Biomechanical Modeling of Pterygium Radiation Surgery: A Retrospective Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pterygium Surgery with Beta Irradiation
2.2. Constitutive Material Model
2.3. Patient-Specific Radiation Surgery Simulation
- (i)
- Mesh warping: In our earlier work [23], we showed that a model with patient-specific geometry of the human cornea can be obtained by warping a spherical finite element mesh such that its anterior and posterior surfaces match the respective surfaces of the tomography measurements. Thereby, the tomography surfaces are expressed as the coefficients obtained from Zernike expansion (up to the twelfth order, and over the central 8.0 mm optical zone of the cornea), and the inside mesh nodes proportionally follow the deformation of the respective surface nodes. This way, the template mesh was warped to match the patient’s cornea, without producing distorted elements (which is crucial for finite element analysis).
- (ii)
- Calculation of initial stress distribution: Since the Pentacam Scheimpflug camera measures corneal geometry in vivo, whereby the corneal tissue is under mechanical stress, the shape in the absence of acting forces is a priori not known. An iterative approach to calculate the initial stress distribution in the model, as was previously published [30,31], was employed in this study.
- (iii)
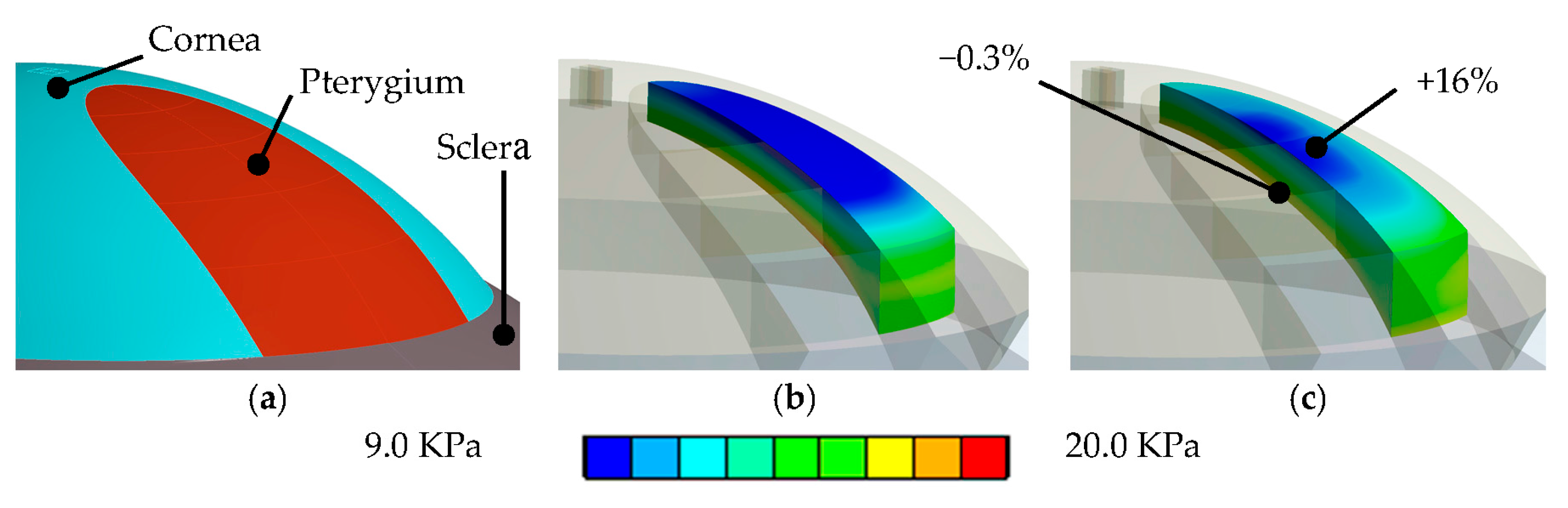
- Surgery simulation: A specific, three-dimensional, finite element model was created in the finite element software package ANSYS 17.1 (ANSYS Inc., Canonsburg, PA, USA). The model represents the full cornea, plus a 4-mm wide rim of scleral tissue. The model was fixed at the edge of the scleral rim, and a pressure of 15 mmHg on the models inside represented the intraocular pressure. The anterior surface of the model cornea is prepared such that a specific part exactly corresponds to the shape and position of the subject’s pterygium (see Figure 1b). Pressure was applied to that specific part, modeling the pulling forces of the retracting pterygium, tangentially to the corneal surface and towards the limbus (see Figure 2). This simulation approach reproduces the pulling effects placed onto the corneal surface when radiation-induced tissue shrinking in pterygium tissue occurs. The pterygium tissue itself was not modelled.
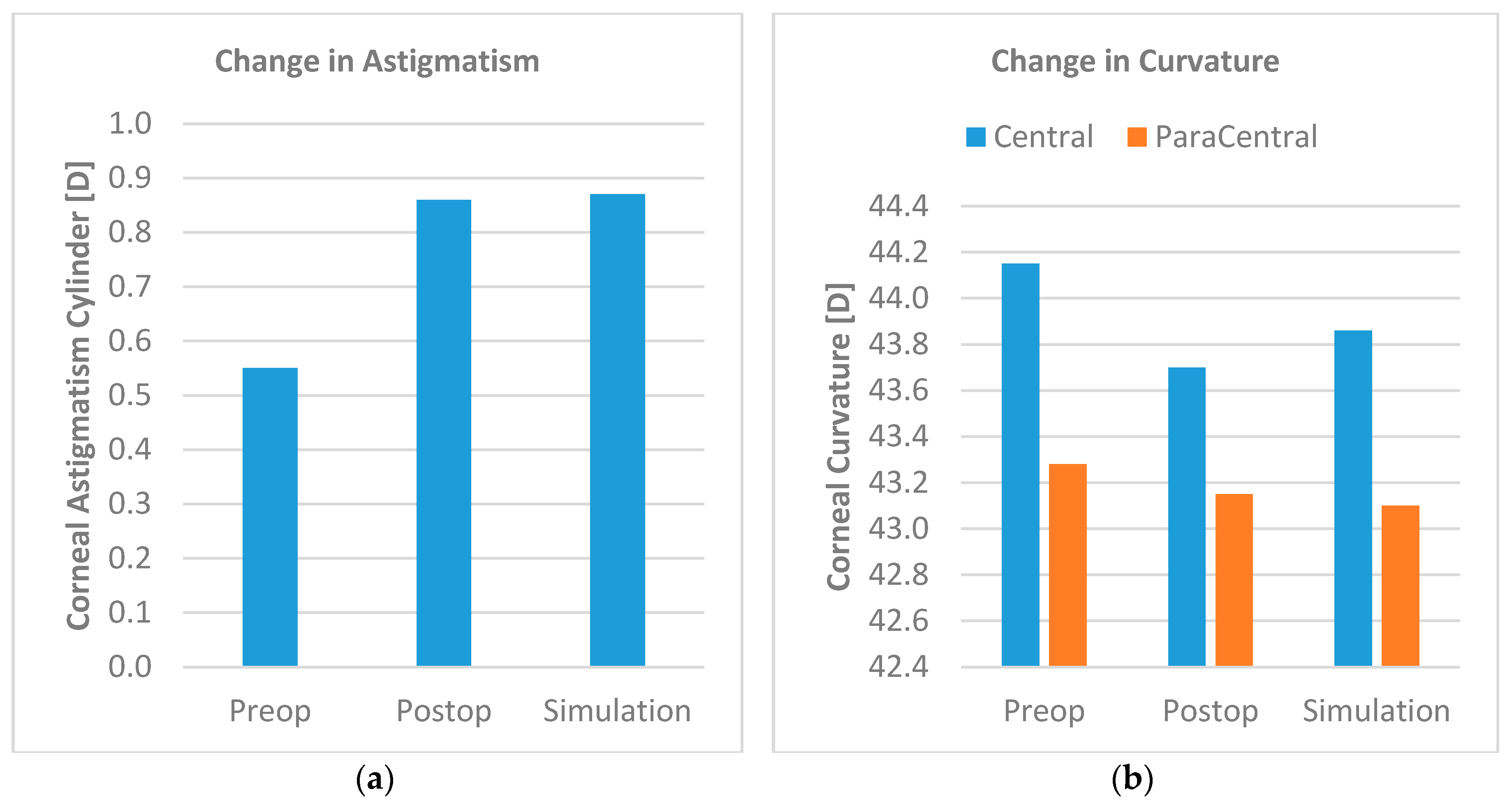
3. Results
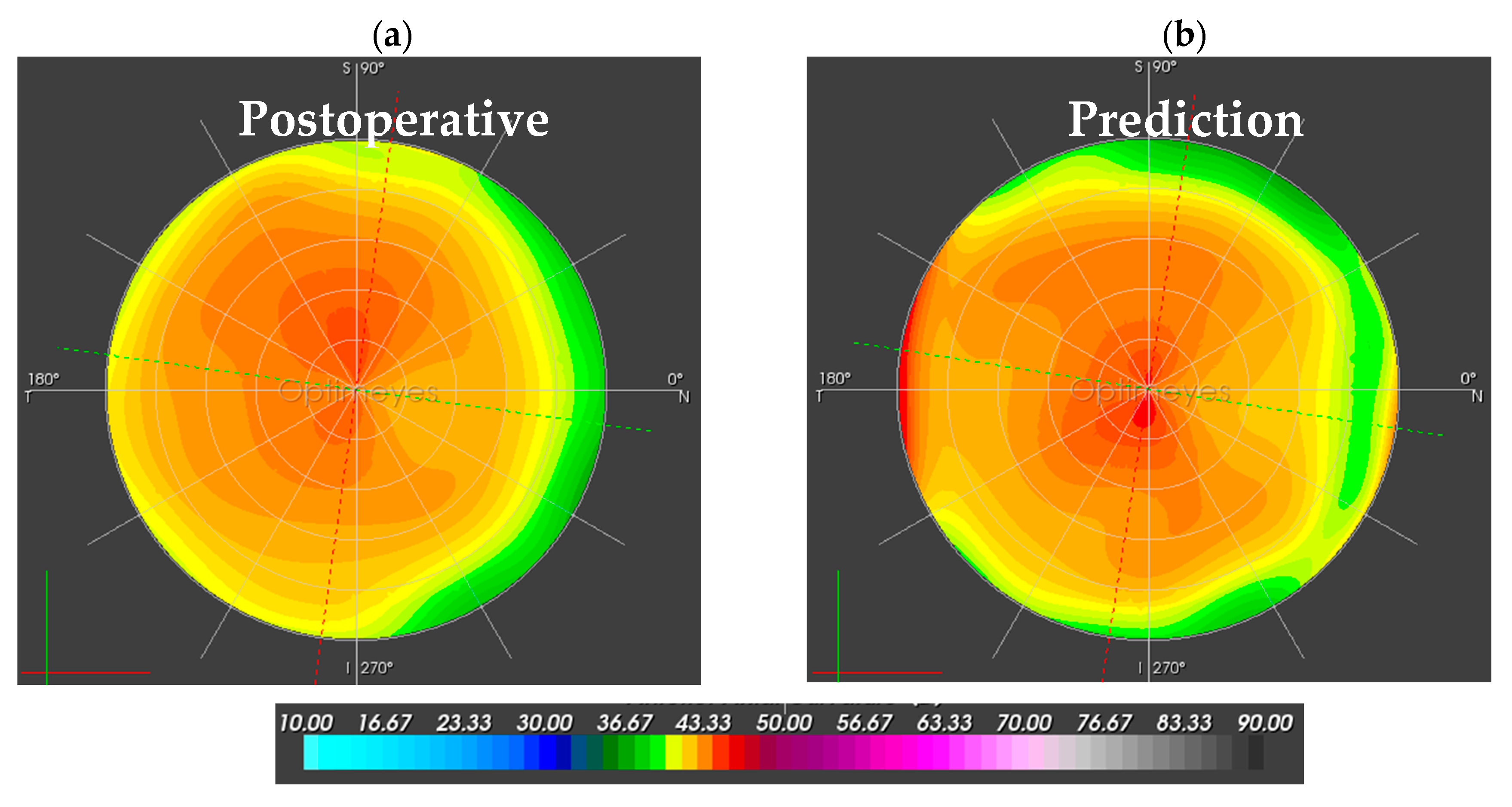
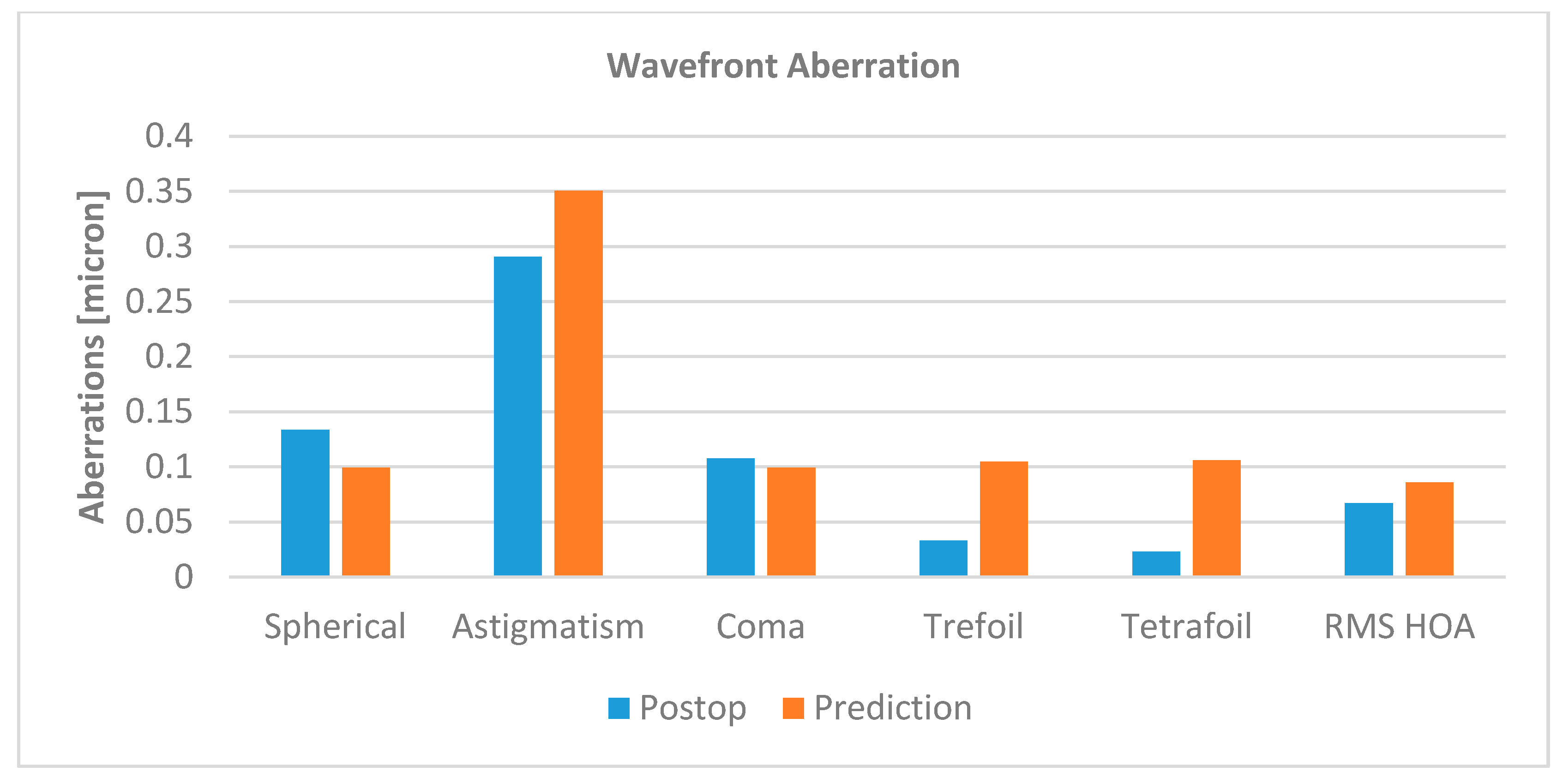
3.1. Corneal Function
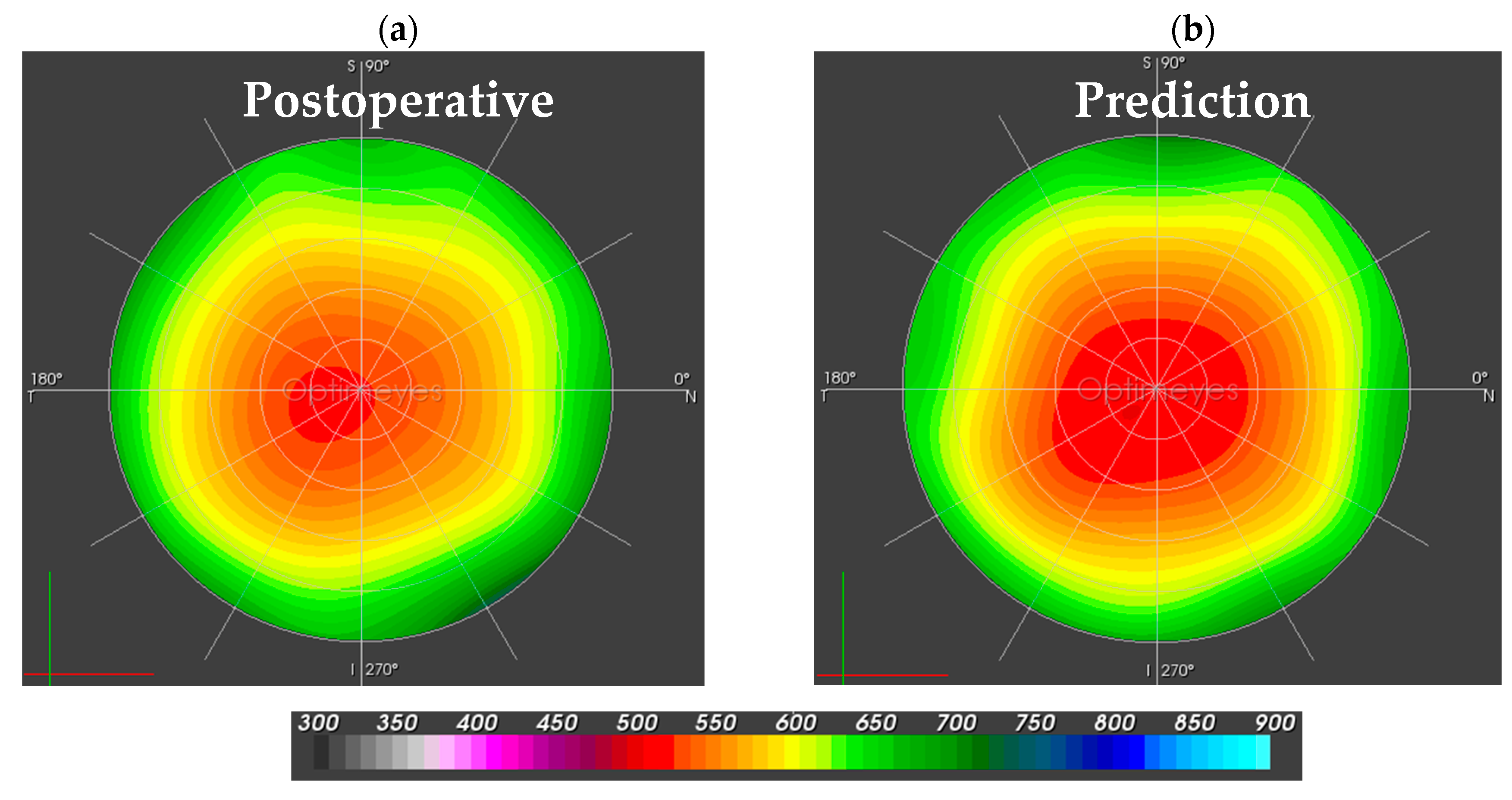
3.2. Corneal Biomechanics
4. Discussion and Conclusions
Author Contributions
Conflicts of Interest
References
- Taylor, H.R. Etiology of climatic droplet keratopathy and pterygium. Br. J. Ophthalmol. 1980, 64, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S. Postoperative irradiation of pterygia: Ten more years of experience. Radiology 1978, 128, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Coronea, M.T.; Di Girolamo, N.; Wakefield, D. The pathogenesis of pterygia. Curr. Opin. Ophthalmol. 1999, 10, 282–288. [Google Scholar] [CrossRef]
- Moran, D.J.; Hollows, F.C. Pterygium and ultraviolet radiation: A positive correlation. Br. J. Ophthalmol. 1984, 68, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Pajic, B.; Pugnale-Verillotte, N.; Greiner, R.H.; Pajic, D.; Eggspühler, A. Résultat de la thérapie au strontium-yttrium-90 des ptérygions. J. Fr. Ophthalmol. 2002, 25, 473–479. [Google Scholar]
- Pajic, B.; Pallas, A.; Aebersold, D.; Gruber, G.; Greiner, R.H. Prospective Study on Exclusive, Nonsurgical Strontium-/Yttrium-90 Irradiation of Pterygia. Strahlenther. Onkol. 2004, 180, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Pajic, B.; Greiner, R.H. Long term results of non-surgical, exclusive Strontium-/Yttrium-90 Beta-irradiation of pterygia. Radiother. Oncol. 2005, 74, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kria, L.; Ohira, A.; Amemiya, T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-β and tumor necrosis factor-α in the pterygium. Acta Histochem. 1996, 98, 195–201. [Google Scholar] [CrossRef]
- Nakagami, T.; Watanabe, I.; Murakami, A.; Okisaka, S.; Ebihara, N. Expression of Stem Cell Factor in Pterygium. Jpn. J. Ophthalmol. 2000, 44, 193–197. [Google Scholar] [CrossRef]
- Vastardis, I.; Pajic, B.; Greiner, R.; Pajic-Eggspuehler, B.; Aebersold, D. Prospective study of exclusive Strontium-/Yttrium-90 β- irradiation of primary and recurrent pterygia with no prior surgical excision: Clinical outcome of long-term follow-up. Strahlenther. Onkol. 2009, 185, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.W.; Chew, J.; Yang, H.; Tan, D.; Beuerman, R. Expression of insulin-like growth factor binding protein- 3 in pterygium tissue. Br. J. Ophthalmol. 2006, 90, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Bahrassa, F.; Datta, R. Postoperative beta radiation treatment of pterygium. Int. J. Radiat Oncol. Biol. Phys. 1983, 9, 679–684. [Google Scholar] [CrossRef]
- Bernstein, M.; Unger, S.M. Experiences with surgery and strontium 90 in the treatment of pterygium. Am. J. Ophthalmol. 1960, 49, 1024–1029. [Google Scholar] [CrossRef]
- De Keizer, R.J.W. Pterygium excision with or without postoperative irradiation, a double blind study. Doc. Ophthalmol. 1982, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Frucht-Perry, J.; Siganos, C.S.; Ilsar, M. Intraoperative application of topical mitomycin C for pterygium surgery. Ophthalmology 1996, 103, 674–677. [Google Scholar] [CrossRef]
- Hayasaka, S.; Noda, S.; Yukari, Y.; Setogawa, T. Postoperative installation of Mitomycin C in the treatment of recurrent pterygium. Ophthalmic Surg. 1989, 20, 580–583. [Google Scholar] [PubMed]
- Paryani, S.B.; Scott, W.P.; Wells, J.W., Jr.; Johnson, D.W.; Chobe, R.J.; Kuruvilla, A.; Schoeppel, S.; Deshmukh, A. Management of pterygium with surgery and radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 101–103. [Google Scholar] [CrossRef]
- Rachmiel, R.; Leiba, H.; Levartovsky, S. Results of treatment with topical mitomycin C 0.02% following excision of primary pterygium. Br. J. Ophthalmol. 1995, 79, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Shusko, A.; Hovanesian, J.A. Pterygium excision with conjunctival autograft and subconjunctival amniotic membrane as antirecurrence agents. Can. J. Ophthalmol. 2016, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Pajic, B.; Vastardis, I.; Rajkovic, P.; Pajic-Eggspuehler, B.; Aebersold, DM.; Cvejic, Z. A mathematical approach to human pterygium shape. Clin. Ophthalmol. 2016, 10, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Studer, H.P.; Larrea, X.; Riedwyl, H.; Büchler, P. Biomechanical Model of Human Cornea Based on Stromal Microstructure. J. Biomech. 2010, 43, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Studer, H.P.; Büchler, P.; Ridewly, H. Importance of Multiple Loading Scenarios for the Identification of Material Coefficients of the Human Cornea. CMBBE 2012, 15, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Studer, H.P.; Riedwyl, H.; Amstutz, C.A.; Hanson, J.V.; Büchler, P. Patient-specific finite-element simulation of the human cornea: A clinical validation study on cataract surgery. J. Biomech. 2013, 46, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Studer, H.P.; Pradhan, K.R.; Reinstein, D.Z.; Businaro, E.; Archer, T.J.; Gobbe, M.; Roberts, C.J. Biomechanical Modeling of Femtosecond Laser Keyhole Endokeratophakia Surgery. J. Refract. Surg. 2015, 31, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Whitford, C.; Studer, H.; Boote, C.; Meek, K.M.; Elsheikh, A. Biomechanical Model of the Human Cornea: Considering Shear Stiffness and Regional Variation of Collagen Anisotropy and Density. JMBBM 2015, 42, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Markert, B.; Ehlers, W.; Karajan, N. A general polyconvex strain-energy function for fiber-reinforced materials. Proc. Appl. Math. Mech. 2005, 5, 245–246. [Google Scholar] [CrossRef]
- Aghamohammadzadeh, H.; Newton, R.; Meek, K. X-ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure 2004, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Wang, D.; Pye, D. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. 2007, 23, 808–818. [Google Scholar] [PubMed]
- Elsheikh, A.; Anderson, K. Comparative study of corneal strip extensometry and inflation tests. J. R. Soc. Interface 2008, 2, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.M.; van der Heide, D.; Chernyak, D. Computational modeling of mechanical anisotropy in the cornea and sclera. J. Cataract Refract. Surg. 2005, 31, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, A.; Manganiello, F. A model for the human cornea: Constitutive formulation and numerical analysis. Biomech. Model. Mechanobiol. 2006, 5, 237–246. [Google Scholar] [CrossRef] [PubMed]


| 0.06 | 0.13 | 24.0 | 0.08 | 95.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajic, B.; Aebersold, D.M.; Eggspuehler, A.; Theler, F.R.; Studer, H.P. Biomechanical Modeling of Pterygium Radiation Surgery: A Retrospective Case Study. Sensors 2017, 17, 1200. https://doi.org/10.3390/s17061200
Pajic B, Aebersold DM, Eggspuehler A, Theler FR, Studer HP. Biomechanical Modeling of Pterygium Radiation Surgery: A Retrospective Case Study. Sensors. 2017; 17(6):1200. https://doi.org/10.3390/s17061200
Chicago/Turabian StylePajic, Bojan, Daniel M. Aebersold, Andreas Eggspuehler, Frederik R. Theler, and Harald P. Studer. 2017. "Biomechanical Modeling of Pterygium Radiation Surgery: A Retrospective Case Study" Sensors 17, no. 6: 1200. https://doi.org/10.3390/s17061200
APA StylePajic, B., Aebersold, D. M., Eggspuehler, A., Theler, F. R., & Studer, H. P. (2017). Biomechanical Modeling of Pterygium Radiation Surgery: A Retrospective Case Study. Sensors, 17(6), 1200. https://doi.org/10.3390/s17061200






