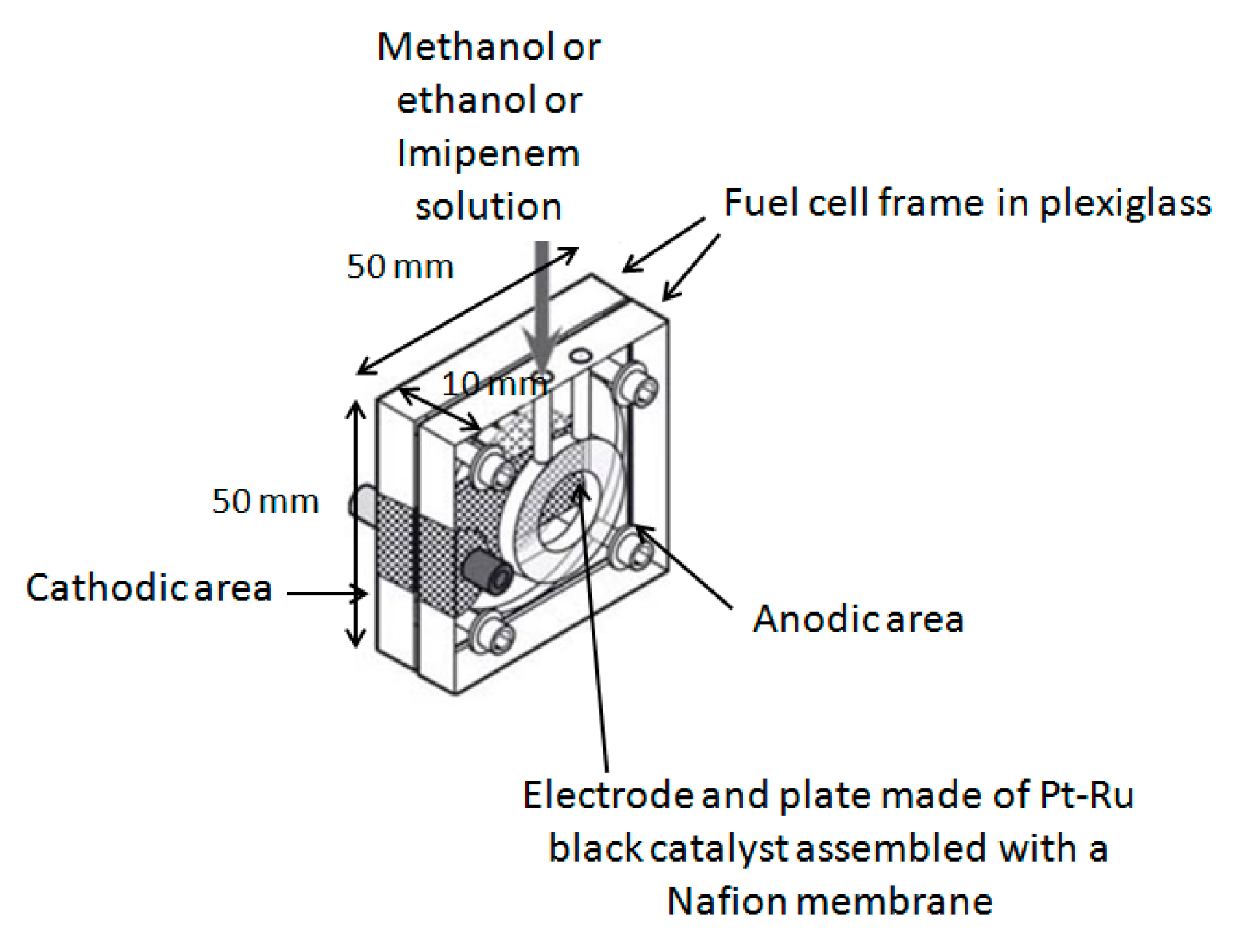
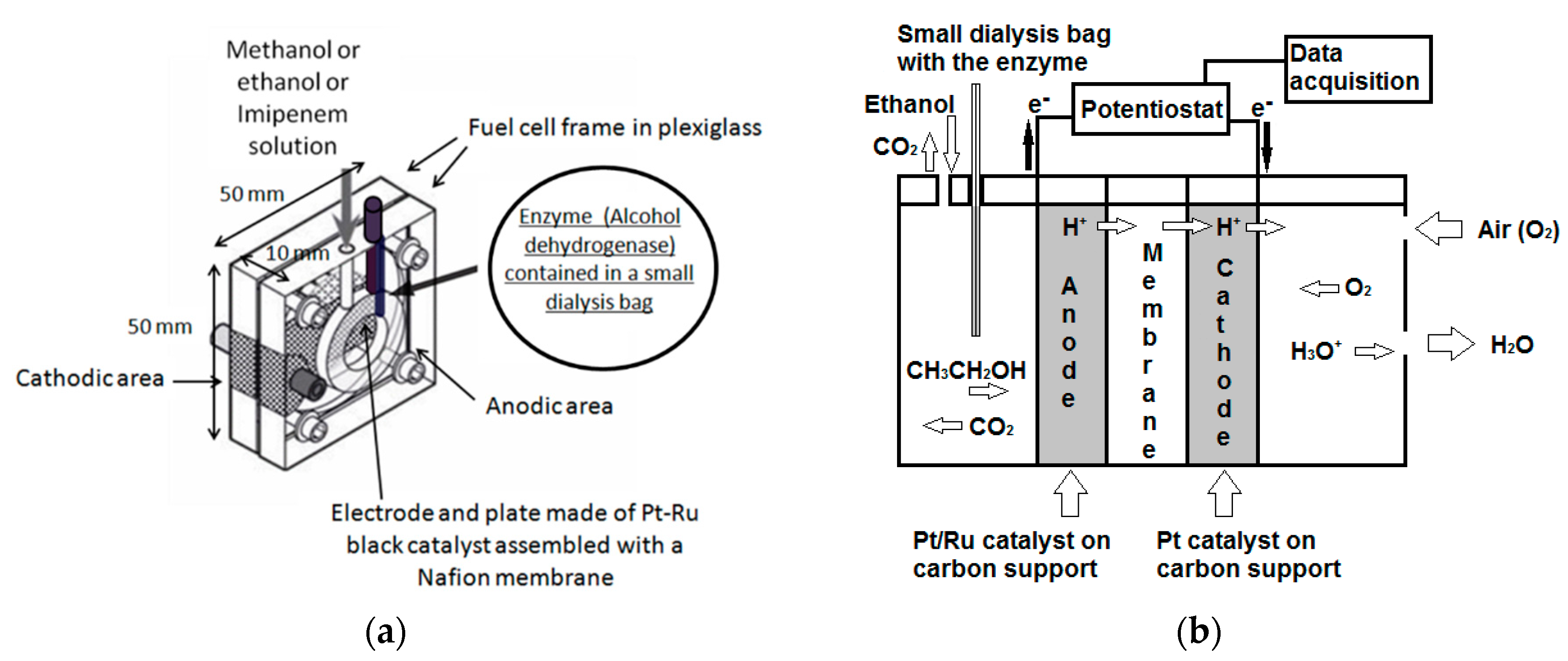
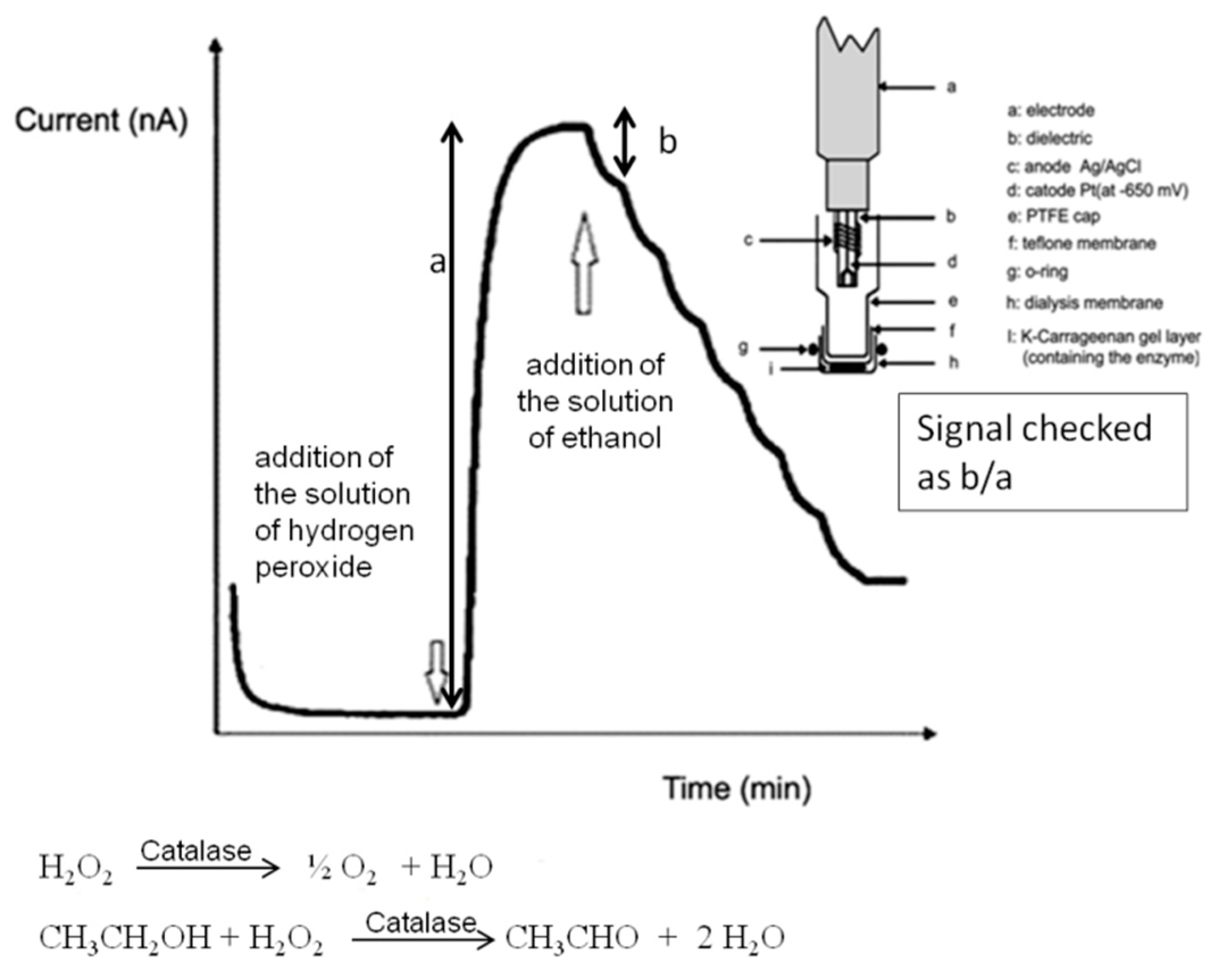
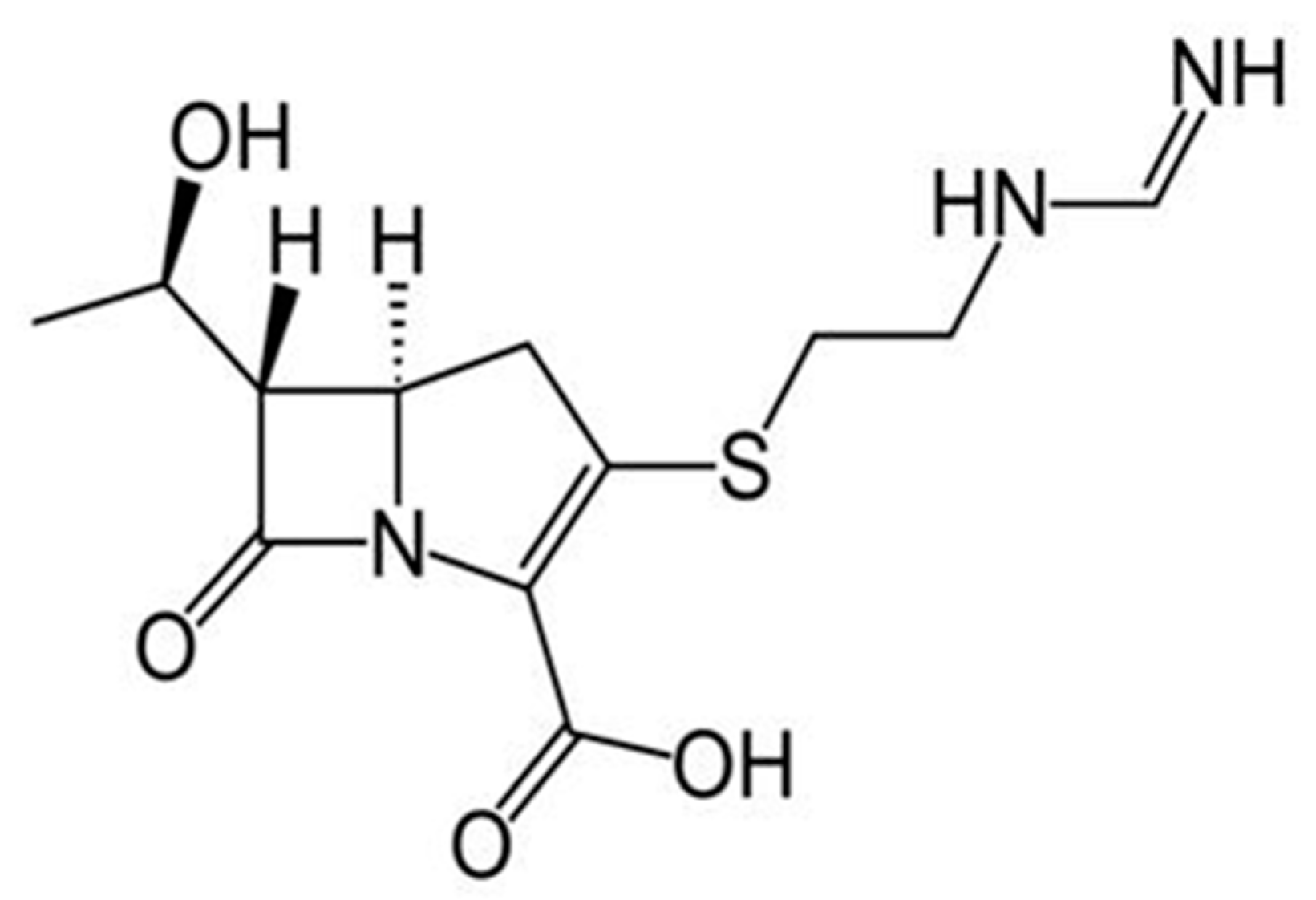
1. Introduction
Researches of different types have been performed in the past by some authors with the aim of using different fuel-cell-based devices for analytical [
1,
2,
3,
4,
5,
6,
7] (and energetic [
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]) purposes. These researches have effectively shown the possibility of this kind of application, but only by means of particular and complex types of fuel cell, not useful for common application to real samples. However, our research group recently performed the first researches of this type, by using a simple and suitable direct methanol fuel cell (DMFC) [
25,
26]. In a further study [
27], the performances of our fuel cell were made more efficient for the determination of ethanol by immobilizing an enzyme into the device [
27]. Our group has continued this analytical research, devoted to enzymatic (or non-enzymatic) DMFC applications for analytical purposes. New unpublished results, reported in the present paper, concern several further analytical applications in pharmaceutical and forensic fields. Firstly, we continued to investigate the possibility of checking, in a simple and inexpensive way, the alcohol content of some drugs available in drugstores. By using a non-enzymatic DMFC device, the ethanol content of several pharmaceutical dyes was easily checked. The obtained results were compared both with the ethanol content declared by the producer and with the obtained data, by analyzing the same samples using a conventional amperometric catalase enzyme sensor, recently prepared in our laboratory and already used to validate results obtained using DMFC device for ethanol determination in beverages [
26]. It was also attempted to utilize the fuel cell to validate the ethanol content of denatured commercial ethanol, usually available in drugstores. In this case, however, great interferences occurred due of the presence of red dye (Reactive Red 120) and denatonium benzoate, both of which are contained in this kind of commercial product. The best opportunity was the determination of a particular antibiotic (imipenem), which is an active agent having a -C(CH
3)H-OH functional group in its molecule, contained in some pharmaceutical formulations. In this case, we used the enzymatic DMFC device for the measurement. The enzyme introduced in the anodic cell section gave the possibility of improving the analytical performances of the catalytic fuel cell, as, for instance, in the case of imipenem, and as was observed in the case of ethanol [
27]. To this purpose, the alcohol dehydrogenase enzyme was immobilize in a dialysis membrane small bag located in the anodic area of the fuel cell. The enzyme with its catalytic action increased the sensitivity of the method and dramatically reduced the response time of the cell. Owing to the presence of the same enzyme in the fuel cell, it was also possible to evaluate the ethanol content in some human saliva and serum samples, a measurement that may be of interest to determine the alcohol level in specific human biological fluids usually checked in support of rapid “breath testing” of drivers.
3. Results and Discussion
In
Table 1, the main analytical data obtained by both the simple fuel cell and the enzymatic fuel cell for ethanol determination (in the potentiostatic mode) are summarized and compared.
It can be observed that the linearity range and LOD (Limit of Detection) values are better for the enzymatic fuel cell and, above all, that the response time is short enough for the latter device, while the life time of the device is longer for the more inexpensive and simple non-enzymatic catalytic fuel cell. By using the simple DMFC device, the ethanol content of several pharmaceutical dyes were determined for a fast, simple, and cheap control of these kind of products. The main results are given in
Table 2.
The obtained data are compared, in the same
Table 2, both with the ethanol content data declared by the producer and with data obtained by analyzing the same samples by using the conventional amperometric catalase enzyme sensor as described in the previous paragraph.
Table 2 shows the analytical validity of the values obtained with the fuel cell; if they are compared to the nominal values, the agreement is always of the same order as in the case of the test performed by the conventional catalase biosensor. On the other hand, the results of the F-test, shown in
Table 3, show the precision for the two methods of analysis is always “not significant”, except in one case.
While in the present research we analyzed several pharmaceutical dyes, almost accidentally, we adopted the fuel cell to determine the more common based ethanol pharmaceutical product, i.e., the “disinfectant (or denatured) ethanol”. Curiously, we found that the values of this product were markedly lower than the expected ones, as shown in
Table 4.
Table 4, presents a comparison between the values (expressed as percentage by volume) in three different commercial denatured ethanol samples purchased in drugstores, experimentally found by the simple DMFC device, and the nominal (% by volume) concentrations values claimed on the label. The values determined by DMFC were consistently lower than the nominal ones. To check if the ethanol content was the same as the claimed value, and if therefore this difference was to be attributed to the analytical method used, the considered denatured alcohol samples were simultaneously analyzed by catalase enzymatic biosensor method, i.e., a conventional method several times well validated in previous papers [
27,
45,
46,
47] (comparing it also with chromatographic tests [
45]). The obtained results are displayed in
Table 4. The data prove that the even lower ethanol content, which in all cases was highlighted by the fuel cell, must surely be attributed to the fuel cell experimental method. We therefore considered the hypothesis that additives present in the denatured alcohol could exert a noticeable interference, lowering the sensitivity of the fuel cell to ethanol. Information about disinfectant ethanol composition is not abundant; usually, the composition of denatured alcohol is not reported in detail on the label (particularly concerning the effective percentage of all the components of the mixture), because sometimes this is covered by patent. The values reported in the literature are generally the following: for denatured EtOH samples, a content not less than 83% by volume has been prescribed for “disinfectant ethanol” by the G.U. European (374/42 of 22 December 2004 i.e., the modified of Commission Regulation No 3199/93) [
48]. Moreover, various other substances are added to commercially so-called “denatured alcohol”. These additions are usually: firstly, b-denatonium benzoate, in addition to a red dye (C.I. Red 24, C.I. 18208), or similar (C.I. Red 120), and lastly thiophene and methyl ethyl ketone. Therefore, all these substances were individually tested by us. We experimentally checked whether, individually, these compounds were able to give any response by themselves by using fuel cell, or if they were able to alter the response of the fuel cell to ethanol. The experimental results indicated that the b-denatonium benzoate, the red dye (C.I. Red 120), thiophene, and methyl ethyl ketone give practically no signal (at least at the concentration of about 10
−3 mol·L
−1), but all bring to lower responses of the fuel cell to ethanol, if the latter was also present in the cell. In conclusion, a comparison with reference analytical data, found both by using the conventional amperometric biosensor and official nominal values, confirmed that additives contained in denatured ethanol give interferences, lowering the response of the fuel cell to ethanol; this is probably because these components, especially denatonium, or Red Dye 120, can complicate the catalyst (ruthenium ions), as reported in the literature for compound of the same type [
49]. Other successive applications have been performed on different kind of complex-relevant samples, by measuring the SC again, although in this case using the enzymatic device. In fact, in a previous study [
27], when simple ethanol solutions were measured it was possible to demonstrate that, if alcohol dehydrogenase was introduced in the fuel cell, this enzyme was able to produce a small increase in the sensitivity of the method. In more detail, it can fasten the ethanol breakdown process [
27] and therefore enhance the analytical performances of the fuel cell, as can be seen from the response time reported in
Table 1.
Table 1 shows that the enzyme alcohol dehydrogenase dramatically reduces the response time of the cell to ethanol, which is of extreme importance for the analytical applications. We therefore considered it useful to check if, with the new enzymatic fuel cell, the same benefits already obtained in the case of simple ethanol sample analysis could also be obtained in the case of the analysis of a particular antibiotic, i.e., imipenem (see
Figure 4). To this purpose, in
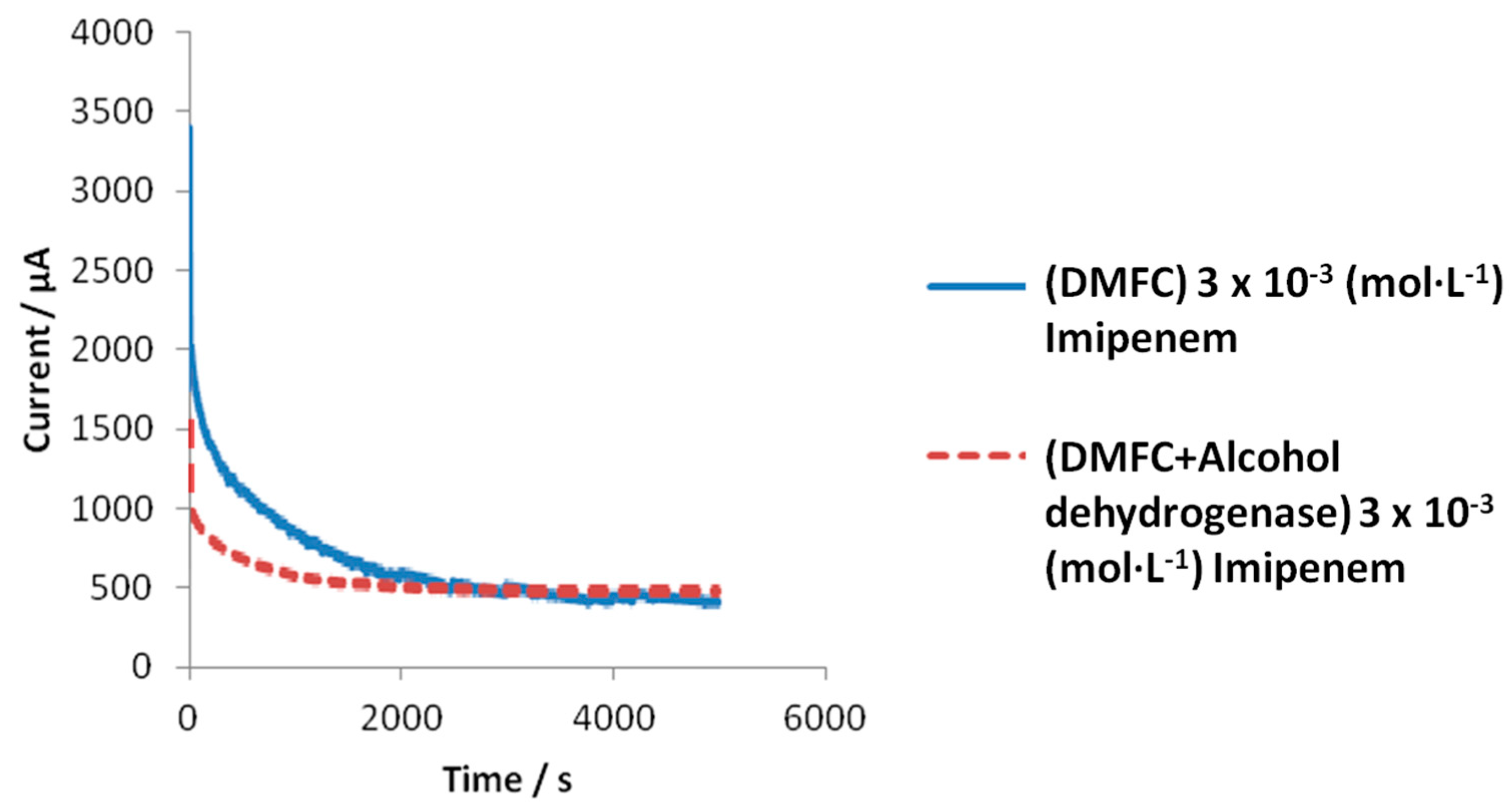
Figure 5 a comparison is displayed between the experimental response behavior to imipenem, both of the enzymatic (alcohol dehydrogenase) and non-enzymatic DMFC devices, in potentiostatic mode at Optimized Anodic Potential applied (OAP); in both cases, the SC values vs. time were plotted until the steady state, for a typical imipenem concentration.
It can be observed immediately that the current reaches the steady state more rapidly in the presence of the enzyme alcohol dehydrogenase than when this enzyme is absent. In
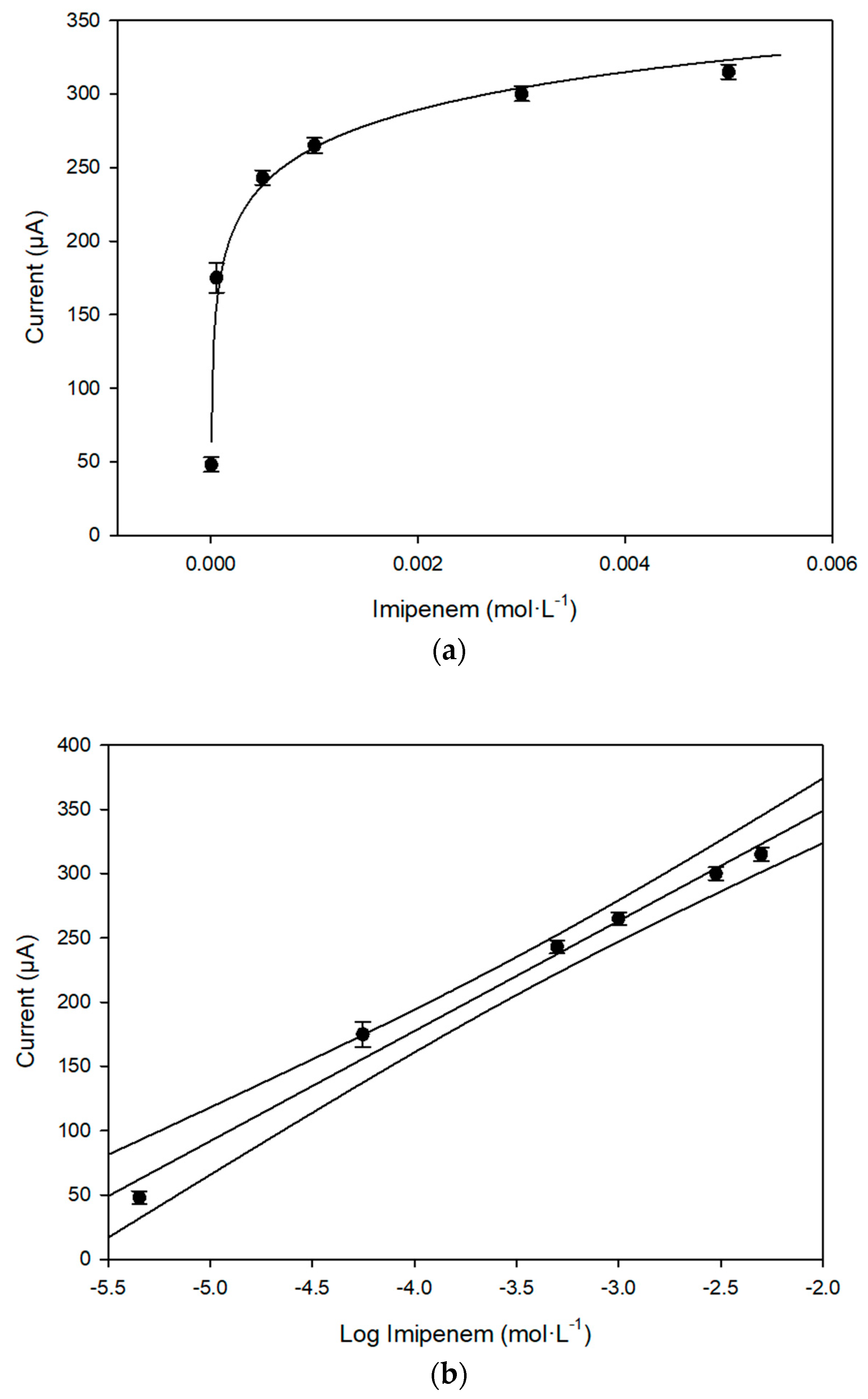
Figure 6a,b, the behavior of the response of the enzymatic fuel cell, on increasing imipenem concentration, and the corresponding calibration curve, in a semilogarithmic scale, are reported.
In
Table 5, a comparison of the main analytical data, including the equation of the calibration curves to imipenem, by using enzymatic or non-enzymatic fuel cell, is reported.
It can be confirmed that on analyzing imipenem, the introduction of the enzyme significantly reduces the response time of the device. Furthermore, in
Table 6, some selectivity data related to other types of antibiotics are reported. The selectivity of the fuel cell results is undoubtedly good.
Additionally, using the enzymatic fuel cell, an application was carried out on a relevant pharmaceutical formulation containing imipenem, by determining the content of this antibiotic in pharmaceutical products. Data obtained by applying the standard addition test method are reported in
Table 7.
It can be concluded that the recovery data are acceptable. It was also ascertained that the fuel cell method is robust, as small variations of parameters such as temperature, pH, ionic strength, etc., do not appreciably influence the results of the measurements. Finally, a forensic application of the enzymatic fuel cell was tried. At present, there are some ethyl tests for the saliva of drivers available, and it was also established [
50,
51] that the alcohol concentration in saliva is comparable to that in blood or serum. For this purpose, the colorimetric test [
52,
53] is the most applied. It is usually considered as qualitative, at most semi-quantitative, as it is affected by many interferences, such as those from ascorbic acid, polyphenols, uric acid, oxalic acid, bilirubin, tannic acid, mercaptans, and generally strong oxidants [
53]. This colorimetric test it is based on the following reactions:
The ethanol detection range usually falls between 0.02% (light green) and 0.30% (gray-green) (i.e., between about 4.3 × 10
−3 mol·L
−1 and 5.4 × 10
−2 mol·L
−1); therefore, as the linearity range of our enzymatic fuel cell, between about 5 × 10
−4 mol·L
−1 and 6 × 10
−1 mol·L
−1, widely covers the whole range of the current colorimetric test, it was decided to simulate ethyl test, with the enzymatic fuel cell, by determining the ethanol content both in saliva and in human serum samples, to which ethanol was added until final concentrations of 1.8 × 10
−3 mol·L
−1 and 1.0 × 10
−2 mol·L
−1, respectively, were reached; this means practically that these enzymatic fuel cell tests were carried out with biological samples having about the lowest and the highest respective concentrations provided usually also by ethyl-colorimetric test. The first results obtained are summarized in
Table 8.
Data in
Table 8 prove an acceptable repeatability of the measurements, while the
t-test shows that the difference between nominal and experimental values is always insignificant. Therefore, although still requiring further investigations, the method is accurate enough.