Abstract
Floral scent is important in plant reproduction and also has aesthetic implications. However, the accurate determination of aroma is presently limited by the available collection and analysis tools. In this study, the floral scents of four crabapple taxa exhibiting faint, weak, clear, and strong scent intensities were comparatively analyzed by electronic nose (E-nose) and gas chromatography–mass spectrometry (GC–MS). The E-nose was able to effectively group the different taxa in the principal component analysis in correspondence with scent intensity. GC–MS analysis identified a total of 60 volatile compounds. The content of nitrogen-containing compounds and aliphatics and the number of unique components of the more aromatic taxa was significantly higher than the less aromatic taxa. α-Cedrene, β-cedrene, 5-methyl-1,3-dihydro-2H-benzimidazol-2-one, benzyl alcohol, linalool, and 4-pyrrolidinopyridine contributed significantly to taxon separation. The pattern recognition results confirmed that the E-nose results corroborated the GC–MS results. Furthermore, partial least squares regression analysis between the aromatic constituents and sensors indicated that particular sensors were highly sensitive to N-containing compounds, aliphatics, and terpenes. In conclusion, the E-nose is capable of discriminating crabapple taxa of different scent intensities in both a qualitative and quantitative respect, presenting a rapid and accurate reference approach for future applications.
1. Introduction
Floral scent plays an important role in the reproductive processes of many plants, as well as in guaranteeing the yield and quality of many economically valuable plants. It also enhances the aesthetic properties of ornamental plants and cut flowers. Traditional scent analysis in plants has typically relied on sensory evaluation methods that perceive types and intensity of odors. Odor intensity is very complex, due to the effect of the odor detection threshold (ODT). ODT is the lowest concentration of a certain odor compound that is perceivable by the human olfactory system. The threshold of a chemical compound may change due to its shape, polarity, partial charges, or the addition of other compounds [1]. Therefore, sensory evaluation has various limitations, including strong subjectivity and poor repeatability.
Chromatographic techniques, such as gas chromatography–mass spectrometry (GC–MS), solid-phase micro extraction (SPME), and headspace analysis, have increasingly been used to identify and quantify the aromatic components of fragrant plants, such as Silene latifolia [2], rose [3], Luculia pinceana [4], and Osmanthus fragrans [5]. However, these methods often fail to provide a global fingerprint of the scent sample, as the compounds detected are typically dependent on the selected sample pretreatment method. Furthermore, they are hampered by their complex technology, high running costs, and prolonged analysis time. In response to chromatographic technologies, an electronic nose (E-nose) has emerged as an olfactory simulation test tool that allows for the high-throughput analysis of volatile organic compounds in a complex matrix [6]. Using specific nano sensor arrays which reflect the changes in conductivity produced by the adsorption of compounds together with pattern recognition software, a global fingerprint of the volatile components in a sample can be obtained. In contrast to chromatographic techniques, which focus on the separation and detection of individual chemical components, the E-nose relies on response and recognition technology, which can rapidly identify and separate complex odors [6]. In addition, the E-nose is able to accurately distinguish the odor of complex samples at a low cost without the need to quantitatively analyze each individual component in the test sample, as is required for GC–MS. Thus far, the E-nose has primarily been used in food processing [7], evaluating the shelf-life of fruits, vegetables [8,9], meat, and aquatic products [10,11], and determining the authenticity of tobacco, alcohol, and other beverages [12,13]. Scholars have recently attempted to train the E-nose to predict the response of the human sensory system to particular odors. For instance, the E-nose was trained to predict odor pleasantness [14] and was found to correlate well with the human data (above the 0.60 level) for single-component odorants [15]. However, none of the models in the pattern recognition software are able to accurately predict the human values or pleasantness for more than a few descriptors. Hence, the prediction of human sensory ratings from instrumental measurements is still arguably the greatest challenge of sensor-based machine olfaction. With regard to floral scent detection, the E-nose has been applied to germplasm differentiation [16,17], flowering stage distinction [18], and flower organ differentiation [19]. If the E-nose can be applied to the fast screening of the interested taxon, such as a strong scent taxon or a special floral scent type, and accurately mapped to sensory evaluation, it would have very important applications in the evaluation of floral scent.
E-nose technology has not been previously applied to the evaluation of scent intensity in crabapple. In this study, using headspace solid-phase micro extraction coupled with gas chromatography–mass spectrometry (HS–SPME)-GC–MS in conjunction with the E-nose, we evaluated the scent characteristics of crabapple (Malus, Rosaceae) taxa of different scent intensities. The aims of the study were as follows: (1) to evaluate the main differences in compounds among the different taxa; (2) to assess the ability of the E-nose to distinguish the different taxa; and (3) to explore the relationship between the detected compounds and the different E-nose sensors in order to assess the potential functionality of the E-nose in floral fingerprinting, scent type classification, and scented flower breeding.
2. Materials and Methods
2.1. Plant Material
More than one hundred Malus taxa scent intensities are evaluated by 30 trained assessors (unpublished) using a 6-point scale method [20]. Based on the sensory evaluation results, four taxa with higher ornamental value, M. ‘Hillieri’, M. sylvestris, M. ‘Van Eseltine’, and M. ‘Brandywine’, had different scent intensities, categorized as faint, weak, clear, and strong, respectively.
Fresh early-flowering inflorescences of these four Malus taxa were collected from the National Crabapple Germplasm Genetic Database (Yangzhou City, Jiangsu Province, China). Each taxon was represented by three different plants. The plants were situated more than 50 m apart, and 10 inflorescences per plant were randomly selected for analysis. Inflorescences from the different taxa were separately placed into deionized water before being transported to the Nanjing Forestry University (Nanjing, China), where they were maintained at room temperature (25 ± 1 °C).
2.2. Floral Scent Determination
On the following day, the experiments were carried out at 8:00 a.m.–11:00 a.m. Approximately 4 g of fully expanded flowers were placed into a 200 mL capped SPME vial. After approximately 30 min equilibration between the flower and the headspace, the floral scent was analyzed using the E-nose and HS–SPME-GC–MS. Three replicates were tested for each taxon (4 g flowers from 10 inflorescences per replicate).
2.2.1. E-Nose Analysis
A PEN3 portable E-nose (Airsense Company, Schwerin, German) was used in the experiment. The basic structure of this device consists of a sensor array unit, a sampling apparatus, and pattern-recognition software (Win Muster v.1.6). The sensor array includes 10 metal oxide semiconductor (MOS) sensors, the characteristics of which are indicated in Table 1 [21,22]. The sensor response is reflected as resistivity (Ohm) and relies on the changes in conductivity produced by the adsorption of chemical molecules in the gas state and on the subsequent surface chemical reaction or physical effect.

Table 1.
Sensors used in this study and their main application in PEN3.
During the measurement process, the headspace gas was pumped into the sensor chamber at a constant rate of 150 mL·min−1. The measurement phase lasted 50 s, which is sufficient to reach a stable state. The interval time was 10 s. In this study, the stabilized response sensor values were selected at 46–48 s to analyze the pattern recognition. To return the sensors to the baseline, a 300 s cleaning phase was undertaken after each measurement. Three replicates were tested for each taxon.
2.2.2. HS-SPME-GC-MS Analysis
HS-SPME extraction was performed using a 65 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) SPME filed portable sampler(Supelco, Bellefonte, PA, USA), which could be capable of retaining volatile compounds for up to two weeks without significant loss [23]. The fiber was exposed to the headspace of the capped vial to absorb volatile compounds for 0.5 h at room temperature (25 ± 1 °C). Following volatile component absorption, the needle of the SPME was inserted into the GC. In addition, an empty capped vial was used as a blank control, and the samples were injected into the GC in a random fashion. All scent of the samples was extracted at the same time by 12 SPME filed portable samplers, then stored in dry ice, and measured in one day.
The GC system (Thermo Fisher Scientific, Waltham, MA, USA) was equipped with a DB-5MS fused silica capillary column (5% phenylmethyl siloxane, 30 m × 0.25 mm i.d.; 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Following HS-SPME extraction, the fiber that had been exposed to the headspace was inserted into the GC injector port for desorption at 250 °C for 5 min in splitless mode. Helium was used as the carrier gas at a constant flow rate of 1.0 mL·min−1. The column oven temperature program was as follows: 50 °C for 1 min, increasing thereafter at 4 °C·min−1 to 120 °C and then held for 1 min, followed by an increase at 1.5 °C·min−1 to 140 °C, and then a final increase at 12 °C·min−1 to 230 °C, with no hold. The temperature of the transfer line and ion source were 230 and 210 °C, respectively. The electron ionization potential of the mass detector was 70 eV and the scan range was from 35 to 450 amu. Linear retention indices (LRI) of the volatile compounds were calculated using an alkane series standard (C5–C30) (Sigma, St. Louis, MO, USA) under the same conditions. Identification of volatile compounds was made by comparing the mass spectra with the National Institute of Standards and Technology (NIST) 12 library (similarity > 75%) and previous reports on linear retention indices, as well as published index data (SuperScent: http://bioinf-applied.charite.de/superscent/index.php?site=scentsearch; PubChem: http://pubchem.ncbi.nlm.nih.gov/; ScentBase: http://www2.dpes.gu.se/SCENTbase.html). Therefore, in our study, no standard was used, and that the identifications are tentative, based only on MS similarity and LRI. Each taxon sample has three replicates, and mean values with relative standard deviations (mean standard deviation, %) were reported. The relative contents of each volatile constituent were calculated by normalizing the peak area (Xcalibur 3.1 (Thermo Fisher Scientific, Waltham, MA, USA)).
2.3. Data Analysis
A biplot, which is the combination of a score and loading plot, was used to illustrate the principal component analysis (PCA) and partial least squares regression (PLSR) results. The non-supervised PCA was used to reveal the distribution of the samples and determine the factors that contributed most to the data separation. This method was used to evaluate the separation of the different taxa based on the E-nose sensors, as well as the contribution of the compounds to the observed data separation. PLSR was used to assess the correlation among the different taxa, E-nose sensors, and volatile compounds. The jack-knife method was used to assess the significance of the variables. PCA, PLSR, and jack-knife significance testing were performed in The Unscrambler software v. 10.4 (CAMO, Oslo, Norway; http://www.camo.com/). The metabolite variance between the different taxa was analyzed using SPSS v. 19.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Discrimination of the Different Taxa Using the E-Nose
PCA was used to evaluate the separation of the different taxa by the E-nose (Figure 1). The first two principal components (PCs) accounted for 98% of the total variance. The four taxa were clearly separated in the plot and were located in both the positive and negative axes of PC1 (91%). The scent intensity showed an increasing trend in a negative direction along the x-axis.
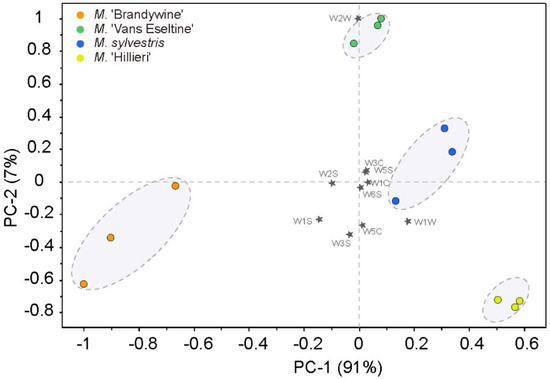
Figure 1.
PCA biplot based on the E-nose data of the flowers of the four Malus taxa.
The contribution of the sensors to the PCA discrimination was assessed (Figure 1). Sensors W1W, W1S, and W2S influenced PC1 more heavily, whereas sensors W2W, W3S, and W5C made a significant contribution to PC2, as indicated by their longer projections on the axes. The sensor W1W is sensitive to terpenes and sulfurous organic compounds, W1S is sensitive to broad-range methane, and W2S is sensitive to alcohols. Sensors W2W, W3S, and W5C are sensitive to sulfurous organic compounds, methane, and nitrogen oxides, respectively (Table 1). This indicated that terpenes, S-containing compounds, aliphatics, N-containing compounds, and alcohols were probably responsible for the observed separation.
3.2. Discrimination of the Different Taxa Using GC–MS
3.2.1. Identification and Comparison of the Volatile Compounds among the Different Taxa
To determine the significant volatile components, the identified aromatic compounds and their relative contents (%) were summarized (Figure 2, Table 2). A total of 60 volatile compounds were putatively identified in the four taxa. There were significant differences in the types and relative contents of volatile compounds in the flowers from the different taxa. The main volatile components in M. ‘Brandywine’ was 5-methyl-1,3-dihydro-2H-benzimidazol-2-one (constituting 23.8% of the total content). The primary volatile components in M. ‘Van Eseltine’ also included 5-methyl-1,3-dihydro-2H-benzimidazol-2-one, as well as linalool and benzyl alcohol (constituting 50.1% of the total). Benzyl alcohol also constituted the primary volatile compounds in in M. sylvestris, along with α-cedrene and 4-pyrrolidinopyridine, constituting 46.3% of the total. Similarly, in M. ‘Hillieri’, the main volatiles were benzyl alcohol and α-cedrene (51.7% of the total content).
Figure 2.
Total ionic chromatogram of the volatile compounds emitted from the flowers of the four Malus taxa.

Table 2.
Volatile compounds identified in the flowers of four Malus taxa using SPME-GC-MS.
The relative contents of the different chemical classes (aliphatics, benzenoids, terpenes, N-containing compounds, and S-containing compounds) among the four taxa were calculated and compared, and the results are shown in Figure 3. Interestingly, Terpenes, benzenoids and N-containing compounds were the highest in all four taxa. Among the most aromatic taxa (‘Brandywine’ and ‘Van Eseltine’), the content of N-containing compounds and aliphatics was significantly higher than in the other less aromatic taxa (Figure 3A). The volatile composition of M. sylvestris and M. ‘Hillieri’ was similar, and these two taxa shared 23 compounds in common and also exhibited a greater diversity of compounds than ‘Brandywine’ and ‘Van Eseltine’ (Figure 3B). Seven compounds were shared between the four taxa (dodecane, linalool, α-cedrene, β-cedrene, geranylacetone, 4-pyrrolidinopyridine, and cocarboxylase). ‘Brandywine’, which is the most aromatic taxon, possessed 13 unique compounds, which was higher than the other taxa (Figure 3B).
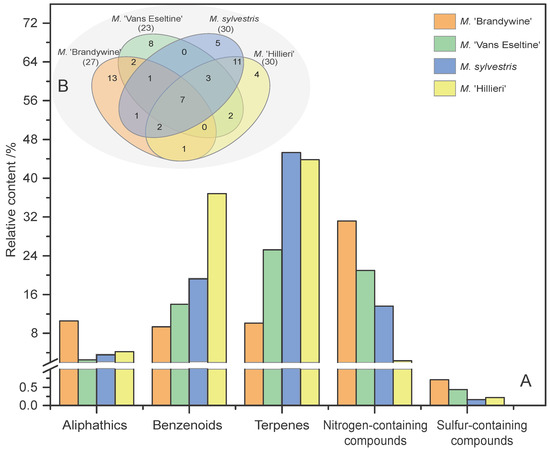
Figure 3.
Statistical analysis of the volatile compounds present in the flowers of the four Malus taxa. (A) Compounds of different chemical classes among the four taxa. (B) Venn diagram indicating the similarities and differences in total volatile compounds among the different taxa. The numbers in related overlapping areas indicate the compounds shared between the different taxa.
3.2.2. PCA Based on the GC–MS Data
To determine the volatile compounds that play a critical role in differentiating scent intensity, the 60 compounds identified using SPME–GC–MS were subjected to PCA. The four taxa could be clearly discriminated based on the first two PCs, which explained 91% of the total variance (Figure 4). Scent intensity increased in a positive direction along the x-axis. Several compounds were found to contribute significantly to the discrimination between the four taxa. 5-methyl-1,3-dihydro-2H-benzimidazol-2-one, benzyl alcohol, and α-cedrene contributed greatly to PC1, whereas linalool, benzyl alcohol, 4-pyrrolidinopyridine, α-cedrene, and β-cedrene showed a high contribution to PC2. These six compounds were predominantly N-containing compounds, terpenes, and alcohols, which corroborates our results obtained in Section 3.1, above. The relative contents of these six compounds were high, accounting for 33% to 48% of the total content. These findings allowed for an evaluation of the correlation between the effective volatile compounds and the sensors.
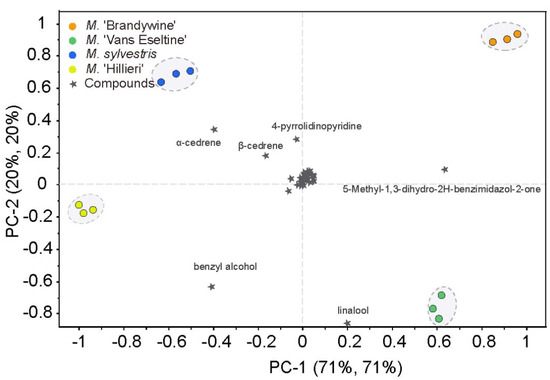
Figure 4.
PCA biplot based on the scent compounds of the flowers of the four Malus taxa.
3.3. Correlation between E-Nose and GC–MS
PLSR was used to compare the E-nose measurements and volatile compounds detected by GC–MS. The regression coefficients obtained from the jack-knife significance testing indicated that some of the X-variables (compounds) were significantly correlated (p ≤ 0.05) with one or more of the 10 sensors (Figure 5). In PC1, aliphatics, N-containing compounds, S-containing compounds, and sensors W5C, W1S, W2S, W2W, and W3S were all located in the right section of the plot and explained between 50% and 100% of the cross-validated variance, indicating that these variables were significantly positively correlated (p ≤ 0.05).
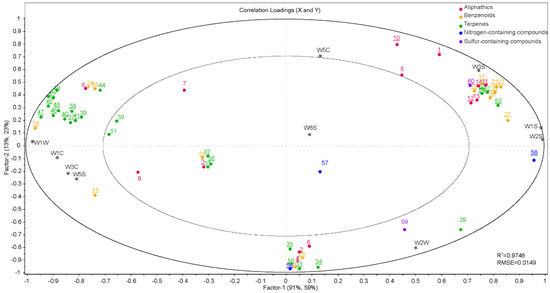
Figure 5.
PLSR correlation loadings plot of the sensory attributes, E-nose sensors, and selected compounds of the four Malus taxa. The numbers 1–60 correspond to the compound codes indicated in Table 2.
Similarly, terpenes were significantly correlated with sensors W1C, W3C, W5S, and W1W (p ≤ 0.05). In addition, benzenoids (alcohols) were also positively associated with sensors W5C, W1S, W2S, W2W, W3S and sensors W1C, W3C, W5S, W1W (p ≤ 0.05). However, most benzenoid alcohols are concentrated on the left-hand side of the plot, particularly near sensor W2S (which is sensitive to alcohol), while other benzenoid alcohols are distributed on the right-hand side of the plot.
4. Discussion
4.1. Correlation between the Scent Discrimination of the E-Nose and Sensory Evaluation
The human olfactory system is highly nonlinear in many respects [38,39], and cross-adaptation, masking, and other processes involved in the human perception of odors further complicate the signal processing in olfaction [40,41]. In addition, sensory evaluation is subjective and depends on the long-term accumulation of human practices and behavior, and thus data standardization is challenging. This study found that the PCA based on the E-nose data and sensory aromatic intensities of the taxa did not exhibit a simple linear correspondence (Figure 1). Furthermore, the contribution of the sensors W1S, W2S, and W1W, which are sensitive to broad range methane, alcohols, and terpenes as well as sulfur organic compounds, respectively, was higher. E-nose recognition is based on the multi-dimensional response values of the sensor to the aromatic components, thereby obtaining an overall fingerprint of the volatile components in a sample rather than the qualitative and quantitative results of one or more components [6]. Therefore, the intensity of the floral aroma recognized by the E-nose considers both the compound and its concentration concurrently.
4.2. Correlation between the E-Nose and GC–MS Analysis
As GC–MS is widely used in volatiles analysis, it is thus necessary to evaluate the correlation between the E-nose and the GC–MS results. A comparative evaluation of E-nose and GC–MS allowed for an assessment of the contribution of the compounds detected by the E-nose. Different types of E-nose instruments and sensors have various sensitivities to each component [6]. Data were collected by a particular E-nose, and a prediction model was then established to provide a basis for future research on the same material. In this study, we discovered that specific sensors were highly correlated with majority of compounds in crabapple, thereby producing different sensor behaviors and leading to successful scent type differentiation. For example, N-containing compounds, terpenes, and S-containing compounds were highly associated with sensors W5C, W1W, W2S, respectively. This provided a reference for the establishment of the rapid detection of crabapple flower fragrance in the future.
4.3. The Contribution of Compounds to Flower Aroma of Crabapple
The sensory characteristics of floral scent are not only related to the aromatic components and their proportions, but also to the aromatic threshold. The odor detection threshold (ODT) refers to the minimum concentration of a certain volatile compound that is perceivable by the human olfactory system and is the quantitative representation of the intensity of a fragrance [2]. The smaller the threshold, the stronger the aromatic intensity. The threshold for monomeric aromatic substances may change due to the addition of other aromatic substances, i.e., different components may exhibit mutual masking or coordinated enhancement [42]. Studies have shown that a mixture of components with a low ODT has coordinated enhancement, while a mixture with a high ODT will have masking properties [42]. In terms of the aromatic substances detected in this study, the ODTs of 42 of the compounds were found in the literature [24,25,26,27,28,29,30,31,32,33,34,35,36], 32 of which had an ODT less than 1 ppm. Among the six compounds that contributed most highly to the aromatic properties of the taxa, the aromatic thresholds of 5-methyl-1,3-dihydro-2H-benzimidazol-2-one and 4-pyrrolidinopyridine are unknown; the threshold of benzyl alcohol is 5; while the other three compounds (α-cedrene, β-cedrene, and linalool) exhibit extremely low thresholds (ODT < 1 ppm), indicating that these four compounds contribute greatly to the aromatic intensity. Although M. ‘Brandywine’ did not have the greatest number of compounds, this taxon did possess the highest numbers of compounds with low ODTs (i.e., high aromatic intensities), while M. ‘Hillieri’ possessed the least. These findings explain the greater aromatic intensity of M. ‘Brandywine’. Typically, ODT is not included in PCA analyses; however, if the ODT values of all the compounds were measured and combined with the compound detection data in the pattern recognition process, the accuracy of the classification results would be further improved. In addition, there were no obvious differences between the compounds detected in this study and other related studies on apples and crabapples [39,43,44,45,46]. However, the main compounds, constituting the primary compounds detected in these studies, were not completely identical. This phenomenon can primarily be attributed to different sample pretreatment methods. Soxhlet extraction, steam distillation, simultaneous distillation–extraction (SDE), and supercritical fluid extraction (SFE) can be used for fragrance analysis; however, these methods can influence the results and also require many reagents. SPME, dynamic-headspace sampling (DHS), and purge and trap (P&T) can also affect the results due to the selective adsorption of compounds by extraction coating. No standard method for determining floral fragrance thus exists.
5. Conclusions
This study is the first to evaluate the volatile constituents of crabapple flowers using E-nose technology. The results indicated that among the more aromatic taxa, the contents of N-containing compounds and aliphatics were significantly higher than the less aromatic taxa. Furthermore, the most aromatic taxon M. ‘Brandywine’ possessed a significantly higher number of unique compounds than the other taxa. α-Cedrene, β-cedrene, 5-methyl-1,3-dihydro-2H-benzimidazol-2-one, benzyl alcohol, linalool, and 4-pyrrolidinopyridine were found to contribute greatly to the separation of the different taxa. The E-nose was capable of identifying the different crabapple taxa based on their sensory characteristics, and the sensors W1W, W1S, W2S, W2W, W3S, and W5C played an important role in distinguishing the taxa. The correlation between the aromatic constituents and sensors indicated that particular sensors were more sensitive to N-containing compounds, aliphatics, and terpenes. Based on the results obtained in this study, volatile profiling by GC–MS and E-nose in combination with multivariate statistical analysis constitutes a promising tool for an overall quality evaluation of crabapple flower scent.
Author Contributions
W.Z. and J.F. designed the reported study, evaluated the results, prepared and reviewed the manuscript. J.F. is responsible for the entire experiment, analyzed the results, and prepared the manuscript. T.Z., D.Z. (Dandan Zhang) and L.Z. helped J.F. to conduct the experiments. D.Z. (Donglin Zhang) revised the manuscript. W.Z., G.W. and F.C. contributed to planning the reported research, evaluate the results, review and approve the manuscript. All authors both read and approved the manuscript.
Funding
This project is funded by the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD), the National Germplasm Center of Crabapple in China (164010065) and Jiangsu Provincial Science and Technology Department (BE2017375-2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagata, Y.; Takeuchi, N. Measurement of odor threshold by triangle odor bag method. Odor Meas. Rev. 2003, 118, 118–127. [Google Scholar]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, K.; Kovacheva, N. Traditional Rosa damascena flower harvesting practices evaluated through GC/MS metabolite profiling of flower volatiles. Food Chem. 2011, 129, 1851–1859. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.; Wan, Y.; Li, T.; Liu, X.; Sun, Z.; Li, Z. Volatile organic compounds emissions from Luculia pinceana flower and its changes at different stages of flower development. Molecules 2016, 21, 531. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Mai, R.Z.; Zou, J.J.; Zhang, H.Y.; Zeng, X.L.; Zheng, R.R.; Wang, C. Analysis of aroma-active compounds in three sweet osmanthus (Osmanthus fragrans) cultivars by GC-olfactometry and GC-MS. J. Zhejiang Univ. Sci. B 2014, 15, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrezméndez, N.; Vallejocordoba, B.; Gonzálezcórdova, A.F.; Nevárezmoorillón, G.V.; Riverachavira, B. Evaluation of aroma generation of Lactococcus lactis with an electronic nose and sensory analysis. J. Dairy Sci. 2008, 91, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Buratti, S.; Spinardi, A.; Mannino, S.; Mignani, I. Electronic nose as a non-destructive tool to characterise peach cultivars and to monitor their ripening stage during shelf-life. Postharvest Biol. Technol. 2008, 47, 181–188. [Google Scholar] [CrossRef]
- Plotto, A.; Ducamp, M.N.; Lebrun, M.; Goodner, K.; Baldwin, E. Discrimination of mango fruit maturity by volatiles using the electronic nose and gas chromatography. Postharvest Biol. Technol. 2008, 48, 122–131. [Google Scholar] [CrossRef]
- Natale, C.D.; Olafsdottir, G.; Einarsson, S.; Martinelli, E.; Paolesse, R.; D’Amico, A. Comparison and integration of different electronic noses for freshness evaluation of cod-fish fillets. Sens. Actuators B 2001, 77, 572–578. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Chanie, E.; Westad, F.; Jonsdottir, R.; Thalmann, C.R.; Bazzo, S.; Haugen, J.E. Prediction of microbial and sensory quality of cold smoked Atlantic salmon (Salmo salar) by electronic nose. J. Food Sci. 2005, 70, S563–S574. [Google Scholar] [CrossRef]
- Lozano, J.; Fernández, M.J.; Fontecha, J.L.; Aleixandre, M.; Santos, J.P.; Sayago, I.; Arroyo, T.; Cabellos, J.M.; Gutiérrez, F.J.; Horrillo, M.C. Wine classification with a zinc oxide saw sensor array. Sens. Actuators B 2006, 120, 166–171. [Google Scholar] [CrossRef]
- Aleixandre, M.; Lozano, J.; Gutiérrez, J.; Sayago, I.; Fernández, M.J.; Horrillo, M.C. Portable e-nose to classify different kinds of wine. Sens. Actuators B 2008, 131, 71–76. [Google Scholar] [CrossRef]
- Bult, J.H.; Schifferstein, H.N.; Roozen, J.P.; Boronat, E.D.; Voragen, A.G.; Kroeze, J.H. Sensory evaluation of character impact components in an apple model mixture. Chem. Sens. 2002, 27, 485–494. [Google Scholar] [CrossRef]
- Khan, R.M.; Luk, C.H.; Flinker, A.; Aggarwal, A.; Lapid, H.; Haddad, R.; Sobel, N. Predicting odor pleasantness from odorant structure: Pleasantness as a reflection of the physical world. J. Neurosci. 2007, 27, 10015–10023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, Y.; Zhang, Q.; Liu, X.; Li, F.; Chen, K. Fragrance discrimination of Chinese cymbidium, species and cultivars using an electronic nose. Sci. Hortic. A 2014, 172, 271–277. [Google Scholar] [CrossRef]
- Fujioka, K.; Shirasu, M.; Manome, Y.; Ito, N.; Kakishima, S.; Minami, T.; Tominaga, T.; Shimozono, F.; Iwamoto, T.; Ikeda, K.; et al. Objective display and discrimination of floral odors from Amorphophallus titanum, bloomed on different dates and at different locations, using an electronic nose. Sensors 2012, 12, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.; Bhattacharyya, N.; Ghosh, A.; Tudu, B.; Bandyopadhyay, R.; Ghosh, A.; Biswas, S.P.; Majumdar, S. Fragrance profiling of Jasminum sambac Ait. flowers using electronic nose. IEEE Sens. J. 2017, 17, 160–168. [Google Scholar] [CrossRef]
- Su, Y.K.; An, H.R.; Park, P.M.; Yun, S.B.; Kwon, O.K.; Park, S.Y.; Park, P.H. Analysis of floral scent patterns in flowering stages and floral organs of Maxillaria using an electronic nose. Flower Res. J. 2016, 24, 171–180. [Google Scholar] [CrossRef]
- Morinaka, Y.; Handa, T.; Takeuchi, H.; Ayabe, S.; Saito, S. Validity of the sensory evaluation scales for fresh flower scent. J. Jpn. Soc. Hortic. 2008, 70, 636–649. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J.; Gao, L. Qualification and quantisation of processed strawberry juice based on electronic nose and tongue. Food Sci. Technol. 2015, 60, 115–123. [Google Scholar] [CrossRef]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Electronic nose technique potential monitoring mandarin maturity. Sens. Actuators B 2006, 113, 347–353. [Google Scholar] [CrossRef]
- Chen, Y.; Pawliszyn, J. Solid-phase microextraction field sampler. Anal. Chem. 2004, 76, 6823–6828. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media, 3rd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2003. [Google Scholar]
- Aaby, K.; Haffner, K.; Skrede, G. Aroma quality of Gravenstein apples influenced by regular and controlled atmosphere storage. Food Sci. Technol. 2002, 35, 254–259. [Google Scholar] [CrossRef]
- Larsen, M.; Poll, L. Odour thresholds of some important aroma compounds in strawberries Geruchsschwellen einiger wichtiger Aromastoffe der Erdbeeren. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1992, 195, 120–123. [Google Scholar] [CrossRef]
- Pino, J.A.; Quijano, C.E. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Sci. Technol. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Devos, M.; Patte, F.; Rouault, J.; Laffort, P.; Van Gemert, L.J. Standardized Human Olfactory Thresholds; IRL Press at Oxford Press: Oxford, UK, 1990. [Google Scholar]
- Acree, T.E.; Teranishi, R. Flavor Science: Sensible Principles and Techniques; American Chemical Society: Washington, DC, USA, 1993; pp. 259–286. [Google Scholar]
- Pyysalo, T.; Suihko, M.; Honkanen, E. Odour thresholds of the major volatiles identified in cloudberry (Rubus chamaemorus L.) and arctic bramble (Rubus arcticus L.). Lebensm. Wiss. Technol. 1977, 10, 36–39. [Google Scholar]
- Buttery, R.G. Quantitative and sensory aspects of flavor of tomato and other vegertables and fruits. In Flavor Science: Sensible Principles and Techniques; American Chemical Society: Washington, DC, USA, 1993; pp. 259–286. [Google Scholar]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. The volatile constituents in the peel and pulp of a green Thai mango, Khieo Sawoei cultivar (Mangifera indica L.). Food Sci. Technol. Res. 2001, 7, 72–77. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Fazzalari, F.A. Compilation of Odor and Taste Threshold Data; American Society for Testing and Materials: Philadelphia, PA, USA, 1978. [Google Scholar]
- Yuan, G.; Ren, J.; Ouyang, X.; Wang, L.; Wang, M.; Shen, X.; Zhu, B. Effect of raw material, pressing and glycosidase on the volatile compound composition of wine made from goji berries. Molecules 2016, 21, 1324. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Ling, L.C. Characterization of additional volatile components of tomato. J. Agric. Food Chem. 1971, 19, 524–529. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Keller, A.; Gerkin, R.C.; Guan, Y.; Dhurandhar, A.; Turu, G.; Szalai, B.; Vens, C. Predicting human olfactory perception from chemical features of odor molecules. Science 2017, 361, eaal2014. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Carmel, L.; Sobel, N.; Harel, D. Predicting the receptive range of olfactory receptors. PLoS Comput. Boil. 2008, 4, e18. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Medhanie, A.; Roth, Y.; Harel, D.; Sobel, N. Predicting odor pleasantness with an electronic nose. PLoS Comput. Biol. 2010, 6, e1000740. [Google Scholar] [CrossRef] [PubMed]
- Burl, M.C.; Doleman, B.J.; Schaffer, A.; Lewis, N.S. Assessing the ability to predict human percepts of odor quality from the detector responses of a conducting polymer composite-based electronic nose. Sens. Actuators B 2001, 72, 149–159. [Google Scholar] [CrossRef]
- Bicchi, C.; Joulain, D. Review headspace-gas chromatographic analysis of medicinal and aromatic plants and flowers. Flavour Fragr. J. 1990, 5, 131–145. [Google Scholar] [CrossRef]
- Baraldi, R.; Rapparini, F.; Rossi, F.; Latella, A.; Ciccioli, P. Volatile organic compound emissions from flowers of the most occuring and economically important species of fruit trees. Phys. Chem. Earth B Hydrol. Ocean. Atmos. 1999, 24, 729–732. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, R.; Huang, C.X.; Mao, Z.Q.; Guo, L.; Shen, X. Taxonomic analysis of volatiles emitted by ornamental crabapple flowers. Acta Ecol. Sin. 2014, 34, 213–218. [Google Scholar] [CrossRef]
- Omata, A.; Yomogida, K.; Nakamura, S.; Hashimoto, S.; Koba, S.; Furukawa, K.; Noro, S. Volatile components of apple flowers. Flavour Fragr. J. 1990, 5, 19–22. [Google Scholar] [CrossRef]
- Li, W. HS-SPME-GC-MS analysis of volatile constituents from the flowers and leaves of Malus baccata (Linn.) Borkh. Nat. Prod. Res. Dev. 2012, 24, 490–493. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).