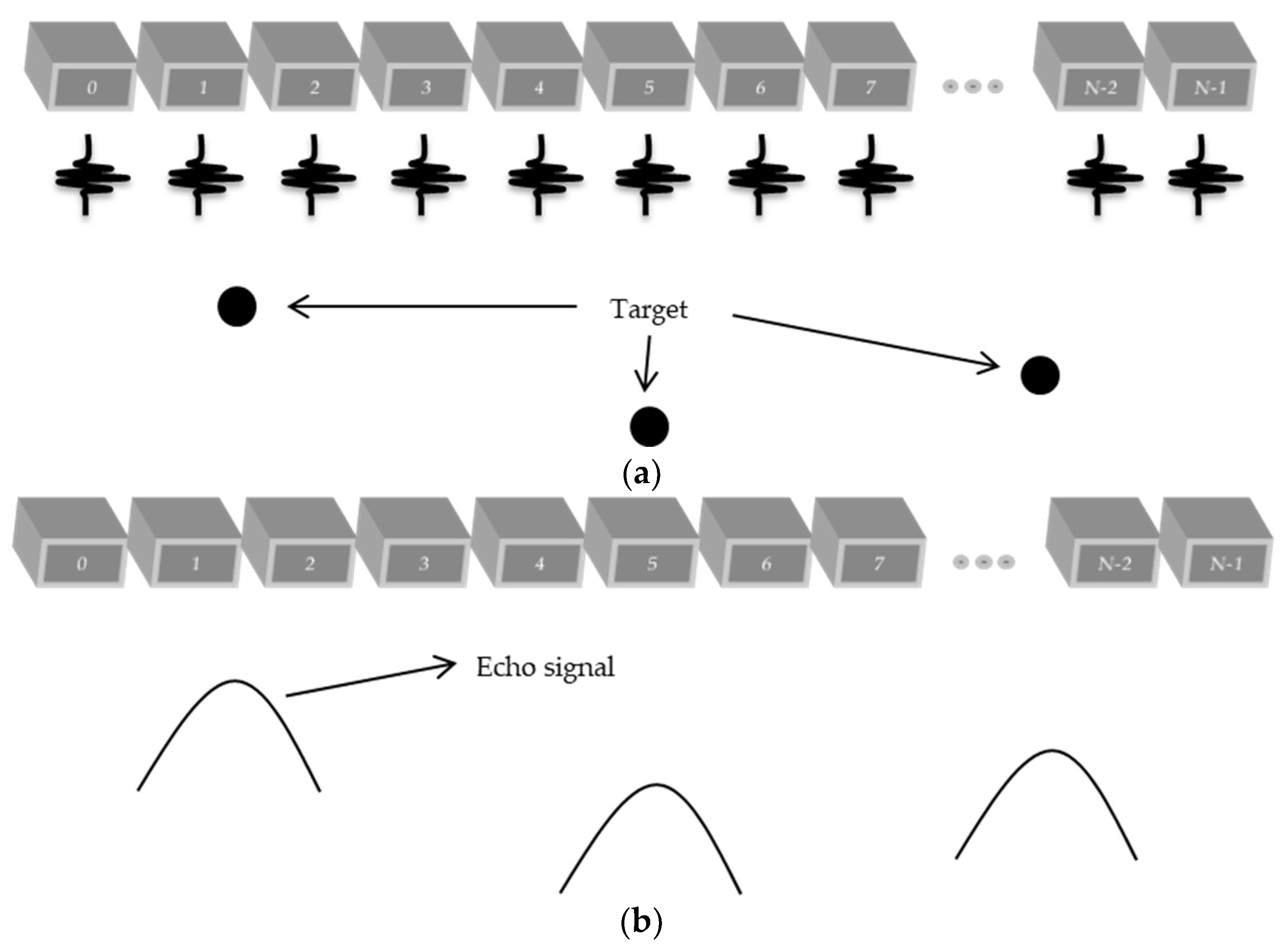
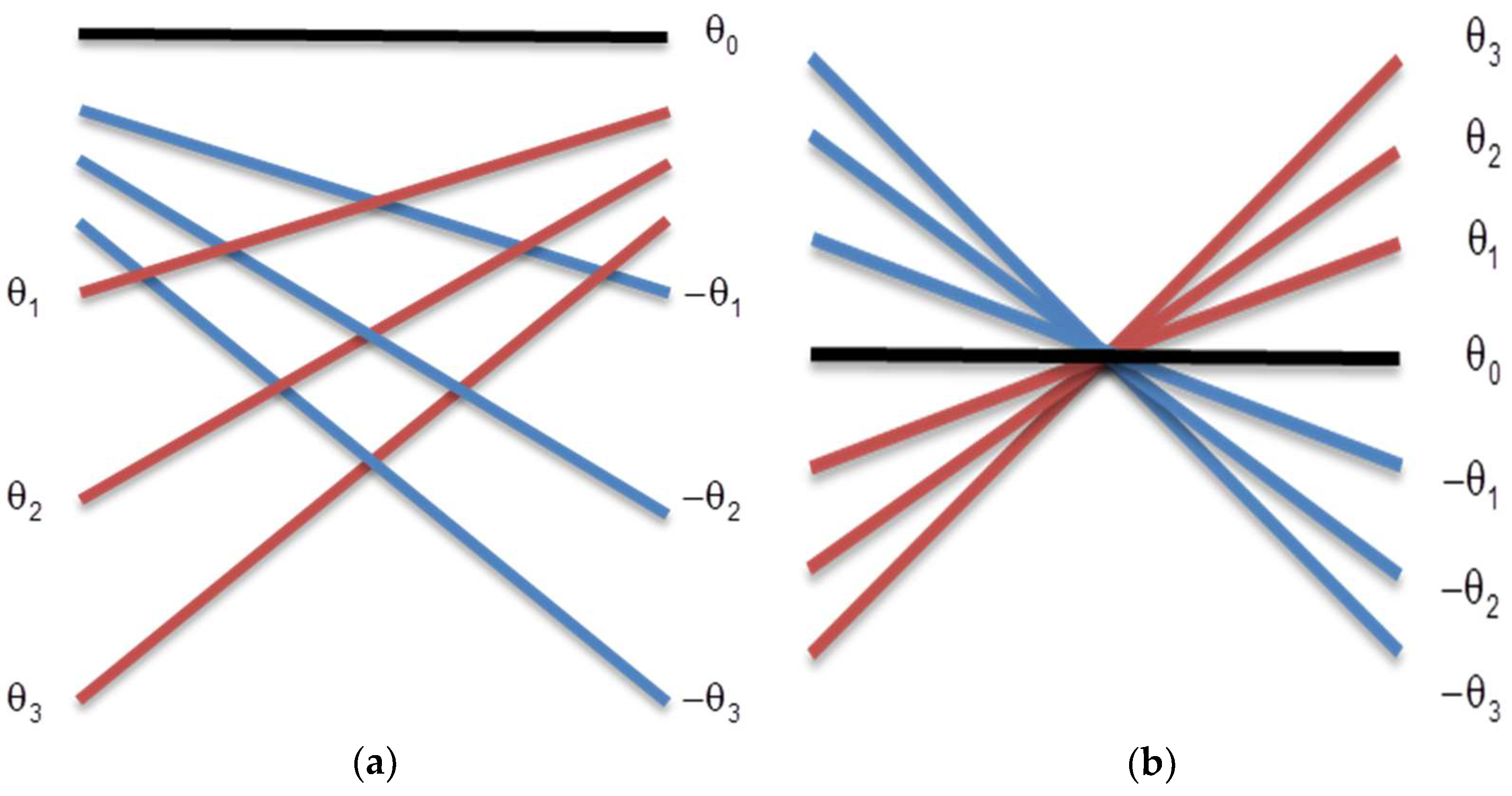
1. Introduction
Currently, images of tissues and human organs might be acquired through several methods such as X-ray, tomography, magnetic resonance, and ultrasound [
1]. Among the quoted methods, the ones that use the resonance and tomography techniques present an elevated quality on the final image exhibited by the equipment. However, the involved costs are higher, complicating the access to these kinds of exams for a major part of the population. Thus, since the beginning of the 2000s, the ultrasound (US) technique has been considered one of the most important imaging techniques to support clinical diagnosis in comparison with other methods that involve an invasive analysis with surgical procedures or images that are generated through ionizing radiations [
2].
The US modality began to be used in the 50s for non-destructive testing focused on the evaluation of the physical properties of certain materials, which might reveal internal information and detect possible discontinuities or flaws [
3,
4,
5]. In 1996, the US was already considered as the second most important modality for acquiring medical images to help in diagnosis, being surpassed only by the X-ray [
3]. It is currently considered the main technique to evaluate soft tissues such as the liver, kidney, and others [
6].
The final quality of the image obtained by US scanners is limited to the technical specifications of the equipment, the propagation of ultrasonic waves through the tissue to be analyzed [
7], and the method used to reconstruct the images, which in the majority of the current equipment, is the traditional Delay and Sum (DAS) [
8].
Many recent studies have focused on the reduction of the rate in which an ultrasonic transducer is excited or on the reduction of the number of signals to be received by the transducer elements (sparse arrays) to allow a higher frame rate (ultrafast image) to evaluate the moving structures in real time [
9,
10].
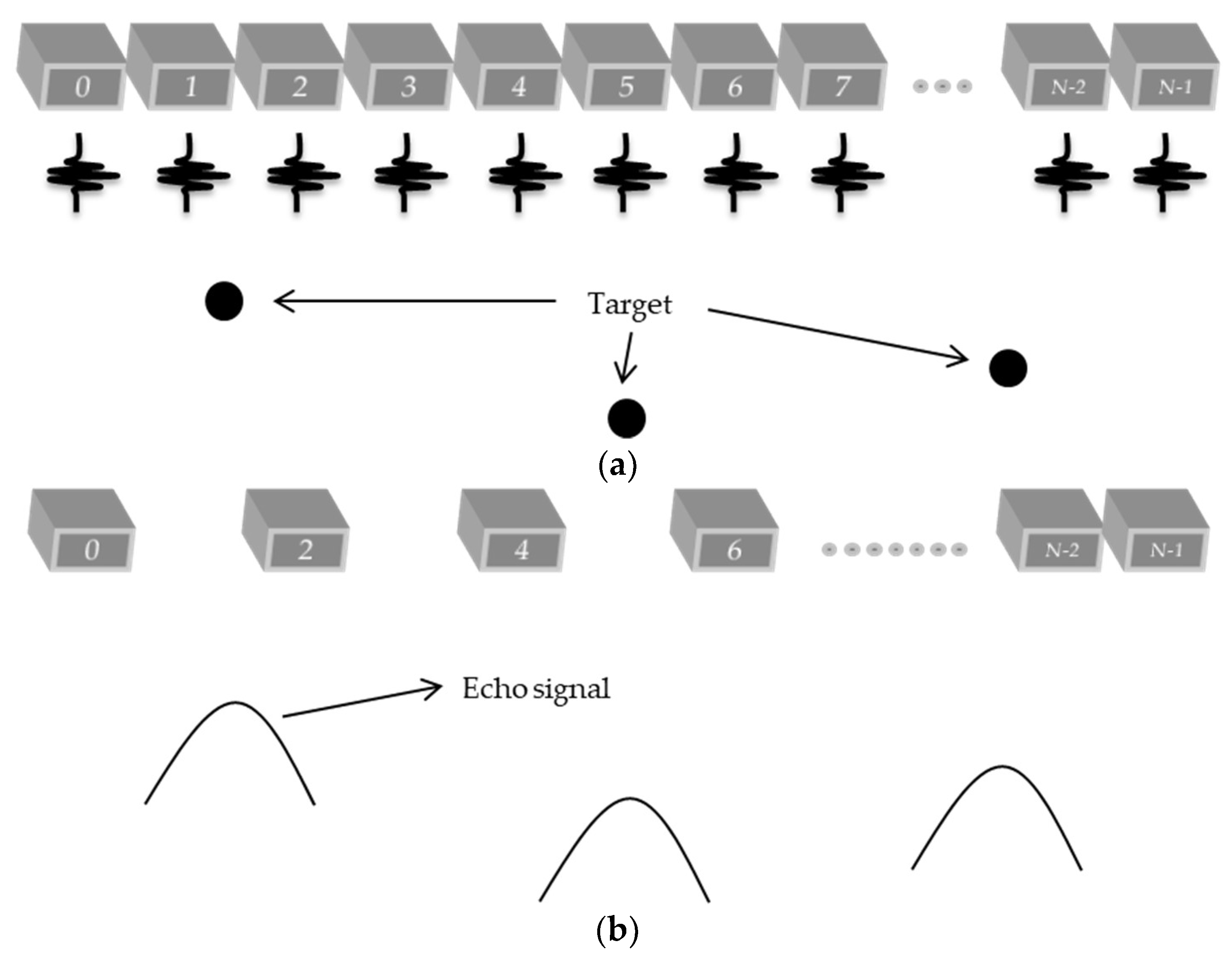
A new technique that has been used to increase the frame rate is the plane wave, where all elements are excited at the same time, taking a picture of the region of interest to be analyzed [
11]. This technique reduces the number of scan lines needed, which consumes much memory to be stored and processing time. The acquisition of the signals in this case is done in a parallel way, and is limited to the number of channels receiving the signals and the acquisition rate of the hardware.
The US equipment should generate a good quality image. However, the reduction in the number of scan lines by the DAS technique does not improve the image quality due to the lack of energy resulting in a poor image quality [
8]. To solve the image quality problem, new techniques must be used to process the signals in order to increase the frame rate and to allow better results in the lateral and axial resolutions [
8].
Therefore, plane wave and sparse arrays have been used separately for many researchers to try to obtain better image quality with lower computational costs than the traditional beamforming techniques. However, to our knowledge, new proposals that combine both techniques have not yet been thoroughly investigated. So, this work proposes the use of a new technique to reconstruct US images based on plane waves to excite the transducer elements and the use of sparse arrays on the reception in order to increase the scan rate and to reduce the amount of data to be stored. The technique based on a coherent plane wave was included to increase the image quality due the use of sparse signals. The Stolt-migration technique was used as a processing base and an interpolation method was applied to reconstruct the ultrasound images. The proposed method was evaluated by the contrast analysis, lateral and axial resolutions for the images generated with data simulated on Field II [
12,
13], and the images generated by the US Verasonics system using the DAS method.
3. Results
The simulations in the Field II using the phantom described previously were done using plane waves with angle variation ranging from −1.5° to +1.5°, −3.0° to +3.0°, and −15.0° to 15.0°, with steps of 0.5°, 1.0°, and 5.0°, respectively, totaling seven angles for each range with five acquisitions for each angle. Other simulations with angle steps of 2.0°, 3.0°, and 4.0° were also undertaken and evaluated using the lateral and axial resolutions. In order to compare the results, the same phantom used to simulate the DAS technique was used for all tests.
For the experimental data through the Verasonics platform, two analyses were conducted in two distinct areas of the commercial phantom. The sparse condition used in the simulation and experimental data was defined with 65, 44, and 23 active elements. In the first experiment, it used the same angle variation from the simulation approach. In the second experiment, it used a sequence of angles ranging from −0.250 to +0.250°, with steps of 0.125°, totaling five angles with 10 acquisitions for each angle, and was used as a target representing an encapsulated cyst. The figure size for each simulation and the experimental tests were optimized to represent only the region of interest and not the full depth.
The evaluation of the image contrast (
ctr) was done using Equation (11), described by [
26], and by using an internal region of the cyst and the background marked on each image:
where:
µin: is the average value of the rf signal amplitude of the region of interest
µout: is the average value of the rf signal amplitude outside the region of interest
σ2in: is the variance of the rf signal on the area of interest
σ2out: is the variance of the rf signal outside the area of interest
The mean square error (MSE), peak signal to noise ratio (PSNR), signal to noise ratio (SNR) and structural similarity index measure (SSIM), were used in this work to evaluate the image quality of the data processed using all sparse arrays setups [
27,
28,
29].
3.1. Simulation Results
The simulated raw
rf signals received by the transducer when excited with plane waves of zero degree angles (0°) are shown in
Figure 9. The logarithmic compression was limited to −50 dB.
Figure 9a shows the raw data with all 128 elements of the transducer receiving the signal and
Figure 9b–d show the raw data for sparse conditions with 65, 44, and 23 active elements in the reception, respectively.
The simulated results for the raw
rf signals acquired from the synthetic phantom described previously and processed with the Stolt migration and DAS algorithms are shown in
Figure 10.
Figure 10a shows the simulation results for the DAS method using the 128-element linear transducer, with an aperture of 64 elements and focal length at 2.0 cm.
Figure 10b–e show the simulation results for the same transducer excited with plane waves with angles ranging from −1.5° to +1.5° (0.5° step) and the reception with 128 (
Figure 10b), 65 (
Figure 10c), 44 (
Figure 10d), and 23 elements (
Figure 10e), respectively. The simulation results for the plane wave with angles ranging from −15° to +15° (5.0° step) are shown in
Figure 10f–i, with the same number of transducer elements used for data reception as in
Figure 10b–e. It is possible to see that for small angles and sparse conditions (
Figure 10c–e) there was an occurrence of some lateral spreading in the vicinities of the solid targets located at 2 cm and 4 cm depth. When the angle step was higher, it was possible to note that more scattering appeared in solid targets for sparse conditions (as shown in
Figure 10g–i), decreasing the image quality and resolution.
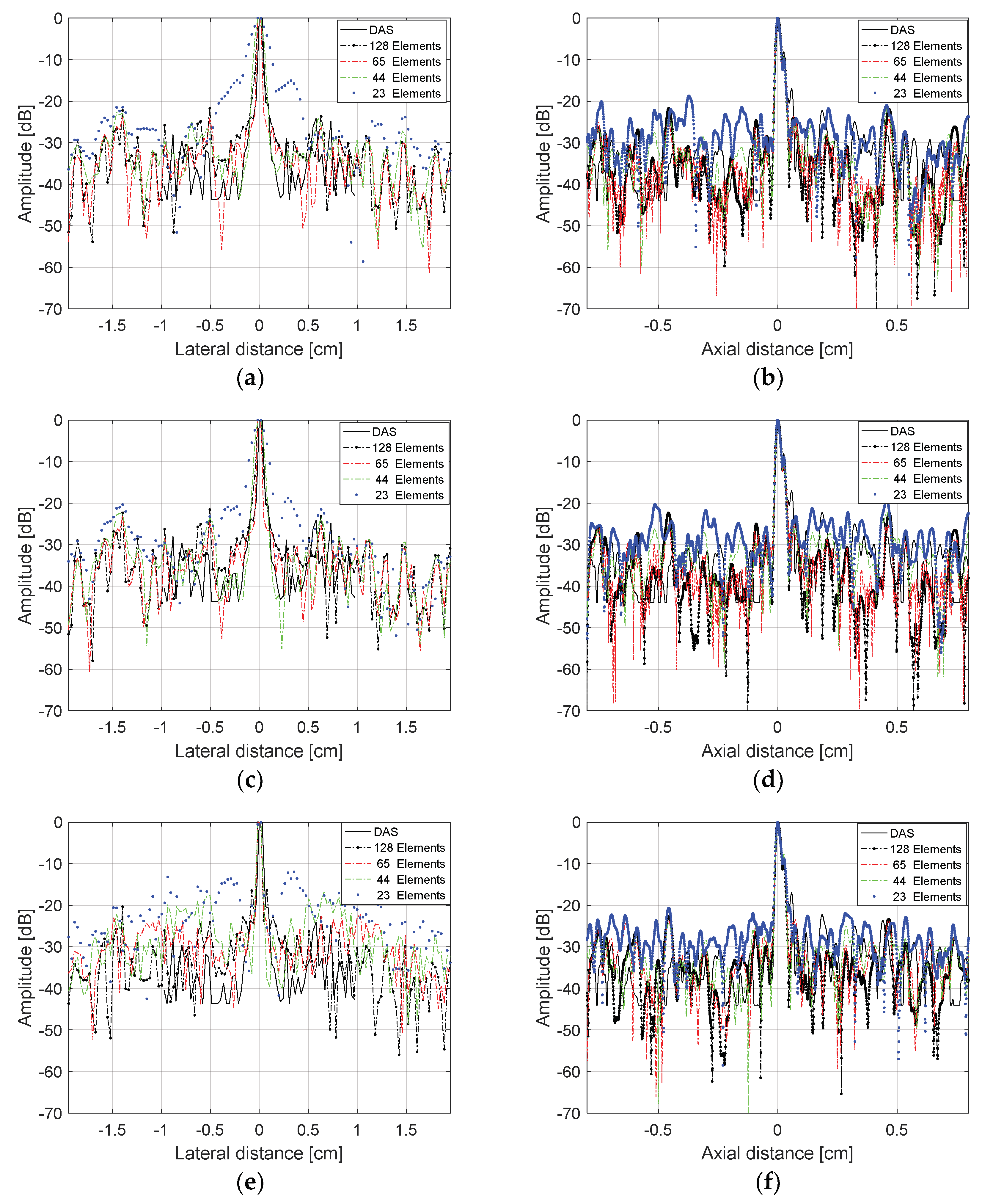
The evaluation of the lateral and axial resolutions for the point located at 2 cm depth in
Figure 10 were done using the Full Width at Half Maximum (FWHM) criteria at −6 dB [
30].
Figure 11 shows the lateral and axial resolutions measured using the DAS method with the transducer excited with plane waves in three different angles ranges: −1.5° to +1.5° (0.5° steps), −3.0° to +3.0° (1.0° step), and −15.0° to +15° (5.0° step). In this case, we evaluated the reception with all 128 elements (non-sparse conditions) and with 65, 44, and 23 elements (sparse conditions). Analyzing
Figure 11a,c,e, it is possible to see that there was a thinning of the central lobes and a soft reduction of the lateral lobes, decreasing the lateral resolution results when the sparsity was higher.
Figure 11b,d,f show that the axial resolution results for the solid target at 2 cm depth, where it was possible to see that the central lobes had lower changes as the sparsity increased, keeping the axial resolutions almost constant.
Numerically, by the FWHM criteria, it is possible to compare the results acquired for the lateral and axial resolutions for the sparse configurations and with all elements from the transducer.
Table 3 presents the lateral and axial resolutions, respectively, where it was possible to see a small difference between the lateral and axial resolution graphics. The traditional DAS method with 64 elements of aperture was used as a reference to compare with the Stolt migration method using the plane wave excitation at different angle ranges and receiving with all 128 elements (non-sparse mode) and with 65, 44, and 23 elements (sparse mode).
The contrast and image quality analysis for
Figure 12 using the DAS method and plane wave excitation with an angle range of −1.5° to +1.5°, 0.5° step, without any scattering close to the target at 2.0 cm, are shown in
Table 3 and
Table 4.
3.2. Experimental Results
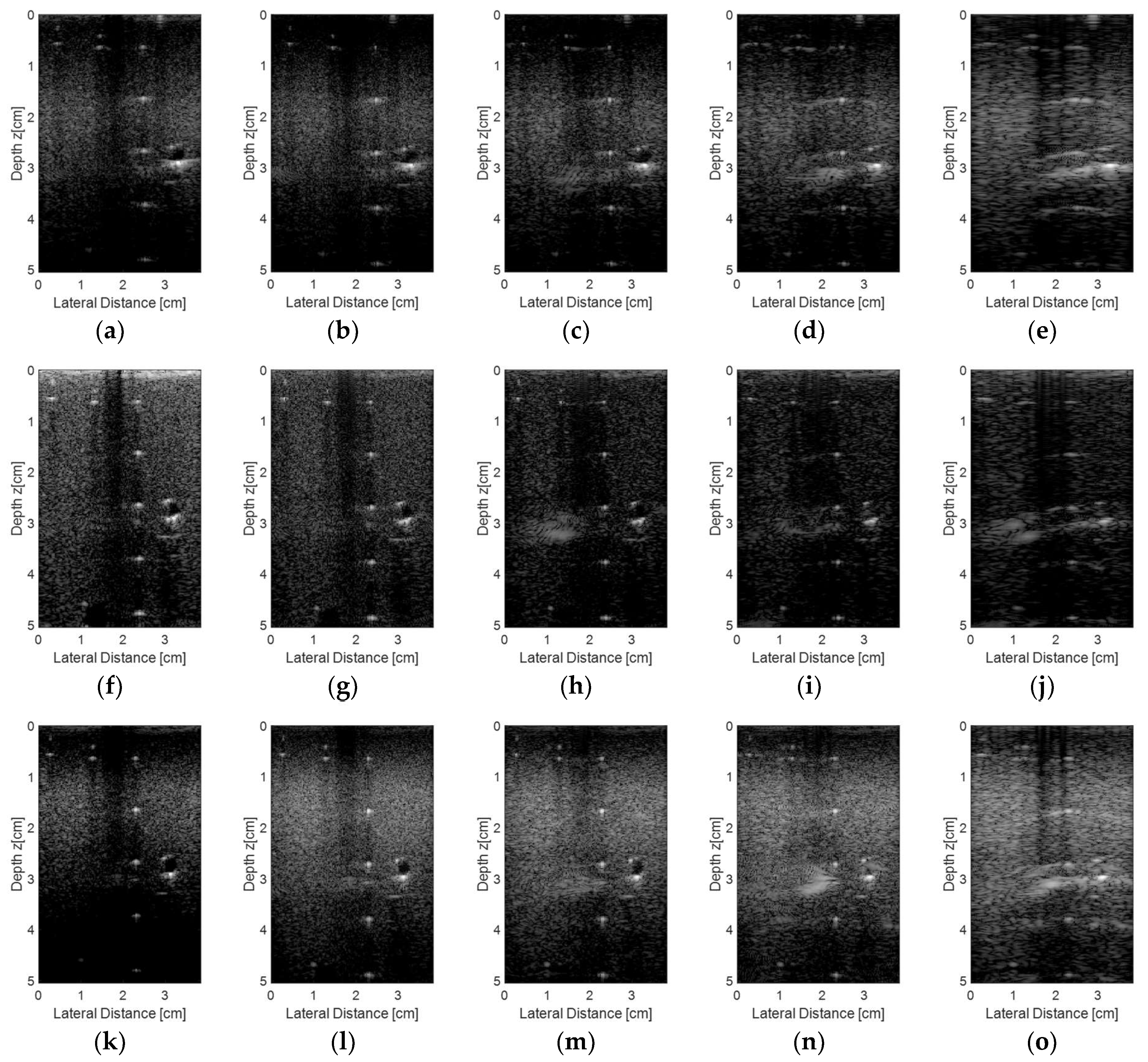
Figure 13 shows the experimental images obtained using the Verasonics Vantage 128™ system using the proprietary VDAS [
31] method to process the data (
Figure 13a,f,k) and with the linear L11-4v transducer excited with plane waves at different angles ranges (−1.5 to 1.5°, 0.5° step; −3.0° to +3.0°, 1.0° step; and −4.5 to 4.5°, 1.5° step). During the reception, we used all 128 transducer elements (
Figure 13b,g,l), 65 elements (
Figure 13c,h,m), 44 elements (
Figure 13d,i,n), and 23 elements (
Figure 13e,j,o).
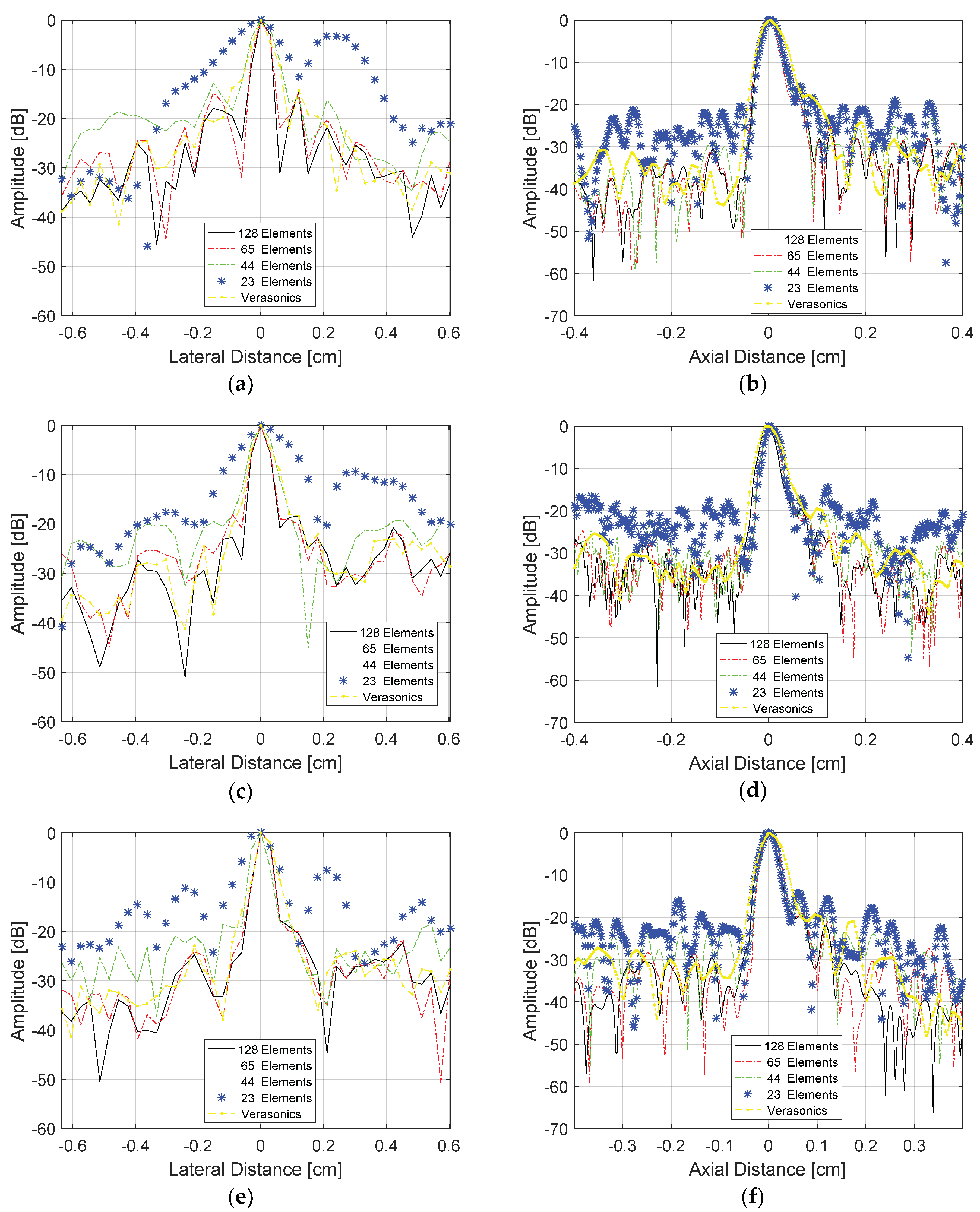
Figure 14 shows the lateral and axial resolution analysis for the target located at a depth of 1.7 cm as shown in
Figure 13, and
Table 5 shows the percentage errors. The transducer was excited with plane waves with angles in the range of −1.5 to +1.5° and 0.5° step (
Figure 14a,b), −3.0 and +3.0° and 1.0° step (
Figure 14c,d), and −4.5 to +4.5° with 1.5° step (
Figure 14e,f). For all cases, it was considered that the data generated by the image from the Verasonics system (proprietary VDAS method) and the results with the data processed with all 128 elements of the transducer and the sparse array case with 65 active elements, which showed the best results with lower percentage errors for FWHM, as presented in
Table 6. All images were processed for the dynamic range of −60 dB.
Analyzing the results shown in
Table 6, it is possible to see that the lowest lateral error was obtained with angles in the range −3.0 to +3.0° and 1.0° step when compared with the reference image. However, the highest resolution was achieved with angles in the range −1.5 to +1.5° and 0.5° step, indicating that it is possible to obtain good quality images with a small angle variation.
Table 7 shows the image quality analysis in terms of Peak-SNR, higher SNR, lower MSE, and higher SSIM for the sparse condition with 65 active elements that showed the best results, using as a reference the images generated with all 128 elements being excited and receiving the data in the same angle range.
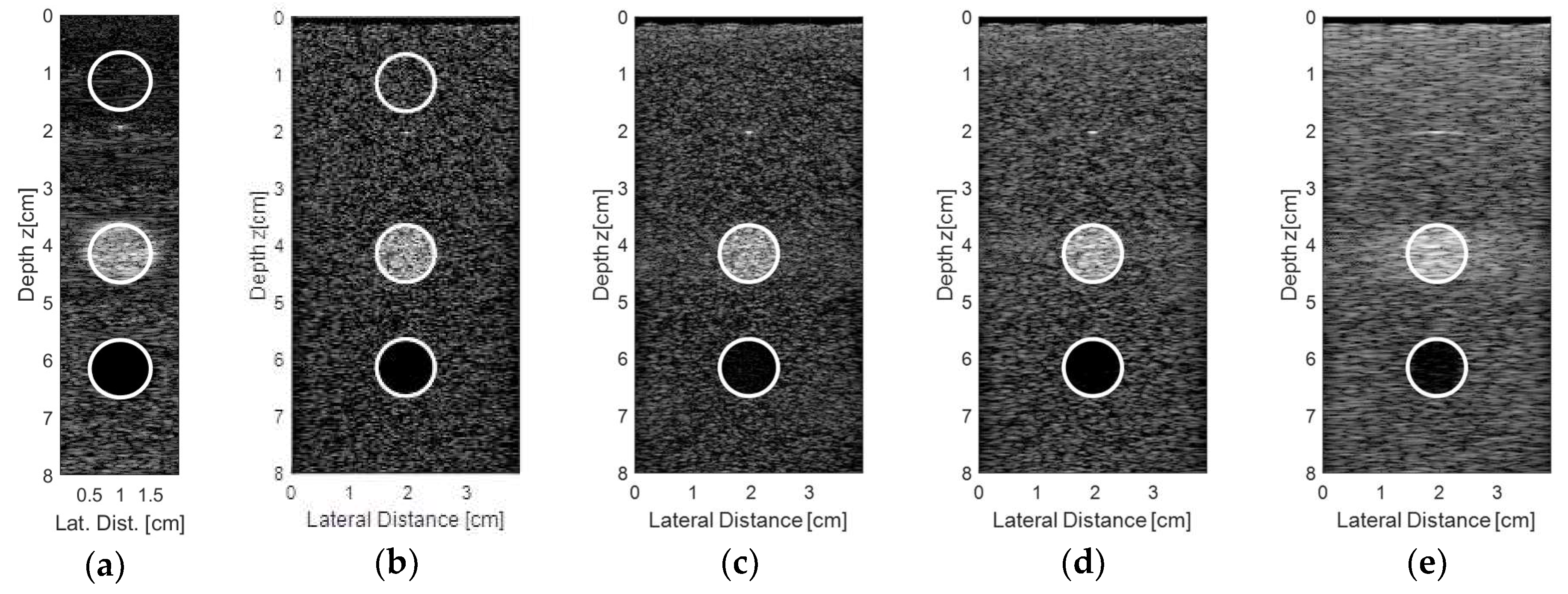
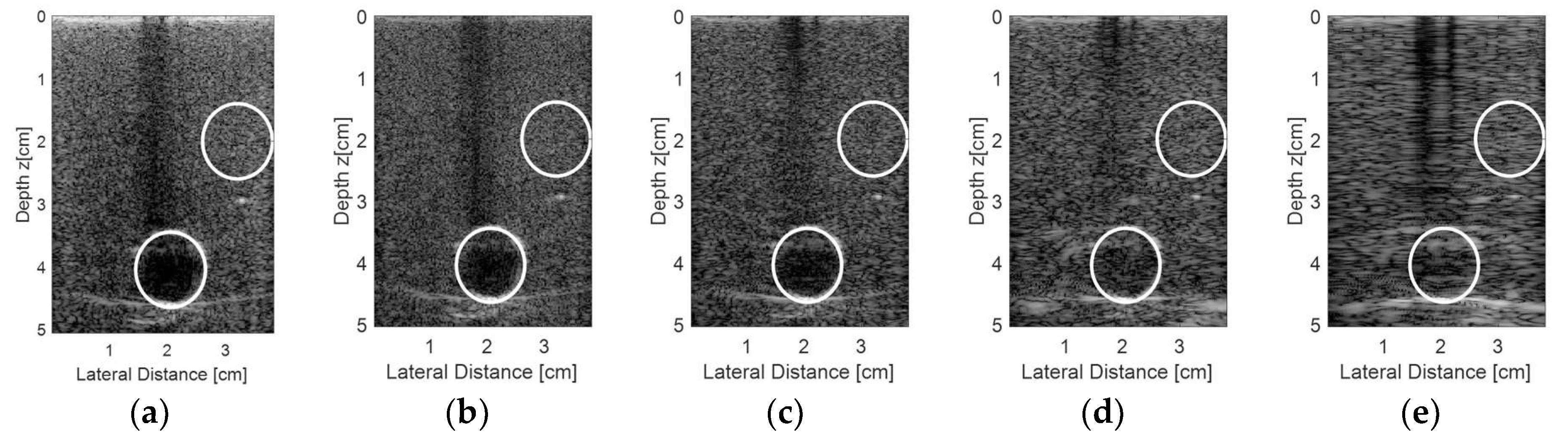
Figure 15 shows the results for the second experiment, considering other regions from the commercial phantom and new acquisition data. A target was chosen with a diameter of 10 mm, representing an encapsulated cyst, and the transducer elements were excited with plane waves with angles in the range of −0.250° to 0.250° and 0.125° step, with 10 frames for each step. The resulting and processed images using the proprietary Verasonics VDAS method (
Figure 15a) and using the Stolt migration method for all 128 elements being excited and receiving the data (
Figure 15b) and for the sparse conditions with 65 (
Figure 15c), 44 (
Figure 15d), and 23 active elements (
Figure 15e) are presented.
Analyzing the images shown in
Figure 15, it was possible to see that the best result for the sparse condition was obtained with 65 active elements (
Figure 15c) when compared with the Verasonics image (
Figure 15a) and the image obtained with 128 active elements (
Figure 15b).
Figure 16 shows the regions of interest for the contrast analysis of the images shown in
Figure 15 and the results are presented in
Table 8. The results of the sparse condition with 65 active elements were in very close agreement with the non-sparse condition (all 128 elements being excited and receiving the data).
Table 9 shows the image quality results in terms of higher similarity for the images shown in
Figure 15 using the Verasonics image (
Figure 15a) as a reference. The best results for the sparse condition was obtained with 65 active elements, with higher SNR and SSIM, and lower Peak-SNR and MSE.
4. Discussion
In this work, the generation of US images for the ultrafast modality with the combination of plane wave and sparse arrays techniques was evaluated. The utilization of sparse arrays allowed the reduction of data to be acquired and subsequently stored in temporary buffers for a posteriori processing.
Separately, the plane wave imaging and the sparse array imaging are well known and widely used techniques. However, in this work we combined and evaluated the two methods with different sparse conditions and several angle inclinations to improve the computational efficiency. The tests were done using 128 elements for transmission and 128, 65, 44, and 23 elements sparsely distributed for reception. The simulated data were compared with images obtained with the DAS method and the experimental data were compared with those acquired from Verasonics proprietary VDAS. The obtained results using the FWHM criteria at −6 dB showed that the images generated by the proposed method were similar in terms of resolutions (axial and lateral) and contrast, mainly using 65 active elements, to the simulated and the Verasonics commercial ones, indicating that the sparse reception proposed method is suitable for ultrasound imaging. Korukonda et al. [
32] presented a work using the two methods for noninvasive vascular elastography. However, in that approach, only seven elements were applied in transmission and all 128 transducer elements were used to receive the
rf data.
The simulations in Field II [
12,
25] showed good quality images with high lateral and axial resolutions. However, the simulated raw data had a higher number of samples (12,900 × 128) than the experimental data (2048 × 128) acquired with the Verasonics Vantage 128 platform, so that the same quality images were not possible to achieve with the experimental data.
Analyzing the results of the simulations in Field II as shown in
Table 3, it was possible to see that the lowest percentage errors when compared to the DAS method for the lateral resolution (0.04%) and the axial resolution (−5.56%) were obtained with the transducers being excited with plane waves with angles from −15.0° to +15.0° (5.0° step) and −9.0° to +9.0° (3.0° step), respectively, and receiving the data in the sparse condition with 23 active elements. However, the images obtained (see
Figure 10i, for example) showed a lot of scattering and it was not possible to visualize the targets adequately. Thus, although it presented a higher percentage error for the lateral and axial resolutions (5.51 % and −16.53 %, respectively), in the proposed method, images with the best quality were obtained with the transducer being excited with plane waves in an angle range of −1.5° to +1.5°, 0.5° step, and 65 active elements (
Figure 10c) with lateral and axial resolutions of 0.4612 mm and 0.2348 mm, respectively.
The simulated results of the proposed method using the sparse condition with 65 active elements of a transducer with a total of 128 elements were comparable to the results obtained by other authors who used different techniques, targets, and phantoms to reach the best resolution.
Rindal and Austeng [
33] obtained, for some sequences of plane waves and DAS methods, lateral resolutions of 0.56 to 0.82 mm and axial resolutions of 0.40 to 0.56 mm. With the usage of the minimum variance technique (MV), they obtained better results to the order of 0.07 to 0.12 mm for the lateral resolution and 0.41 mm to 0.42 mm for the axial resolution. They did not used the sparse condition in their work.
Using a DAS method, Mozaffarzadeh and collaborators [
34] obtained lateral resolution values of 0.3931, 0.5825, and 0.8613 mm for a small depth of 25 mm. Even for a better resolution, according to the author, it was considered that the FWHM criteria for −3 dB, different from the metrics used in this work, which was −6 dB, as indicated by Harput et al. [
30].
Comparing the results with other works that used the Stolt migration, Garcia et al. [
22] showed that as the depth increased, the lateral resolution became worse. In comparative terms, for the same depth of the data simulated in this work (2 cm) they obtained lateral resolution values between 0.5 and 0.6 mm, considering all elements of the transducer, while in this work, the sparse condition was used.
The contrast analysis for the simulated data (
Figure 12 and
Table 4) in the sparse (65 active elements) and non-sparse (128 active elements) conditions were similar, being 7.03 dB and 6.49 dB for the solid target and 6.35 dB and 6.33 dB for the cyst, respectively. The same happened for the experimental results (
Figure 16 and
Table 8) with −1.66 dB for the sparse and −1.78 dB for the non-sparse condition. Analyzing the results shown in
Table 4, it was possible to see that the obtained contrast was higher when using the DAS method to process the data from the solid target, and for the cyst, it was higher when using the plane wave method.
The image quality analysis for the plane wave method was done using the DAS method with 64 elements of aperture as a reference (non-sparse mode).
Table 5 shows all of the analysis for the sparse condition processed with an angle range from −1.5° to +1.5° and 0.5° step. The sparse condition with 65 active elements showed the lowest Peak SNR and MSE and higher SNR, but the SSIM criteria was not so good. This happened due to the size of the images generated by each method (size for the DAS and size for the proposed method).
The contrast results in this work were close to the ones obtained by Matrone et al. [
35], where the contrast was 1.3 and 1.8 dB. Analyzing the results obtained by Garcia et al. [
22], it was possible to verify that the contrast values were close for variations up to 10 angles, regardless of the method used to reconstruct the images, and were comparable to those obtained in this work by using small angulation ranges. Furthermore, by evaluating the images generated by Verasonics and the ones processed by the sparse arrays method proposed here, it was possible to notice similar or even better results.
Experimentally, Kotowick et al. [
36] obtained for a CIRS model 40 Multi-Purpose Multi-Tissue Ultrasound Phantom using DAS, plane wave compounding (PWC), and synthetic aperture (SA) in an adaptative system with lateral resolution values higher than 1.0 mm. In this work, we obtained a lateral resolution of 0.5815 mm (
Table 6) with sparse arrays using 65 active elements and an angle range of −1.5° to +1.5°, 0.5° step. The axial resolution obtained in this work (0.4270 mm for the 65 active elements sparse condition) was also better than those obtained by the authors, which was 0.53 mm with SA and 0.64 mm for PWC.
Comparing the quality of the images generated by the proposed method in this work to the Verasonics VDAS method, it was possible to see that they were similar as it was possible to see all the phantom targets (
Figure 13 and
Figure 15). Increasing the angle range through the FWHM analysis, it was possible to verify that the resolution of the generated images was better in most cases, but some scattering began to appear, thus degrading the image quality.
The use of plane waves without angulation and sparse data in the reception was difficult in the reconstruction of detailed images, even with linear interpolation because there was a great loss of information. The effect was higher when the targets were in the direction of sparse elements. With the use of angulation, the effect of losing information was compensated and it was possible to reconstruct a detailed image, even when the sparse data were in the same direction of a small target. The tests showed good results when the angulation was increased, improving the attenuation between the central and secondary lobes. However, the scattering was a limiting factor for image quality when using sparse conditions with less than 65 active elements in the reception.
The Stolt migration method used in this work was shown to be proper, easy to implement, and fast to process the data. It was possible to clearly distinguish the points and cysts on the generated images. Even for the sparse case, the recomposition of the missing data using a linear interpolation presented good results.
5. Conclusions
Analyzing the obtained results in this work, it was possible to conclude that the proposed use of the sparse condition, with a reduction in the number of active elements of the transducer to receive the signal, will lead to a reduction in the hardware cost and complexity, mainly in the circuits aimed to amplify the signals, the high-speed analog to digital converters and filters.
The use of plane waves without angles to excite the transducer elements might result in a low-quality image, where it is difficult to identify the image contours, mainly when receiving data using sparse arrays. Thus, the acquisition of multiple frames and the angulation used to excite the transducer elements tends to compensate the information lost by the use of sparse conditions, improving the lateral and axial resolutions besides the contrast.
The use of new processing techniques for the reconstruction of US real time images has been established as a decisive factor to reach the ultrafast modality in USs. The migration method chosen to process the data depends on the inclusion of a direct or inverse FFT, which generates good results when combined with the acquisition of multiple frames and angulation.
The technique proposed in this work, using sparse arrays with a low number of frames and angles, was shown to be viable in generating ultrasound images with good quality and higher lateral and axial resolutions. Analyzing the image quality, our best results were obtained, for both simulation and experimental dataset, with 65 elements for the sparse condition and angle step of 0.5°. However, other new processing techniques to reduce the spreading should be included in the future to improve the final image quality.