, Y.S. Development of a flexible non-metal electrode for cell stimulation and recording. Sensors 2016, 16, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zhmud, B. Lube-Tech093-ViscosityBlendingEquations; Lube: The European Lubricants Industry Magazine: Stockholm, Sweden, 2014; pp. 2–5. [Google Scholar]
- Tonello, S.; Abate, G.; Borghetti, M.; Marziano, M.; Serpelloni, M.; Uberti, D.L.; Lopomo, N.F.; Memo, M.; Sardini, E. Wireless Point-of-Care Platform with Screen-Printed Sensors for Biomarkers Detection. IEEE Trans. Instrum. Meas. 2017, 66, 2448–2455. [Google Scholar] [CrossRef]
- Tonello, D.U.S.; Marziano, M.; Abate, G.; Kilic, T.; Memo, M.; Carrara, E.S.S.; Lopomo, N.F.; Serpelloni, M. Enhanced Sensing of Interleukin 8 by Stripping Voltammetry: Carbon Nanotubes versus Fullerene. In Proceedings of the European Medical and Biological Engineering Confernce, Tampere, Finland, 11–15 June 2017; pp. 213–218. [Google Scholar]
- Chen, Z.P.; Peng, Z.F.; Jiang, J.H.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. An electrochemical amplification immunoassay using biocatalytic metal deposition coupled with anodic stripping voltammetric detection. Sens. Actuators B Chem. 2008, 129, 146–151. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar] [PubMed]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E.; Beebe, D.J.; Ismagilov, R.F. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef] [PubMed]






| Process Parameters | Ag | AgCl | C | NOA 81 | Nink 1000 |
|---|---|---|---|---|---|
| Sheath gas flow (SCCM) | 20 | 30 | 110 | 80 | 65 |
| Exhaust flow (SCCM) | 570 | 570 | 1000 | 1400 | 800 |
| Atomizer flow (SCCM) | 550 | 530 | 900 | 1360 | 750 |
| Process speed (mm s−1) | 2 | 2 | 2 | 0.75 | 3.5 |
| Plate temperature (°C) | 60 | 65 | 70 | / | 45 |
| Material | Thickness (μm) | Standard Deviation (μm) | Section (μm2) |
|---|---|---|---|
| Ag | 6.8 | ±1 | 854.2 |
| AgCl | 4 | ±0.8 | 392.3 |
| C + MWCNTs | 6.5 | ±0.2 | 365.3 |
| NOA 81 | 25 | ±3 | 1400 |
| WE Diameter | Current Peaks (μA) | Standard Deviation (μA) | Current Density (μA/mm2) |
|---|---|---|---|
| 3 mm | 280.8 | 118.8 | 39.7 |
| 2 mm | 632.0 | 142.0 | 201.3 |
| 1 mm | 82.0 | 27.9 | 104.5 |
| SPEs | AJPEs | |
|---|---|---|
| Bare C | 3.4 ± 0.5 ng/mL | 2.1 ± 0.2 ng/mL |
| MWCNTs | 0.5 ± 0.4 ng/mL | 0.3 ± 0.2 ng/mL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantù, E.; Tonello, S.; Abate, G.; Uberti, D.; Sardini, E.; Serpelloni, M. Aerosol Jet Printed 3D Electrochemical Sensors for Protein Detection. Sensors 2018, 18, 3719. https://doi.org/10.3390/s18113719
Cantù E, Tonello S, Abate G, Uberti D, Sardini E, Serpelloni M. Aerosol Jet Printed 3D Electrochemical Sensors for Protein Detection. Sensors. 2018; 18(11):3719. https://doi.org/10.3390/s18113719
Chicago/Turabian StyleCantù, Edoardo, Sarah Tonello, Giulia Abate, Daniela Uberti, Emilio Sardini, and Mauro Serpelloni. 2018. "Aerosol Jet Printed 3D Electrochemical Sensors for Protein Detection" Sensors 18, no. 11: 3719. https://doi.org/10.3390/s18113719
APA StyleCantù, E., Tonello, S., Abate, G., Uberti, D., Sardini, E., & Serpelloni, M. (2018). Aerosol Jet Printed 3D Electrochemical Sensors for Protein Detection. Sensors, 18(11), 3719. https://doi.org/10.3390/s18113719








