Dual-Mode Electro-Optical Techniques for Biosensing Applications: A Review
Abstract
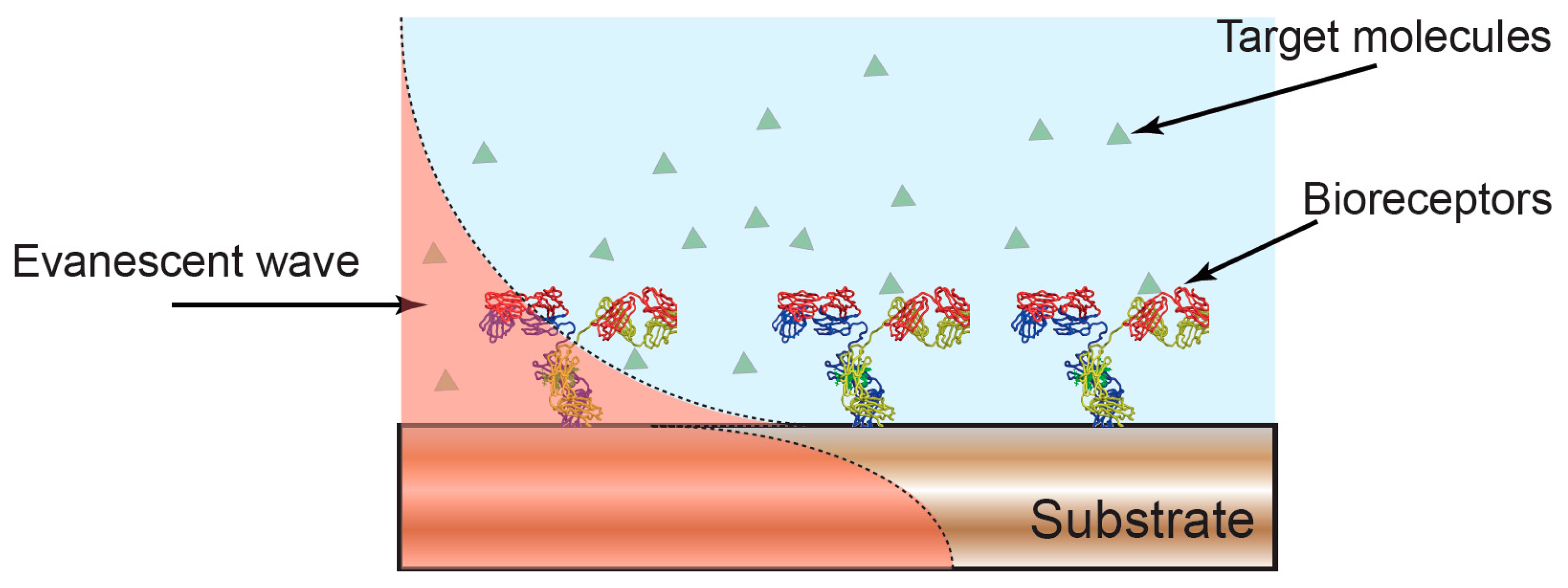
1. Introduction
2. Electro-Optical Multi-Domain Techniques
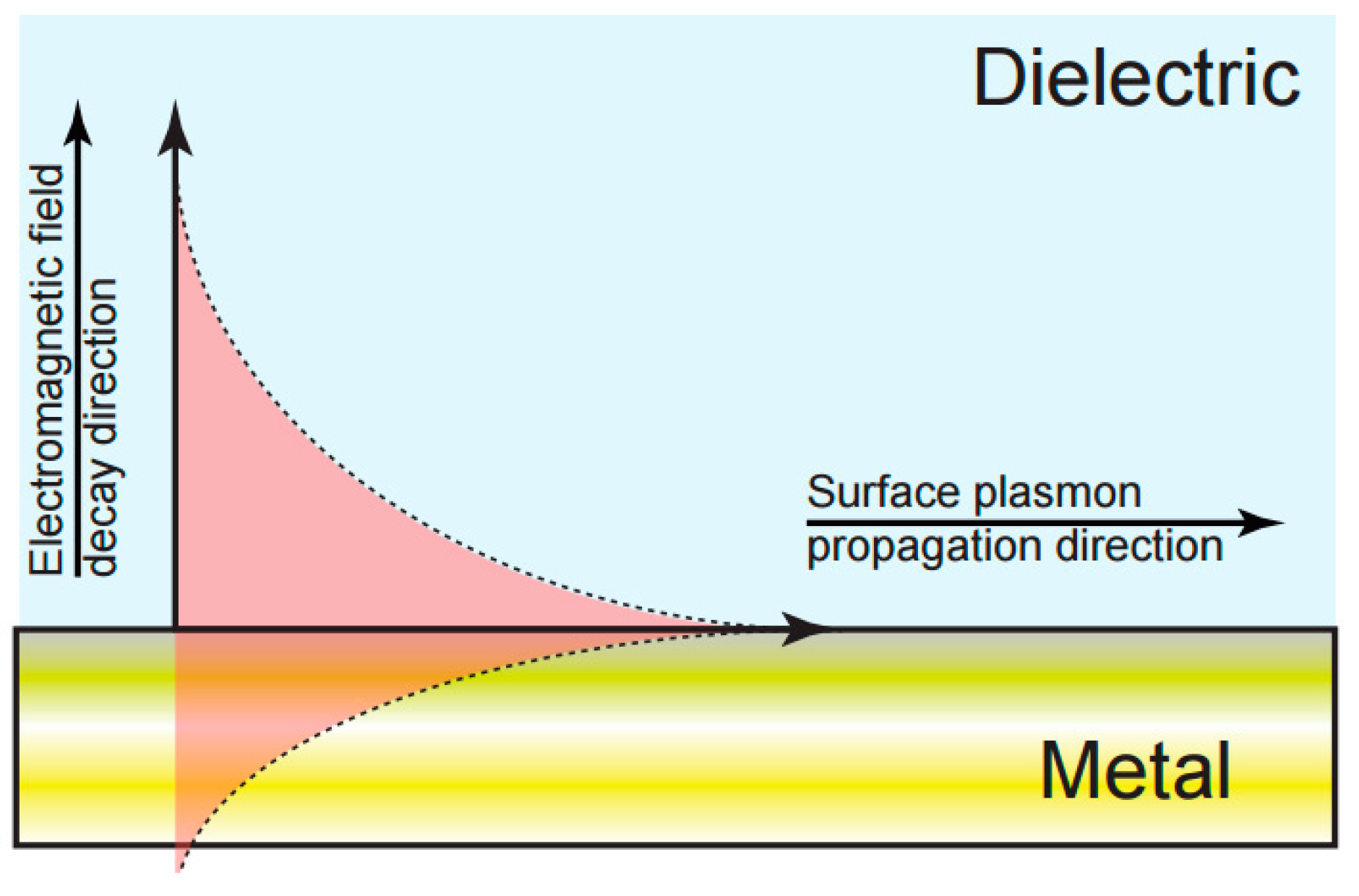
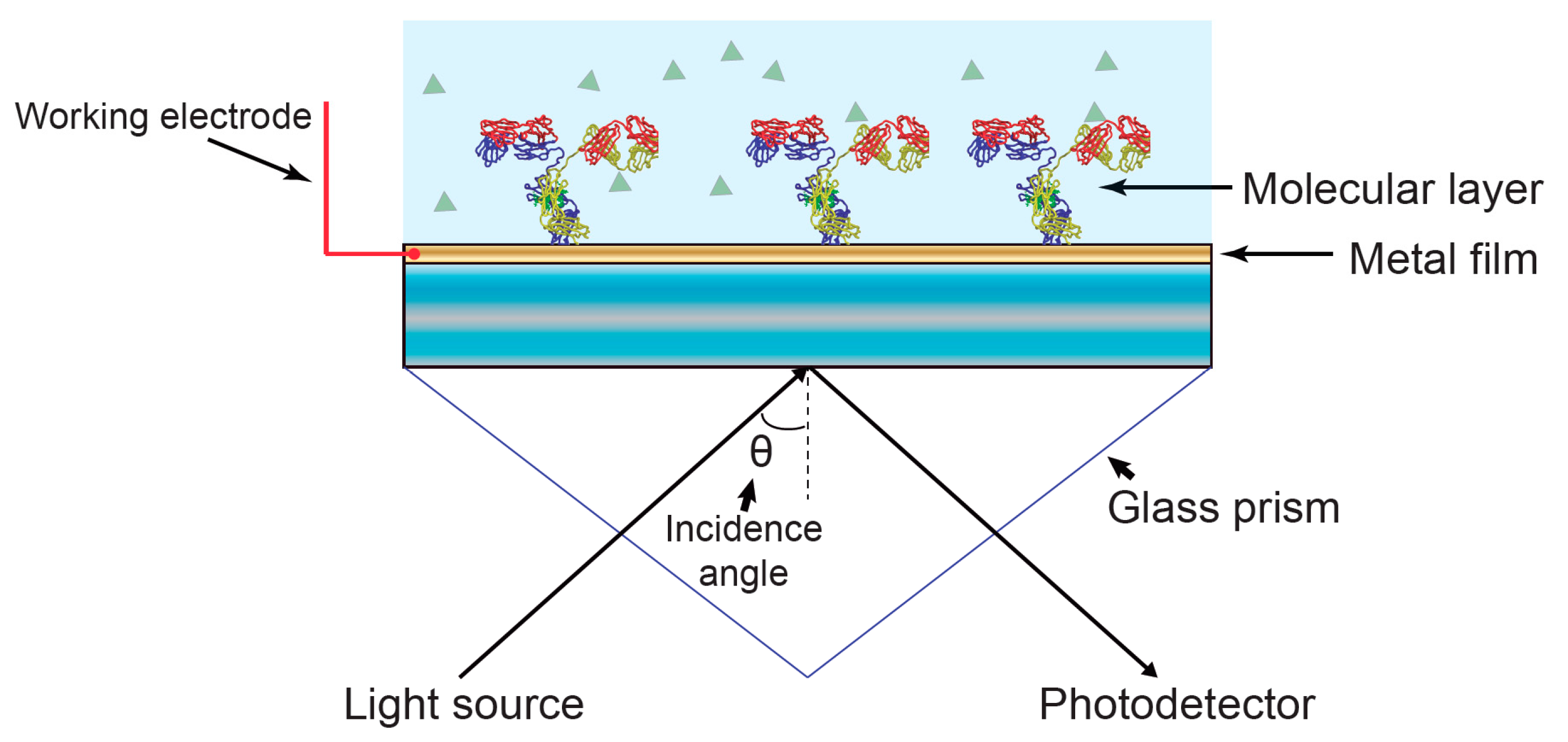
2.1. Electrochemical Surface Plasmon Resonance (EC-SPR)
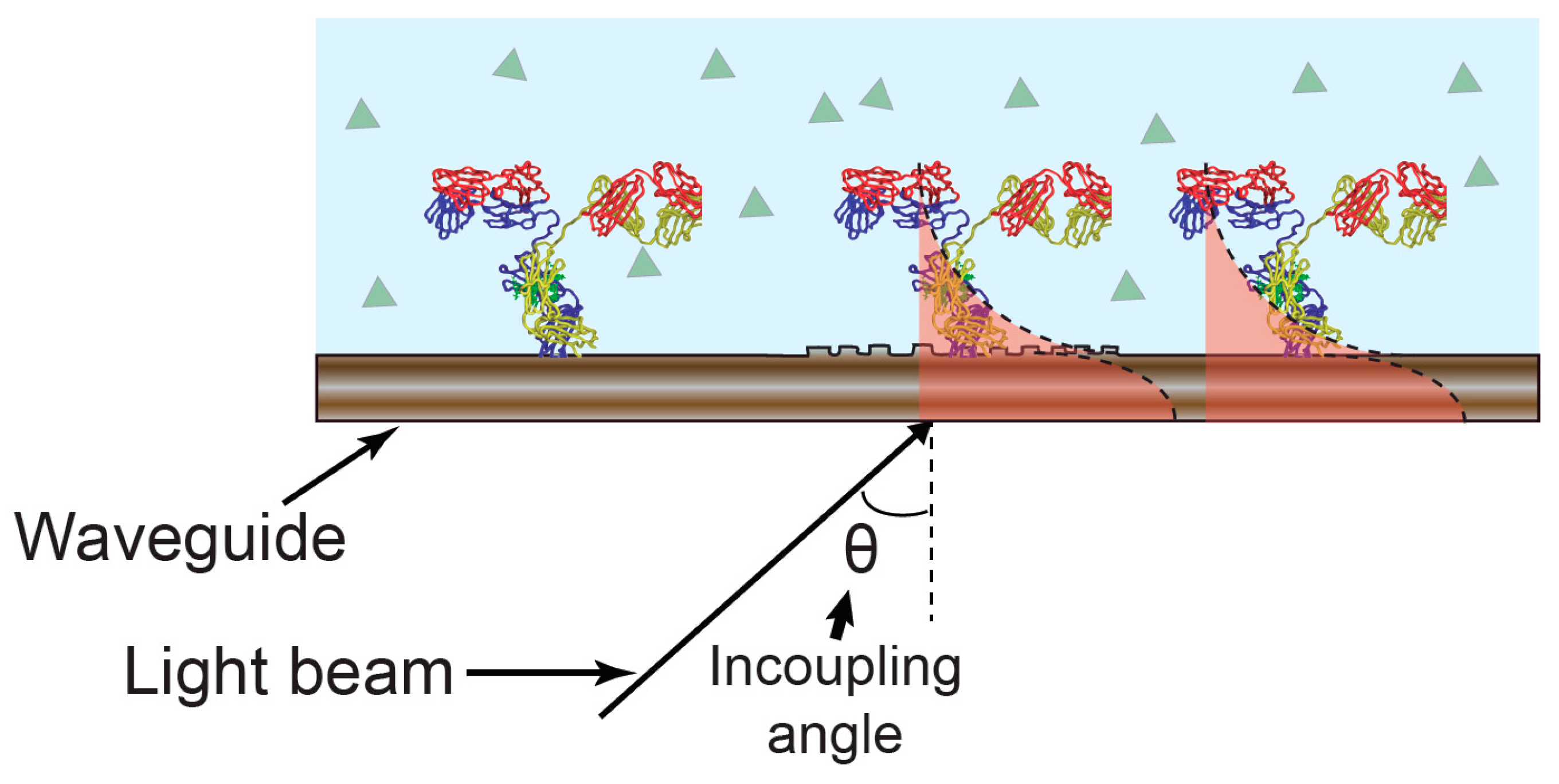
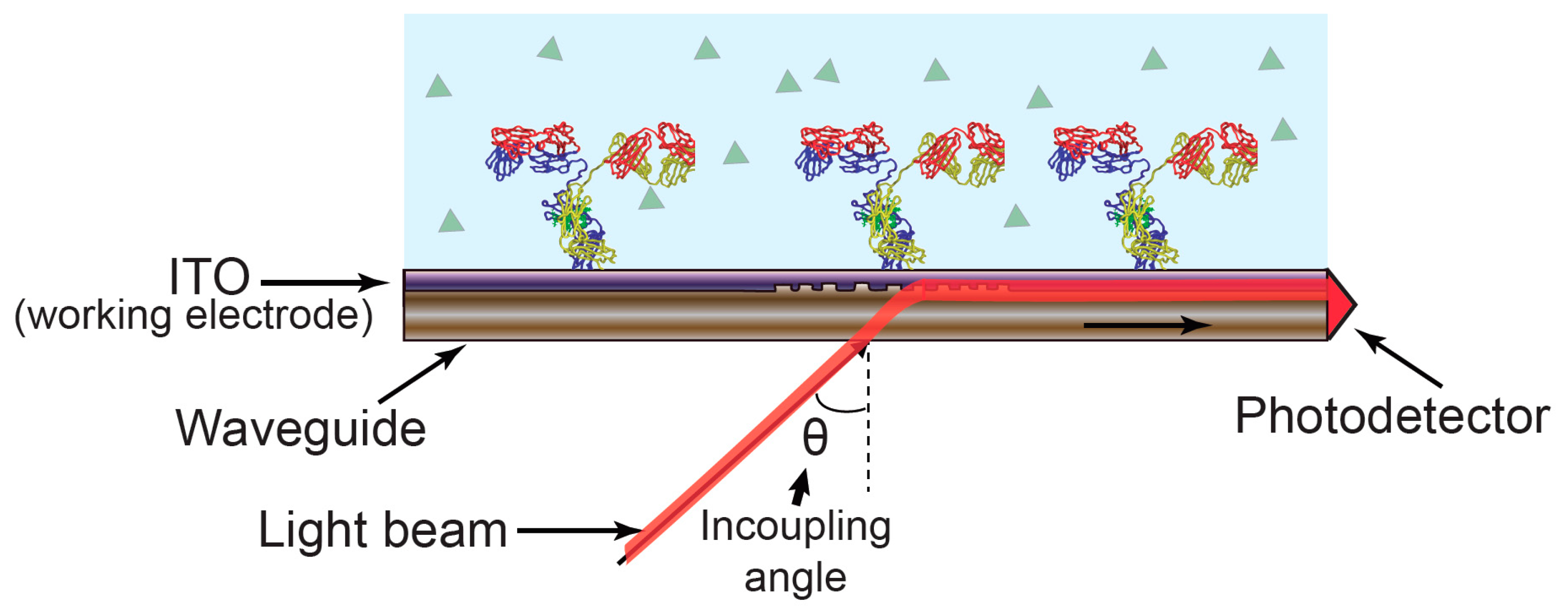
2.2. Electrochemical Optical Waveguide Lightmode Spectroscopy (EC-OWLS)
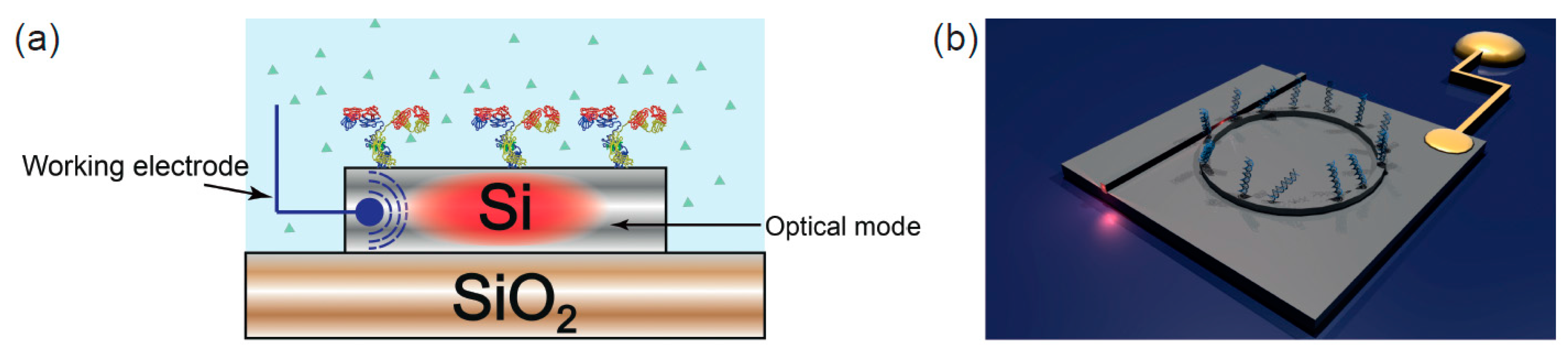
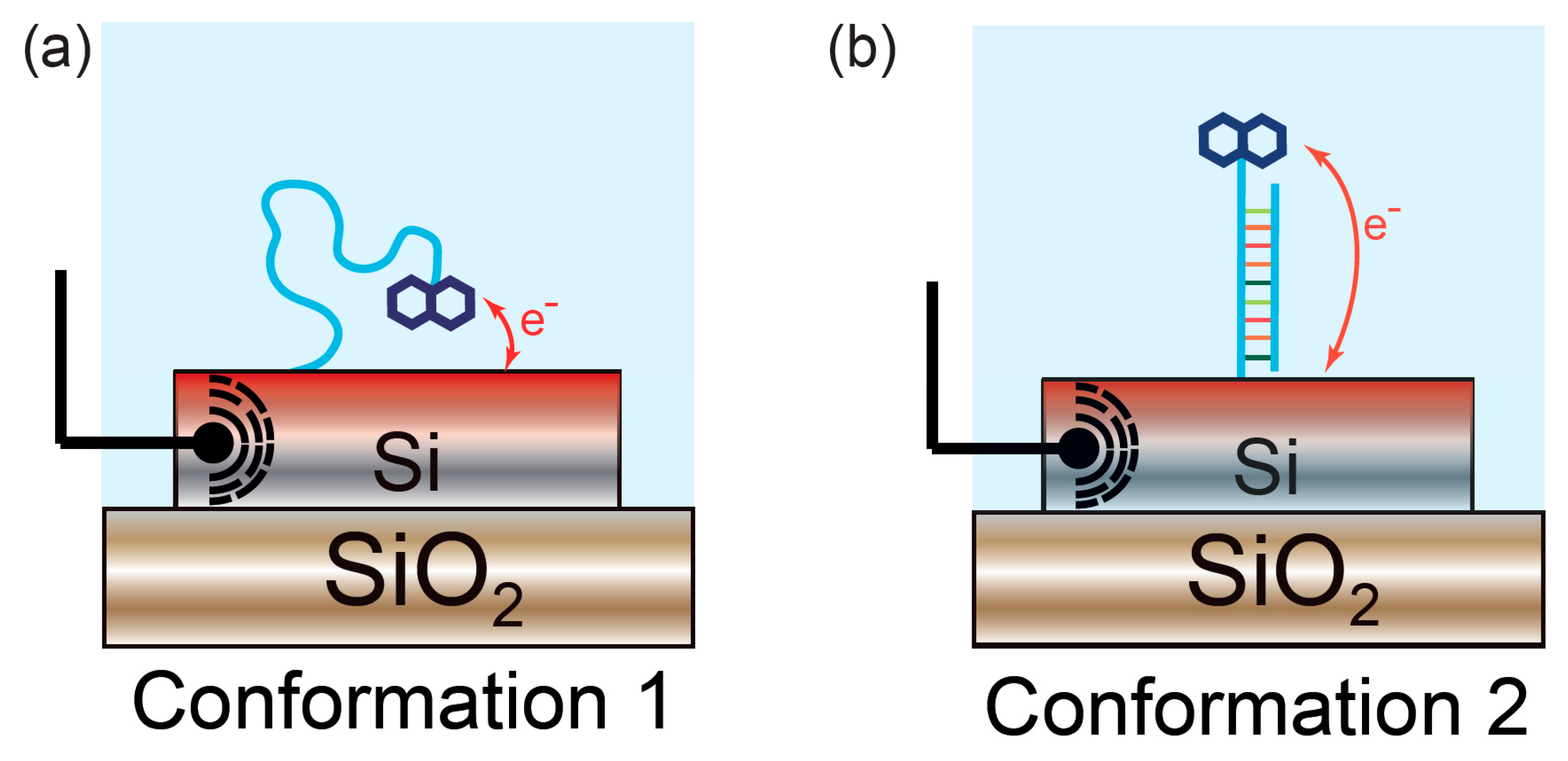
2.3. Electrophotonic Silicon Biosensing
2.4. Comparison between Optical Dual-Mode Sensing Techniques
2.4.1. Intrinsic Detection Limit
2.4.2. Integration
2.4.3. Tailoring of Light-Matter Interaction for the Understanding of Biomolecular Processes
2.4.4. 2D Imaging of Biointeractions on a Surface
2.4.5. Comparison Summary
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Borrebaeck, C.A.K. Precision diagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer 2017, 17, 199–204. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, B.; Hearty, S.; Leonard, P.; O’Kennedy, R. Cardiac biomarkers and the case for point-of-care testing. Clin. Biochem. 2009, 42, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Deacon, S.E.; Nowak, D.; George, S.E.; Szymonik, M.P.; Tang, A.A.S.; Tomlinson, D.C.; Davies, A.G.; McPherson, M.J.; Walti, C. Label-free electrochemical impedance biosensor to detect human interleukin-8 in serum with sub-pg/ml sensitivity. Biosens. Bioelectron. 2016, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.D.; Vanjari, S.R.K.; Sharma, C.S.; Singh, S.G. Ultrasensitive, Label Free, Chemiresistive Nanobiosensor Using Multiwalled Carbon Nanotubes Embedded Electrospun SU-8 Nanofibers. Sensors 2016, 16, 1354. [Google Scholar] [CrossRef]
- Yuan, J.; Oliver, R.; Aguilar, M.-I.; Wu, Y. Surface Plasmon Resonance Assay for Chloramphenicol. Anal. Chem. 2008, 80, 8329–8333. [Google Scholar] [CrossRef] [PubMed]
- Maraldo, D.; Rijal, K.; Campbell, G.; Mutharasan, R. Method for Label-Free Detection of Femtogram Quantities of Biologics in Flowing Liquid Samples. Anal. Chem. 2007, 79, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Yalow, R.S.; Berson, S.A. Immunoassay of Endogenous Plasma Insulin in Man. J. Clin. Investig. 1960, 39, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Daves, M.; Mattiuzzi, C. Interference of medical contrast media on laboratory testing. Biochem. Med. 2014, 24, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mehta, G.; Srivastava, S. Label-free detection techniques for protein microarrays: Prospects, merits and challenges. Proteomics 2009, 1002, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rodda, E.; Furlong, D.N.; Niikura, K.; Okahata, Y. Quartz Crystal Microbalance Study of DNA Immobilization and Hybridization for Nucleic Acid Sensor Development. Anal. Chem. 1997, 69, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, C.N.; Evans, J.A.; Chien, L.-C.; Flowers, N. Nucleic Acid Hybridization Detected by Piezoelectric Resonance. Anal. Lett. 1988, 21, 1099–1114. [Google Scholar] [CrossRef]
- Luo, X.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 42, 5944–5962. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D. (Bio)sensors for measurement of analytes implicated in food safety: A review. Trends Anal. Chem. 2002, 21, 96–115. [Google Scholar] [CrossRef]
- Grieshaber, D.; Mackenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors -Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Kehl, F.; Ehrentreich-Forster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2012, 6, 463–487. [Google Scholar] [CrossRef]
- Taillaert, D.; Bogaerts, W.; Bienstman, P.; Krauss, T.F.; Van Daele, P.; Moerman, I.; Verstuyft, D.; De Mesel, K.; Baets, R. An out-of-plane grating coupler for efficient butt-coupling between compact planar waveguides and single-mode fibers. IEEE J. Quantum Electron. 2002, 38, 949–955. [Google Scholar] [CrossRef]
- Bluestein, B.I.; Walczak, I.M.; Chen, S.Y. Fiber optic evanescent wave immunosensors for medical diagnostics. Trends Biotechnol. 1990, 8, 161–168. [Google Scholar] [CrossRef]
- Walczak, I.M.; Love, W.F.; Cook, T.A.; Slovacek, R.E. The application of evanescent wave sensing to a high-sensitivity fluoroimmunoassay. Biosens. Bioelectron. 1992, 7, 39–48. [Google Scholar] [CrossRef]
- Duval, D.; Lechuga, L.M. Optical Waveguide Biosensors. In Photonics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 323–365. ISBN 9781119011804. [Google Scholar]
- Gavela, A.F.; García, D.G.; Ramirez, J.C.; Lechuga, L.M. Last advances in silicon-based optical biosensors. Sensors 2016, 16, 285. [Google Scholar] [CrossRef]
- Iqbal, M.; Gleeson, M.A.; Spaugh, B.; Tybor, F.; Gunn, W.G.; Hochberg, M.; Baeher-Jones, T.; Bailey, R.C.; Gunn, L.C. Label-Free Biosensor Arrays Based on Silicon Ring Resonators and High-Speed Optical Scanning Instrumentation. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 654–661. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Gylfason, K.B.; Kaźmierczak, A.; Dortu, F.; Bañuls Polo, M.J.; Maquieira Catala, A.; Kresbach, G.M.; Sohlström, H.; Moh, T.; Vivien, L.; et al. A packaged optical slot-waveguide ring resonator sensor array for multiplex label-free assays in labs-on-chips. Lab Chip 2010, 10, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.L.; De Vos, K.; Claes, T.; Komorowska, K.; Van Thourhout, D.; Bienstman, P. Silicon-on-Insulator Microring Resonator Sensor Integrated on an Optical Fiber Facet. IEEE Photonics Technol. Lett. 2011, 23, 890–892. [Google Scholar] [CrossRef]
- Yablonovitch, E. Photonic band-gap structures. J. Opt. Soc. Am. B 1993, 10, 283. [Google Scholar] [CrossRef]
- Troia, B.; Paolicelli, A.; Leonardis, F.D.; Passaro, V.M.N. Photonic Crystals for Optical Sensing: A Review; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar] [PubMed]
- Baba, A.; Taranekar, P.; Ponnapati, R.R.; Knoll, W.; Advincula, R.C. Electrochemical Surface Plasmon Resonance (EC-SPR) and Waveguide Enhanced Glucose Biosensing with N-Alkylaminated Polypyrrole/Glucose Oxidase Multilayers. ACS Appl. Mater. Interface 2010, 2, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Bearinger, J.P.; Vörös, J.; Hubbell, J.A.; Textor, M. Electrochemical optical waveguide lightmode spectroscopy (EC-OWLS): A pilot study using evanescent-field optical sensing under voltage control to monitor polycationic polymer adsorption onto indium tin oxide (ITO)-coated waveguide chips. Biotechnol. Bioeng. 2003, 82, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Juan-Colás, J.; Parkin, A.; Dunn, K.E.; Scullion, M.G.; Krauss, T.F.; Johnson, S.D. The electrophotonic silicon biosensor. Nat. Commun. 2016, 7, 12769. [Google Scholar] [CrossRef] [PubMed]
- Juan Colás, J. Dual-Mode Electro-Photonic Silicon Biosensors, 1st ed.; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-60500-5. [Google Scholar]
- Grover Shah, V.; Ray, S.; Karlsson, R.; Srivastava, S. Calibration-free concentration analysis of protein biomarkers in human serum using surface plasmon resonance. Talanta 2015, 144, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Lazar, J.; Rosencrantz, R.R.; Elling, L.; Schnakenberg, U. Simultaneous Electrochemical Impedance Spectroscopy and Localized Surface Plasmon Resonance in a Microfluidic Chip: New Insights into the Spatial Origin of the Signal. Anal. Chem. 2016, 88, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Chabot, V.; Miron, Y.; Grandbois, M.; Charette, P.G. Long range surface plasmon resonance for increased sensitivity in living cell biosensing through greater probing depth. Sens. Actuators B 2012, 174, 94–101. [Google Scholar] [CrossRef]
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making it stick: Convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, H.E.; Altenburg, B.S.F.; Kooyman, R.P.H.; Greve, J. Determination of thickness and dielectric constant of thin transparent dielectric layers using surface plasmon resonance. Opt. Commun. 1991, 82, 425–432. [Google Scholar] [CrossRef]
- Treviño, J.; Calle, A.; Rodríguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Determination of human growth hormone in human serum samples by surface plasmon resonance immunoassay. Talanta 2009, 78, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Hanken, D.G.; Corn, R.M. Electric Fields and Interference Effects inside Noncentrosymmetric Multilayer Films at Electrode Surfaces from Electrochemically Modulated Surface Plasmon Resonance Experiments. Anal. Chem. 1997, 69, 3665–3673. [Google Scholar] [CrossRef]
- Boussaad, S.; Pean, J.; Tao, N.J. High-resolution multiwavelength surface plasmon resonance spectroscopy for probing conformational and electronic changes in redox proteins. Anal. Chem. 2000, 72, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Chalovich, J.M.; Eisenberg, E. NIH Public Access. Biophys. Chem. 2005, 257, 2432–2437. [Google Scholar] [CrossRef]
- Zhai, P.; Guo, J.; Xiang, J.; Zhou, F. Electrochemical Surface Plasmon Resonance Spectroscopy at Bilayered Silver/Gold Films. J. Phys. Chem. C 2007, 111, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, J.; Zhou, F.; Wang, J.; Tao, N. Quantification of Redox-Induced Thickness Changes of 11-Ferrocenylundecanethiol Self-Assembled Monolayers by Electrochemical Surface Plasmon Resonance. J. Phys. Chem. B 2004, 108, 7206–7212. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Shan, X.; Chang, X.; Tao, N. Flow-through Electrochemical Surface Plasmon Resonance: Detection of intermediate reaction products. J. Electroanal. Chem. 2010, 649, 37–41. [Google Scholar] [CrossRef]
- Salamifar, S.E.; Lai, R.Y. Application of electrochemical surface plasmon resonance spectroscopy for characterization of electrochemical DNA sensors. Colloids Surf. B 2014, 122, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, W.; Wang, S.P.; Shan, X.N.; Li, J.H.; Tao, N.J. Plasmonic-Based Electrochemical Impedance Spectroscopy: Application to Molecular Binding. Anal. Chem. 2012, 84, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kurrat, R.; Ramsden, J.J.; Prenosil, J.E. Kinetic model for serum albumin adsorption: Experimental verification. J. Chem. Soc. Faraday Trans. 1994, 90, 587–590. [Google Scholar] [CrossRef]
- Kurrat, R.; Prenosil, J.E.; Ramsden, J.J. Kinetics of Human and Bovine Serum Albumin Adsorption at Silica–Titania Surfaces. J. Colloid Interface Sci. 1997, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kurrat, R.; Textor, M.; Ramsden, J.J.; Böni, P.; Spencer, N.D. Instrumental improvements in optical waveguide light mode spectroscopy for the study of biomolecule adsorption. Rev. Sci. Instrum. 1997, 68, 2172–2176. [Google Scholar] [CrossRef]
- Vörös, J.; Ramsden, J.; Csúcs, G.; Szendro, I.; De Paul, S.; Textor, M.; Spencer, N. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Piehler, J.; Brandenburg, A.; Brecht, A.; Wagner, E.; Gauglitz, G. Characterization of grating couplers for affinity-based pesticide sensing. Appl. Opt. 1997, 36, 6554–6562. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Schmutz, P.; Petronis, S.; Textor, M.; Keller, B.; Vörös, J. Locally Addressable Electrochemical Patterning Technique (LAEPT) applied to poly(L-lysine)-graft-poly(ethylene glycol) adlayers on titanium and silicon oxide surfaces. Biotechnol. Bioeng. 2005, 91, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Szendrő, I.; Erdélyi, K.; Fábián, M.; Puskás, Z.; Adányi, N.; Somogyi, K. Combination of the optical waveguide lightmode spectroscopy method with electrochemical measurements. Thin Solid Films 2008, 516, 8165–8169. [Google Scholar] [CrossRef]
- Erdélyi, K.; Frutos, A.G.; Ramsden, J.J.; Szendro, I.; Voirin, G. Grating-Based Optical Biosensors. In Handbook of Biosensors and Biochips; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; ISBN 9780470061565. [Google Scholar]
- Ngankam, A.P.; Van Tassel, P.R. In situ layer-by-layer film formation kinetics under an applied voltage measured by optical waveguide lightmode spectroscopy. Langmuir 2005, 21, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Boulmedais, F.; Tang, C.S.; Keller, B.; Vörös, J. Controlled Electrodissolution of Polyelectrolyte Multilayers: A Platform Technology Towards the Surface-Initiated Delivery of Drugs. Adv. Funct. Mater. 2006, 16, 63–70. [Google Scholar] [CrossRef]
- Gabi, M.; Sannomiya, T.; Larmagnac, A.; Puttaswamy, M.; Vo, J.; Vörös, J.J. Influence of applied currents on the viability of cells close to microelectrodes. Integr. Biol. 2009, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, M.; Reuter, F.; Vörös, J. Review of transducer principles for label-free biomolecular interaction analysis. Biosensors 2011, 1, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Brusatori, M.A.; Tie, Y.; Van Tassel, P.R. Protein Adsorption Kinetics under an Applied Electric Field: An Optical Waveguide Lightmode Spectroscopy Study. Langmuir 2003, 19, 5089–5097. [Google Scholar] [CrossRef]
- Adányi, N.; Németh, E.; Halász, A.; Szendrő, I.; Váradi, M. Application of electrochemical optical waveguide lightmode spectroscopy for studying the effect of different stress factors on lactic acid bacteria. Anal. Chim. Acta 2006, 573–574, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Delai, M.; Szendro, I.; Guillaume-Gentil, O.; Vörös, J.; Zambelli, T. Simultaneous OWLS and EIS monitoring of supported lipid bilayers with the pore forming peptide melittin. Sens. Actuators B 2012, 161, 600–606. [Google Scholar] [CrossRef]
- Bogaerts, W.; Baets, R.; Dumon, P.; Wiaux, V.; Beckx, S.; Taillaert, D.; Luyssaert, B.; Campenhout, J.V.; Bienstman, P.; Thourhout, D. Van Nanophotonic Waveguides in Silicon-on-Insulator Fabricated with CMOS Technology. J. Lightwave Technol. 2005, 23, 401–412. [Google Scholar]
- Bañuls, M.-J.; Puchades, R.; Maquieira, Á. Chemical surface modifications for the development of silicon-based label-free integrated optical (IO) biosensors: A review. Anal. Chim. Acta 2013, 777, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tu, X.; Kim, K.W.; Kee, J.S.; Shin, Y.; Han, K.; Yoon, Y.-J.; Lo, G.-Q.; Park, M.K. Highly sensitive Mach–Zehnder interferometer biosensor based on silicon nitride slot waveguide. Sens. Actuators B 2013, 188, 681–688. [Google Scholar] [CrossRef]
- Kindt, J.T.; Bailey, R.C. Biomolecular Analysis with Microring Resonators: Applications in Multiplexed Diagnostics and Interaction Screening. Curr. Opin. Chem. Biol. 2013, 17, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, M.S.; Bailey, R.C. High-Q Optical Sensors for Chemical and Biological Analysis. Anal. Chem. 2012, 84, 793–821. [Google Scholar] [CrossRef] [PubMed]
- Washburn, A.L.; Shia, W.W.; Lenkeit, K.A.; Lee, S.-H.; Bailey, R.C. Multiplexed cancer biomarker detection using chip-integrated silicon photonic sensor arrays. Analyst 2016, 141, 5358–5365. [Google Scholar] [CrossRef] [PubMed]
- Flavel, B.S.; Gross, A.J.; Garrett, D.J.; Nock, V.; Downard, A.J. A simple approach to patterned protein immobilization on silicon via electrografting from diazonium salt solutions. ACS Appl. Mater. Interfaces 2010, 2, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Mahouche-Chergui, S.; Gam-Derouich, S.; Mangeney, C.; Chehimi, M.M. Aryl diazonium salts: A new class of coupling agents for bonding polymers, biomacromolecules and nanoparticles to surfaces. Chem. Soc. Rev. 2011, 40, 4143–4166. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.C.; Polsky, R.; Wheeler, D.R.; Brozik, S.M. Maleimide-activated aryl diazonium salts for electrode surface functionalization with biological and redox-active molecules. Langmuir 2008, 24, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Magnusson, R. Theory and applications of guided-mode resonance filters. Appl. Opt. 1993, 32, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Tibuleac, S.; Magnusson, R. Reflection and transmission guided-mode resonance filters. J. Opt. Soc. Am. A 1997, 14, 1617–1626. [Google Scholar] [CrossRef]
- Ramaiah, K.S.; Raja, V.S.; Bhatnagar, A.K.; Tomlinson, R.D.; Pilkington, R.D.; Hill, A.E.; Chang, S.J.; Su, Y.K.; Juang, F.S. Optical, structural and electrical properties of tin doped indium oxide thin films prepared by spray-pyrolysis technique. Semicond. Sci. Technol. 2000, 15, 676–683. [Google Scholar] [CrossRef]
- Lakshmanan, T.K. Optical and Electrical Properties of Semiconducting Cadmium Oxide Films. J. Electrochem. Soc. 1963, 110, 548–551. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Shan, X.; Foley, K.J.; Tao, N. Electrochemical surface plasmon resonance: Basic formalism and experimental validation. Anal. Chem. 2010, 82, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chakravarty, S.; Lai, W.-C.; Lin, C.-Y.; Chen, R.T. Methods to array photonic crystal microcavities for high throughput high sensitivity biosensing on a silicon-chip based platform. Lab Chip 2012, 12, 2309–2312. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; del Río, J.S.; Moreno, M.; Blanco, F.J.; Mayora, K.; Domínguez, C.; Lechuga, L.M. Optical biosensor microsystems based on the integration of highly sensitive Mach–Zehnder interferometer devices. J. Opt. A 2006, 8, S561. [Google Scholar] [CrossRef]
- Triggs, G.J.; Wang, Y.; Reardon, C.P.; Fischer, M.; Evans, G.J.O.; Krauss, T.F. Chirped guided-mode resonance biosensor. Optica 2017, 4, 229–234. [Google Scholar] [CrossRef]
- Claes, T.; Bogaerts, W.; Bienstman, P. Vernier-cascade label-free biosensor with integrated arrayed waveguide grating for wavelength interrogation with low-cost broadband source. Opt. Lett. 2011, 36, 3320–3322. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, L.; Li, M.; He, J.J. Optical sensor based on vernier-cascade of ring resonator and echelle diffraction grating. IEEE Photonics Technol. Lett. 2012, 24, 954–956. [Google Scholar] [CrossRef]
- Kirk, J.T.; Fridley, G.E.; Chamberlain, J.W.; Christensen, E.D.; Hochberg, M.; Ratner, D.M. Multiplexed inkjet functionalization of silicon photonic biosensors. Lab Chip 2011, 11, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ming, H. Review of surface plasmon resonance and localized surface plasmon resonance sensor? Photonic Sens. 2012, 2, 37–49. [Google Scholar] [CrossRef]
- Ramsden, J.J. Review of new experimental techniques for investigating random sequential adsorption. J. Stat. Phys. 1993, 73, 853–877. [Google Scholar] [CrossRef]
- Hoste, J.-W.; Werquin, S.; Claes, T.; Bienstman, P. Conformational analysis of proteins with a dual polarisation silicon microring. Opt. Express 2014, 22, 2807–2820. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Sohlström, H.; Holgado, M.; Casquel, R. Slot-waveguide biochemical sensor. Opt. Lett. 2007, 32, 3080–3082. [Google Scholar] [CrossRef] [PubMed]
- Claes, T.; Molera, J.G.; De Vos, K.; Schacht, E.; Baets, R.; Bienstman, P. Label-Free Biosensing With a Slot-Waveguide-Based Ring Resonator in Silicon on Insulator. IEEE Photonics J. 2009, 1, 197–204. [Google Scholar] [CrossRef]
- Di Falco, A.; O’Faolain, L.; Krauss, T.F. Chemical sensing in slotted photonic crystal heterostructure cavities. Appl. Phys. Lett. 2009, 94, 63503. [Google Scholar] [CrossRef]
- Scullion, M.G.; Krauss, T.F.; Di Falco, A. Slotted photonic crystal sensors. Sensors 2013, 13, 3675–3710. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Wang, S.; Nagaraj, V.J.; Liu, Q.; Wu, J.; Tao, N. Label-free measuring and mapping of binding kinetics of membrane proteins in single living cells. Nat. Chem. 2012, 4, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, H.; Shan, X.; Wang, W.; Liu, X.; Wang, S.; Tao, N. Label-Free Tracking of Single Organelle Transportation in Cells with Nanometer Precision Using a Plasmonic Imaging Technique. Small 2015, 11, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.E.; Coskun, A.F.; Galarreta, B.C.; Huang, M.; Herman, D.; Ozcan, A.; Altug, H. Handheld high-throughput plasmonic biosensor using computational on-chip imaging. Light Sci. Appl. 2014, 3, e122. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. SPR and SPR imaging: Recent trends in developing nanodevices for detection and real-time monitoringof biomolecular events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.T.; Laing, L. Microplate-based, label-free detection of biomolecular interactions: Applications in proteomics. Expert Rev. Proteom. 2006, 3, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Triggs, G.J.; Fischer, M.; Stellinga, D.; Scullion, M.G.; Evans, G.J.O.; Krauss, T.F. Spatial resolution and refractive index contrast of resonant photonic crystal surfaces for biosensing. IEEE Photonics J. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Jahns, S.; Bräu, M.; Meyer, B.-O.; Karrock, T.; Gutekunst, S.B.; Blohm, L.; Selhuber-Unkel, C.; Buhmann, R.; Nazirizadeh, Y.; Gerken, M. Handheld imaging photonic crystal biosensor for multiplexed, label-free protein detection. Biomed. Opt. Express 2015, 6, 3724–3736. [Google Scholar] [CrossRef] [PubMed]
| Technology | Degradation on the Intrinsic Detection Limit by EC Integration | Integration for LOC Devices | Tailoring of Light-Matter Interaction | 2D Imaging | Commercial Availability |
|---|---|---|---|---|---|
| EC-SPR | Not degraded [3] | Complex [4] | Not possible [5] | ~5 times lower resolution than SPR [4,6,7,8,9] | Yes |
| EC-OWLS | Degraded [10,11] | Complex, no reports found | Achievable to some extend [10] | Capable, but no reports found | Yes |
| Electro-photonic approach | Minimally degraded [1,2] | Easy | Complete freedom [1,2] | Capable with minimal degradation | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan-Colás, J.; Johnson, S.; Krauss, T.F. Dual-Mode Electro-Optical Techniques for Biosensing Applications: A Review. Sensors 2017, 17, 2047. https://doi.org/10.3390/s17092047
Juan-Colás J, Johnson S, Krauss TF. Dual-Mode Electro-Optical Techniques for Biosensing Applications: A Review. Sensors. 2017; 17(9):2047. https://doi.org/10.3390/s17092047
Chicago/Turabian StyleJuan-Colás, José, Steven Johnson, and Thomas F. Krauss. 2017. "Dual-Mode Electro-Optical Techniques for Biosensing Applications: A Review" Sensors 17, no. 9: 2047. https://doi.org/10.3390/s17092047
APA StyleJuan-Colás, J., Johnson, S., & Krauss, T. F. (2017). Dual-Mode Electro-Optical Techniques for Biosensing Applications: A Review. Sensors, 17(9), 2047. https://doi.org/10.3390/s17092047





