TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects
Abstract
1. Introduction
2. Fabrication of TiO2-Based Nanoheterostructures
2.1. CVD and ALD Methods
2.2. Solid Phase Reactions
2.3. Electrochemical Deposition
2.4. Chemical Deposition
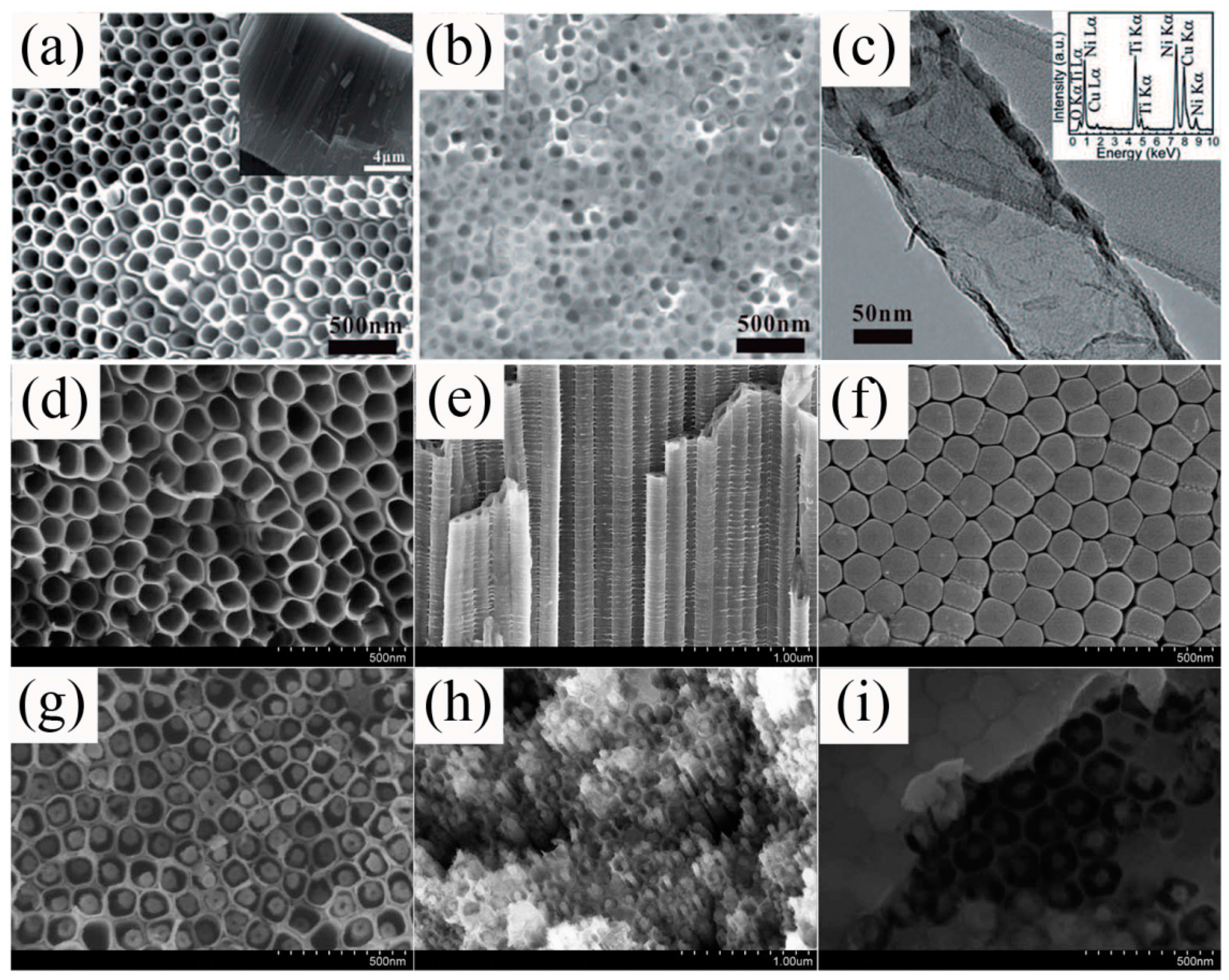
2.5. Hydrothermal/Solvothermal Technique
2.6. Sol-Gel Method
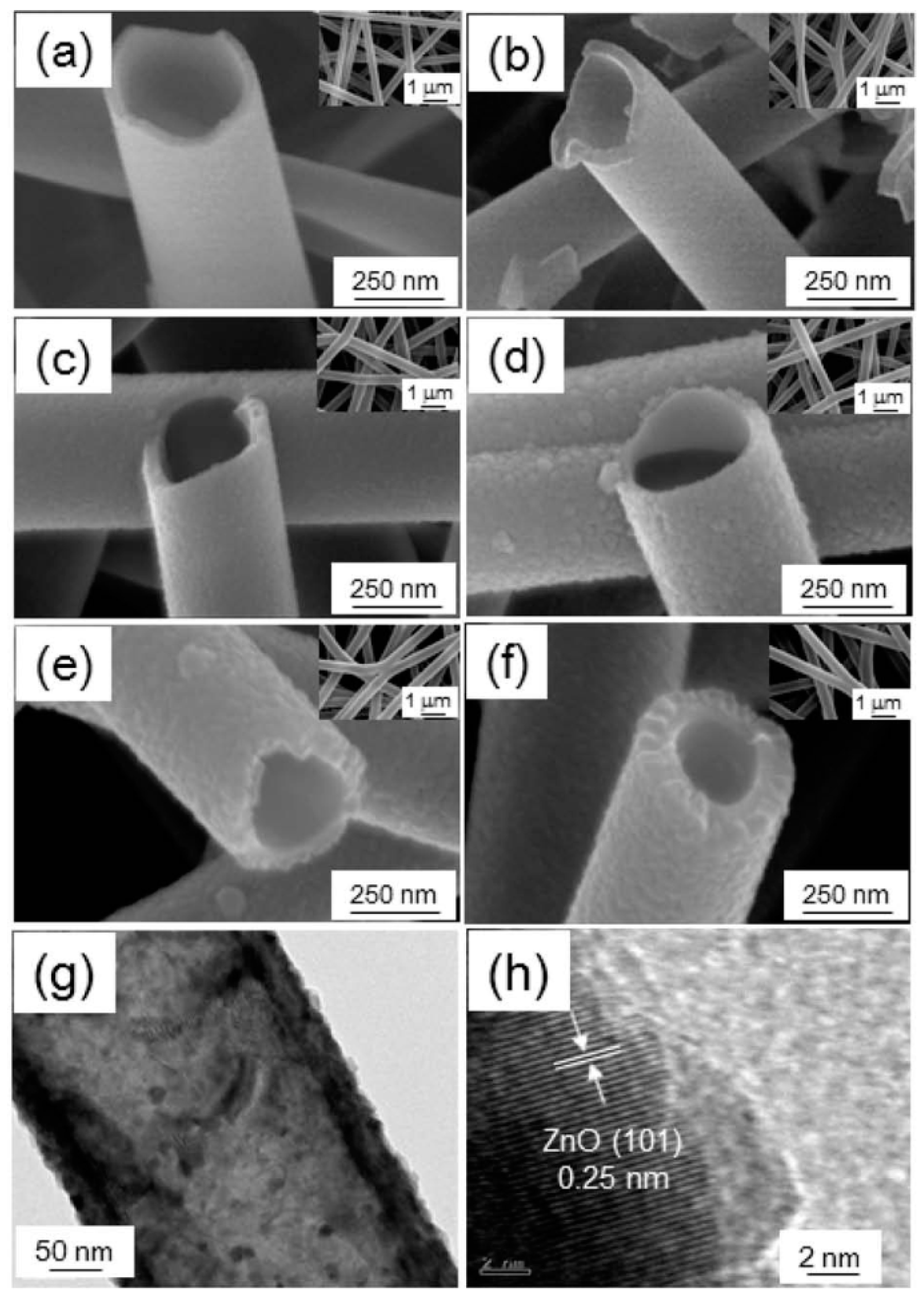
2.7. Electrospinning
3. Diverse Nanoheterostructural Gas Sensors
3.1. Semiconductor/Semiconductor Nanoheterostructures
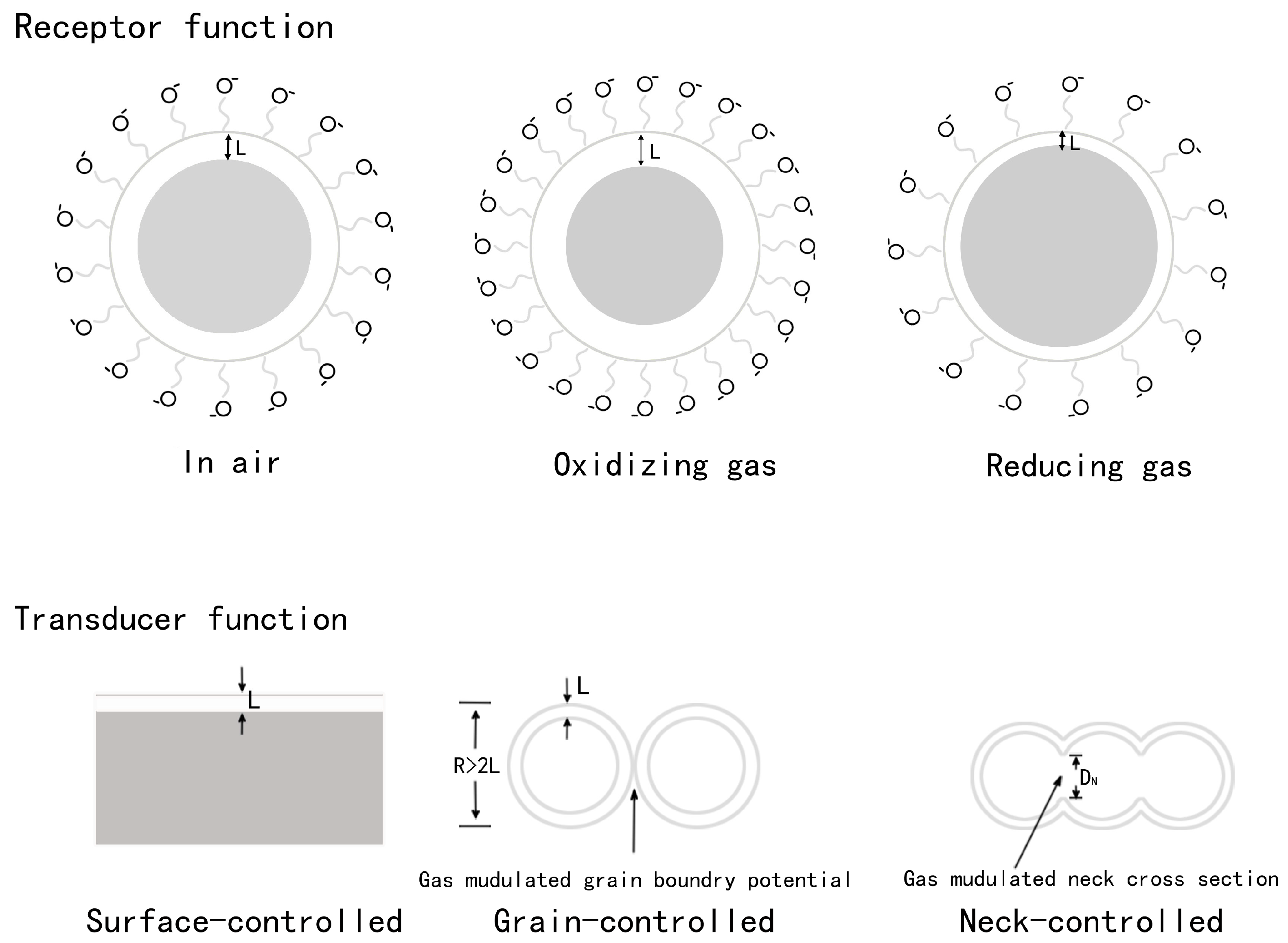
3.1.1. Sensing Mechanism
3.1.2. The Influence of Morphology on Sensing Performance
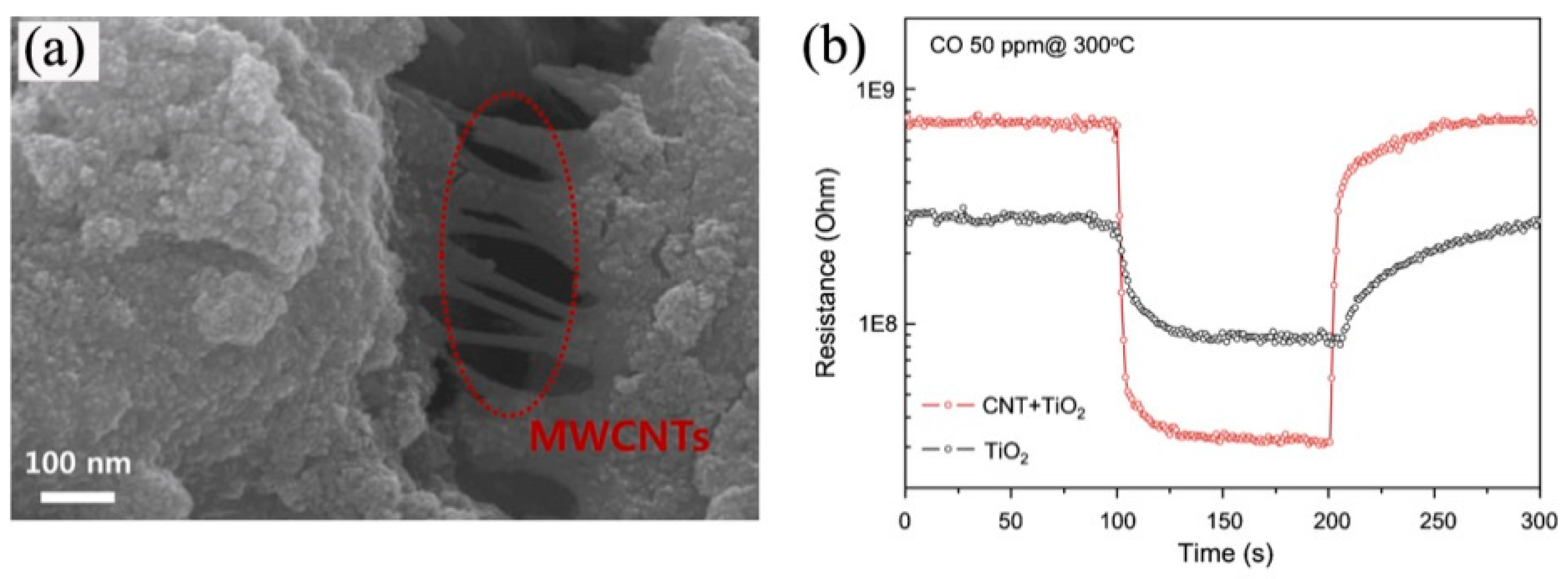
3.2. Carbon-Group-Materials/Semiconductor Nanoheterostructures
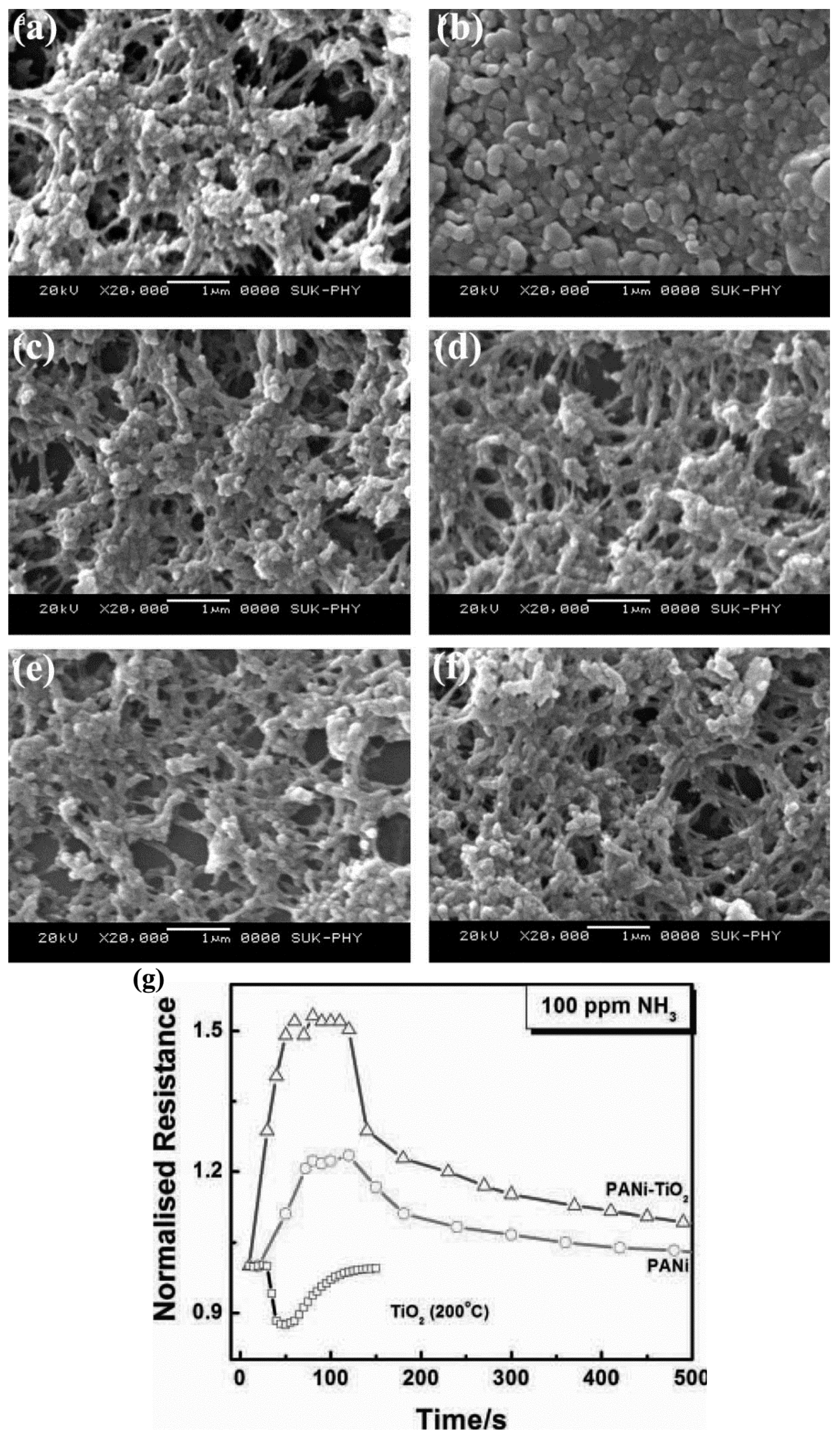
3.3. Organic/Inorgnic Nanoheterostructures
4. Conclusions
- (1)
- Coupling TiO2 by with other semiconducting materials to form heterostructures could result in enhanced sensitivity and selectivity, faster response/recovery times, and/or lower operational temperatures than pure TiO2. The enhanced sensing properties could be related to the heterojunctions formed at the interface between two semiconductors, the synergetic effect and catalytic effect. Additionally, the influence of the nanoheterostructures’ morphology on sensing performance should also be taken into account, where the gas sensitivity and the response/recovery time affected by gas absorption and gas diffusion can be promoted by the specific surface area and the special morphology of structures. As compared with other types of nanoheterostructure-based sensors, the minimization of detectable levels of the semiconductor/semiconductor nanoheterostructure-based sensors might not bear comparison with the carbon-group-materials/semiconductor nanoheterostructures, and their operation temperatures might be higher than that of noble metal/semiconductor nanoheterostructures and organic/inorganic nanoheterostructures, however, their most excellent stability make the semiconductor/semiconductor nanoheterostructures more acceptable when applied in many fields.
- (2)
- Combining carbon-group-materials with TiO2 is considered an efficient way to improve the gas sensing performance of TiO2. It has been demonstrated that the absorptivity, conductivity, and/or electrochemical reactions of some small gas molecules with carbon/TiO2 nano- heterostructures could be promoted, thus the nanoheterostructures can display remarkably improved sensing performance. Remarkably, carbon-group-material/semiconductor nano- heterostructure-based sensors display the most outstanding minimum detectable levels as compared with other nanoheterostructures, which could be attributed to their large surface area and the high electrical conductivity of the carbon-group-materials.
- (3)
- Conducting polymer-functionalized TiO2 nanoheterostructures have also been demonstrated to be some of most promising materials for gas detection. These nanoheterostructures possess fast and reversible responses at room temperature due to the formation of organic/inorganic heterojunctions. It is noticeable that defined organic material modified semiconductor sensors exhibit outstanding sensing selectivity to a single gas species because of their exclusive chemical and electronic conditions. As a type of typical room temperature sensor, the controllable sensing selectivity of organic/inorganic nanoheterostructures is an outstanding characteristic that other types of nanoheterostructures do not possess.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Docherty, C.J.; Lin, C.T.; Joyce, H.J.; Nichola, R.J.; Herz, L.M.; Li, L.J.; Johnston, M.B. Extreme sensitivity of graphene photoconductivity to environmental gases. Nat. Commun. 2012, 3, 1228. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wladyka, C.; Fujii, J.; Sockanathan, S. Prdx4 is a compartment-specific H2O2 sensor that regulates neurogenesis by controlling surface expression of GDE2. Nat. Commun. 2015, 6, 7006. [Google Scholar] [CrossRef]
- Lehner, P.; Staudinger, C.; Borisov, S.M.; Klimant, I. Ultra-sensitive optical oxygen sensors for characterization of nearly anoxic systems. Nat. Commun. 2014, 5, 4460. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mei, L.; Chen, Y.; Li, Q.; Wang, T.; Xu, Z.; Duan, X.; Zheng, W. α-Fe2O3 nanochains: Ammonium acetate-based ionothermal synthesis and ultrasensitive sensors for low-ppm-level H2S gas. Nanoscale 2013, 5, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Gao, J.; Wang, L.; Kan, K.; Xie, Y.; Shen, P.; Li, L.; Shi, K. Role of the heterojunctions in In2O3-composite SnO2 nanorod sensors and their remarkable gas-sensing performance for NOx at room temperature. Nanoscale 2015, 7, 14643–14651. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.W.G.; Prades, J.D.; Mayrhofer, L.; Hernandez-Ramirez, F.; Järvi, T.T.; Moseler, M.; Waag, A.; Shen, H. Highly selective SAM-nanowire hybrid NO2 sensor: Insight into charge transfer dynamics and alignment of frontier molecular orbitals. Adv. Funct. Mater. 2014, 24, 595–602. [Google Scholar] [CrossRef]
- Leite, E.R.; Weber, I.T.; Longo, E.; Varela, J.A. A new method to control particle size and particle size distribution of SnO2 nanoparticles for gas sensor applications. Adv. Mater. 2000, 12, 965–968. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Peng, Q.; Li, Y. Vanadium pentoxide nanobelts: Highly selective and stable ethanol sensor materials. Adv. Mater. 2005, 17, 764–767. [Google Scholar] [CrossRef]
- Izu, N.; Hagen, G.; Schönauer, D.; Röder-Roith, U.; Moos, R. Application of V2O5/WO3/TiO2 for Resistive-Type SO2 Sensors. Sensors 2011, 11, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Weppner, W. Solid-state electrochemical gas sensor. Sens. Actuators 1987, 12, 107–119. [Google Scholar] [CrossRef]
- Miura, N.; Nakatou, M.; Zhuiykov, S. Impedancemetric gas sensor based on zirconia solid electrolyte and oxide sensing electrode for detecting total NOx at high temperature. Sens. Actuators B Chem. 2003, 93, 221–228. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Reddy, K.; Zhong, Z.; Fan, X. Graphene nanoelectronic heterodyne sensor for rapid and sensitive vapour detection. Nat. Commun. 2014, 5, 4376. [Google Scholar] [CrossRef]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhanen, T. Ultrafast Graphene Oxide Humidity Sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Umar, A.; Wang, S.; Wang, Y.; Tian, T.; Shang, Y.; Fan, Y.; Qi, Q.; Xu, D.; Jiang, L. Supramolecular fabrication of multilevel graphene-based gas sensors with high NO2 sensibility. Nanoscale 2015, 7, 10259–10266. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kwon, O.S.; Park, S.J.; Park, E.Y.; You, S.A.; Yoon, H.; Jang, J. Fabrication of ultrafine metal-oxide-decorated carbon nanofibers for DMMP sensor application. ACS Nano 2011, 5, 7992–8001. [Google Scholar] [CrossRef] [PubMed]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductor thin film. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Muhr, H.J.; Krumeich, F.; Schönholzer, U.P.; Bieri, F.; Niederberger, M.; Gauckler, L.J.; Nesper, R. Vanadium oxide nanotubes-a new flexible vanadate nanophase. Adv. Mater. 2000, 12, 231–234. [Google Scholar] [CrossRef]
- Shi, L.; Naik, A.J.T.; Goodall, J.B.M.; Tighe, C.; Gruar, R.; Binions, R.; Parkin, I.; Darr, J. Highly sensitive ZnO nanorod- and nanoprism-based NO2 gas sensors: Size and shape control using a continuous hydrothermal pilot plant. Langmuir 2013, 29, 10603–10609. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Heo, Y.U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D hierarchical rutile TiO2 and metal-free organic sensitizer producing dye-sensitized solar cells 8.6% conversion efficiency. Sci. Rep. 2014, 4, 5769. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Peter, Y.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Zarifi, M.H.; Farsinezhad, S.; Abdolrazzaghi, M.; Daneshmand, M.; Shankar, K. Selective microwave sensors exploiting the interaction of analytes with trap states in TiO2 nanotube arrays. Nanoscale 2016, 8, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Ding, S.; Yuan, J.; Lou, X.W.; Kim, D.H. Hierarchically structured one-dimensional TiO2 for protein immobilization, direct electrochemistry, and mediator-free glucose sensing. ACS Nano 2011, 5, 7617–7626. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.J.; Li, C.M.; Zang, J.F.; Cui, X.Q.; Qiao, Y.; Guo, J. New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Qi, Y.; Lun, N.; Liu, N. Large scale synthesis and gas-sensing properties of anatase TiO2 three-dimensional hierarchical nanostructures. Langmuir 2010, 26, 12841–12848. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.D.; Rothschild, A.; Lee, B.H.; Kim, D.Y.; Jo, S.M.; Tuller, H.L. Ultrasensitive chemiresistors based on electrospun TiO2 nanofibers. Nano Lett. 2006, 6, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Sakai, R.; Sato, S.; Fukawa, T.; Ikehara, T.; Maeda, R.; Mihara, T. Sensing of vaporous organic compounds by TiO2 porous films covered with polythiophene layers. Adv. Funct. Mater. 2012, 22, 469–476. [Google Scholar] [CrossRef]
- Wang, Y.; Du, G.; Liu, H.; Liu, D.; Qin, S.; Wang, N.; Hu, C.; Tao, X.; Jiao, J.; Wang, J.; et al. Nanostructured sheets of Ti-O nanobelts for gas sensing and antibacterial applications. Adv. Funct. Mater. 2008, 18, 1131–1137. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. Electronic properties of Nano-porous TiO2- and ZnO-thin films- comparison of simulations and experiments. J. Ceram. Proc. Res. 2004, 5, 343–354. [Google Scholar]
- Dai, J.; Yang, J.; Wang, X.H.; Zhang, L.; Li, Y.J. Enhanced visible-light photocatalytic activity for selective oxidation of amines into imines over TiO2(B)/anatase mixed-phase nanowires. Appl. Surf. Sci. 2015, 349, 343–352. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Muscat, J.; Swamy, V.; Harrison, N.M. First-principles calculations of the phase stability of TiO2. Phys. Rev. B 2002, 65, 224112. [Google Scholar] [CrossRef]
- Altomare, M.; Dozzi, M.V.; Chiarello, G.L.; Di Paola, A.; Palmisano, L.; Selli, E. High activity of brookite TiO2 nanoparticles in the photocatalytic abatement of ammonia in water. Catal. Today 2015, 252, 184–189. [Google Scholar] [CrossRef]
- Nisar, J.; Topalian, Z.; De Sarkar, A.; Österlund, L.; Ahuja, R. TiO2-based gas sensor: A possible application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, K. Gas sensing mechanism of TiO2-based thin films. Vacuum 2004, 74, 335–338. [Google Scholar] [CrossRef]
- Göpel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B Chem. 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubes: Recent advances in synthesis and gas sensing properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-doped TiO2 nanotubes for wide-range hydrogen sensing. Nanoscal. Res. Lett. 2014, 9, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lü, R.; Zhou, W.; Shi, K.; Yang, Y.; Wang, L.; Pan, K.; Tian, C.; Ren, Z.; Fu, H. Alumina decorated TiO2 nanotubes with ordered mesoporous walls as high sensitivity NOx gas sensors at room temperature. Nanoscale 2013, 5, 8569–8576. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Comini, E.; Ferrucci, A.P.; Gasparotto, A.; Maccato, C.; Maragno, C.; Sberveglieri, G.; Tondello, E. First example of ZnO-TiO2 nanocomposites by chemical vapor deposition: Structure, morphology, composition, and gas sensing performances. Chem. Mater. 2007, 19, 5642–5649. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, G.N.; Bambole, D.R.; Bodade, A.B.; Padole, P.R. Characterization of nanosized TiO2 based H2S gas sensor. J. Mater. Sci. 2006, 41, 4860–4864. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Hu, Z.; Zhou, Z.; Deng, Y. Ultrasensitive NH3 gas sensor from polyaniline nanograin enchased TiO2 fibers. J. Phys. Chem. C 2010, 114, 9970–9974. [Google Scholar] [CrossRef]
- Zhang, J.; Strelcov, E.; Kolmakov, A. Visible light assisted gas sensing with TiO2 nanowires. arXiv 2015, 1501, 01877. [Google Scholar]
- Alferov, Z.I. The double heterostructure: The concept and its applications in physics, electronics, and technology (Nobel lecture). ChemPhysChem 2001, 2, 500–513. [Google Scholar] [CrossRef]
- Layek, A.; Middya, S.; Dey, A.; Das, M.; Datta, J.; Ray, P.P. Synthesis of ZnO composited TiO2 nanoparticle and its application in dye sensitized solar cells: A novel approach in enhancing open-circuit voltage. Mater. Lett. 2014, 126, 214–216. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. One-pot facile synthesis of Janus-structured SnO2-CuO composite nanorods and their application as anode materials in Li-ion batteries. Nanoscale 2013, 5, 4662–4668. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 core-shell nanostructures for enhanced photoelectrochemical water splitting under solar light illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.; Yong, K. CuO/ZnO heterostructured nanorods: Photochemical synthesis and the mechanism of H2S gas sensing. J. Phys. Chem. C 2012, 116, 15682–15691. [Google Scholar] [CrossRef]
- Deepagan, V.G.; You, D.G.; Um, W.; Ko, H.; Kwon, S.; Choi, K.Y.; Yi, G.R.; Lee, J.Y.; Lee, D.S.; Kim, K.; et al. Long-circulating Au-TiO2 nanocomposite as a sonosensitizer for ROS-mediated eradication of cancer. Nano Lett. 2016, 16, 6257–6264. [Google Scholar] [CrossRef]
- Leonardi, S.G.; Aloisio, D.; Donato, N.; Russo, P.A.; Ferro, M.C.; Pinna, N.; Neri, G. Amperometric sensing of H2O using Pt–TiO2/reduced graphene oxide nanocomposites. ChemElectroChem 2014, 1, 617–624. [Google Scholar] [CrossRef]
- Pandey, N.K.; Tiwari, K.; Roy, A. ZnO-TiO2 nanocomposite: Characterization and moisture sensing studies. Bull. Mater. Sci. 2012, 35, 347–352. [Google Scholar] [CrossRef]
- Hu, L.; Fong, C.C.; Zhang, X.; Chan, L.L.; Lam, P.K.; Chu, P.K.; Wong, K.Y.; Yang, M. Au nanoparticles decorated TiO2 nanotube arrays as a recyclable sensor for photo-enhanced electrochemical detection of bisphenol A. Environ. Sci. Technol. 2016, 50, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Wu, B.; Kan, K.; Xu, S.; Xie, Y.; Li, L.; Shi, K. Designed synthesis of In2O3 beads@TiO2-In2O3 composite nanofibers for high performance NO2 sensor at room tempreture. ACS Appl. Mater. Interfaces 2015, 7, 27152–27159. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, F.; Deng, J.; Wang, L.; Zhang, T. Branch-like hierarchical heterostructure (α-Fe2O3/TiO2): A novel sensing material for trimethylamine gas sensor. ACS Appl. Mater. Interfaces 2013, 5, 12310–12316. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Tang, J.; Wu, Z.; Selomulya, C.; Wang, H.; Wei, J.; Wang, Y.; Zheng, G.; Zhao, D. Bio-inspired porous antenna-like nanocube/nanowire heterostructure as ultra-sensitive cellular interfaces. NPG Asia Mater. 2014, 6, e117. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Ko, H.; Lee, S.; Kim, H.W.; Lee, C. Enhanced ethanol sensing properties of TiO2/ZnO core-shell nanorod sensors. Appl. Phys. A 2014, 115, 1223–1229. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, H.; Gao, X.; Yang, J.; Wang, L. Brookite TiO2 decorated a-Fe2O3 nanoheterostructures with rod morphologies for gas sensor application. J. Mater. Chem. A 2014, 2, 7935–7943. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Torad, N.L.; Yamauchi, Y. Polymeric micelle assembly for the direct synthesis of platinum-decorated mesoporous TiO2 toward highly selective sensing of acetaldehyde. ACS Appl. Mater. Interfaces 2014, 6, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Da, P.; Li, W.; Lin, X.; Wang, Y.; Tang, J.; Zheng, G. Surface plasmon resonance enhanced real-time, photoelectrochemical protein sensing by Au nanoparticle-decorated TiO2 nanowires. Anal. Chem. 2014, 86, 6633–6639. [Google Scholar] [CrossRef] [PubMed]
- Buso, D.; Post, M.; Cantalini, C.; Mulvaney, P.; Martucci, A. Gold nanoparticle-doped TiO2 semiconductor thin films: Gas sensing properties. Adv. Funct. Mater. 2008, 18, 3843–3849. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Leonardi, S.G.; Pullar, R.C.; Seabra, M.P.; Neri, G.; Labrinchaa, J.A. Sensing properties and photochromism of Ag-TiO2 nano-heterostructures. J. Mater. Chem. A 2016, 4, 9600–9613. [Google Scholar] [CrossRef]
- Nechita, V.; Schoonman, J.; Musa, V. Ethanol and methanol sensing characteristics of Nb-doped TiO2 porous thin films. Phys. Status Solidi A 2012, 209, 153–159. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Comini, E.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Novel synthesis and gas sensing performances of CuO-TiO2 nanocomposites functionalized with Au nanoparticles. J. Phys. Chem. C 2011, 115, 10510–10517. [Google Scholar] [CrossRef]
- Katoch, A.; Kim, J.H.; Kim, S.S. TiO2/ZnO inner/outer double-layer hollow fibers for improved detection of reducing gases. ACS Appl. Mater. Interfaces 2014, 6, 21494–21499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Han, S.; Lin, J.; Wang, R. Interface dominated high photocatalytic properties of electrostatic self-assembled Ag2O/TiO2 heterostructure. Phys. Chem. Chem. Phys. 2010, 12, 15119–15123. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.G.; Chougule, M.A.; Sen, S.; Patil, V.B. Development of nanostructured polyaniline-titanium dioxide gas sensors for ammonia recognition. J. Appl. Polym. Sci. 2012, 125, 1418–1424. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.; Xie, T.; Yuan, Z.; Liu, C.; Jiang, Y. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens. Actuators B Chem. 2015, 223, 149–156. [Google Scholar] [CrossRef]
- Kusior, A.; Radecka, M.; Rekas, M.; Lubecka, M.; Zakrzewska, K.; Reszka, A.; Kowalski, B.J. Sensitization of gas sensing properties in TiO2/SnO2 nanocomposites. Procedia Eng. 2012, 47, 1073–1076. [Google Scholar] [CrossRef]
- Feng, C.; Xu, G.; Liu, H.; Lv, J.; Zheng, Z.; Wu, Y. Facile fabrication of Pt/graphene/TiO2 NTAs based enzyme sensor for glucose detection. J. Electrochem. Soc. 2014, 161, B1–B8. [Google Scholar] [CrossRef]
- Yang, Q.; Long, M.; Tan, L.; Zhang, Y.; Ouyang, J.; Liu, P.; Tang, A. Helical TiO2 nanotube arrays modified by Cu-Cu2O with ultrahigh sensitivity for non-enzymatic electro-oxidation of glucose. ACS Appl. Mater. Interfaces 2015, 7, 12719–12730. [Google Scholar] [CrossRef] [PubMed]
- Huo, K.; Li, Y.; Chen, R.; Gao, B.; Peng, C.; Zhang, W.; Hu, L.; Zhang, X.; Chu, P.K. Recyclable non-enzymatic glucose sensor based on Ni/NiTiO3/TiO2 nanotube arrays. ChemPlusChem 2015, 80, 576–582. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Li, Z.Y.; Yang, X.J.; Chen, Y.J.; Ma, J.M. Design of a highly sensitive ethanol sensor using a nano-coaxial p-Co3O4/n-TiO2 heterojunction synthesized at low temperature. Nanoscale 2013, 5, 10916–10926. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ma, C.; Li, L.; Zhang, L.; Yu, J. A photoelectrochemical sensor for hydrogen sulfide in cancer cells based on the covalently and in situ grafting of CdS nanoparticles onto TiO2 nanotubes. J. Electroanal. Chem. 2016, 783, 176–181. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Park, S.; Lee, W.I.; Lee, C. Effects of functionalization of TiO2 nanotube array sensors with Pd nanoparticles on their selectivity. Sensors 2014, 14, 15849–15860. [Google Scholar] [CrossRef] [PubMed]
- Bulakhe, R.N.; Patil, S.V.; Deshmukh, P.R.; Shinde, N.M.; Lokhande, C.D. Fabrication and performance of polypyrrole (Ppy)/TiO2 heterojunction for room temperature operated LPG sensor. Sens. Actuators B Chem. 2013, 181, 417–423. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Xu, L.; Cao, X.; Li, X.; Huang, Y.; Meng, C.; Wang, Z.; Zhu, W. Ag2O/TiO2/V2O5 one-dimensional nanoheterostructures for superior solar light photocatalytic activity. Nanoscale 2014, 6, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- Ashok, C.H.; Rao, K.V. Microwave-assisted synthesis of CuO/TiO2. J. Mater. Sci. Mater. Electron. 2016, 27, 8816–8825. [Google Scholar] [CrossRef]
- Buvailo, A.I.; Xing, Y.; Hines, J.; Dollahon, N.; Borguet, E. TiO2/LiCl-based nanostructured thin film for humidity sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ong, W.L.; Zhu, L.; Ho, G.W. TiO2 fibers supported β-FeOOH nanostructures as efficient visible light photocatalyst and room temperature sensor. Sci. Rep. 2015, 5, 10601. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Zhao, M. Self-assembly of TiO2/polypyrrole nanocomposite ultrathin films and application for an NH3 gas sensor. Int. J. Environ. Anal. Chem. 2007, 87, 539–551. [Google Scholar] [CrossRef]
- Yue, J.; Chen, Z.; Yifeng, E.; Chen, L.; Zhang, J.; Song, Y.; Zhai, Y. Preparation TiO2 core-shell nanospheres and application as efficiency drug detection sensor. Nanoscal. Res. Lett. 2014, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; André, R.; Sahoo, J.K.; Jochum, F.D.; Theato, P.; Natalio, F.; Berger, R.; Branscheid, R.; Kolbc, U.; Tremel, W. Hydrogen peroxide sensors for cellular imaging based on horse radish peroxidase reconstituted on polymer-functionalized TiO2 nanorods. Nanoscale 2011, 3, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, M.; Zhao, G.; Shi, H.; Fan, L.; Zhao, S. Fabrication of a novel and simple microcystin-LR photoelectrochemical sensor with high sensitivity and selectivity. Environ. Sci. Technol. 2012, 46, 11955–11961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, W.; Wang, S.; Li, Z.; Zeng, Y.; Yan, S.; Sun, Y. Simple synthesis and photoelectrochemical characterizations of polythiophene/Pd/TiO2 composite microspheres. ACS Appl. Mater. Interfaces 2014, 6, 20197–20204. [Google Scholar] [CrossRef] [PubMed]
- Epifani, M.; Díaz, R.; Force, C.; Comini, E.; Andreu, T.; Zamani, R.R.; Arbiol, J.; Siciliano, P.; Faglia, G.; Morante, J.R. Colloidal counterpart of the TiO2-supported V2O5 system: A case study of oxide-on-oxide deposition by wet chemical techniques. synthesis, vanadium speciation, and gas-sensing enhancement. J. Phys. Chem. C 2013, 117, 20697–20705. [Google Scholar] [CrossRef]
- Lee, S.W.; Takahara, N.; Korposh, S.; Yang, D.H.; Toko, K.; Kunitake, T. Nanoassembled thin film gas sensors. III. sensitive detection of amine odors using TiO2/poly(acrylic acid) ultrathin film quartz crystal microbalance sensors. Anal. Chem. 2010, 82, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Meng, C.; Zhou, Y.; Gao, Z.; Li, X.; Cao, X.; Xu, L.; Zhu, W. A novel ethanol gas sensor based on TiO2/Ag0.35V2O5 branched nanoheterostructures. Sci. Rep. 2016, 6, 33062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Huang, Y.; Li, X.; Cao, X.; Xu, L.; Meng, C.; Wang, Z.; Zhu, W. Ag0.35V2O5/TiO2 branched nanoheterostructures:Facilef abrication and efficient visible light photocatalytic activity. Mater. Lett. 2014, 128, 358–361. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Meng, C.; Gao, Z.; Cao, X.; Li, X.; Xu, L.; Zhu, W.; Peng, X.; Zhang, B.; et al. A high-response ethanol gas sensor based on one-dimensional TiO2/V2O5 branched nanoheterostructures. Nanotechnology 2016, 27, 425503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Liu, L.; Zhu, C.; Liu, X.; Su, Q. Visible light photocatalysis of V2O5/TiO2 nanoheterostructures prepared via electrospinning. Mater. Lett. 2012, 75, 95–98. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Xu, L.; Meng, C.; Zhu, W. Ag/TiO2 nanofiber heterostructures: Highly enhanced photocatalysts under visible light. J. Appl. Phys. 2013, 113, 174311. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zheng, W.; Wang, W.; Huang, H.; Wang, C.; MacDiarmid, A.G.; Wei, Y. Highly sensitive and stable humidity nanosensors based on LiCl doped TiO2 electrospun nanofibers. J. Am. Chem. Soc. 2008, 130, 5036–5037. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Zhang, L.; Zhang, H.; Li, C.M.; Lei, Y. Preparation of TiO2-Pt hybrid nanofibers and their application for sensitive hydrazine detection. Nanoscale 2011, 3, 1149–1157. [Google Scholar] [CrossRef]
- Du, P.; Song, L.; Xiong, J.; Li, N.; Xi, Z.; Wang, L.; Jin, D.; Guo, S.; Yuan, Y. Coaxial electrospun TiO2/ZnO core–sheath nanofibers film: Novel structure for photoanode of dye-sensitized solar cells. Electrochim. Acta 2012, 78, 392–397. [Google Scholar] [CrossRef]
- Du, X.; Wang, Y.; Mu, Y.; Gui, L.; Wang, P.; Tang, Y. A new highly selective H2 sensor based on TiO2/PtO-Pt dual-layer films. Chem. Mater. 2002, 14, 3953–3957. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensor. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z. Enhanced gas sensing properties by SnO2 nanosphere functionalized TiO2 nanobelts. J. Mater. Chem. 2012, 22, 3544–3548. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Z.; Liu, C.; Zhang, Y.; Tu, Z.; Yang, X.; Yang, F.; Wen, Z.; Zhu, L.; Liu, R.; et al. Synthesis of TiO2 decorated Co3O4 acicular nanowire arrays and their application as an ethanol sensor. J. Mater. Chem. A 2015, 3, 2794–2801. [Google Scholar] [CrossRef]
- Zhu, C.L.; Yu, H.L.; Zhang, Y.; Wang, T.S.; Ouyang, Q.Y.; Qi, L.H.; Chen, Y.J.; Xue, X.Y. Fe2O3/TiO2 tube-like nanostructures: Synthesis, structural transformation and the enhanced sensing properties. ACS Appl. Mater. Interfaces 2012, 4, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ji, S.; Li, H.; Kang, X.; Chang, S.; Wang, Y.; Yu, G.; Lu, J.; Claverie, J.; Sang, Y.; et al. High-energy faceted SnO2-coated TiO2 nanobelt heterostructure for near-ambient temperature-responsive ethanol sensor. ACS Appl. Mater. Interfaces 2015, 7, 24950–24956. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.R.; Shendy, S.K.; Ebadzadeh, T. Synthesis of TiO2/SnO2 core shell nanocomposite by chemical route and its gas sensing properties. Indian J. Phys. 2012, 86, 9–13. [Google Scholar] [CrossRef]
- Deng, J.; Yu, B.; Lou, Z.; Wang, L.; Wang, R.; Zhang, T. Facile synthesis and enhanced ethanol sensing properties of the brush-like ZnO-TiO2 heterojunctions nanofibers. Sens. Actuators B Chem. 2013, 184, 21–26. [Google Scholar] [CrossRef]
- Lou, Z.; Deng, J.; Wang, L.; Wang, R.; Fei, T.; Zhang, T. A class of hierarchical nanostructures: ZnO surfacefunctionalized TiO2 with enhanced sensing properties. RSC Adv. 2013, 3, 3131–3136. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Lou, Z.; Zhang, T. Design of CuO-TiO2 heterostructure nanofibers and their sensing performance. J. Mater. Chem. A 2014, 2, 9030–9034. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z. Sensitivity improvement of TiO2-doped SnO2 to volatile organic compounds. Phys. E Low-Dimens. Syst. Nanostructures 2010, 43, 633–638. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.; Kang, X.; Sang, Y.; Liu, H. Hierarchically assembled ZnO nanorods on TiO2 nanobelts for high performance gas sensor. Energy Environ. Focus 2014, 3, 404–410. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z.; Tsukimoto, S.; Saito, M.; Ikuhara, Y. Selective detection of formaldehyde gas using a Cd-doped TiO2-SnO2 sensor. Sensors 2009, 9, 9029–9038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Dong, B.; Yuan, Q.; Hea, Y.; Wolfbeis, O.S. Rational tailoring of ZnSnO3/TiO2 heterojunctions with bioinspired surface wettability for high-performance humidity nanosensors. Nanoscale 2015, 7, 4149–4155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, Z.; Liu, H. Humidity sensing properties of La3+/Ce3+-doped TiO2- 20 wt.% SnO2 thin films derived from sol-gel method. J. Rare Earth 2010, 28, 123–127. [Google Scholar]
- Wang, Y.; Jia, W.; Strout, T.; Schempf, A.; Zhang, H.; Li, B.; Cui, J.; Lei, Y. Ammonia gas sensor using polypyrrole-coated TiO2/ZnO nanofibers. Electroanalysis 2009, 21, 1432–1438. [Google Scholar] [CrossRef]
- Radecka, M.; Kusior, A.; Lacz, A.; Trenczek-Zajac, A.; Lyson-Sypien, B.; Zakrzewska, K. Nanocrystalline TiO2/SnO2 composites for gas sensors. Therm. Anal. Calorim. 2012, 108, 1079–1084. [Google Scholar] [CrossRef]
- Waghmare, S.D.; Shinde, D.V.; Zate, M.K.; Konda, R.; Mane, R.S.; Han, S.H. Enhanced gas sensitivity in TiO2 nanoneedles grown on upright SnO2 nanoplates. Scr. Mater. 2013, 68, 735–738. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, C.S. Characteristics of the TiO2/SnO2 thick film semiconductor gas sensor to determine fish freshness. J. Ind. Eng. Chem. 2011, 17, 237–242. [Google Scholar] [CrossRef]
- Comini, E.; Guidi, V.; Frigeri, C.; Riccò, I.; Sberveglieri, G. CO sensing properties of titanium and iron oxide nanosized thin films. Sens. Actuators B Chem. 2001, 77, 16–21. [Google Scholar] [CrossRef]
- Bodade, A.B.; Bende, A.M.; Chaudhari, G.N. Synthesis and characterization of CdO-doped nanocrystalline ZnO: TiO2-based H2S gas sensor. Vacuum 2008, 82, 588–593. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Park, S.E.; Lee, C. Synthesis and hydrogen gas sensing properties of TiO2-decorated CuO nanorods. Bull. Korean Chem. Soc. 2015, 36, 2458–2463. [Google Scholar] [CrossRef]
- Devi, G.S.; Hyodo, T.; Shimizu, Y.; Egashira, M. Synthesis of mesoporous TiO2-based powders and their gas-sensing properties. Sens. Actuators B Chem. 2002, 87, 122–129. [Google Scholar] [CrossRef]
- Imawan, C.; Solzbacher, F.; Steffes, H.; Obermeier, E. TiOx-modified NiO thin films for H2 gas sensors: Effects of TiOx-overlayer sputtering parameters. Sens. Actuators B Chem. 2000, 68, 184–188. [Google Scholar] [CrossRef]
- Kim, H.S.; Jin, C.H.; Park, S.H.; Lee, C.M. Structural, luminescent, and NO2 sensing properties of SnO2-core/V2O5-shell nanorods. J. Electroceram. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Wlodarski, W.; Li, Y. Nanocrystalline V2O5-TiO2 thin-films for oxygen sensing prepared by sol-gel process. Sens. Actuators B Chem. 2001, 77, 484–490. [Google Scholar] [CrossRef]
- Trinchi, A.; Li, Y.X.; Wlodarski, W.; Kaciulis, S.; Pandolfi, L.; Viticoli, S.; Comini, E.; Sberveglieri, G. Investigation of sol–gel prepared CeO2-TiO2 thin films for oxygen gas sensing. Sens. Actuators B Chem. 2003, 95, 145–150. [Google Scholar] [CrossRef]
- Lee, H.C.; Hwang, W.S. Substrate effects on the oxygen gas sensing properties of SnO2/TiO2 thin films. Appl. Surf. Sci. 2006, 253, 1889–1897. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.R.; Qiao, L.; Liu, L.X.; Su, Q.; Zhu, C.Q.; Liu, X.Q. Synthesis of one-dimensional TiO2/V2O5 branched heterostructures and their visible light photocatalytic activity towards Rhodamine B. Nanotechnology 2011, 22, 225702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, X.; Xu, L.; Liao, L.; Liu, W.; Ho, J.; Xiao, X.; Jiang, C.; Li, J. Hydrogen gas sensor based on metal oxide nanoparticles decorated graphene transistor. Nanoscale 2015, 7, 10078–10084. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Sung, M.G.; Lee, J.; Baik, K.Y.; Kwon, Y.K.; Lee, M.S.; Hong, S. Universal parameters for carbon nanotube network-based sensors: Can nanotube sensors be reproducible? ACS Nano 2011, 5, 4373–4379. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Wang, Y.; Chai, J.; Gao, R.; Yang, Z.; Kong, E.S.W.; Zhang, Y. Gas sensor based on p-phenylenediamine reduced graphene oxide. Sens. Actuators B Chem. 2012, 163, 107–114. [Google Scholar] [CrossRef]
- Mu, H.; Wang, K.; Zhang, Z.; Xie, H. Formaldehyde graphene gas sensors modified by thermally evaporated tin oxides and tin compound films. J. Phys. Chem. C 2015, 119, 10102–10108. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, C.; Wang, J.; Sun, L.; Shi, K.; Zhou, W.; Fu, H. Facile synthesis of novel 3D nanoflower-like CuxO/multilayer graphene composites for room temperature NOx gas sensor application. Nanoscale 2014, 6, 7369–7378. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.H.; Fu, Z.X. Ascorbic acid amperometric sensor using a graphene-wrapped hierarchical TiO2 nanocomposite. Chem. Pap. 2015, 69, 655–661. [Google Scholar] [CrossRef]
- Jain, R.; Dhanjai. TiO2-multi walled carbon nanotubes hybrid film sensor for sensing of antiprotozoal agent satranidazole in solubilzed system. J. Electrochem. Soc. 2013, 160, H474–H480. [Google Scholar] [CrossRef]
- Trocino, S.; Donato, A.; Latino, M.; Donato, N.; Leonardi, S.G.; Neri, G. Pt-TiO2/MWCNTs hybrid composites for monitoring low hydrogen concentrations in air. Sensors 2012, 12, 12361–12373. [Google Scholar] [CrossRef]
- Ueda, T.; Takahashi, K.; Mitsugi, F.; Ikegami, T. Preparation of single-walled carbon nanotube/TiO2 hybrid atmospheric gas sensor operated at ambient temperature. Diam. Relat. Mater. 2009, 18, 493–496. [Google Scholar] [CrossRef]
- Ghadiry, M.; Gholami, M.; Lai, C.K.; Ahmad, H.; Chong, W.Y. Ultra-sensitive humidity sensor based on optical properties of graphene oxide and nano-anatase TiO2. PLoS ONE 2016, 11, e0153949. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Chen, F.; Wei, C. Simple one-pot polyol synthesis of Pd nanoparticles, TiO2 microrods and reduced graphene oxide ternary composite for sensing NH3 gas at room temperature. Sens. Actuators B Chem. 2017, in press. [Google Scholar]
- Amiri, M.T.; Ashkarran, A.A. Fabrication, characterization and enhanced sensing performance of graphene-TiO2 gas sensor device. J. Mater. Sci. Mater. Electron. 2017, 28, 9435–9441. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, X.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres forselective room-temperature gas sensors. Sens. Actuators B Chem. 2016, 230, 330–336. [Google Scholar] [CrossRef]
- Tian, J.; Yang, G.; Jiang, D.; Su, F.; Zhang, Z. A hybrid material consisting of bulk-reduced TiO2, graphene oxide and polyaniline for resistance based sensing of gaseous ammonia at room temperature. Microchim. Acta 2016, 183, 2871–2878. [Google Scholar] [CrossRef]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia sensor based on polypyrrole-graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Cai, L.; Cao, Y.; Gan, L.; Liu, S.; Wang, Z.; Zhang, H.; Li, L. TiO2-decorated graphenes as efficient photoswitches with high oxygen sensitivity. Chem. Sci. 2011, 2, 1860–1864. [Google Scholar] [CrossRef]
- Sánchez, M.; Rincón, M.E. Sensor response of sol–gel multiwalled carbon nanotubes-TiO2 composites deposited by screen-printing and dip-coating techniques. Sens. Actuators B Chem. 2009, 140, 17–23. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, W. Electrodeposition of TiO2 nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis 2009, 21, 988–993. [Google Scholar] [CrossRef]
- Ampelli, C.; Spadaro, D.; Neri, G.; Donato, N.; Latino, M.; Passalacqua, R.; Perathoner, S.; Centi, G. Development of hydrogen leak sensors for fuel cell transportation. Chem. Eng. Trans. 2012, 26, 333–338. [Google Scholar]
- Lee, J.S.; Ha, T.J.; Hong, M.H.; Park, C.S.; Park, H.H. The effect of multiwalled carbon nanotube doping on the CO gas sensitivity of TiO2 xerogel composite film. Appl. Surf. Sci. 2013, 269, 125–128. [Google Scholar] [CrossRef]
- Kim, H.; Hong, M.H.; Jang, H.W.; Yoon, S.J.; Park, H.H. CO gas sensing properties of direct-patternable TiO2 thin films containing multi-wall carbon nanotubes. Thin Solid Films 2013, 529, 89–93. [Google Scholar] [CrossRef]
- Liou, W.J.; Lin, H.M. Nanohybrid TiO2/carbon black sensor for NO2 gas. China Part. 2007, 5, 225–229. [Google Scholar] [CrossRef]
- Llobet, E.; Espinosa, E.H.; Sotter, E.; Ionescu, R.; Vilanova, X.; Torres, J.; Felten, A.; Pireaux, J.J.; Ke, X.; Van Tendeloo, G.; et al. Carbon nanotube-TiO2 hybrid films for detecting traces of O2. Nanotechnology 2008, 19, 375501. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Karimi-Maleh, H. Determination of 6-mercaptopurine in the presence of uric acid using modified multiwall carbon nanotubes-TiO2 as a voltammetric sensor. Drug Test. Anal. 2011, 4, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Prasad, G.K.; Radhakrishnan, T.P.; Sravan Kumar, D.; Ghanshyam Krishna, M. Ammonia sensing characteristics of thin film based on polyelectrolyte templated polyaniline. Sens. Actuators B Chem. 2005, 106, 626–631. [Google Scholar]
- Wrenn, C. Real-time measurement of ammonia gas. Occup. Health Saf. 2000, 69, 64–67. [Google Scholar] [PubMed]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Ultralow power consumption gas sensors based on self-heated individual nanowires. Appl. Phys. Lett. 2008, 93, 123110. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, F.; Prades, J.D.; Tarancon, A.; Barth, S.; Casals, O.; Jimenez-Diaz, R.; Pellicer, E.; Rodriguez, J.; Morante, J.R.; Juli, M.A.; et al. Insight into the role of oxygen diffusion in the sensing mechanisms of SnO2 nanowires. Adv. Funct. Mater. 2008, 18, 2990–2994. [Google Scholar] [CrossRef]
- Hoffmann, M.W.; Gad, A.E.; Prades, J.D.; Hernandez-Ramirez, F.; Fiz, R.; Shen, H.; Mathur, S. Solar diode sensor: Sensing mechanism and applications. Nano Energy 2013, 2, 514–522. [Google Scholar] [CrossRef]
- Lu, B.; Liu, M.; Shi, H.; Huang, X.; Zhao, G. A novel photoelectrochemical sensor for bisphenol A with high sensitivity and selectivity based on surface molecularly imprinted polypyrrole modified TiO2 nanotubes. Electroanalysis 2013, 25, 771–779. [Google Scholar] [CrossRef]
- Dou, Y.; Han, J.; Wang, T.; Wei, M.; Evans, D.G.; Duan, X. Fabrication of MMO-TiO2 one-dimensional photonic crystal and its application as a colorimetric sensor. J. Mater. Chem. 2012, 22, 14001–14007. [Google Scholar] [CrossRef]
- Sonker, R.K.; Sabhajeet, S.R. Yadav, B.C. TiO2-PANI nanocomposite thin film prepared by spin coating technique working as room temperature CO2 gas sensing. J. Mater. Sci. Mater. Electron. 2016, 27, 11726–11732. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C.; Sabhajeet, S.R. Preparation of PANI doped TiO2 nanocomposite thin film and its relevance as room temperature liquefied petroleum gas sensor. J. Mater. Sci. Mater. Electron. 2017, in press. [Google Scholar]
- Cui, S.; Yang, L.; Wang, J.; Wang, X. Fabrication of a sensitive gas sensor based on PPy/TiO2 nanocomposites films by layer-by-layer self-assembly and itsapplication in food storage. Sens. Actuators B Chem. 2016, 233, 337–346. [Google Scholar] [CrossRef]
- Chandra, R.M.; Reddy, P.S.P.; Rao, T.S.; Pammi, S.V.N.; Kumar, K.S.; Babu, V.V.; Kumar, Ch.K.; Hemalatha, K.P.J. Enhanced visible-light photocatalysis and gas sensor properties of polythiophene supported tin doped titanium nanocomposite. J. Phys. Chem. Solids 2017, 105, 99–105. [Google Scholar] [CrossRef]
- Patil, U.V.; Ramgir, N.S.; Debnath, A.K.; Karmakar, N.; Aswal, D.K.; Kothari, D.C.; Gupta, S.K. NH3 sensing properties polyaniline: TiO2 nanorods heterostructure. AIP Conf. Proc. 2016, 1731, 050033. [Google Scholar]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A room temperature ammonia gas sensor based on cellulose/TiO2/PANI composite nanofibers. Colloids Surf. A 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, S.; Fu, J. Examining the use of TiO2 to enhance the NH3 sensitivity of polypyrrole films. Appl. Polym. Sci. 2010, 118, 3351–3356. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Pang, Z.; Du, Y.; Xia, X.; Wei, Q.; Huang, F. Ammonia sensing behaviors of TiO2-PANI/PA6 composite nanofibers. Sensors 2012, 12, 17046–17057. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.G.; Patil, S.L.; Chougule, M.A.; Raut, B.T.; Godase, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. New method for fabrication of CSA doped PANi-TiO2 thin-film ammonia sensor. IEEE Sens. 2011, 11, 2980–2985. [Google Scholar] [CrossRef]
- Su, P.G.; Wang, C.P. Flexible humidity sensor based on TiO2 nanoparticles-polypyrrole-poly- [3-(methacrylamino) propyl] trimethyl ammonium chloride composite materials. Sens. Actuators B Chem. 2008, 129, 538–543. [Google Scholar] [CrossRef]
- Su, P.G.; Huang, L.N. Humidity sensors based on TiO2 nanoparticles/polypyrrole composite thin films. Sens. Actuators B Chem. 2007, 123, 501–507. [Google Scholar] [CrossRef]
- Oprea, A.; Barsan, N.; Weimar, U. Ammonia detection mechanism with polyacrylic acid sensitive layers: Field effect transduction. Sens. Actuators B Chem. 2005, 111, 577–581. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Toyoki Kunitake, A.; Nakao, A. Zirconia−titania nanofilm with composition gradient. Nano Lett. 2002, 2, 669–672. [Google Scholar] [CrossRef]
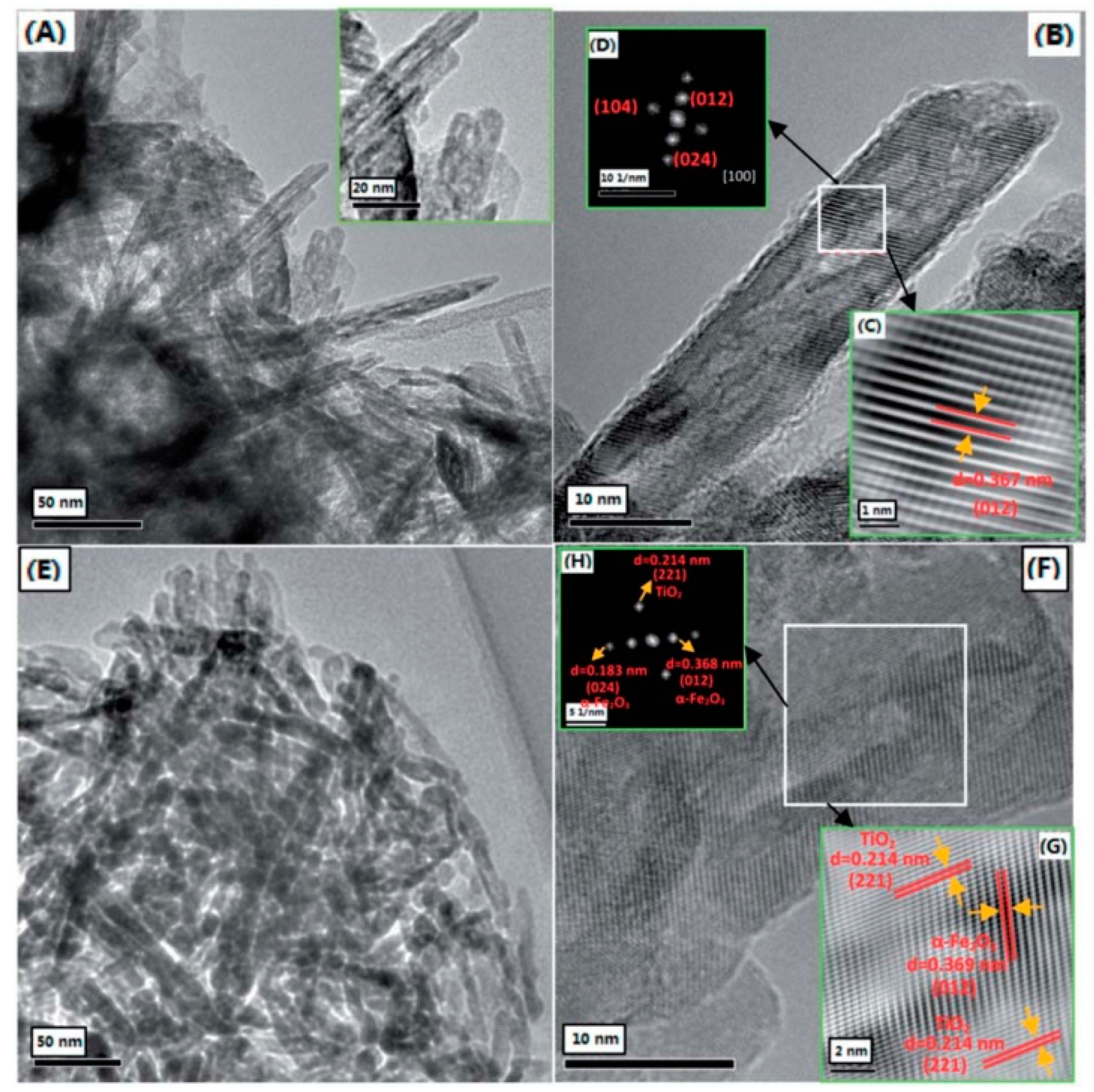
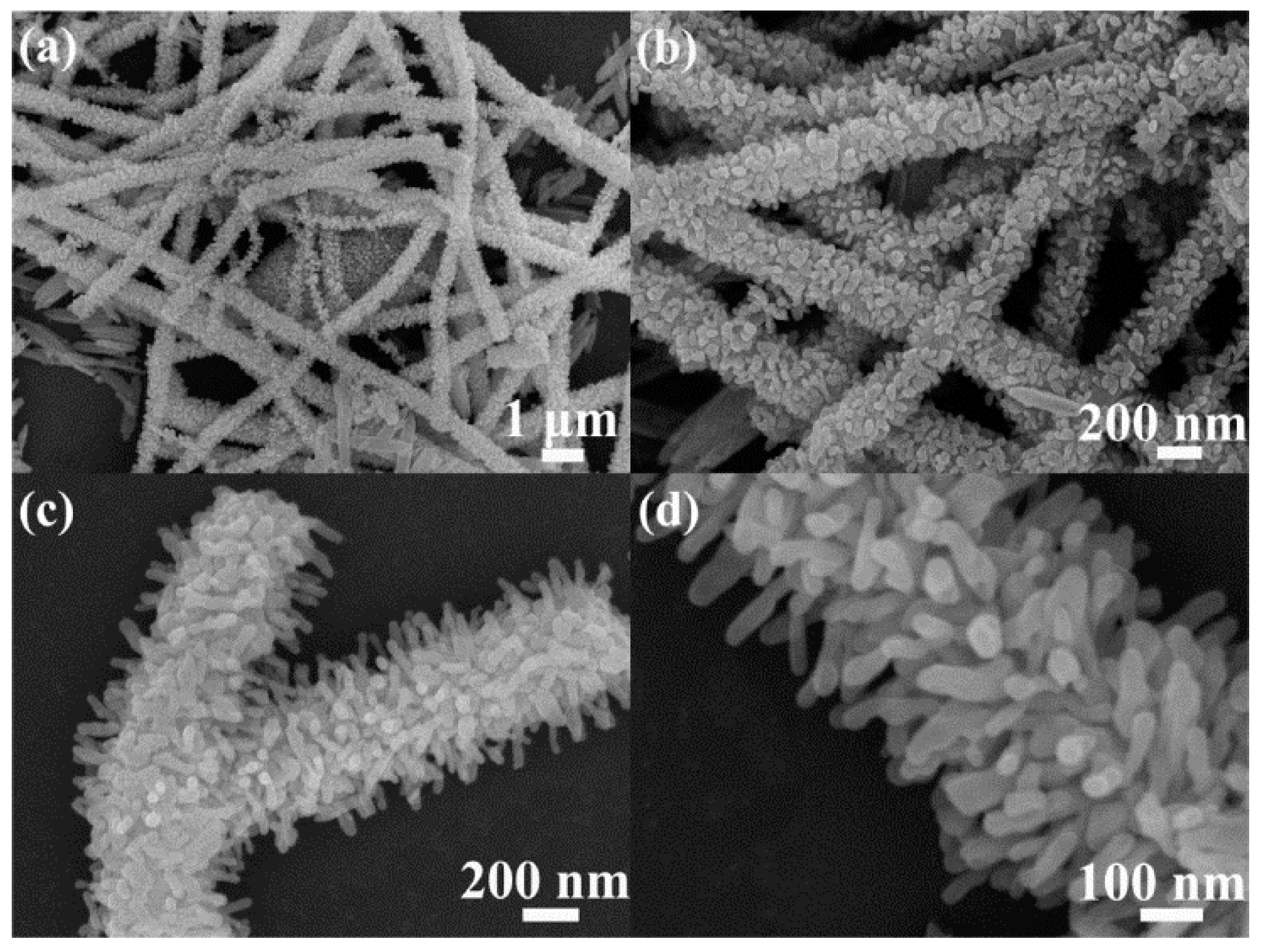
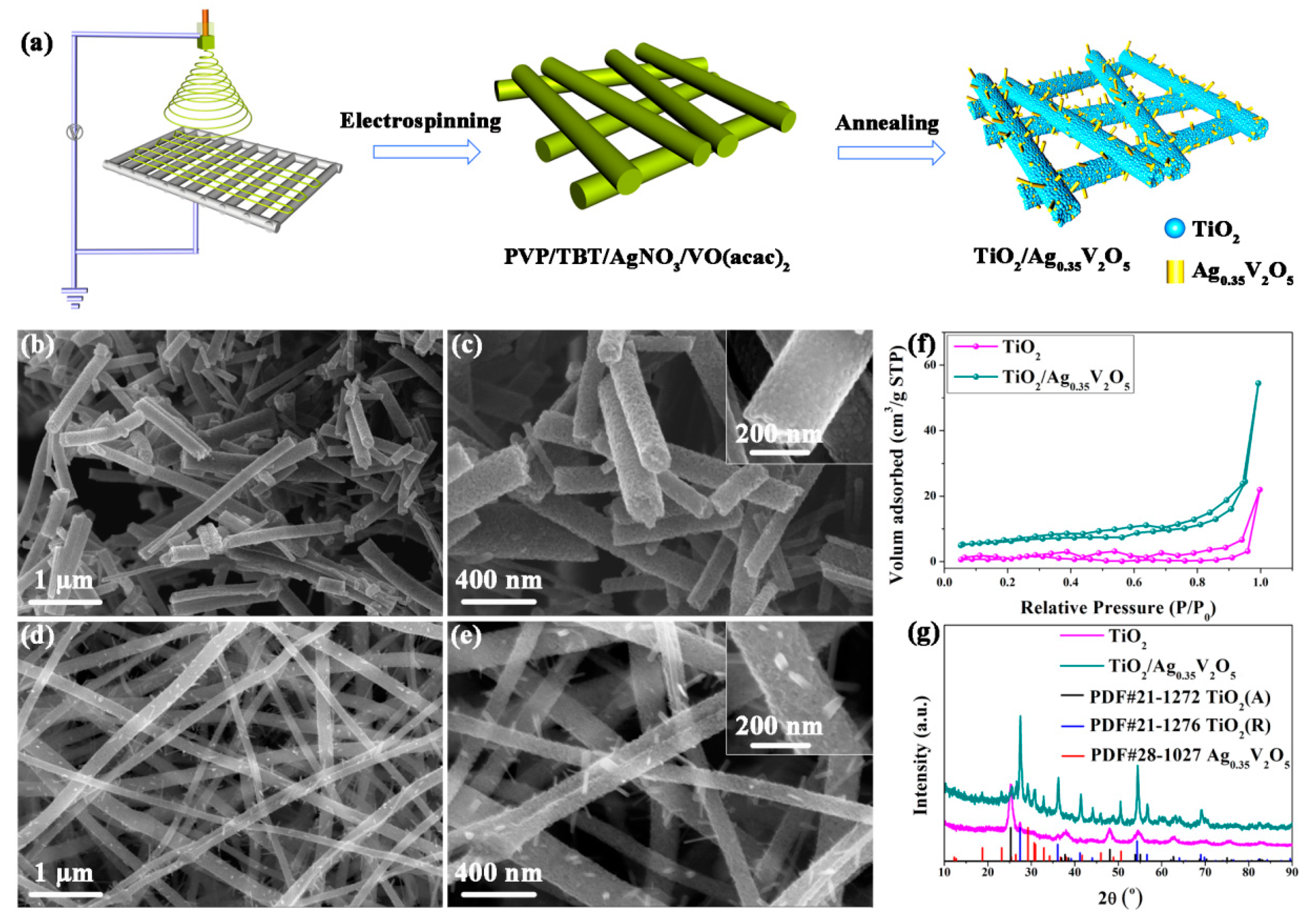
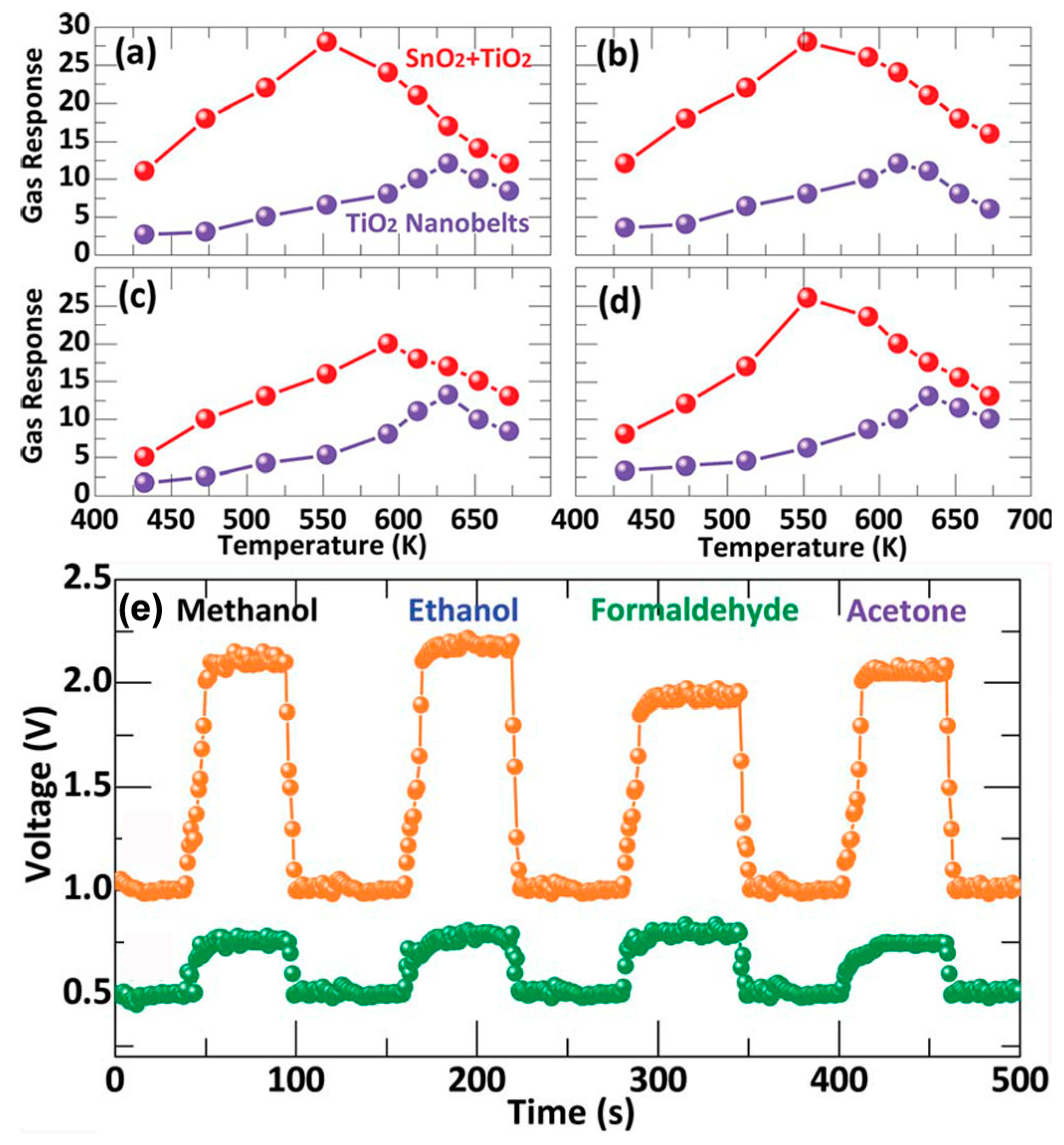
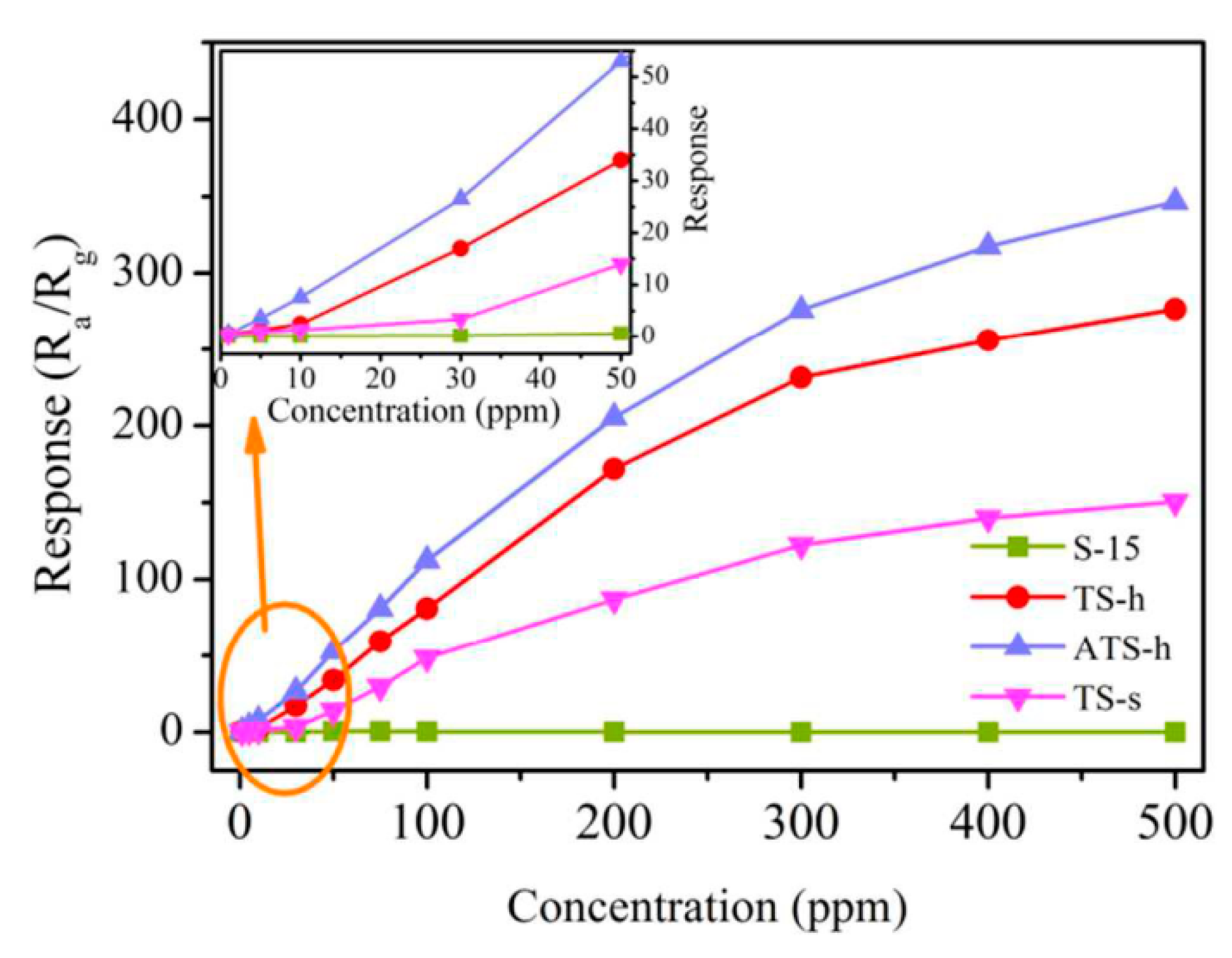
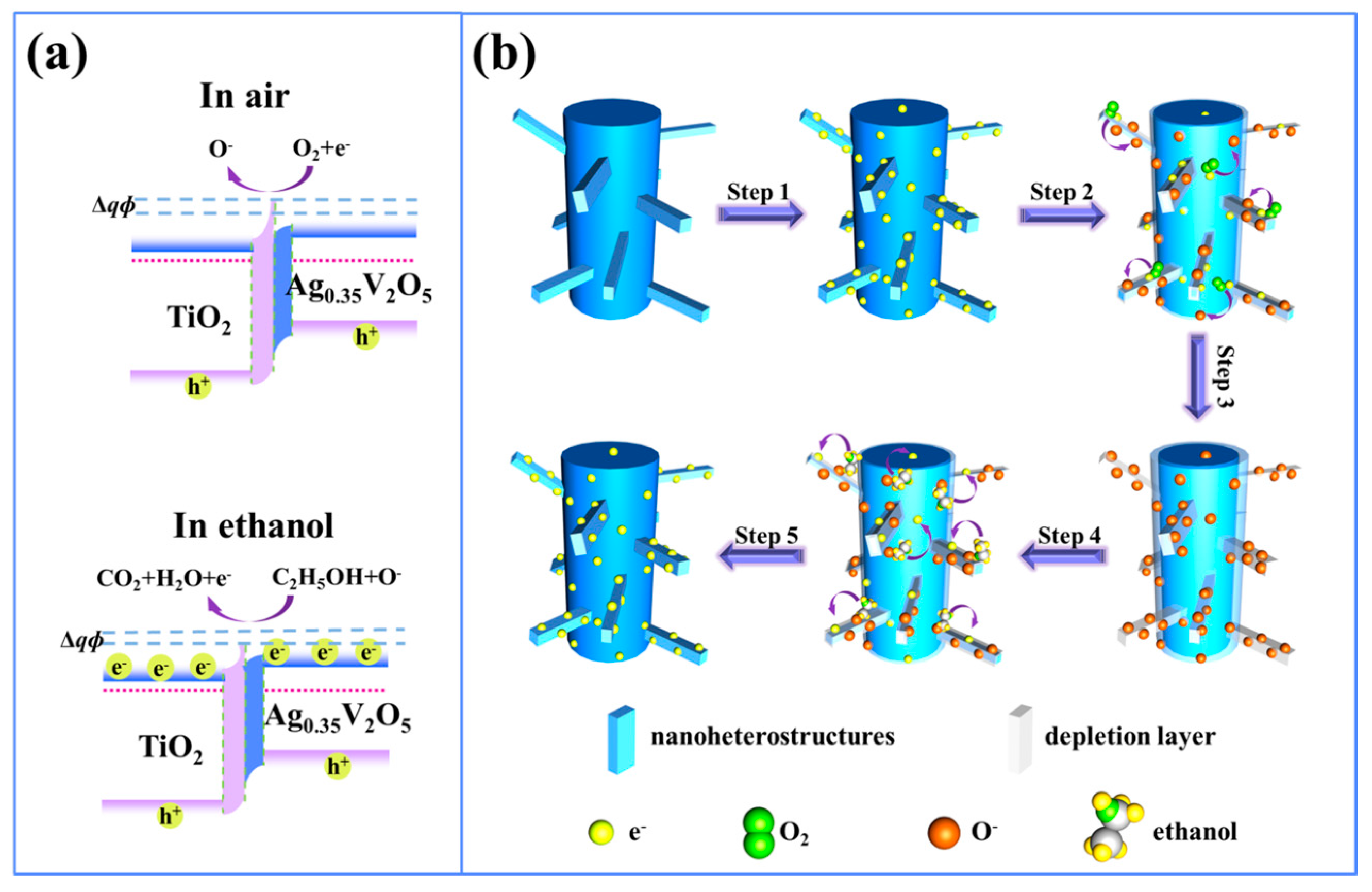
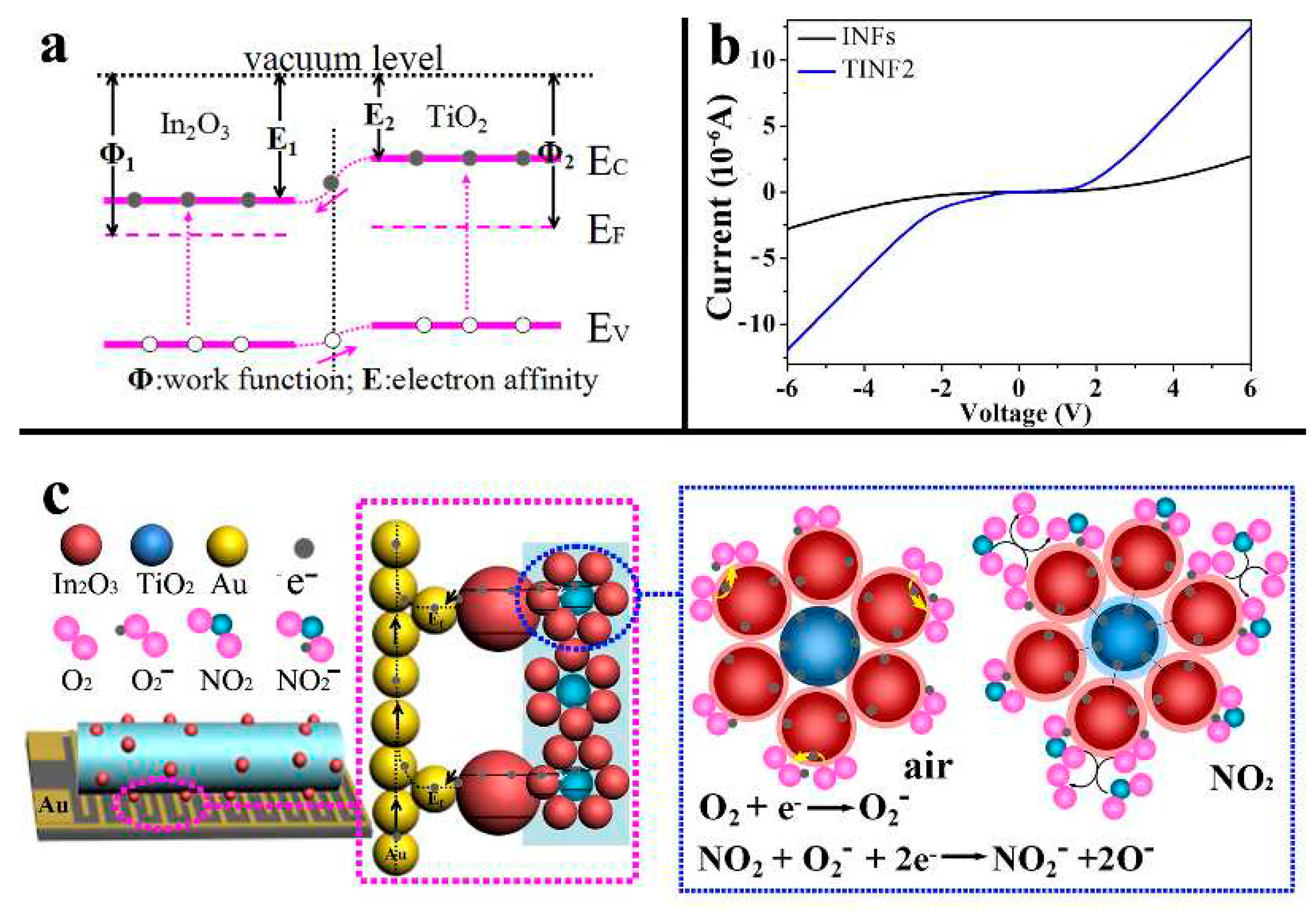
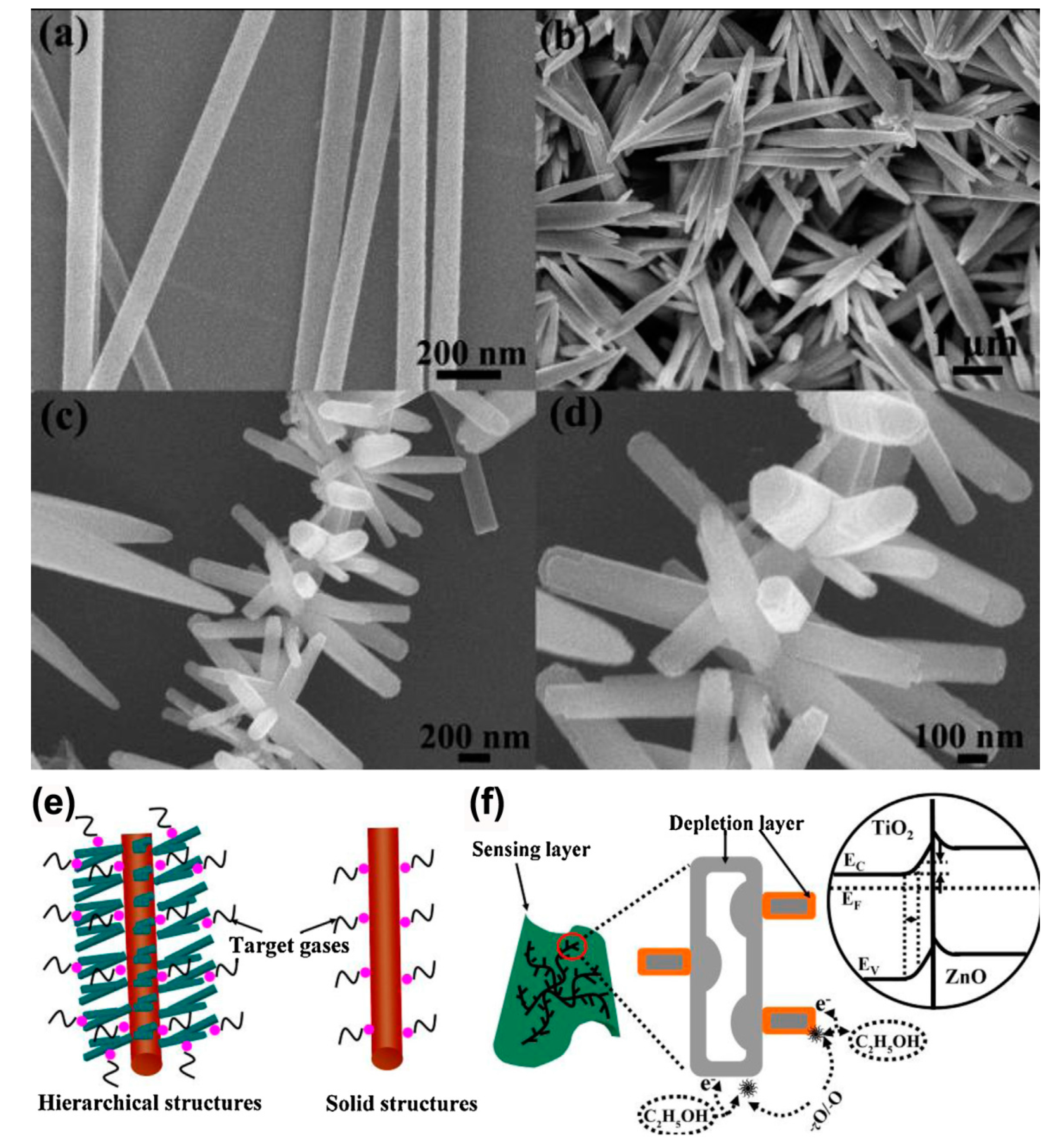



| TiO2-Based Nanoheterostructures | Fabrication Method | Size | Detection Gas | Detection Range | Response | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Operation Temperature (°C) | Sensitivity | Response/Recovery Time | Concentration | ||||||
| TiO2/Co3O4 acicular nanowires | hydrothermal + pulsed laser deposition | length: 1–3 μm; diameter: 200 nm | ethanol | 10–500 ppm | 160 | Rg/Ra: 65 | 100 ppm | [106] | |
| nano-coaxial p-Co3O4/n-TiO2 heterojunction | electronchemical anodization + hydrothermal process | nanotubes diameter: ~150 nm; core nanorods diameter: ~50 nm | ethanol | 260 | Ra/Rg: 40 | 1.4/7.2 s | 100 ppm | [80] | |
| Fe2O3/TiO2 tube-like nanostructures | hydrothermal + chemical deposition | diameter: 120 nm; length: 400 nm; outer wall thickness: 23.5 nm | ethanol | 0.5–500 ppm | 270 | Ra/Rg: 19.4 | 500 ppm | [107] | |
| SnO2-coated TiO2 nanobelts | hydrothermal | TiO2 nanobelts: length: over ten micrometers; width: 100–200 nm; thickness: 20–40 nm; SnO2 nanoplates: length: 20–200 nm; thickness: 20 nm | ethanol | 10–500 ppm | 43 | Ra/Rg: 11.2 | 40/5 min | 10 ppm | [108] |
| TiO2/SnO2 core shell nanocomposites | hydrothermal treatment + chemical deposition | ethanol | 500–5000 ppm | 200 | Ra/Rg: 12.7 | ≤50/50 s | 1000 ppm | [109] | |
| SnO2 nanospheres functionalized TiO2 nanobelts | hydrothermal process | nanobelts diameter: 50–200 nm; length: several micrometers; nanospheres diameter: ~400 nm | ethanol | 100–800 ppm | 320 | Rg/Ra: 27.5 | 400 ppm | [105] | |
| Ag-TiO2/SnO2 nanocomposites | chemical deposition | diameter: ~100 nm | ethanol | 1–500 ppm | 275 | Ra/Rg: 53 | 3.5/7 s | 50 ppm | [104] |
| TiO2/V2O5 nanoheterostructures | electrospinning | nanobranches diameter: 15–20 nm; nanofibers diameter: 160 nm | ethanol | 20–1000 ppm | 350 | Ra/Rg: 24.6 | 6/7 s | 100 ppm | [97] |
| TiO2/Ag0.35V2O5 branched nanoheterostructures | electrospinning | nanobranches diameter: ~20 nm; nanofibers diameter: ~190 nm | ethanol | 20–1000 ppm | 350 | Ra/Rg: 31.8 | 7/12 s | 100 ppm | [95] |
| brush-like ZnO-TiO2 heterojunctions nanofibers | electrospinning + hydrothermal process | ZnO nanorods diameter: 100–300 nm; TiO2 nanofibers diameter: 100 nm | ethanol | 20–500 ppm | 320 | Ra/Rg: 50.6 | 5/10 s | 500 ppm | [110] |
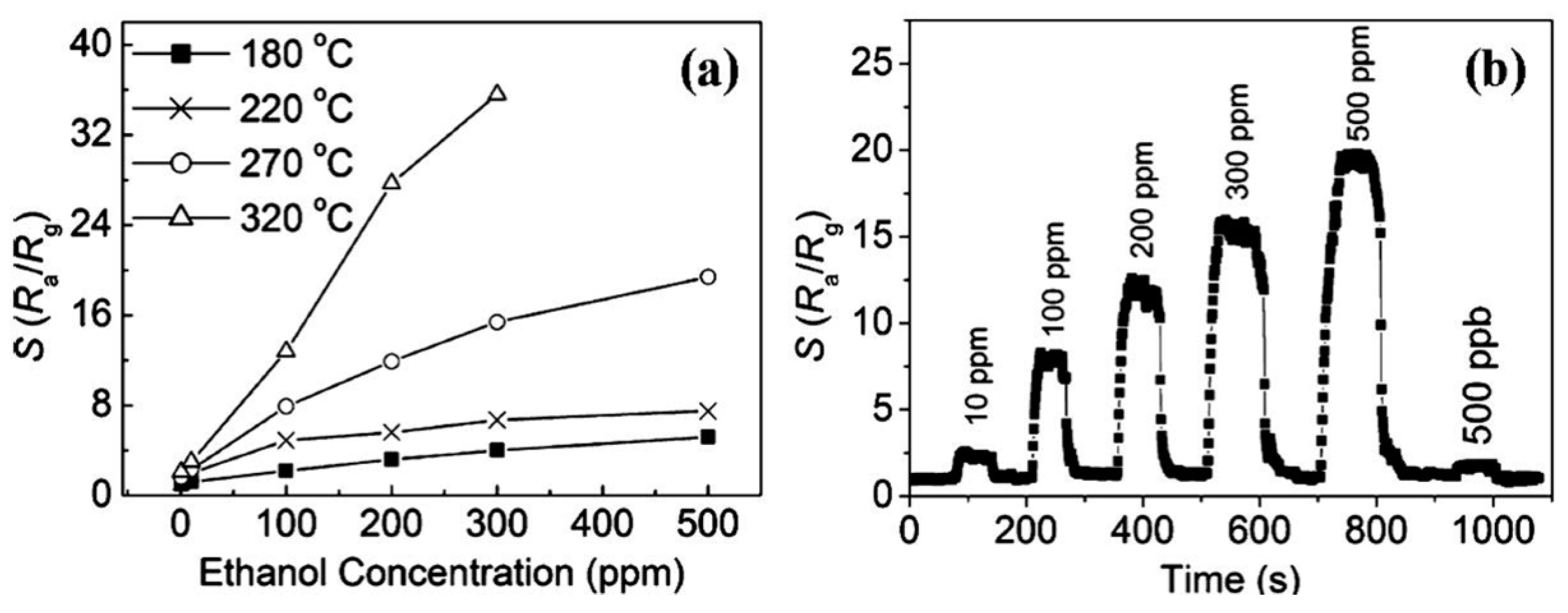
| TiO2/ZnO core-shell nanorods | hydrothermal method + ALD | core width: 120 nm; shell width: 20 nm | ethanol | 5–25 ppm | 150 | Ra/Rg: 2.37 | 100/70 s | 10 ppm | [64] |
| ZnO surface functionalized TiO2 | electrospinning + hydrothermal treatment | nanofibers diameter : 70–100 nm; ZnO nanosheets diameter: 500 nm | ethanol | 10–200 ppm | 280 | Ra/Rg: 15.7 | 5/3 s | 100 ppm | [111] |
| ZnO/TiO2 nanocomposites | CVD | ethanol | 400 | Ra/Rg: 5 | ~1/1 min | 50 ppm | [46] | ||
| brookite TiO2 decorated a-Fe2O3 nanoheterostructures | chemical deposition | length: 50–100 nm; diameter: ~10 nm | butanol | 10–500 ppm | 370 | Ra/Rg: 27.6 | 5/6 s | 100 ppm | [65] |
| ZnO-TiO2 nanocomposites | CVD | diameter: 5–20 nm | acetone | 20–100 ppm | 350 | Ra/Rg: 22.7 | 1/1 min | 100 ppm | [46] |
| ZnO/TiO2 nanocomposites | CVD | acetone | Ra/Rg: 22 | 100 ppm | [46] | ||||
| CuO-TiO2 heterostructure nanofibers | electrospinning + hydrothermal process | TiO2 nanofibers: length: several tens micrometers; diameter: 200 nm; CuO nanocubes: diameter: 40–80 nm | formaldehyde | 5–100 ppm | 200 | Ra/Rg: 15.5 | 50 ppm | [112] | |
| Cd/SnO2/TiO2 composites | sol-gel | formaldehyde | 100–500 ppm | 320 | Ra/Rg: 32 | 25/17 s | 200 ppm | [113] | |
| α-Fe2O3/TiO2 branch-like hierarchical heterostructures | electrospinning + hydrothermal method | diameter: 600 nm; length: several micrometers | trimethylamine | 10–200 ppm | 250 | Ra/Rg: 13.9 | 0.5/1.5 s | 50 ppm | [62] |
| hierarchically assembled ZnO nanorods on TiO2 nanobelts | hydrothermal process | TiO2 nanobelts: width: 50–200 nm; length: several micrometers; ZnO nanorods: length: 500 nm | trimethylamine | 5–500 ppm | 200 | Ra/Rg: 25 | 5 ppm | [114] | |
| SnO2/TiO2 composites | sol-gel | methanol | 50–400 ppm | 360 | Ra/Rg: 60 | 10–15/14–20 s | 200 ppm | [115] | |
| ZnO-TiO2 nanocomposites | physical mixture | humidity | 5%–90% RH | RT (room temperature) | (ΔR)/(Δ%RH): 9.08 MΩ/%RH | [59] | |||
| LiCl/TiO2 electrospun nanofibers | electrospinning | diameter: 150–260 nm | humidity | 11%–95% RH | RT | Ra/Rg: 103 | <3/7 s | 11%–95% RH | [100] |
| ZnSnO3 nanoneedles/TiO2 nanofibers heterojunction | electrospinning + hydrothermal treatment | TiO2 nanofibers diameters: 200–300 nm; ZnSnO3 nanorods: tip diameter: 0.5~1.5 nm; ratio of length to diameter: ~9.6 | humidity | 11%–95% | RT | 2.5/3 s | [116] | ||
| ZnSnO3 nanoparticles/TiO2 nanofibers heterojunction | electrospinning + hydrothermal treatment | TiO2 nanofibers diameters: 200–300 nm; ZnSnO3 nanoparticles diameters: 30–50 nm | humidity | 11%–95% | RT | 3.5/29 s | [116] | ||
| Ce2O3/TiO2/SnO2 thin film | sol-gel | humidity | 15%–95% RH | RT | Ra/Rg: 100 | 40% | [117] | ||
| polypyrrole-coated TiO2/ZnO nanofibers | electrospinning + chemical deposition | TiO2/ZnO core diameter: 100 nm; PPy shell thickness: 7 nm | NH3 | 0.5–450 | RT | ΔR/Ra: 0.35 | 450 ppm | [118] | |
| nanocrystalline TiO2/SnO2 composites | commercial powder | NH3 | 100–5000 ppm | 400 | ΔR/Ra: 0.5 | 1200 ppm | [119] | ||
| SnO2/TiO2nanoneedles | wet chemical method | diameter: 40–80 nm; length: 60–100 nm | NH3 | 150 | (ΔR/Rg) × 100%: 300% | 3/5 min | 1000 ppm | [120] | |
| TiO2/SnO2 thick film | sol | NH3 | 100–1000 ppm | 250 | Ra/Rg: 3 | 400 ppm | [121] | ||
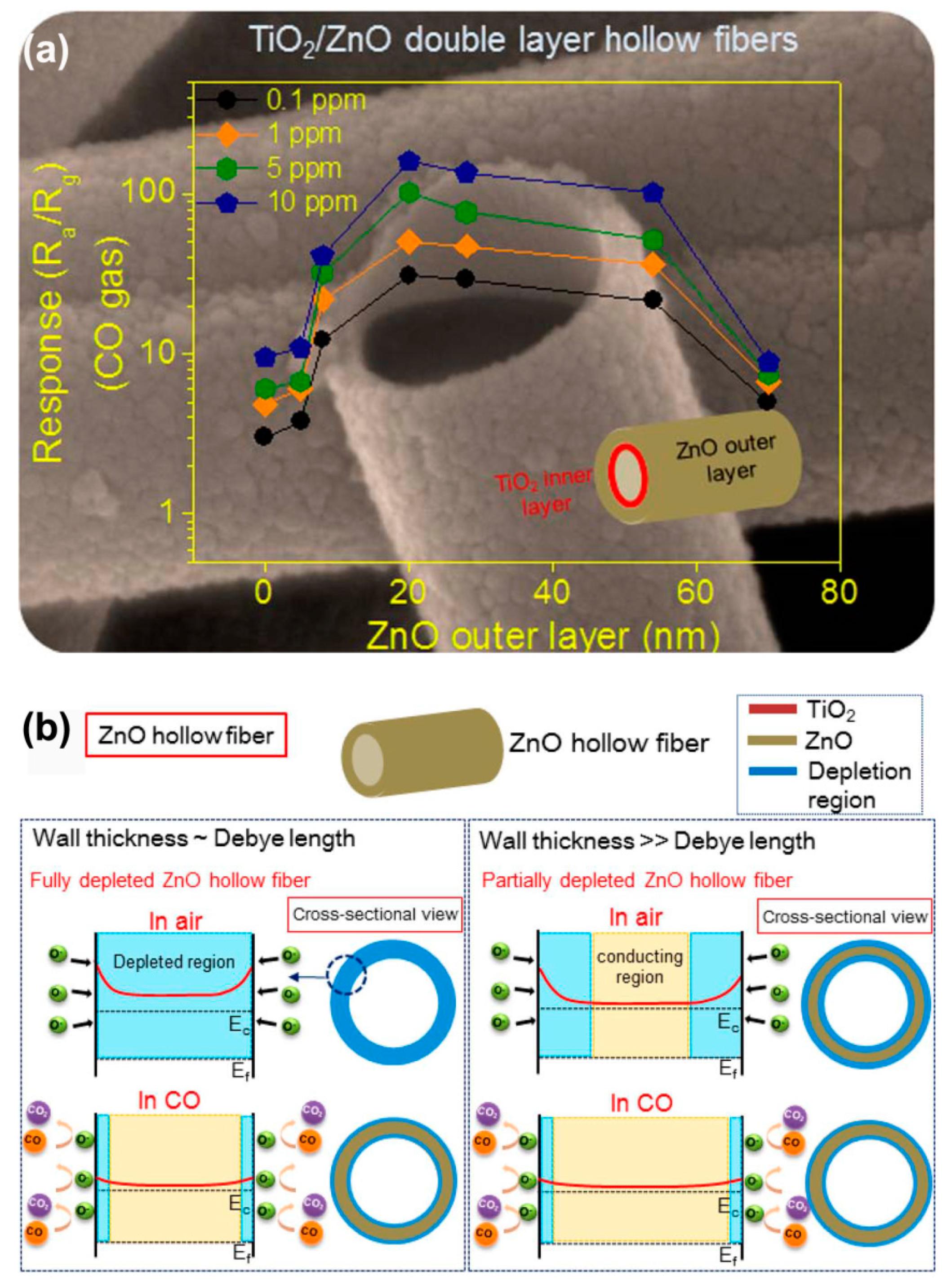
| TiO2/ZnO inner/outer double-layer hollow fibers | electrospinning + ALD | inner diameter: ~320 nm; TiO2 layer thickness: ~30 nm; ZnO outer layer thickness: 20 nm | CO | 0.1–10 ppm | 375 | Ra/Rg: 20.3 | 1 ppm | [72] | |
| TiO2/Fe2O3 nanosized thin film | sputtering | diameter: 20–30 nm | CO | ΔR/Ra: 15 | ~50/- s | 1000 ppm | [122] | ||
| TiO2/Al2O3/Pd composites | sol | H2S | 200–1000 ppm | 225 | log(Ra/Rg): 0.9 | 1000 ppm | [48] | ||
| nanocrystalline CdO/ZnO/TiO2 | pyrolyzation | H2S | 225–250 | ΔR/Ra: 0.8 | 10,000 ppm | [123] | |||
| TiO2 decorated CuO nanorods | thermal evaporative + sputtering | diameters: 50–100 nm; lengths: a few tens of micrometers | H2 | 0.1–5 ppm | 300 | Ra/Rg: 8.57 | 5 ppm | [124] | |
| TiO2/SnO2 nanocomposites | physical mixture | diameter: 8–28 nm | H2 | 50–3000 ppm | 375 | [76] | |||
| TiO2 fibers supported β-FeOOH nanostructures | electrospinning + hydrothermal method | nanofiber diameter: ~500 nm | H2 | 100 500 ppm | RT | Ra/Rg: 52.5 | 500 ppm | [87] | |
| mesoporous Nb2O5/TiO2 | sol-gel | diameter: 4.1 nm | H2 | 450 | Ra/Rg: ~5.5 | ~1/1 min | 500 ppm | [125] | |
| TiO2/NiO thin film | sputtering | H2 | 200 ppm–0.5% | 300 | Rg/Ra: 15 | 2/2.3 min | 1000 ppm | [126] | |
| PtO/Pt/TiO2 thin film | sol-gel | H2 | 1%–10% | 180 | ΔR/Ra × 100%: 40% | ~10/10 min | 2% | [103] | |
| TiO2-In2O3 composite nanofibers | electrospinning | diameters: 250 nm; length: several micrometers | NO2 | 0.3–97 ppm | RT | ΔR/Ra: ~1.25 | ~ 9.5/- s | 1 ppm | [61] |
| SnO2-core/V2O5-shell nanorods | thermal evaporation + sputtering | length: several tens micrometers; SnO2-core thicknesses: 100 nm; V2O5-shell thickness: 10 nm | NO2 | 10–80 ppm | 300 | Rg/Ra: 1.03% | ≤4.5/4.5 min | 10 ppm | [127] |
| Al2O3 decorated anatase TiO2 nanotubes | electrochemical anodization + thermal decomposition | nanotube outer diameter: ≤200 nm; length: several micrometers | NOx | 0.97–97 ppm | RT | ΔR/Ra: 88.04% | 8/- s | 97 ppm | [45] |
| CuO-TiO2-Au nanosystems | CVD + sputtering | O3 | 300 ppb | [71] | |||||
| V2O5/TiO2 thin film | sol-gel | 3–5 nm | O2 | 1 ppm–20.9% | 250 | Rg/Ra: 3.5 | 5/30 min | 120 ppm | [128] |
| CeO2/TiO2 thin film | sol-gel | O2 | 5–10,000 ppm | 420 | Rg/Ra: ~3 | 40–60/80 | 1000 ppm | [129] | |
| SnO2/TiO2 thin film | sputtering | 44–67 nm | O2 | 100–2000 ppm | Rg/Ra: 2 | 1000 ppm | [130] | ||
| TiO2-Based Nanoheterostructures | Fabrication Method | Size | Detection Gas | Detection Range | Response | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Operation Temperature (°C) | Sensitivity | Response/Recovery Time | Concentration | ||||||
| Pd/TiO2/reduced graphene oxide ternary composite | one-pot polyol | NH3 | 5–150 ppm | RT | (ΔR/Ra) × 100%: 39.9% | 100 ppm | [142] | ||
| PPy/graphene nanoplatelets decorated TiO2 nanoparticles | sol-gel + chemical polymerization | TiO2 nanoparticles diameter: 10–30 nm | NH3 | 1–200 ppm | RT | (ΔR/Ra) × 100%: 102.2% | 36/16 s | 50 ppm | [146] |
| CNTs/TiO2 nanocomposites | screen-printing + dip-coating techniques | NH3 | RT | ΔR/Ra: 93 | 9/2 min | 1% | [148] | ||
| Pt/TiO2/MWCNTs nanocomposites | sol-gel | H2 | 5%–100% | 50 | (ΔR/Ra) × 100%: 30% | 70% | [149] | ||
| CNTs/Pt-TiO2 NTs | anodization | diameter: 100 nm;length: 14 um | H2 | 0.5%–3% | 100 | (ΔR/Ra) × 100%: 2% | 1% | [150] | |
| Pt-TiO2/MWCNTs hybrid composites | wet chemical procedure | H2 | 0.5%–3% | 150 | 0.5% | [140] | |||
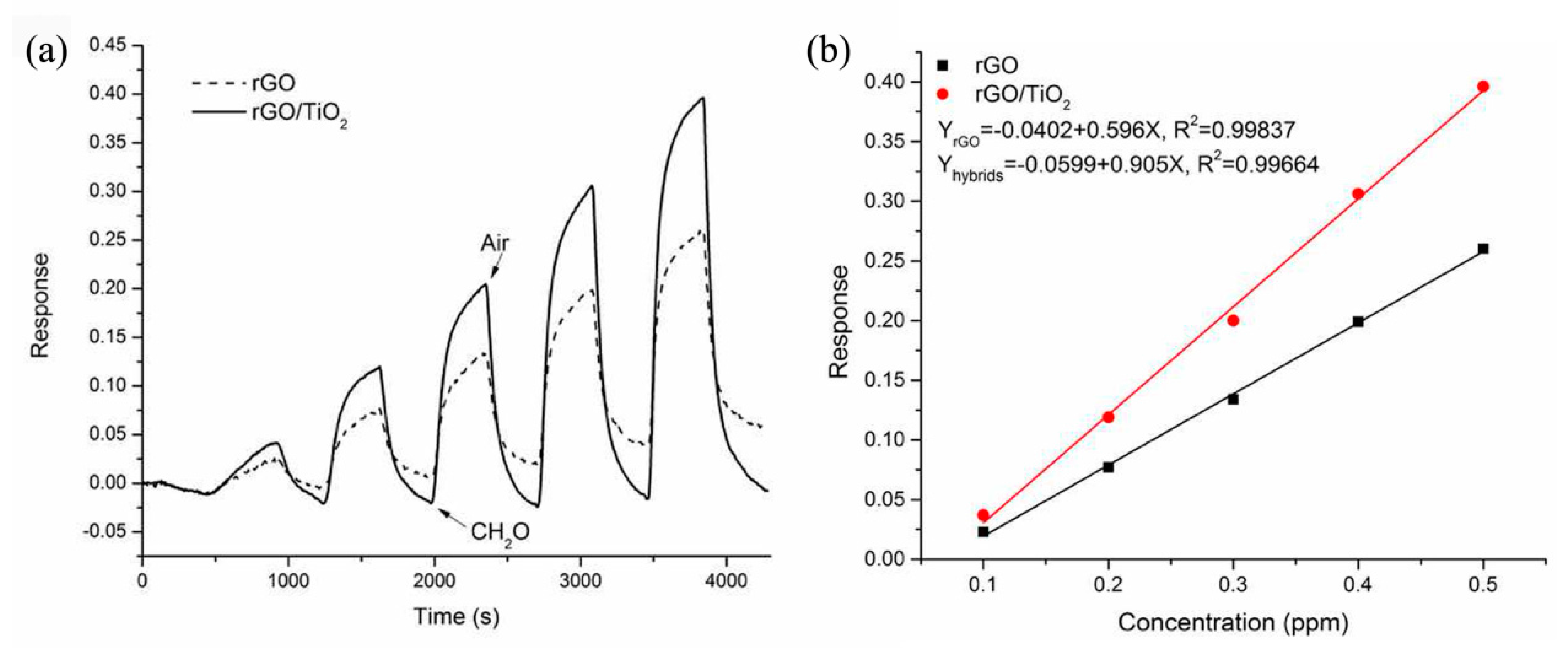
| rGO/TiO2 thin film | formaldehyde | 0.1–1 ppm | RT | (ΔR/Ra) × 100%: 0.64 | 70/126 s | 1 ppm | [75] | ||
| MWCNTs/TiO2 nanocomposites | sol-gel | diameter: 20–40 nm | CO | 350 | Ra/Rg: 15.8 | 4/16 s | 50 ppm | [151] | |
| MWCNTs/TiO2 thin film | sol-gel | CO | 400 | Ra/Rg: 89.2 | 5.16/2.72 s | 100 ppm | [152] | ||
| graphene-TiO2 nanocomposite | sol–gel | TiO2 nanoparticles: ~35 nm | CO2 | 500–15,000 ppm | 200 | Rg/Ra: 1.34 | 10,000 ppm | [144] | |
| TiO2/carbon black | sol-gel | NO2 | 1–100 ppm | 150 | ΔR/Ra × 100%: 7% | 100 ppm | [153] | ||
| single-walled carbon nanotube/TiO2 hybrid | length of carbon nanotubes: 20–50 nm | NO | 50 ppb–1 ppm | RT | (ΔR/Ra) × 100%: 9% | 50 ppb | [140] | ||
| CNT/TiO2 hybrid films | sol-gel | O2 | 10 ppm | 350 | ΔR/Ra: 6.5 | 8/- s | 10 ppm in CO2 | [154] | |
| grapheme oxide/nano-anatase TiO2 | ~5 nm | humidity | 35%–95% | power loss/ΔRH: ~0.47 dB/%RH | 0.74/0.91 s | [141] | |||
| TiO2-Based Nanoheterostructures | Fabrication Method | Size | Detection Gas | Detection Range | Response | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Operation Temperature (°C) | Sensitivity | Response/Recovery Time | Concentration | ||||||
| TiO2/PPy nanocomposites | in situ chemical polymerization | 33–67 nm | NH3 | 20–140 ppm | RT | (ΔR/Ra) × 100%: 7.95% | 19/85 s | 141 ppm | [88] |
| PPy-coated TiO2/ZnO nanofibers | electrospinning + chemical deposition | TiO2/ZnO core diameter: 100 nm; PPy shell thickness: 7 nm | NH3 | 0.5–450 ppm | RT | ΔR/Ra: 0.35 | 450 ppm | [118] | |
| PPy/TiO2 nanocomposites | layer by layer self-assembly technology. | NH3 | 10–1600 ppm | RT | frequency shift (ΔF): 50 Hz | ~100/200 s | 10 ppm | [165] | |
| PPy/TiO2 | in situ polymerization | NH3 | 20–500 ppm | RT | ΔR/Ra: 0.13 | 100 ppm | [169] | ||
| PANi/TiO2 nanofibers | electrospinning | diameter: 600 nm | NH3 | >50 ppt | RT | ΔR/Ra: 0.018 | <10/10 s | 200 ppt | [49] |
| PANi/TiO2 thin film heterojunction | chemical polymerization + sol-gel | NH3 | 20–100 ppm | RT | (ΔR/Ra) × 100%: ~11% | 41/- s | 100 ppm | [74] | |
| polyaniline/TiO2 nanorods heterostructure | hydrothermal method | NH3 | 5–100 ppm | RT | (Rg/Ra) × 100%: 610% | 40/60 s | 100 ppm | [167] | |
| cellulose/TiO2/PANi composite nanofibers | electrospinning | NH3 | 10–250 ppm | RT | ΔR/Ra: 0.584 | 10 ppm | [168] | ||
| TiO2-PANi/PA6 nanofibers | electrospinning + sputtering | NH3 | 50–250 ppm | RT | ΔR/Ra: 18.3 | <50/50 s | 250 ppm | [170] | |
| PANi/TiO2 nanocomposite thin film | in situ self-assembly technique | diameter: 90 nm | NH3 | 20–140 ppm | RT | ΔR/Ra: 0.3 | 2–3/~60 s | 1 ppm | [88] |
| CSA/PANi/TiO2 thin film | sol-gel | NH3 | 20–100 ppm | RT | ΔR/Ra: 0.75 | 49/413 s | 100 ppm | [171] | |
| TiO2–PANi nanocomposite thin film | spin coating | ~20 nm | CO2 | 53–1000 ppm | RT | Rg/Ra: 53 | 9.2/5.7 min | 1000 ppm | [164] |
| PANi doped TiO2 nanocomposite thin film | spin coating | 21 nm | LPG | RT | Rg/Ra: 2.37 | 2.6/2.4 min | 2000 ppm | [164] | |
| TiO2/PPy/poly 3-[(methacryoylamino) propyl trimethylammonium chloride] (PMAPTAC) nanocomposite thin film | in situ photopolymerization | humidity | 13–90% RH | RT | log Z: ~6 | 30/45 s | 60% | [172] | |
| TiO2/PPy nanocomposite film | in situ photopolymerization | humidity | 30–84% RH | RT | log Z: ~5.5 | 40/20 s | 30% | [173] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, T.; Zhou, Y.; Meng, C.; Zhu, W.; Liu, L. TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects. Sensors 2017, 17, 1971. https://doi.org/10.3390/s17091971
Wang Y, Wu T, Zhou Y, Meng C, Zhu W, Liu L. TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects. Sensors. 2017; 17(9):1971. https://doi.org/10.3390/s17091971
Chicago/Turabian StyleWang, Yuan, Tao Wu, Yun Zhou, Chuanmin Meng, Wenjun Zhu, and Lixin Liu. 2017. "TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects" Sensors 17, no. 9: 1971. https://doi.org/10.3390/s17091971
APA StyleWang, Y., Wu, T., Zhou, Y., Meng, C., Zhu, W., & Liu, L. (2017). TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects. Sensors, 17(9), 1971. https://doi.org/10.3390/s17091971




