Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optical Gas Sensor Fabrication
2.2. Characterization of the Hybrid Optical Gas Sensor
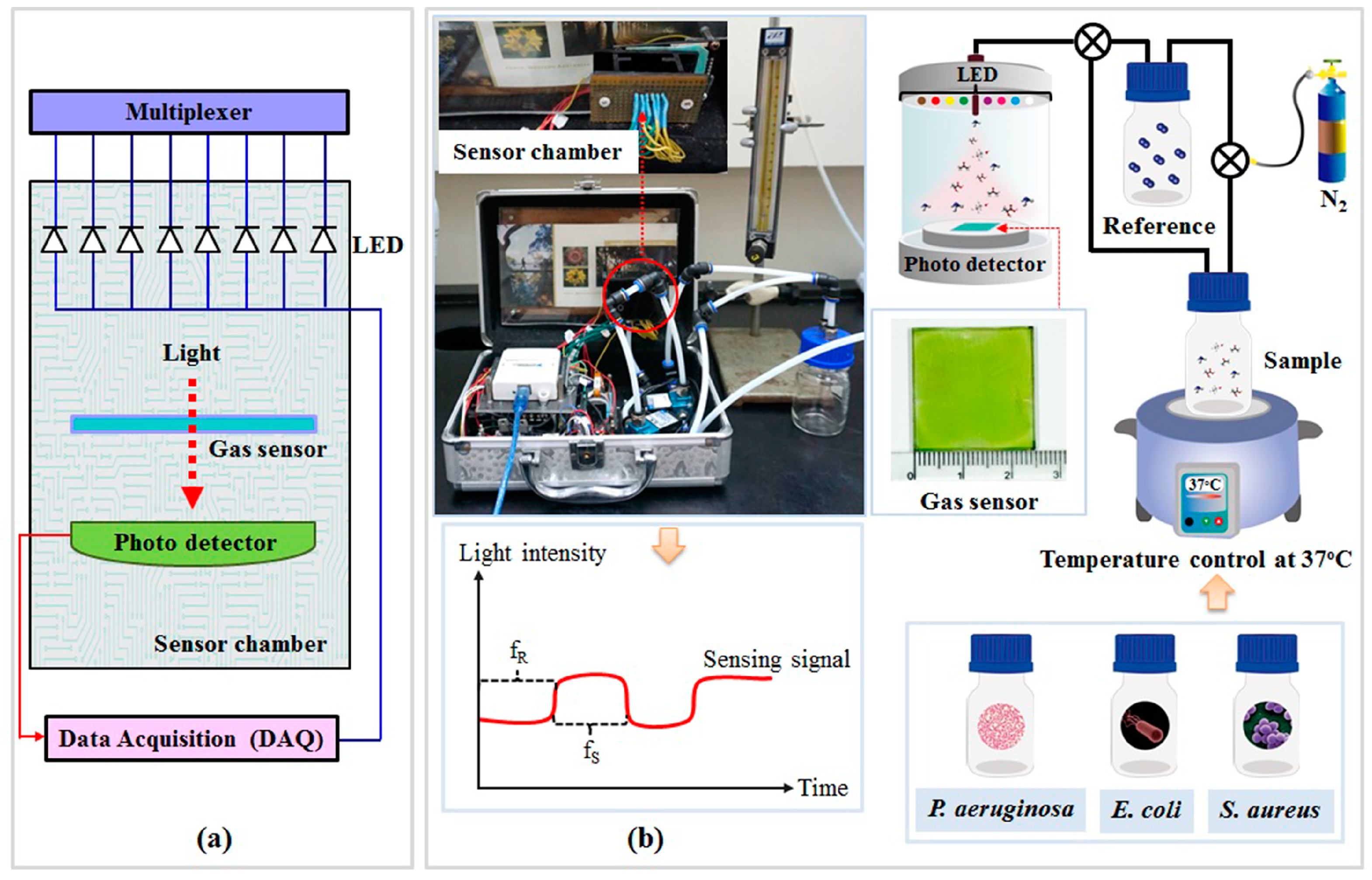
2.3. Optical Artificial Nose System
2.4. Bacterial Cultures
3. Results and Discussion
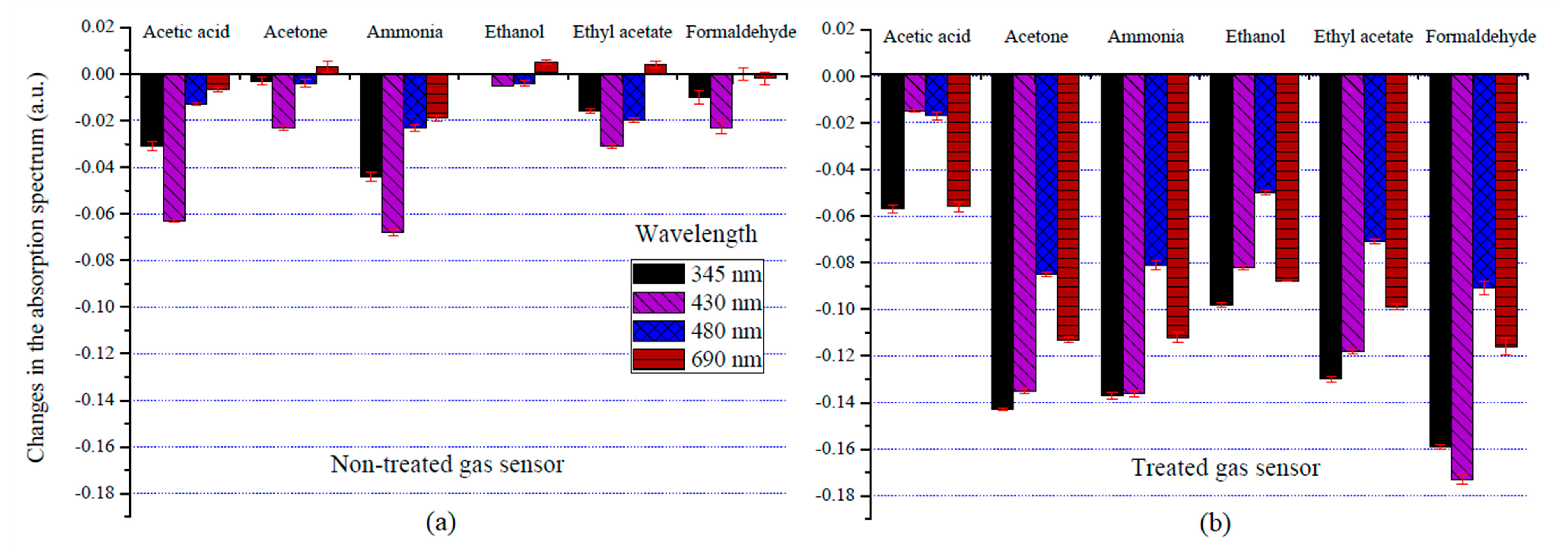
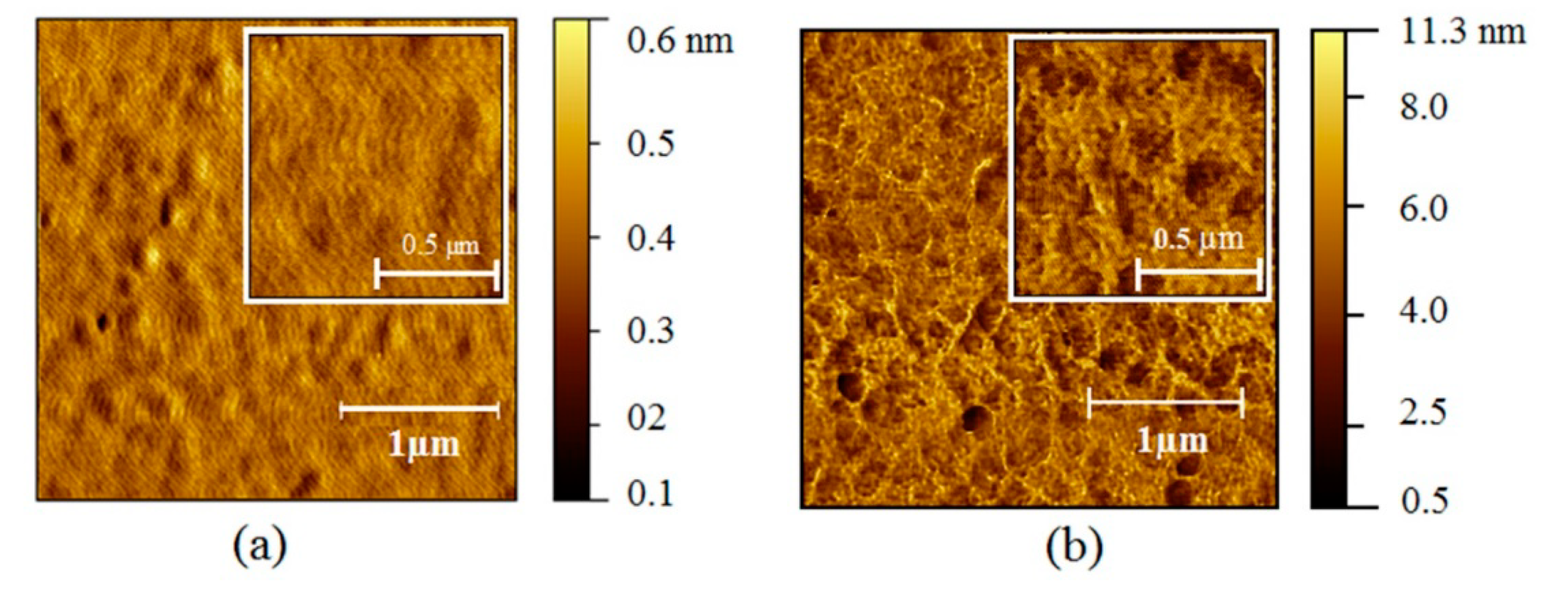
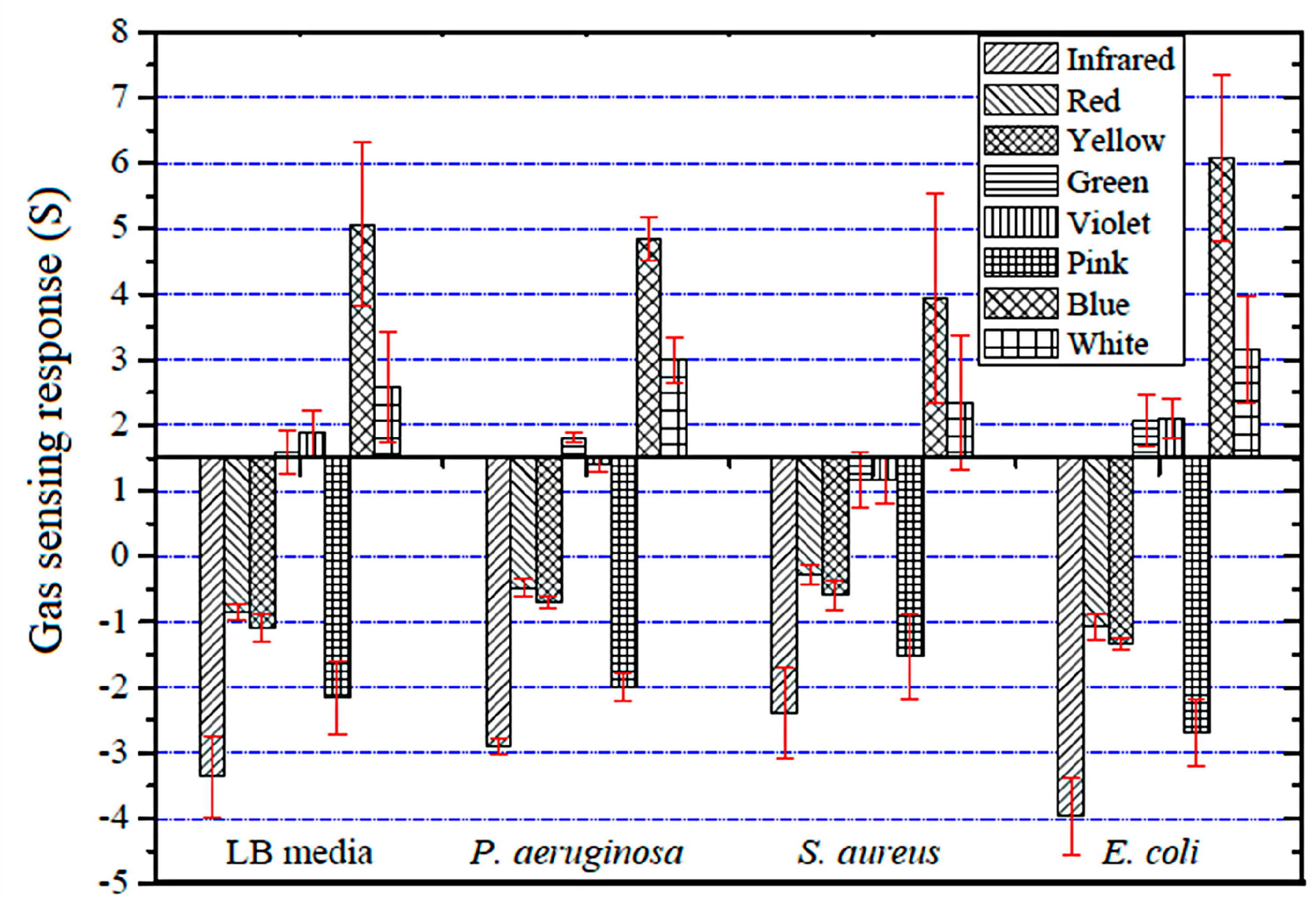
3.1. Optical Gas Sensor Characterization
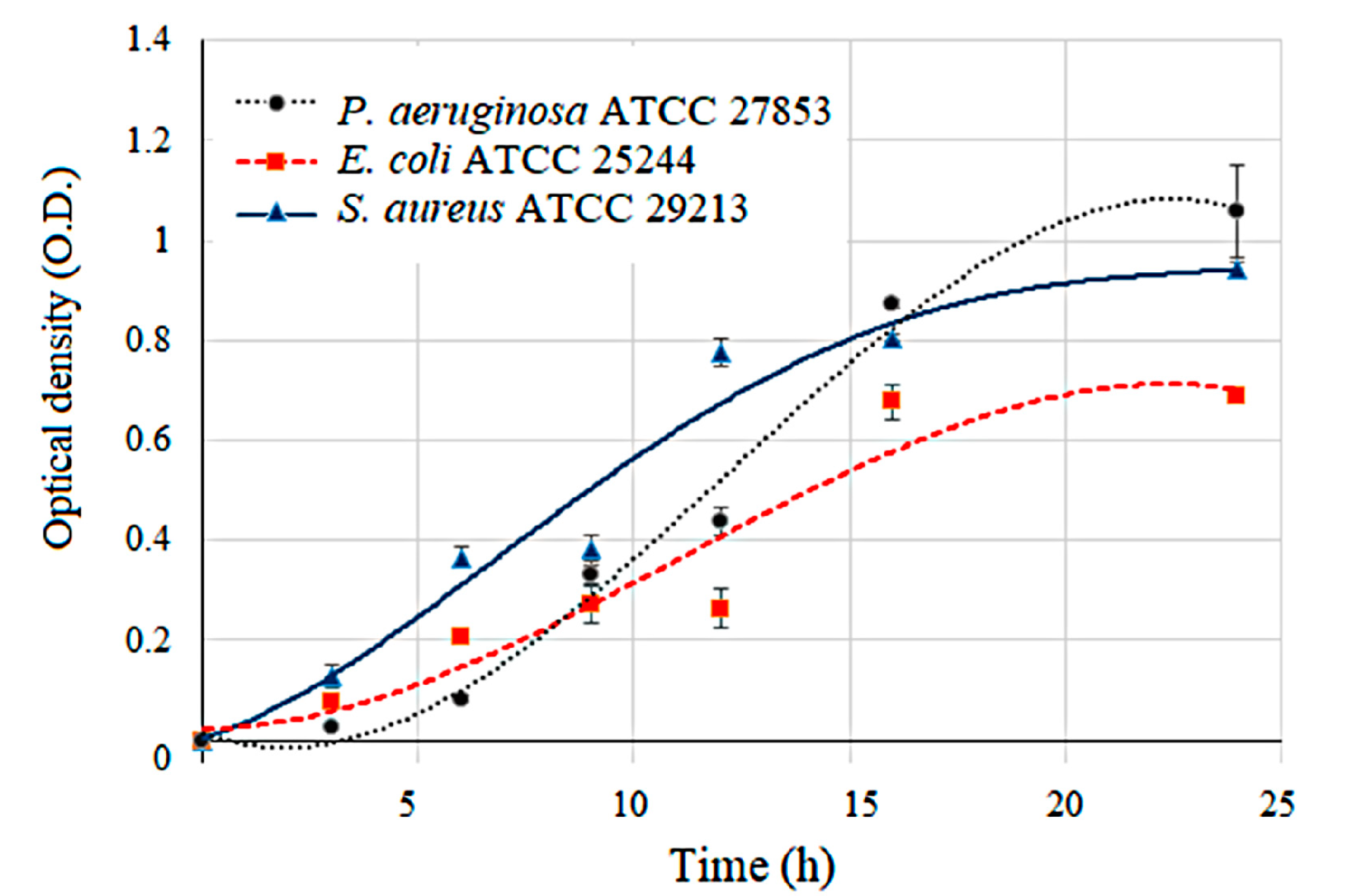
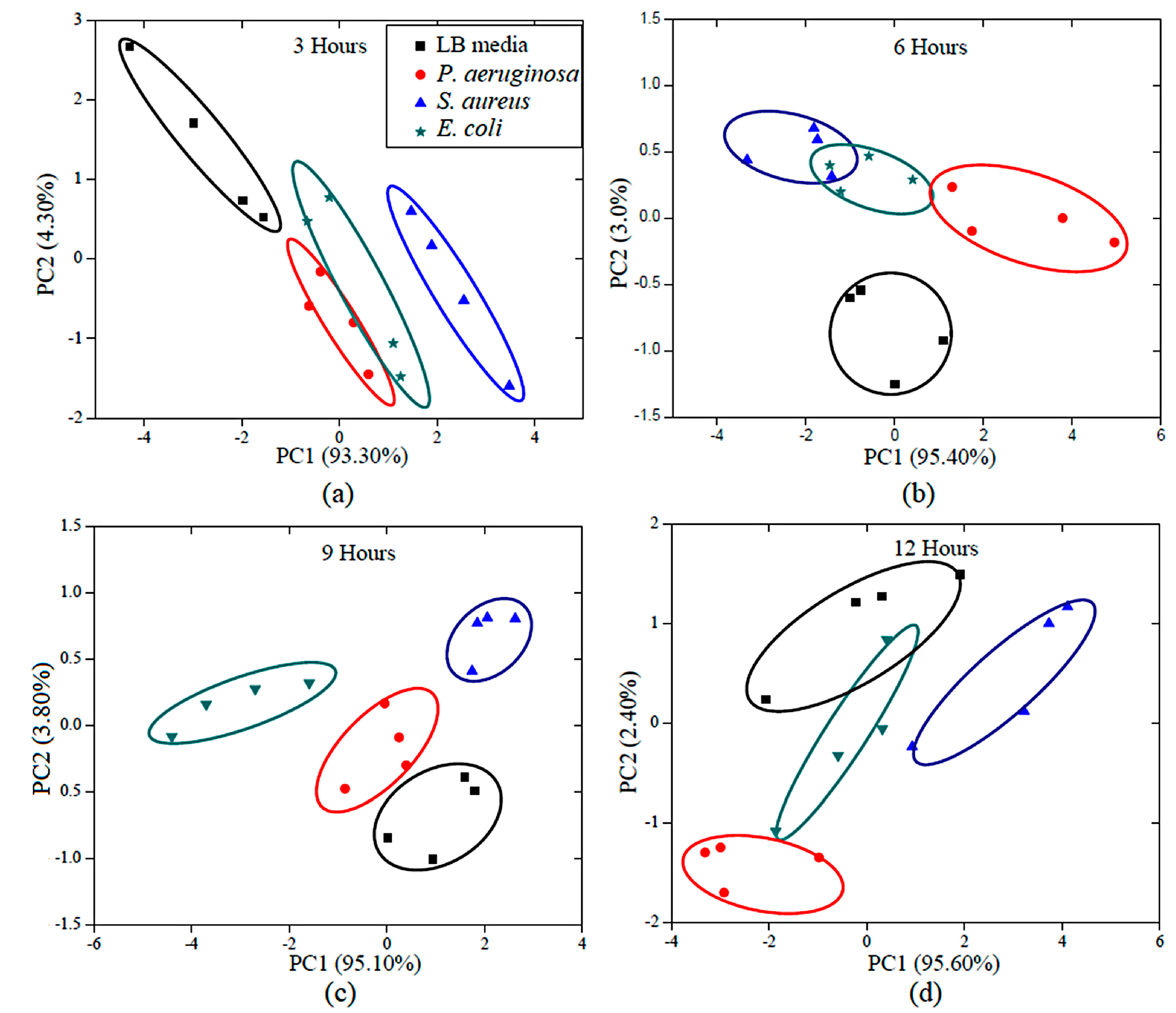
3.2. Bacterial Growth and Pattern Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abeyrathne, C.D.; Huynh, D.H.; McIntire, T.W.; Nguyen, T.C.; Nasr, B.; Zantomio, D.; Chana, G.; Abbott, I.; Choong, P.; Catton, M.; et al. Lab on a chip sensor for rapid detection and antibiotic resistance determination of Staphylococcus aureus. Analyst 2016, 141, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Mellata, M.; Johnson, J.R.; Curtiss, R. Escherichia coli isolates from commercial chicken meat and eggs cause sepsis, meningitis and urinary tract infection in rodent models of human infections. Zoonoses Public Health 2018, 65, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Cho, O.H.; Jung, M.; Suk, K.S.; Lee, J.H.; Park, J.S.; Ryu, K.N.; Kim, S.H.; Lee, S.O.; Choi, S.H.; et al. Clinical characteristics and outcomes of hematogenous vertebral osteomyelitis caused by gram-negative bacteria. J. Infect. 2014, 69, 42–50. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.K.; Yarwood, J.M.; Schlievert, P.M. Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 2001, 55, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Herwald, H.; Mörgelin, M.; Björck, L. Contact activation by pathogenic bacteria: A virulence mechanism contributing to the pathophysiology of sepsis. Scand. J. Infect. Dis. 2003, 35, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, N.; Xu, Y.; Huang, K.; Luo, Y.; Xu, W. Point-of-care and visual detection of P. aeruginosa and its toxin genes by multiple LAMP and lateral flow nucleic acid biosensor. Biosens. Bioelectron. 2016, 81, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Rijal, K.; Mutharasan, R. A method for DNA-based detection of E. coli O157: H7 in a proteinous background using piezoelectric-excited cantilever sensors. Analyst 2013, 138, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Inglis, T.J.J.; Hahne, D.R.; Merritt, A.J.; Clarke, M.W. Volatile-sulfur-compound profile distinguishes Burkholderia pseudomallei from Burkholderia thailandensis. J. Clin. Microbiol. 2015, 53, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Smolinska, A.; Van Berkel, J.J.B.N.; Fijten, R.R.R.; Stobberingh, E.E.; Boumans, M.L.L.; Moonen, E.J.; Wouters, E.F.M.; Dallinga, J.W.; Van Schooten, F.J. Identification of microorganisms based on headspace analysis of volatile organic compounds by gas chromatography-mass spectrometry. J. Breath Res. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, K.; Jaremek, M.; Pohle, R.; Von Sicard, O.; Stuetz, E. In Monitoring of bacterial growth and rapid evaluation of antibiotic susceptibility by headspace gas analysis. Procedia Eng. 2014, 332–335. [Google Scholar] [CrossRef]
- Zscheppank, C.; Wiegand, H.L.; Lenzen, C.; Wingender, J.; Telgheder, U. Investigation of volatile metabolites during growth of Escherichia coli and Pseudomonas aeruginosa by needle trap-GC-MS. Anal. Bioanal. Chem. 2014, 406, 6617–6628. [Google Scholar] [CrossRef] [PubMed]
- Thorn, R.M.S.; Reynolds, D.M.; Greenman, J. Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J. Microbiol. Meth. 2011, 84, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Sterk, P.J.; Schultz, M.J. Volatile metabolites of pathogens: A systematic review. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Tait, E.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Identification of volatile organic compounds produced by bacteria using HS-SPME-GC-MS. J. Chromatogr. Sci. 2014, 52, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Yan, J.; Duan, S.; Wang, L.; Jia, P.; Zhang, S. Enhancing electronic nose performance based on a novel QPSO-KELM model. Sensors 2016, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Li, Q.; Gu, Y.; Jia, J. Classification of multiple Chinese liquors by means of a QCM-based e-nose and MDS-SVM classifier. Sensors 2017, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, M.; Izquierdo, J.; Braga, G.; Dirani, E.; Pereira-da-Silva, M.; Rodríguez, E.; Fonseca, F. Enhanced sensitivity of gas sensor based on poly (3-hexylthiophene) thin-film transistors for disease diagnosis and environment monitoring. Sensors 2015, 15, 9592–9609. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.A.; Al-Shatti, L.A.; Alhajraf, A.F.; Al-Hammad, N.; Al-Awadi, B. The detection of foodborne bacteria on beef: The application of the electronic nose. SpringerPlus 2013, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Taverna, G.; Bellini, A.; Eusebio, L.; Buffi, N.; Lazzeri, M.; Guazzoni, G.; Bozzini, G.; Seveso, M.; Mandressi, A.; et al. Application and uses of electronic noses for clinical diagnosis on urine samples: A review. Sensors 2016, 16, 1708. [Google Scholar] [CrossRef] [PubMed]
- Korposh, S.O.; Takahara, N.; Lee, S.W.; Kunitake, T. Fabrication of optical gas sensors using porphyrin-based nano-assembled thin films: A Comparison with bulk materials. In Proceedings of the 2007 7th IEEE International Conference on Nanotechnology (IEEE-NANO), Hong Kong, China, 2–5 August 2007; pp. 1037–1040. [Google Scholar] [CrossRef]
- Semeano, A.T.S.; Maffei, D.F.; Palma, S.; Li, R.W.C.; Franco, B.D.G.M.; Roque, A.C.A.; Gruber, J. Tilapia fish microbial spoilage monitored by a single optical gas sensor. Food Control 2018, 89, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Fukuda, K.; Sugano, K.; Matsumoto, J.; Yasuda, M.; Fueda, Y.; Shiomori, K. Improvement of UV-stability of porphyrin-type humidity indicator by the addition of UV-absorbents. Kagaku Kogaku Ronbunshu 2017, 43, 123–128. [Google Scholar] [CrossRef]
- Guesmi, A. New fluoro triazol porphyrin-cellulose: Synthesis, characterization and antibacterial activity. Polym. Adv. Technol. 2016, 27, 1517–1522. [Google Scholar] [CrossRef]
- Kantar, C.; Akal, H.; Kaya, B.; Islamoʇlu, F.; Türk, M.; Şaşmaz, S. Novel phthalocyanines containing resorcinol azo dyes; Synthesis, determination of pKa values, antioxidant, antibacterial and anticancer activity. J. Organomet. Chem. 2015, 783, 28–39. [Google Scholar] [CrossRef]
- Kalimuthu, P.; Sivanesan, A.; John, S.A. Fabrication of optochemical and electrochemical sensors using thin films of porphyrin and phthalocyanine derivatives. J. Chem. Sci. 2012, 124, 1315–1325. [Google Scholar] [CrossRef]
- Capuano, R.; Pomarico, G.; Paolesse, R.; Di Natale, C. Corroles-porphyrins: A teamwork for gas sensor arrays. Sensors 2015, 15, 8121–8130. [Google Scholar] [CrossRef] [PubMed]
- Tonezzer, M.; Quaranta, A.; Maggioni, G.; Carturan, S.; Mea, G.D. Optical sensing responses of tetraphenyl porphyrins toward alcohol vapours: A comparison between vacuum evaporated and spin-coated thin films. Sens. Actuators B 2007, 122, 620–626. [Google Scholar] [CrossRef]
- Wang, N.; Liu, H.; Zhao, J.; Cui, Y.; Xu, Z.; Ye, Y.; Kiguchi, M.; Murakoshi, K. Theoretical investigation on the electron transport path through the porphyrin molecules and chemisorption of CO. J. Phys. Chem. C 2009, 113, 7416–7423. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Lutz, M.; Pogfay, T.; Puntheeranurak, T.; Kerdcharoen, T. Hybrid optical-electrochemical electronic nose system based on Zn-porphyrin and multi-walled carbon nanotube composite. J. Nanosci. Nanotechnol. 2012, 12, 5240–5244. [Google Scholar] [CrossRef] [PubMed]
- Mensing, J.P.; Wisitsoraat, A.; Tuantranont, A.; Kerdcharoen, T. Inkjet-printed sol-gel films containing metal phthalocyanines/porphyrins for opto-electronic nose applications. Sens. Actuators B 2013, 176, 428–436. [Google Scholar] [CrossRef]
- Jaisutti, R.; Osotchan, T. An investigation of molecular interactions between zinc phthalocyanine thin film and various oxidizing gases for sensor applications. Adv. Mater. Res. 2012, 403–408, 48–81. [Google Scholar] [CrossRef]
- Liu, C.J.; Shih, J.J.; Ju, Y.H. Surface morphology and gas sensing characteristics of nickel phthalocyanine thin films. Sens. Actuators B 2004, 99, 344–349. [Google Scholar] [CrossRef]
- Sugumaran, S.; Jamlos, M.F.; Ahmad, M.N.; Bellan, C.S.; Sivaraj, M. Thickness and annealing effects on thermally evaporated InZnO thin films for gas sensors and blue, green and yellow emissive optical devices. Opt. Mater. 2016, 58, 342–352. [Google Scholar] [CrossRef]
- Wang, B.; Zuo, X.; Wu, Y.; Chen, Z.; He, C.; Duan, W. Comparative gas sensing in copper porphyrin and copper phthalocyanine spin-coating films. Sens. Actuators B 2011, 152, 191–195. [Google Scholar] [CrossRef]
- Long, J.; Xu, J.; Yang, Y.; Wen, J.; Jia, C. A colorimetric array of metalloporphyrin derivatives for the detection of volatile organic compounds. Mater. Sci. Eng. B Solid 2011, 176, 1271–1276. [Google Scholar] [CrossRef]
- Zetola, N.M.; Modongo, C.; Mathlagela, K.; Sepako, E.; Matsiri, O.; Tamuhla, T.; Mbongwe, B.; Martinelli, E.; Sirugo, G.; Paolesse, R.; et al. Identification of a large pool of microorganisms with an array of porphyrin based gas sensors. Sensors 2016, 16, 466. [Google Scholar] [CrossRef] [PubMed]
- Haugen, J.E.; Rudi, K.; Langsrud, S.; Bredholt, S. Application of gas-sensor array technology for detection and monitoring of growth of spoilage bacteria in milk: A model study. Anal. Chim. Acta 2006, 565, 10–16. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kladsomboon, S.; Thippakorn, C.; Seesaard, T. Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria. Sensors 2018, 18, 3189. https://doi.org/10.3390/s18103189
Kladsomboon S, Thippakorn C, Seesaard T. Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria. Sensors. 2018; 18(10):3189. https://doi.org/10.3390/s18103189
Chicago/Turabian StyleKladsomboon, Sumana, Chadinee Thippakorn, and Thara Seesaard. 2018. "Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria" Sensors 18, no. 10: 3189. https://doi.org/10.3390/s18103189
APA StyleKladsomboon, S., Thippakorn, C., & Seesaard, T. (2018). Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria. Sensors, 18(10), 3189. https://doi.org/10.3390/s18103189





