Whispering-Gallery Mode Resonators for Detecting Cancer
Abstract
:1. Introduction
2. Fundamentals of Optical Resonators
2.1. Performance Parameters of Optical Resonators
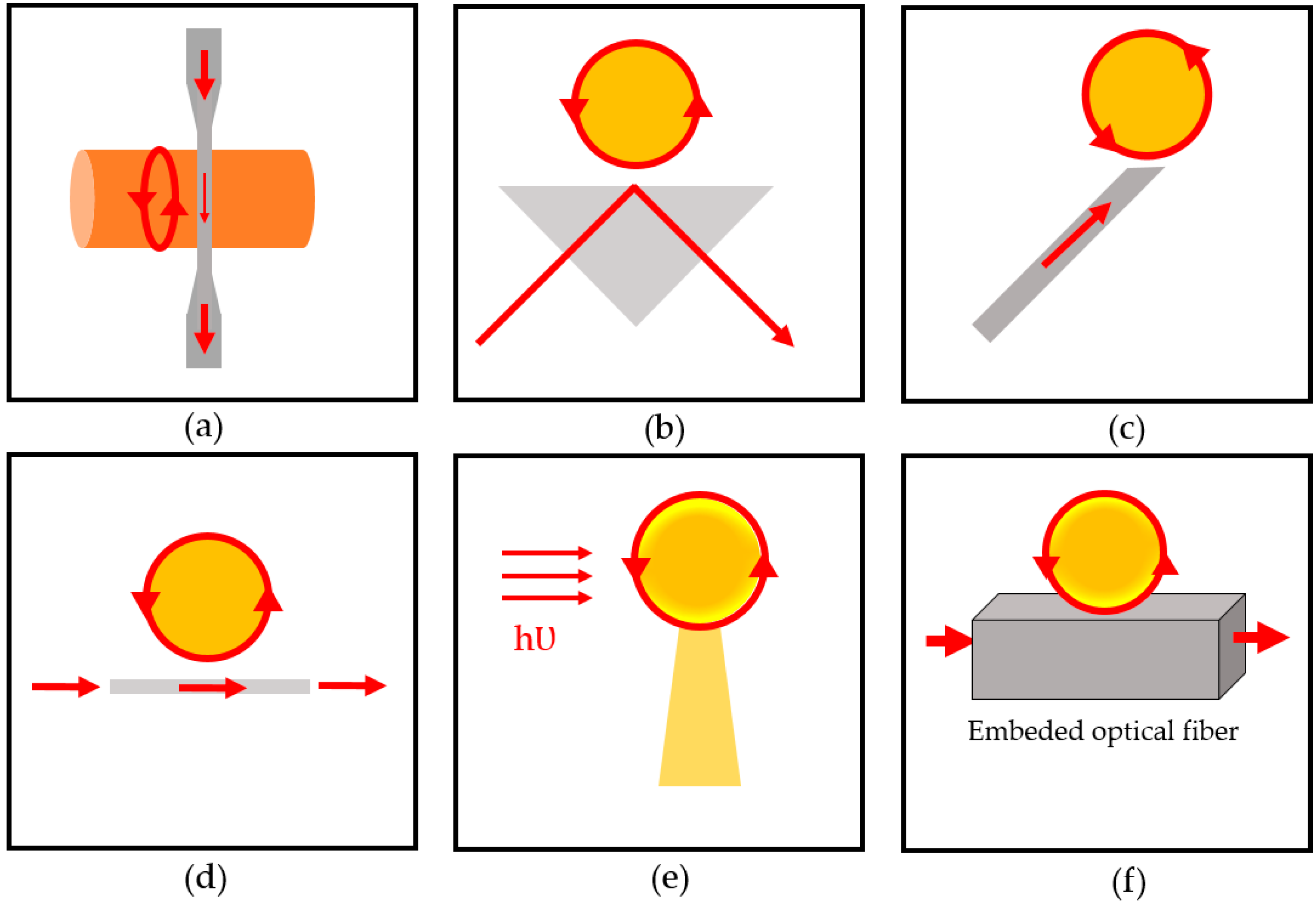
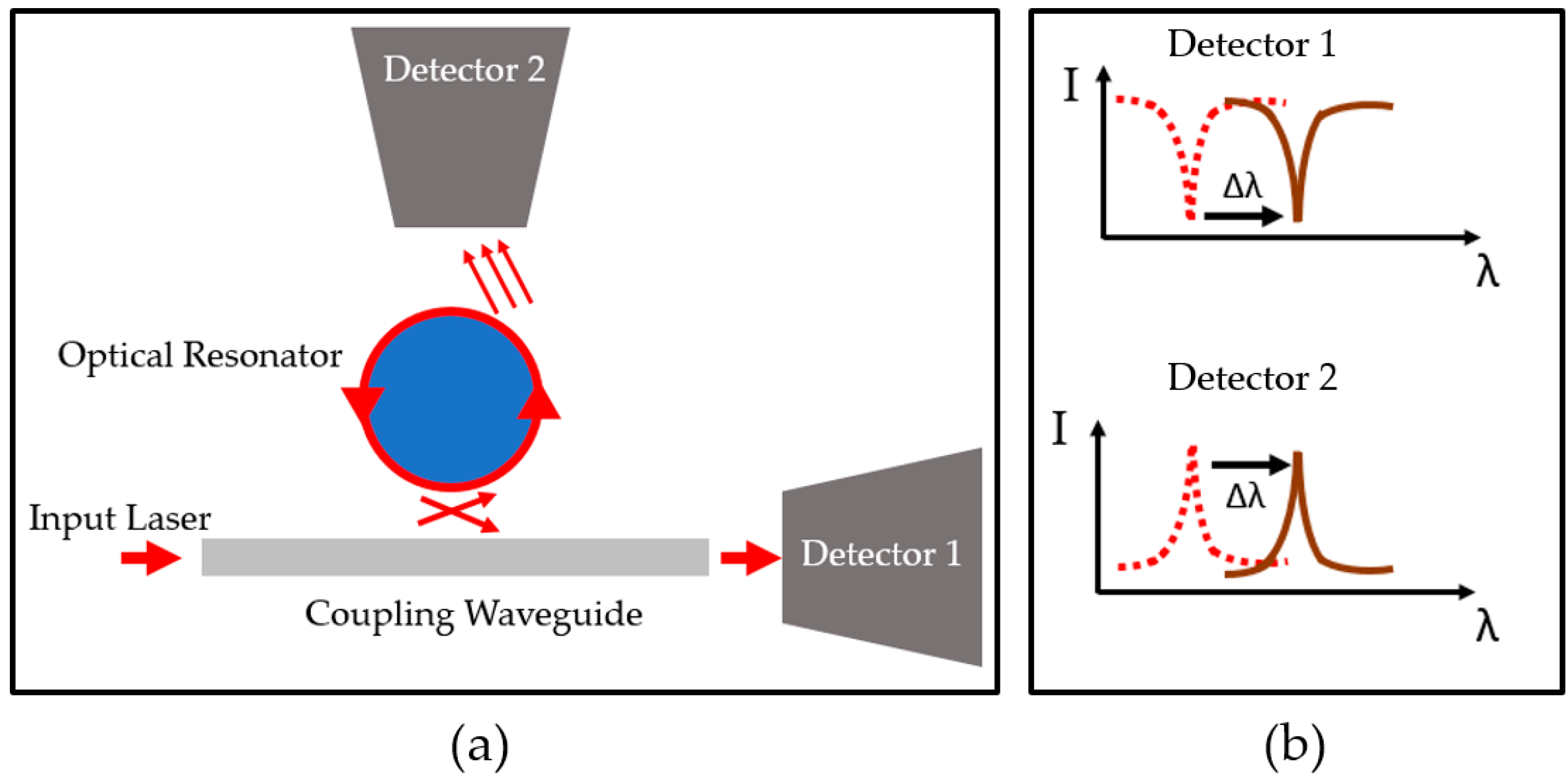
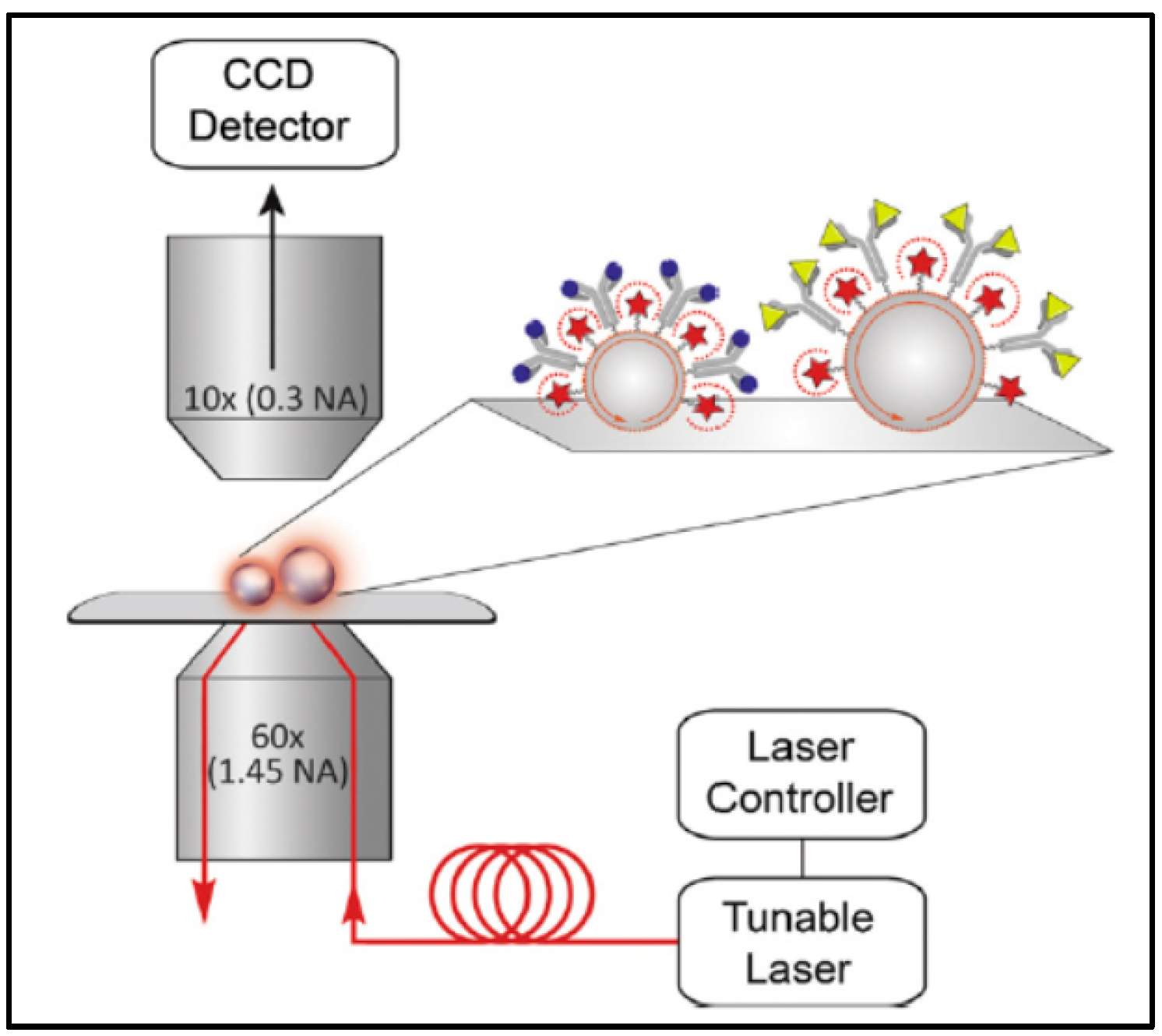
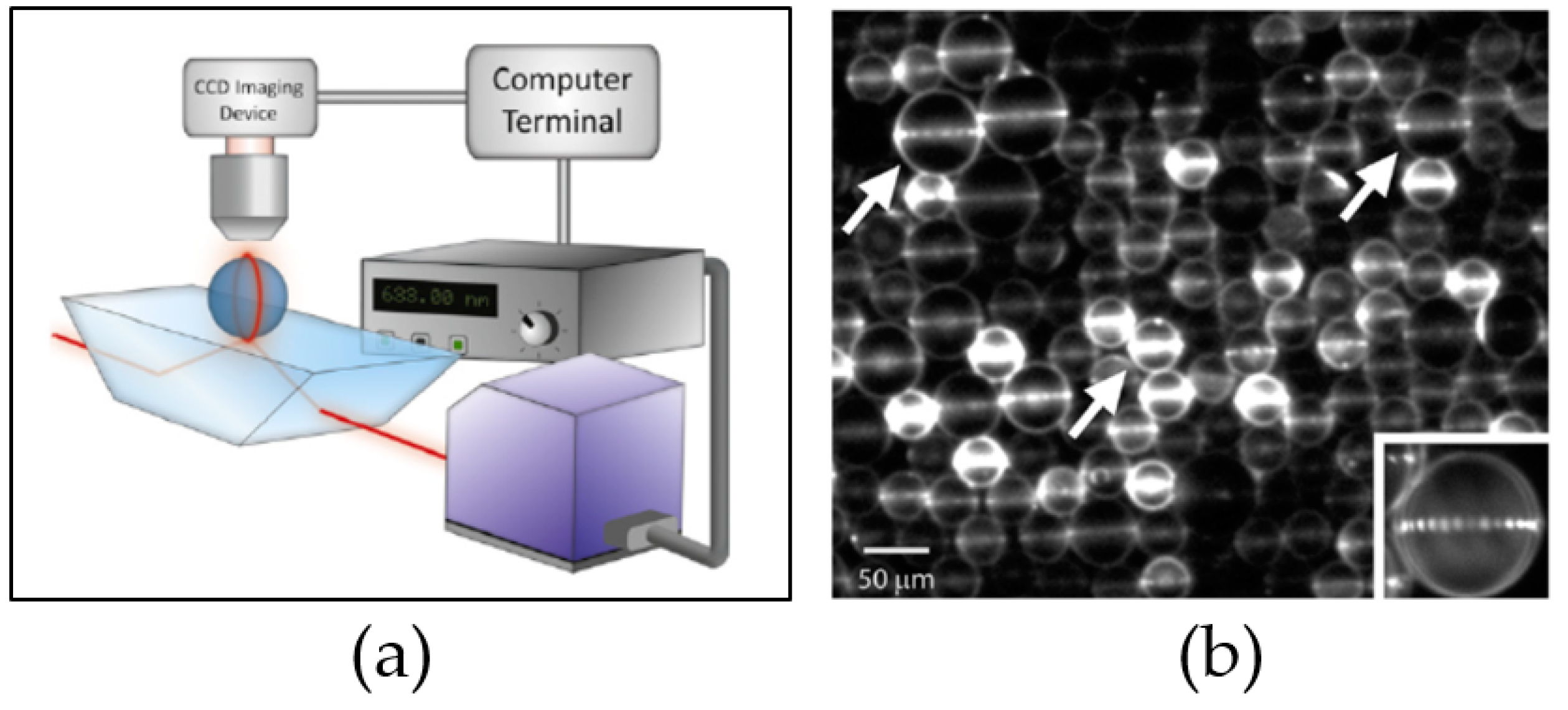
2.2. Light Coupling
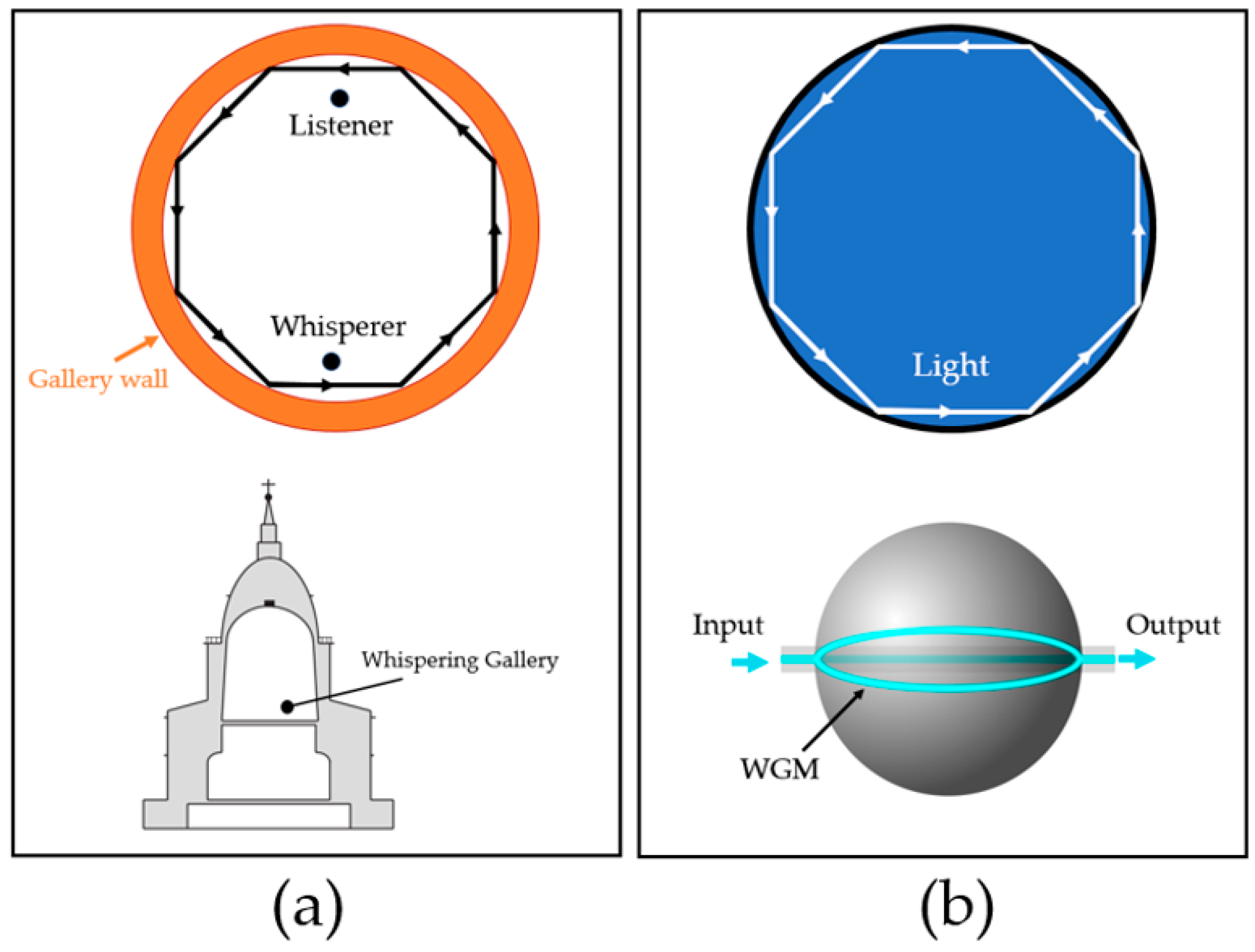
2.3. Whispering-Gallery Mode
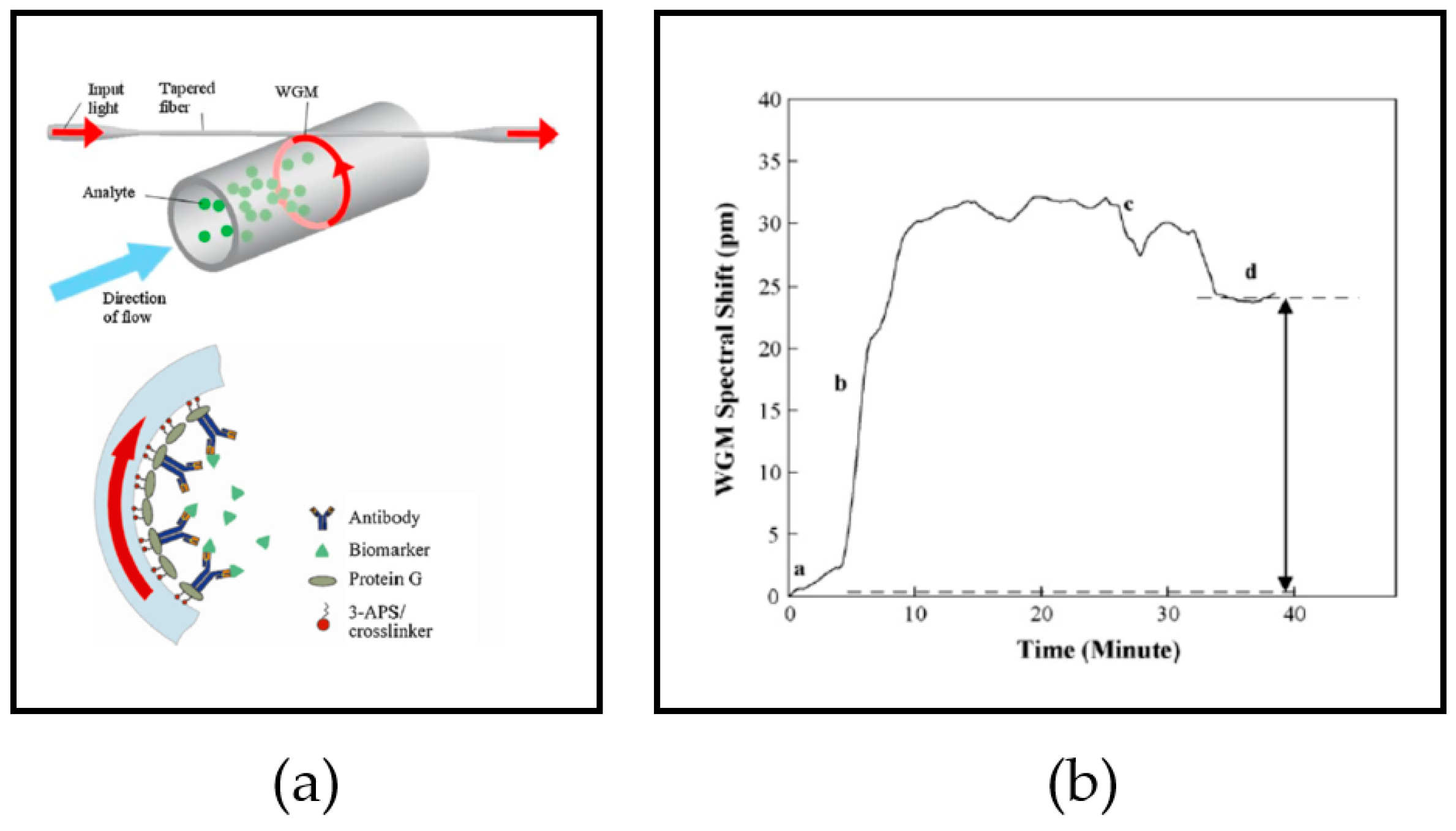
2.4. Detection Mechanism
3. Optical Resonator Types
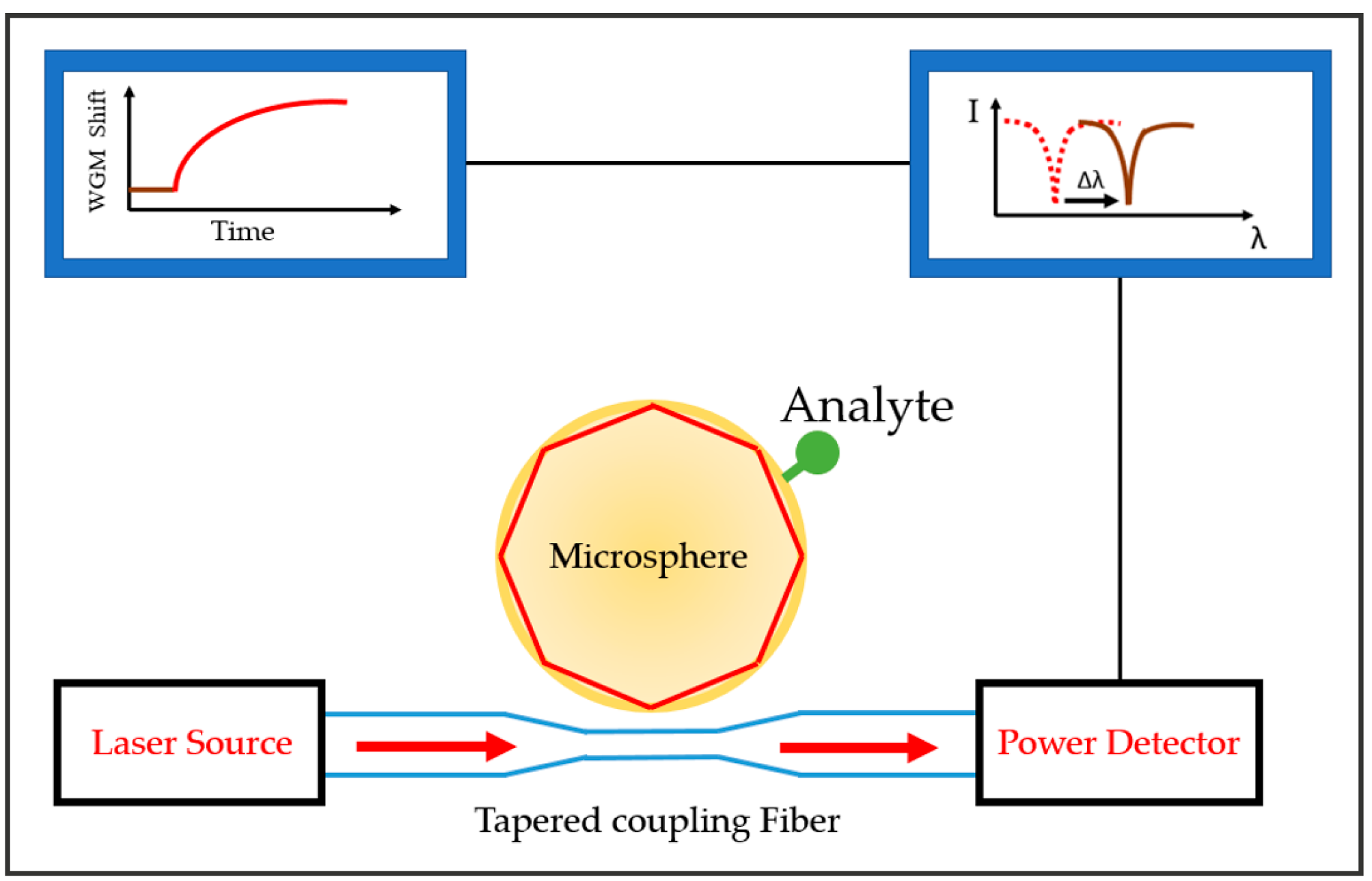
3.1. WGM Microsphere Resonators
3.2. Ring Resonators
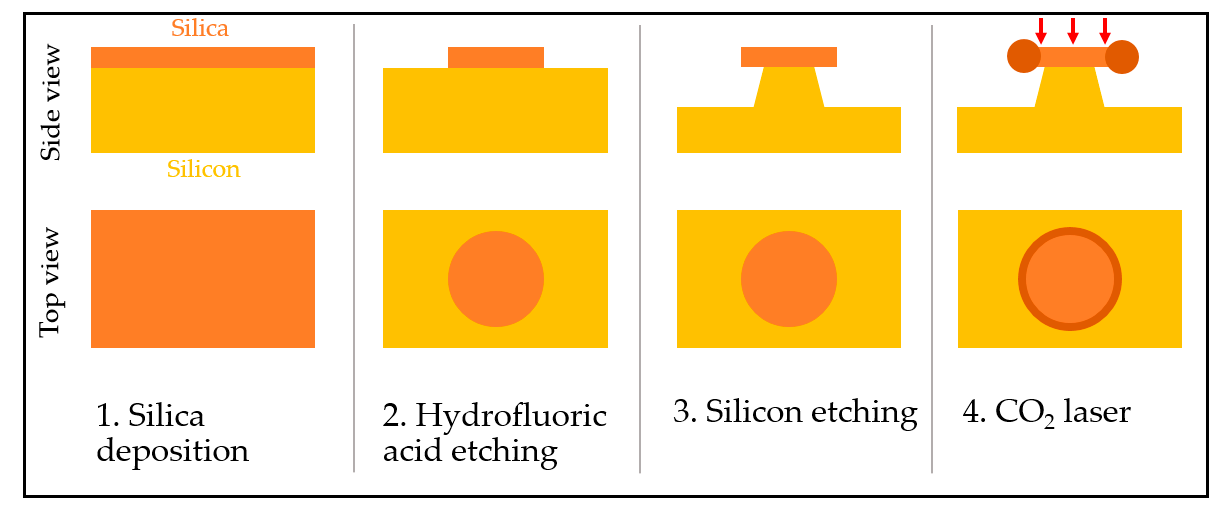
3.2.1. Microring and Microtoroid Optical Resonators
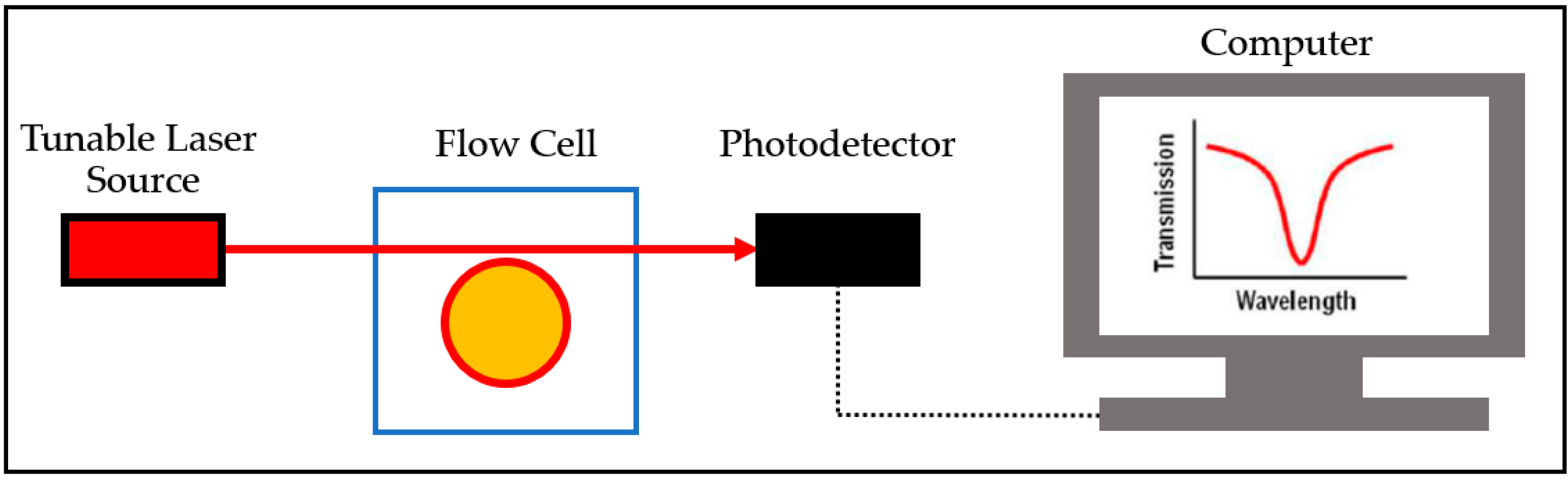
3.2.2. Optofluidic Ring Resonator (OFRR)
4. Pre-Processing
4.1. Sensor Surface Functionalization
4.2. Sample Preparation
5. Application of Optical Resonators for Detecting Cancer
5.1. Cancer Biomarkers
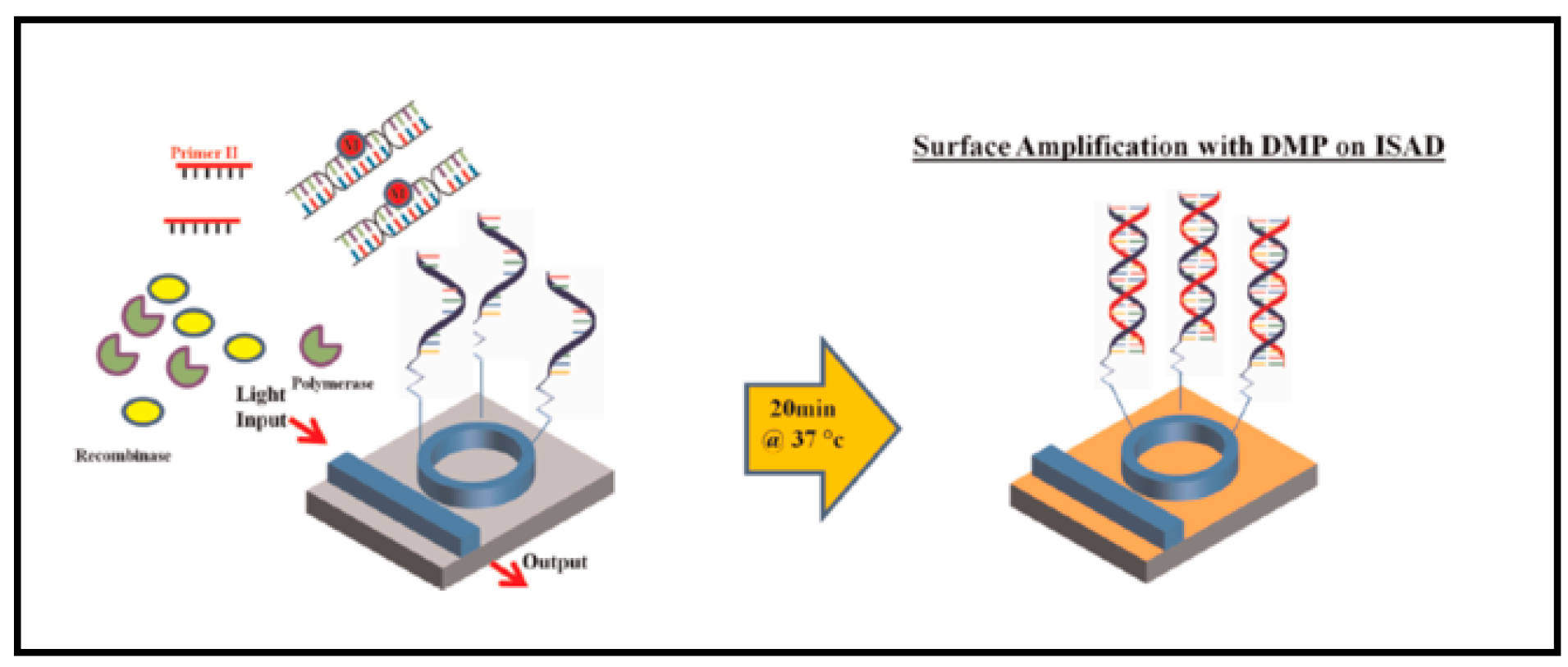
5.2. Optical Resonators for Nucleic Acid Testing (NAT)
5.3. Optical Resonators for Detecting Antigens
5.4. Optical Resonators for Detecting Other Proteins
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| POC | Point-of-care |
| WGM | Whispering-gallery mode |
| Q-factor | Quality factor |
| WGR | WGM resonator |
| CW-WGM | Continuous-wave WGM |
| RIU | Refractive index unit |
| LOD | Limit of detection |
| UV | Ultraviolet |
| CMOS | Complementary metal-oxide semiconductor |
| DUV | Deep UV lithography |
| NIL | Nanoimprinting lithography |
| 3D | Three-dimensional |
| OFRR | Optofluidic ring resonator |
| μTAS | Micro total analysis system |
| LOC | Lab-on-a-chip |
| PCR | Polymerase chain reaction |
| FDA | Food and Drug Administration |
| ELISA | Enzyme-linked immunosorbent assay |
| EtBr | Ethidium bromide |
| RIA | Radioimmunoassay |
| NAT | Nucleic acid testing |
| ISAD | Isothermal solid-phase amplification/detection |
| RPA | Recombinase polymerase amplification |
| WGMI | WGM imaging |
| TRAP | Telomerase repeat amplification protocol |
| APTES | 3-Aminopropyltriethoxysilane |
| PBS | Phosphate-buffered saline |
| GAD | Glutamate decarboxylase |
| BSA | Bovine serum albumin |
| PSA | Prostate-specific antigen |
| PAP | Papanicolaou |
| AFP | Alpha-fetoprotein |
| CA125 | Cancer antigen 125 |
| hCG | Human chorionic gonadotropin |
| CEA | Carcinoembryonic antigen |
| CA15-3 | Cancer antigen 15-3 |
| CA27.29 | Cancer antigen 27-29 |
| MUC-1 | Mucin 1 |
| ING-1 | Inhibitor of growth protein 1 |
| HER2/neu | Human epidermal growth factor receptor 2 |
| ER | Estrogen receptors |
References
- Cancer Research UK Worldwide Cancer Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer (accessed on 28 June 2017).
- American Cancer Society Survival Rates for Hodgkin Lymphoma By Stage. Available online: https://www.cancer.org/cancer/hodgkin-lymphoma/detection-diagnosis-staging/survival-rates.html (accessed on 28 June 2017).
- American Cancer Society How Are Lung Carcinoid Tumors Staged? Available online: https://www.cancer.org/cancer/lung-carcinoid-tumor/detection-diagnosis-staging/staging.html (accessed on 28 June 2017).
- Cancer Research UK Stages of Cancer. Available online: http://www.cancerresearchuk.org/about-cancer/what-is-cancer/stages-of-cancer (accessed on 28 June 2017).
- American Cancer Society Survival Rates for Ovarian Cancer, by Stage. Available online: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 28 June 2017).
- American Cancer Society Survival Rates for Cervical Cancer, by Stage. Available online: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/survival.html (accessed on 28 June 2017).
- American Cancer Society Cervical Cancer Stages. Available online: https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/staged.html (accessed on 28 June 2017).
- Power, E.; Wardle, J. Change in public awareness of symptoms and perceived barriers to seeing a doctor following be clear on cancer campaigns in England. Br. J. Cancer 2015, 112, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Vanture, S.; Cho, M.; Klapperich, C.M.; Wang, C.; Huang, F.W. Awareness, interest, and preferences of primary care providers in using point-of-care cancer screening technology. PLoS ONE 2016, 11, e0145215. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K. Point-of-care testing: An overview and a look to the future (circa 2009, USA). Clin. Lab. Med. 2009, 29, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M. Over-the-counter biosensors: Past, present, and future. Sensors 2008, 8, 5535–5559. [Google Scholar] [CrossRef] [PubMed]
- Sheingold, B.H.; Hahn, J.A. The history of healthcare quality: The first 100 years 1860–1960. Int. J. Afr. Nurs. Sci. 2014, 1, 18–22. [Google Scholar] [CrossRef]
- Rasooly, A. Moving biosensors to point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Soper, S.A.; Brown, K.; Ellington, A.; Frazier, B.; Garcia-Manero, G.; Gau, V.; Gutman, S.I.; Hayes, D.F.; Korte, B.; Landers, J.L.; et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens. Bioelectron. 2006, 21, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Donzella, V.; Crea, F. Optical biosensors to analyze novel biomarkers in oncology. J. Biophotonics 2011, 4, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Ciminelli, C.; Campanella, C.M.; Dell’Olio, F.; Campanella, C.E.; Armenise, M.N. Label-free optical resonant sensors for biochemical applications. Prog. Quantum Electron. 2013, 37, 51–107. [Google Scholar] [CrossRef]
- Web of Science Optical Resonator: Search Result. Available online: http://apps.webofknowledge.com/Search.do?product=WOS&SID=U1nPYbtl65peJQUk6kq&search_mode=GeneralSearch&prID=26722c9c-0bf8-4b30-a7d3-5b764469a8da (accessed on 2 July 2017).
- Bianucci, P. Optical microbottle resonators for sensing. Sensors 2016, 16, 1841. [Google Scholar] [CrossRef] [PubMed]
- Ghali, H.; Chibli, H.; Nadeau, J.L.; Bianucci, P.; Peter, Y.A. Real-time detection of staphylococcus aureus using whispering gallery mode optical microdisks. Biosensors 2016, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Guider, R.; Gandolfi, D.; Chalyan, T.; Pasquardini, L.; Samusenko, A.; Pederzolli, C.; Pucker, G.; Pavesi, L. Sensitivity and limit of detection of biosensors based on ring resonators. Sens. Bio-Sens. Res. 2015, 6, 99–102. [Google Scholar] [CrossRef]
- Armani, D.K.; Kippenberg, T.J.; Spillane, S.M.; Vahala, K.J. Ultra-high-Q toroid microcavity on a chip. Nature 2003, 421, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [PubMed]
- Shin, Y.; Soo, R.A.; Yoon, J.; Perera, A.P.; Yoon, Y.J.; Park, M.K. Rapid and label-free amplification and detection assay for genotyping of cancer biomarker. Biosens. Bioelectron. 2015, 68, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Suter, J.D.; Howard, D.J.; Shi, H.; Caldwell, C.W.; Fan, X. Label-free DNA methylation analysis using opto-fluidic ring resonators. Biosens. Bioelectron. 2010, 26, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Huckabay, H.A.; Wildgen, S.M.; Dunn, R.C. Label-free detection of ovarian cancer biomarkers using whispering gallery mode imaging. Biosens. Bioelectron. 2013, 45, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Shin, Y.; Park, M.K. A simple, low-cost, and rapid device for a DNA methylation-specific amplification/detection system using a flexible plastic and silicon complex. Lab Chip 2014, 14, 4220–4229. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.M.; Kulkarni, R.P.; Fraser, S.E.; Flagan, R.C.; Vahala, K.J. Label-free, single-molecule detection with optical microcavities. Science 2007, 317, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Dale, P.S.; Caldwell, W.C.; Fan, X. Rapid and label-free detection of breast cancer biomarker CA15-3 in clinical human serum samples with optofluidic ring resonator sensors. Anal. Chem. 2009, 81, 9858–9865. [Google Scholar] [CrossRef] [PubMed]
- Suter, J.D.; White, I.M.; Zhu, H.; Shi, H.; Caldwell, C.W.; Fan, X. Label-free quantitative DNA detection using the liquid core optical ring resonator. Biosens. Bioelectron. 2008, 23, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Pechprasarn, S.; Somekh, M.G. Detection limits of confocal surface plasmon microscopy. Biomed. Opt. Express 2014, 5, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sun, X.; Agarwal, A.; Kimerling, L.C. Design guidelines for optical resonator biochemical sensors. J. Opt. Soc. Am. B 2009, 26, 1032. [Google Scholar] [CrossRef]
- Sterr, U.; Legero, T.; Mattei, D.; Kessler, T.; Hagemann, C.; Riehle, F.; Ye, J.; Martin, M.; Zhang, W. Thermal noise in optical reference resonators. In Proceedings of the 2014 IEEE Metrology for Aerospace (MetroAeroSpace), Benevento, Italy, 29–30 May 2014. [Google Scholar]
- Zhou, X.; Zhang, L.; Pang, W. Performance and noise analysis of optical microresonator-based biochemical sensors using intensity detection. Opt. Express 2016, 24, 18197–28208. [Google Scholar] [CrossRef] [PubMed]
- Soria, S.; Berneschi, S.; Brenci, M.; Cosi, F.; Conti, G.N.; Pelli, S.; Righini, G.C. Optical microspherical resonators for biomedical sensing. Sensors 2011, 11, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, I.A.; Testa, G.; Persichetti, G.; Loffredo, F.; Villani, F.; Bernini, R. Plasma functionalization procedure for antibody immobilization for SU-8 based sensor. Biosens. Bioelectron. 2016, 86, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Shin, Y.; Perera, A.P.; Liu, Q.; Kee, J.S.; Han, K.; Yoon, Y.J.; Park, M.K. Label-free, PCR-free chip-based detection of telomerase activity in bladder cancer cells. Biosens. Bioelectron. 2013, 45, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Kee, J.S.; Quah, J.Y.; Netto, V.; Song, J.; Fang, Q.; La Fosse, E.M.; Lo, G.-Q. Label-free aptamer sensor based on silicon microring resonators. Sens. Actuators B 2013, 176, 552–559. [Google Scholar] [CrossRef]
- Arnold, S.; Folan, L.M. Energy transfer and the photon lifetime within an aerosol particle. Opt. Lett. 1989, 14, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, M.L.; Savchenkov, A.A.; Ilchenko, V.S. Ultimate Q of optical microsphere resonators. Opt. Lett. 1996, 21, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Lenferink, A.T.M.; Schipper, E.F.; Kooyman, R.P.H. Improved detection method for evanescent wave interferometric chemical sensing. Rev. Sci. Instrum. 1997, 68, 1582–1586. [Google Scholar] [CrossRef]
- Mazzei, A.; Götzinger, S.; Menezes, L.D.S.; Sandoghdar, V.; Benson, O. Optimization of prism coupling to high-Q modes in a microsphere resonator using a near-field probe. Opt. Commun. 2005, 250, 428–433. [Google Scholar] [CrossRef]
- Ilchenko, V.S.; Yao, X.S.; Maleki, L. Pigtailing the high-Q microsphere cavity: A simple fiber coupler for optical whispering-gallery modes. Opt. Lett. 1999, 24, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sun, X.; Li, X.; Chen, J. Miniature microring resonator sensor based on a hybrid plasmonic waveguide. Sensors 2011, 11, 6856–6867. [Google Scholar] [CrossRef] [PubMed]
- Chandrahalim, H.; Rand, S.C.; Fan, X. Evanescent coupling between refillable ring resonators and laser-inscribed optical waveguides. Appl. Opt. 2017, 56, 4750–4756. [Google Scholar] [CrossRef]
- Qian, S.X.; Snow, J.B.; Tzeng, H.M.; Chang, R.K. Lasing droplets: Highlighting the liquid-air interface by laser emission. Science 1986, 231, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.M.; Wall, K.F.; Long, M.B.; Chang, R.K. Laser emission from individual droplets at wavelengths corresponding to morphology-dependent resonances. Opt. Lett. 1984, 9, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Little, B.E.; Laine, J.P.; Haus, H.A. Analytic theory of coupling from tapered fibers and half-blocks into microsphere resonators. J. Lightwave Technol. 1999, 17, 704–715. [Google Scholar] [CrossRef]
- Cai, M.; Vahala, K. Highly efficient optical power transfer to whispering-gallery modes by use of a symmetrical dual-coupling configuration. Opt. Lett. 2000, 25, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, M.L.; Ilchenko, V.S. Optical microsphere resonators: Optimal coupling to high-Q whispering-gallery modes. J. Opt. Soc. Am. B 1999, 16, 147–154. [Google Scholar] [CrossRef]
- Ulrich, R. Optimum excitation of optical surface waves. J. Opt. Soc. Am. 1971, 61, 1467–1477. [Google Scholar] [CrossRef]
- Matsko, A.A.; Strekalov, S.D.; Ilchenko, V.S.; Maleki, L. Review of applications of whispering-gallery mode resonators in photonics and nonlinear optics. IPN Prog. Rep. 2005, 42, 51. [Google Scholar]
- Raman, C.V.; Sutherland, G.A. Whispering-gallery phenomena at St. Paul’s Cathedral. Nature 1921, 108, 42. [Google Scholar] [CrossRef]
- Garrett, C.G.B.; Kaiser, W.; Bond, W.L. Stimulated emission into optical whispering modes of spheres. Phys. Rev. 1961, 124, 1807–1809. [Google Scholar] [CrossRef]
- Debye, P. Der lichtdruck auf kugeln von beliebigem material. Ann. Phys. 1909, 335, 57–136. [Google Scholar] [CrossRef]
- Horvath, H. Gustav mie and the scattering and absorption of light by particles: Historic developments and basics. J. Quant. Spectrosc. Radiat. Transfer 2009, 110, 787–799. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Observation of optical resonances of dielectric spheres by light scattering. Appl. Opt. 1981, 20, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Ilchenko, V.S.; Matsko, A.B. Optical resonators with whispering-gallery modes-part II: Applications. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 15–32. [Google Scholar] [CrossRef]
- Darmawan, S.; Chin, M.K. Critical coupling, oscillation, reflection, and transmission in optical waveguide-ring resonator systems. J. Opt. Soc. Am. B 2006, 23, 834–841. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Lee, R.K.; Yariv, A. Scattering-theory analysis of waveguide-resonator coupling. Phy. Rev. E 2000, 62, 7389–7404. [Google Scholar] [CrossRef]
- Little, B.E.; Chu, S.T.; Haus, H.A.; Foresi, J.; Laine, J.P. Microring resonator channel dropping filters. J. Lightwave Technol. 1997, 15, 998–1005. [Google Scholar] [CrossRef]
- Hryniewicz, J.V.; Absil, P.P.; Little, B.E.; Wilson, R.A.; Ho, P.T. Higher order filter response in coupled microring resonators. IEEE Photonics Technol. Lett. 2000, 12, 320–322. [Google Scholar] [CrossRef]
- Huston, A.L.; Eversole, J.D. Strain-sensitive elastic scattering from cylinders. Opt. Lett. 1993, 18, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Camli, B.; Kusakci, E.; Lafci, B.; Salman, S.; Torun, H.; Yalcinkaya, A. A microwave ring resonator based glucose sensor. Procedia Eng. 2016, 168, 465–468. [Google Scholar] [CrossRef]
- Yao, X.S.; Maleki, L. Optoelectronic microwave oscillator. J. Opt. Soc. Am. B 1996, 13, 1725–1735. [Google Scholar] [CrossRef]
- Kolli, V.R.; Srinivasulu, T.; Hegde, G.; Badrinarayana, T.; Talabattula, S. Design and analysis of serially coupled double microring resonator based force sensor for 1μN range measurement. Optik-Int. J. Light Electron. Opt. 2017, 131, 1063–1070. [Google Scholar] [CrossRef]
- Arnold, S.; Khoshsima, M.; Teraoka, I.; Holler, S.; Vollmer, F. Shift of whispering-gallery modes in microspheres by protein adsorption. Opt. Lett. 2003, 28, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakkal Abdulla, S.M.; Kauppinen, L.J.; Dijkstra, M.; de Boer, M.J.; Berenschot, J.W.; de Ridder, R.M.; Krijnen, G.J.M. Mechanical tuning of optical race-track ring resonators. Procedia Eng. 2010, 5, 424–427. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, Y.; Hou, C.; Bai, J.; Yang, G. A temperature sensor based on optical microfiber knot resonator. Opt. Commun. 2009, 282, 3817–3819. [Google Scholar] [CrossRef]
- Saito, M.; Shimatani, H.; Koyama, K. Stimulated emission in a deformable fluorescent droplet. In Proceedings of the 2009 IEEE LEOS Annual Meeting Conference Proceedings, Belek-Antalya, Turkey, 4–8 October 2009. [Google Scholar]
- Braginsky, V.B.; Gorodetsky, M.L.; Ilchenko, V.S. Quality-factor and nonlinear properties of optical whispering-gallery modes. Phys. Lett. A 1989, 137, 393–397. [Google Scholar] [CrossRef]
- Savchenkov, A.A.; Ilchenko, V.S.; Byrd, J.; Liang, W.; Eliyahu, D.; Matsko, A.B.; Seidel, D.; Maleki, L. Whispering-gallery mode based opto-electronic oscillators. In Proceedings of the 2010 IEEE International Frequency Control Symposium, Newport Beach, CA, USA, 1–4 June 2010. [Google Scholar]
- Zhang, J.; Grischkowsky, D. Whispering-gallery-mode cavity for terahertz pulses. J. Opt. Soc. Am. B 2003, 20, 1894–1904. [Google Scholar] [CrossRef]
- Climb the Dome. Available online: https://www.stpauls.co.uk/history-collections/history/explore-the-cathedral/climb-the-dome (accessed on 8 July 2017).
- Su, J. Label-free biological and chemical sensing using whispering gallery mode optical resonators: Past, present, and future. Sensors 2017, 17, 540. [Google Scholar] [CrossRef] [PubMed]
- Chung-Yen, C.; Guo, L.J. Design and optimization of microring resonators in biochemical sensing applications. J. Lightwave Technol. 2006, 24, 1395–1402. [Google Scholar] [CrossRef]
- Teraoka, I.; Arnold, S.; Vollmer, F. Perturbation approach to resonance shifts of whispering-gallery modes in a dielectric microsphere as a probe of a surrounding medium. J. Opt. Soc. Am. B 2003, 20, 1937–1946. [Google Scholar] [CrossRef]
- Schwinger, J. The Theory of Obstacles in Resonant Cavities and Waveguides; Radiation Laboratory, Massachusetts Institute of Technology: Cambridge, MA, USA, 1943. [Google Scholar]
- Malmir, K.; Habibiyan, H.; Ghafoorifard, H. An ultrasensitive optical label-free polymeric biosensor based on concentric triple microring resonators with a central microdisk resonator. Opt. Commu. 2016, 365, 150–156. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, F.; Yang, C.; Song, J.; Tang, L.; Li, M.; He, J.-J. Label-free biosensing using cascaded double-microring resonators integrated with microfluidic channels. Opt. Commu. 2015, 344, 129–133. [Google Scholar] [CrossRef]
- Meziane, F.; Raimbault, V.; Hallil, H.; Joly, S.; Conédéra, V.; Lachaud, J.L.; Béchou, L.; Rebière, D.; Dejous, C. Study of a polymer optical microring resonator for hexavalent chromium sensing. Sens. Actuators B 2015, 209, 1049–1056. [Google Scholar] [CrossRef]
- Jin, X.; Dong, Y.; Wang, K. Demonstration and characterization of distributed multiparticle-induced mode splitting in a microsphere resonator. Opt. Commun. 2016, 363, 57–62. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, L.; Kim, C.-W.; Lan, X.; Huang, J.; Ma, Y.; Xiao, H. Integrated chemical vapor sensor based on thin wall capillary coupled porous glass microsphere optical resonator. Sens. Actuators B 2015, 216, 332–336. [Google Scholar] [CrossRef]
- Ballard, Z.; Baaske, M.D.; Vollmer, F. Stand-off biodetection with free-space coupled asymmetric microsphere cavities. Sensors 2015, 15, 8968–8980. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.; Mai, M.; Grossmann, T.; Wienhold, T.; Hauser, M.; Mappes, T.; Kalt, H. High-Q polymer resonators with spatially controlled photo-functionalization for biosensing applications. Appl. Phys. Lett. 2013, 102, 121108. [Google Scholar] [CrossRef]
- Schweinsberg, A.; Hocdé, S.; Lepeshkin, N.N.; Boyd, R.W.; Chase, C.; Fajardo, J.E. An environmental sensor based on an integrated optical whispering gallery mode disk resonator. Sens. Actuators B 2007, 123, 727–732. [Google Scholar] [CrossRef]
- Berneschi, S.; Farnesi, D.; Cosi, F.; Conti, G.N.; Pelli, S.; Righini, G.C.; Soria, S. High Q silica microbubble resonators fabricated by arc discharge. Opt. Lett. 2011, 36, 3521–3523. [Google Scholar] [CrossRef] [PubMed]
- Barucci, A.; Berneschi, S.; Giannetti, A.; Baldini, F.; Cosci, A.; Pelli, S.; Farnesi, D.; Righini, G.C.; Soria, S.; Nunzi Conti, G. Optical microbubble resonators with high refractive index inner coating for bio-sensing applications: An analytical approach. Sensors 2016, 16, 1992. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Goldberg, A.F.G.; Stoltz, B.M. Label-free detection of single nanoparticles and biological molecules using microtoroid optical resonators. Light Sci. Appl. 2016, 5, e16001. [Google Scholar] [CrossRef]
- Madani, A.; Azarinia, H.R. Design and fabrication of all-polymeric photonic waveguides in optical integrated circuits. Optik-Int. J. Light Electron. Opt. 2017, 138, 33–39. [Google Scholar] [CrossRef]
- Testa, G.; Persichetti, G.; Bernini, R. Optofluidic approaches for enhanced microsensor performances. Sensors 2014, 15, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Washburn, A.L.; Luchansky, M.S.; Bowman, A.L.; Bailey, R.C. Quantitative, label-free detection of five protien biomarkers using multiplexed arrays of silicon photonic microring resonators. Anal. Chem. 2010, 82, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Gohring, J.T.; Fan, X. Label free detection of CD4+ and CD8+ T cells using the optofluidic ring resonator. Sensors 2010, 10, 5798–5808. [Google Scholar] [CrossRef] [PubMed]
- Su, J. Label-free single exosome detection using frequency-locked microtoroid optical resonators. ACS Photonics 2015, 2, 1241–1245. [Google Scholar] [CrossRef]
- Zhu, H.; White, I.M.; Suter, J.D.; Fan, X. Phage-based label-free biomolecule detection in an opto-fluidic ring resonator. Biosens. Bioelectron. 2008, 24, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Perera, A.P.; Park, M.K. Label-free DNA sensor for detection of bladder cancer biomarkers in urine. Sens. Actuators B 2013, 178, 200–206. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, X.L.; Liedberg, B.; Liu, A.Q. Gas sensor for volatile organic compounds detection using silicon photonic ring resonator. Procedia Eng. 2016, 168, 1771–1774. [Google Scholar] [CrossRef]
- Yang, G.; White, I.M.; Fan, X. An opto-fluidic ring resonator biosensor for the detection of organophosphorus pesticides. Sens. Actuators B 2008, 133, 105–112. [Google Scholar] [CrossRef]
- Mallika, C.S.; Bahaddur, I.; Srikanth, P.C.; Sharan, P. Photonic crystal ring resonator structure for temperature measurement. Opt-Int. J. Light Electron. Opt. 2015, 126, 2252–2255. [Google Scholar] [CrossRef]
- Koch, B.; Carson, L.; Guo, C.-M.; Lee, C.-Y.; Yi, Y.; Zhang, J.-Y.; Zin, M.; Znameroski, S.; Smith, T. Hurricane: A simplified optical resonator for optical-power-based sensing with nano-particle taggants. Sens. Actuators B 2010, 147, 573–580. [Google Scholar] [CrossRef]
- Zhu, J.; Özdemir, Ş.K.; He, L.; Chen, D.-R.; Yang, L. Single virus and nanoparticle size spectrometry by whispering-gallery-mode microcavities. Opt. Express 2011, 19, 16195–16206. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M.; Zhu, H.; Suter, J.D.; Oveys, H. Overview of novel integrated optical ring resonator bio/chemical sensors. Proc. SPIE 2007, 6452, 64520M. [Google Scholar]
- White, I.M.; Suter, J.D.; Oveys, H.; Fan, X.; Smith, T.L.; Zhang, J.; Koch, B.J.; Haase, M.A. Universal coupling between metal-clad waveguides and optical ring resonators. Opt. Express 2007, 15, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Genalyte Inc. Available online: http://www.genalyte.com/ (accessed on 8 July 2016).
- Tobing, L.Y.M.; Dumon, P. Fundamental principles of operation and notes on fabrication of photonic microresonators. In Photonic Microresonator Research and Applications; Chremmos, I., Schwelb, O., Uzunoglu, N., Eds.; Springer: Boston, MA, USA, 2010; pp. 1–27. [Google Scholar]
- Chung-Yen, C.; Fung, W.; Guo, L.J. Polymer microring resonators for biochemical sensing applications. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 134–142. [Google Scholar] [CrossRef]
- Spencer, D.T.; Bauters, J.F.; Heck, M.J.R.; Bowers, J.E. Integrated waveguide coupled Si3N4 resonators in the ultrahigh-Q regime. Optica 2014, 1, 153–157. [Google Scholar] [CrossRef]
- Spencer, D.T.; Tang, Y.; Bauters, J.F.; Heck, M.J.R.; Bowers, J.E. Integrated Si3N4/SiO2 ultra high Q ring resonators. In Proceedings of the IEEE Photonics Conference 2012, Burlingame, CA, USA, 23–27 September 2012. [Google Scholar]
- Zhu, H.; Suter, J.D.; Fan, X. Label-free optical ring resonator bio/chemical sensors. In Springer Series on Chemical Sensors and Biosensors; Springer: Boston, MA, USA, 2010. [Google Scholar]
- Armani, A.M. Corrections and Clarifications. Science 2011, 334, 1496. [Google Scholar]
- Arnold, S.; Shopova, S.I.; Holler, S. Whispering gallery mode bio-sensor for label-free detection of single molecules: Thermo-optic vs. reactive mechanism. Opt. Express 2010, 18, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Zadeh, M.; Vahala, K.J. Free ultra-high-Q microtoroid: A tool for designing photonic devices. Opt. Express 2007, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kippenberg, T.; Spillane, S.M.; Armani, D.K.; Min, B.; Yang, L.; Vahala, K.J. Fabrication, coupling and nonlinear optics of ultra-high Q micro-sphere and chip-based toroid microcavities. In Optical Microcavities; Vahala, K., Ed.; World Scientific: Singapore, 2004. [Google Scholar]
- Zhang, X.; Armani, A.M. Silica microtoroid resonator sensor with monolithically integrated waveguides. Opt. Express 2013, 21, 23592–23603. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Oveys, H.; Fan, X. Liquid-core optical ring-resonator sensors. Opt. Lett. 2006, 31, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; White, I.M.; Suter, J.D.; Zourob, M.; Fan, X. Integrated refractive index optical ring resonator detector for capillary electrophoresis. Anal. Chem. 2007, 79, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.S.; Park, M.-C.; Kuang, Q.; Deng, Y.; Sood, A.K.; Polla, D.L.; Wang, Z.L. Giant enhancement in UV response of ZnO nanobelts by polymer surface-functionalization. J. Am. Chem. Soc. 2007, 129, 12096–12097. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Gleeson, M.A.; Spaugh, B.; Tybor, F.; Gunn, W.G.; Hochberg, M.; Baehr-Jones, T.; Bailey, R.C.; Gunn, L.C. Label-free biosensor arrays based on silicon ring resonators and high-speed optical scanning instrumentation. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 654–661. [Google Scholar] [CrossRef]
- Sigal, G.B.; Bamdad, C.; Barberis, A.; Strominger, J.; Whitesides, G.M. A self-assembled monolayer for the binding and study of histidine-tagged proteins by surface plasmon resonance. Anal. Chem 1996, 68, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Z.; Naves, L.; Siwak, N.P.; Brown, A.; Culver, J.; Ghodssi, R. Integration of genetically modified virus-like-particles with an optical resonator for selective bio-detection. Nanotechnol. 2015, 26, 205501. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xu, X. Progress on sample pretreatment techniques for polycyclic aromatic hydrocarbons in water system. In Proceedings of the 2011 International Conference on Electric Technology and Civil Engineering (ICETCE), Lushan, China, 22–24 April 2011. [Google Scholar]
- Zhao, H.-P.; Feng, X.-Q.; Gao, H. Ultrasonic technique for extracting nanofibers from nature materials. Appl. Phys. Lett. 2007, 90, 073112. [Google Scholar] [CrossRef]
- Wang, H.-M.; Pan, J.-L.; Chen, C.-Y.; Chiu, C.-C.; Yang, M.-H.; Chang, H.-W.; Chang, J.-S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Mulcahey, L.J.; Taylor, L.T. Supercritical fluid extraction. Microchim. Acta 1992, 108, 115–132. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Sparr Eskilsson, C.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Fluigen Microfluidic Definitions and Advantage. Available online: https://www.fluigent.com/expertises/microfluidic-definitions-advantages/ (accessed on 11 August 2017).
- Yoon, J.; Park, M.K.; Lee, T.Y.; Yoon, Y.J.; Shin, Y. LoMA-B: A simple and versatile lab-on-a-chip system based on single-channel bisulfite conversion for DNA methylation analysis. Lab Chip 2015, 15, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Graybill, R.M.; Para, C.S.; Bailey, R.C. PCR-free, multiplexed expression profiling of microRNAs using silicon photonic microring resonators. Anal. Chem. 2016, 88, 10347–10351. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Perera, A.P.; Kim, K.W.; Park, M.K. Real-time, label-free isothermal solid-phase amplification/detection (ISAD) device for rapid detection of genetic alteration in cancers. Lab Chip 2013, 13, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration Biomarker Qualification Program. Available online: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/default.htm (accessed on 28 June 2017).
- Pasini, A.; Paganelli, G.; Tesei, A.; Zoli, W.; Giordano, E.; Calistri, D. Specific biomarkers are associated with docetaxeland gemcitabine-resistant NSCLC cell lines. Transl. Oncol. 2012, 5, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Crowther, J.R. The ELISA guidebook. Methods Mol. Biol. 2000, 149, 1–413. [Google Scholar]
- Vinagre, J.; Pinto, V.; Celestino, R.; Reis, M.; Populo, H.; Boaventura, P.; Melo, M.; Catarino, T.; Lima, J.; Lopes, J.M.; et al. Telomerase promoter mutations in cancer: An emerging molecular biomarker? Virchows Arch. 2014, 465, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Huckabay, H.A.; Dunn, R.C. Whispering gallery mode imaging for the multiplexed detection of biomarkers. Sens. Actuators B 2011, 160, 1262–1267. [Google Scholar] [CrossRef]
- Washburn, L.C.G.; Bailey, R.C. Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators. Anal. Chem. 2009, 81, 9499–9506. [Google Scholar] [CrossRef] [PubMed]
- Gohring, J.T.; Dale, P.S.; Fan, X. Detection of HER2 breast cancer biomarker using the opto-fluidic ring resonator biosensor. Sens. Actuators B 2010, 146, 226–230. [Google Scholar] [CrossRef]
- Kindt, J.T.; Bailey, R.C. Biomolecular analysis with microring resonators: Applications in multiplexed diagnostics and interaction screening. Curr. Opin. Chem. Biol. 2013, 17, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Orghici, R.; Lutzow, P.; Burgmeier, J.; Koch, J.; Heidrich, H.; Schade, W.; Welschoff, N.; Waldvogel, S. A microring resonator sensor for sensitive detection of 1,3,5-trinitrotoluene (TNT). Sensors 2010, 10, 6788–6795. [Google Scholar] [CrossRef] [PubMed]
- Dantham, V.R.; Holler, S.; Barbre, C.; Keng, D.; Kolchenko, V.; Arnold, S. Label-free detection of single protein using a nanoplasmonic-photonic hybrid microcavity. Nano Lett. 2013, 13, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
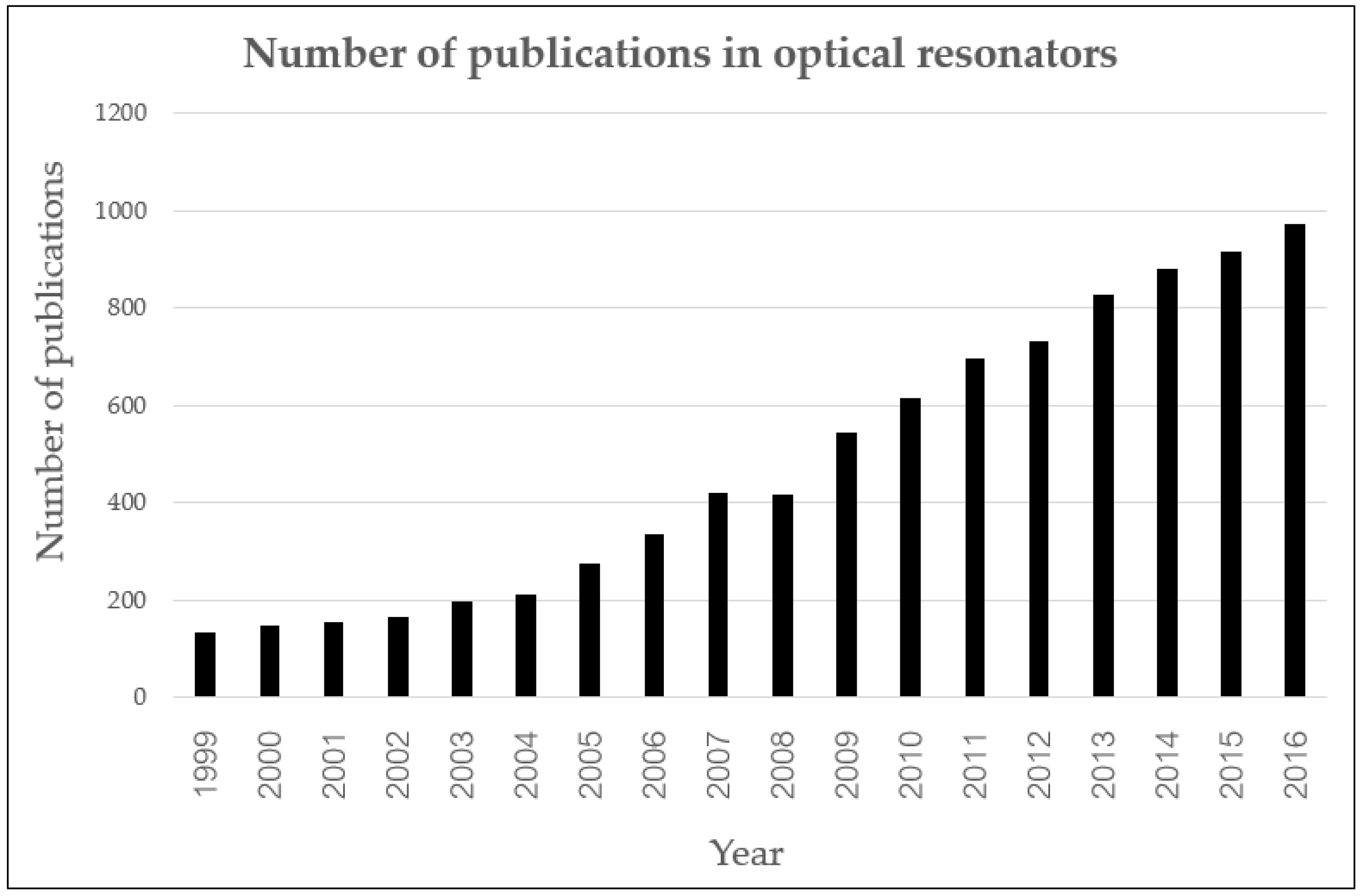


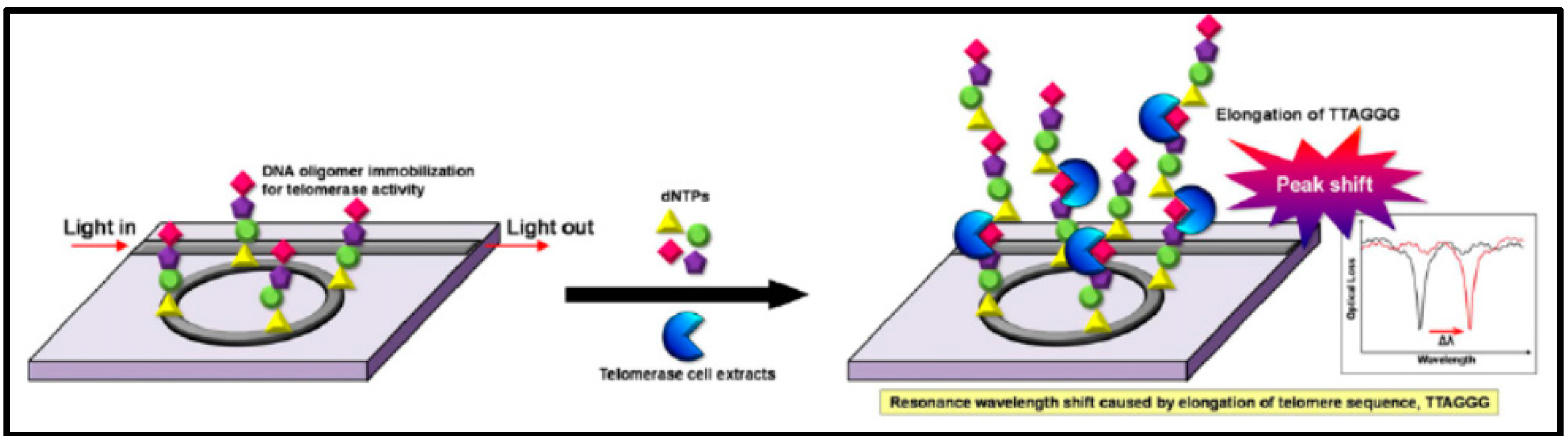
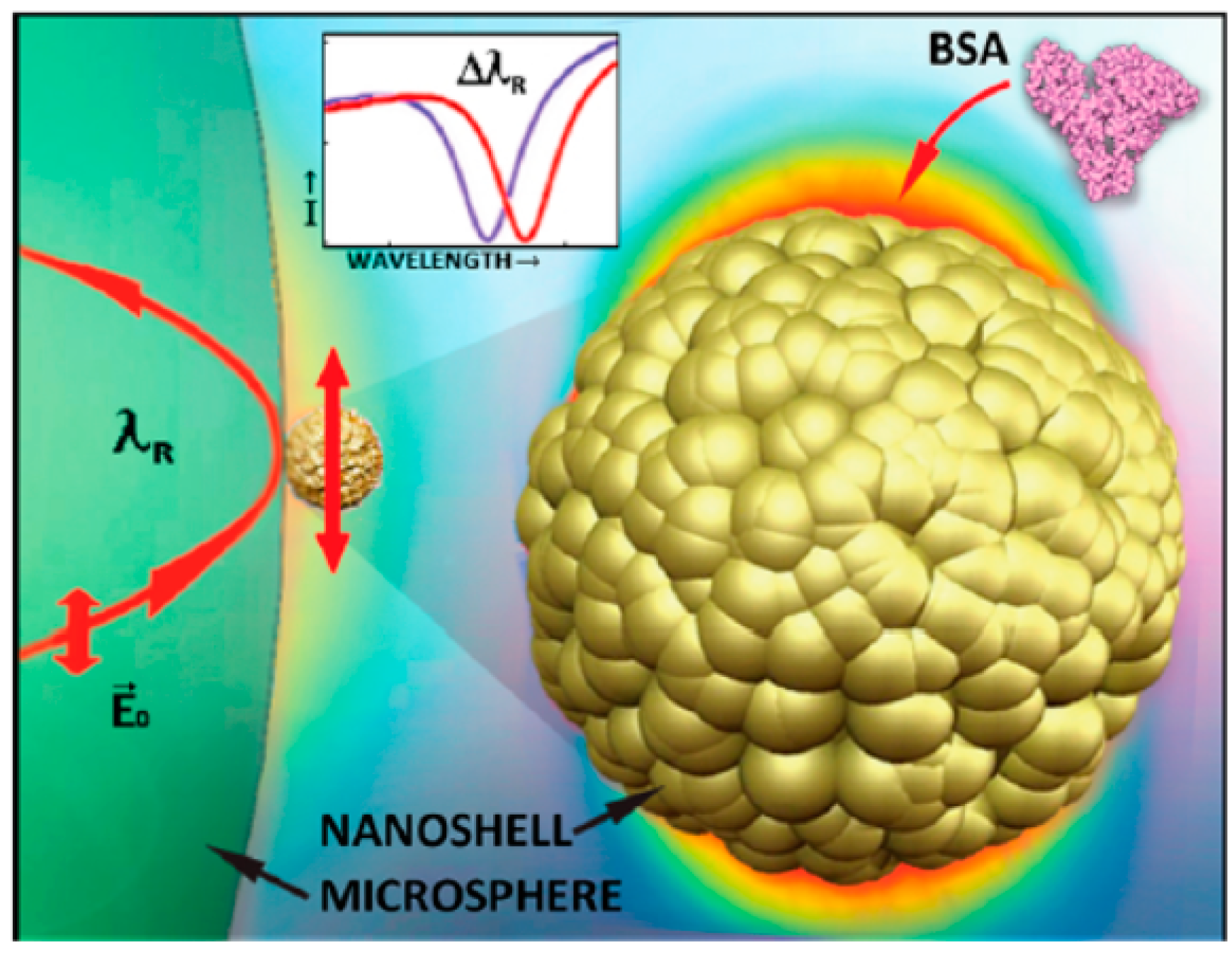
| Number System | TNM System |
|---|---|
| Stage 0 | Tis, N0, M0 |
| Stage I | T1, N0, M0 |
| Stage IA | T1a, N0, M0 |
| Stage IB | T1b, N0, M0 |
| Stage II | T2, N0, M0 |
| Stage IIA | T2a, N0, M0 |
| Stage III | T3, N0, M0 |
| Stage IIIA | T3a, N0, M0 |
| Stage IIIB | T3b, N0, M0 or T1–T3, N1, M0 |
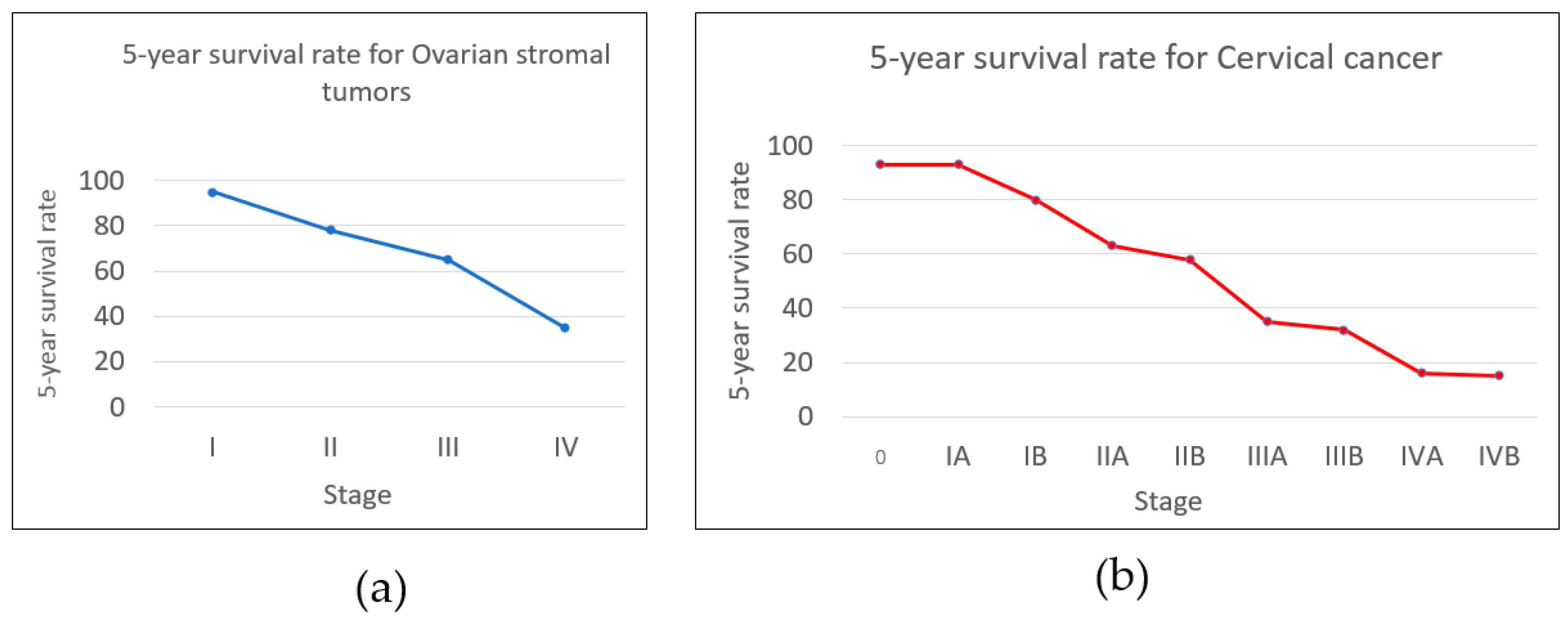
| Stage IV | - |
| Stage IVA | T4, N0, M0 |
| Stage IVB | T1–T3, N0–N3, M1 |
| Tumor Type | Biomarker | Reference |
|---|---|---|
| Prostate | PSA, PAP | [17] |
| Testicular | α-Fetoprotein (AFP), β-human chorionic | [17] |
| Ovarian | CA125, AFP, hCG, p53, CEA | [17] |
| Breast | CA15-3, CA125, CA27.29, CEA BRCA1, CEA BRCA2, MUC-1, CEA, NY-BR-1, ING-1, HER2/NEU, ER/PR | [17] |
| Lung | AFP, CEA, EML4/ALK, EGFR, KRAS | [17,24] |
| Esophageal | SCC | [17,24] |
| Gastric | CA72-4, CEA, CA19-9 | |
| Colon and pancreatic | CEA, CA19-9, CA24-2, p53, EGFR, KRAS, UGT1A1 | [24] |
| Liver | AFP, CEA | [17] |
| Bladder | HRAS, FGFR3 | [135] |
| Trophoblastic | SCC, hCG | [17] |
| Melanoma | Tyrosinase, NY-ESO-1, BRAF | [17] |
| Leukemia | Chromosomal abnormalities | [17] |
| Biomarker | Clinical Relevant Concentration | Type of Optical Resonator | Detection Limit | Time (min) | Ref. |
|---|---|---|---|---|---|
| CA-125 | 35 U/mL | Microsphere resonator | ∼1.5 U/mL | - | [136] |
| CA-15-3 | 30 U/mL | OFRR | 1 U/mL | 20 | [30] |
| EGFR Mutation | 25% of mutant allele | Microring resonator | 1% of mutant allele | 20 | [25] |
| TNF-α | 203 pg/mL | Microsphere resonator | ∼240 pg/mL | - | [136] |
| CEA | 3–5 ng/mL | Microring resonator | 2 ng/mL | 30 | [137] |
| HER2 | 15 ng/mL | OFRR | 1 ng/mL | 15–30 | [138] |
| Parameter | ISAD | RPA | PCR | RT-PCR |
|---|---|---|---|---|
| LOD | 500 fg/μL | 50 pg/μL | 50 pg/μL | 5 pg/μL |
| Analysis time (min) | 20–30 | 40–50 | 120–180 | 60–120 |
| Label | Label-free | EtBr | EtBr | Fluorescence |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongruengkiat, W.; Pechprasarn, S. Whispering-Gallery Mode Resonators for Detecting Cancer. Sensors 2017, 17, 2095. https://doi.org/10.3390/s17092095
Pongruengkiat W, Pechprasarn S. Whispering-Gallery Mode Resonators for Detecting Cancer. Sensors. 2017; 17(9):2095. https://doi.org/10.3390/s17092095
Chicago/Turabian StylePongruengkiat, Weeratouch, and Suejit Pechprasarn. 2017. "Whispering-Gallery Mode Resonators for Detecting Cancer" Sensors 17, no. 9: 2095. https://doi.org/10.3390/s17092095
APA StylePongruengkiat, W., & Pechprasarn, S. (2017). Whispering-Gallery Mode Resonators for Detecting Cancer. Sensors, 17(9), 2095. https://doi.org/10.3390/s17092095





