Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors
Abstract
:1. Introduction
2. Materials and Methods
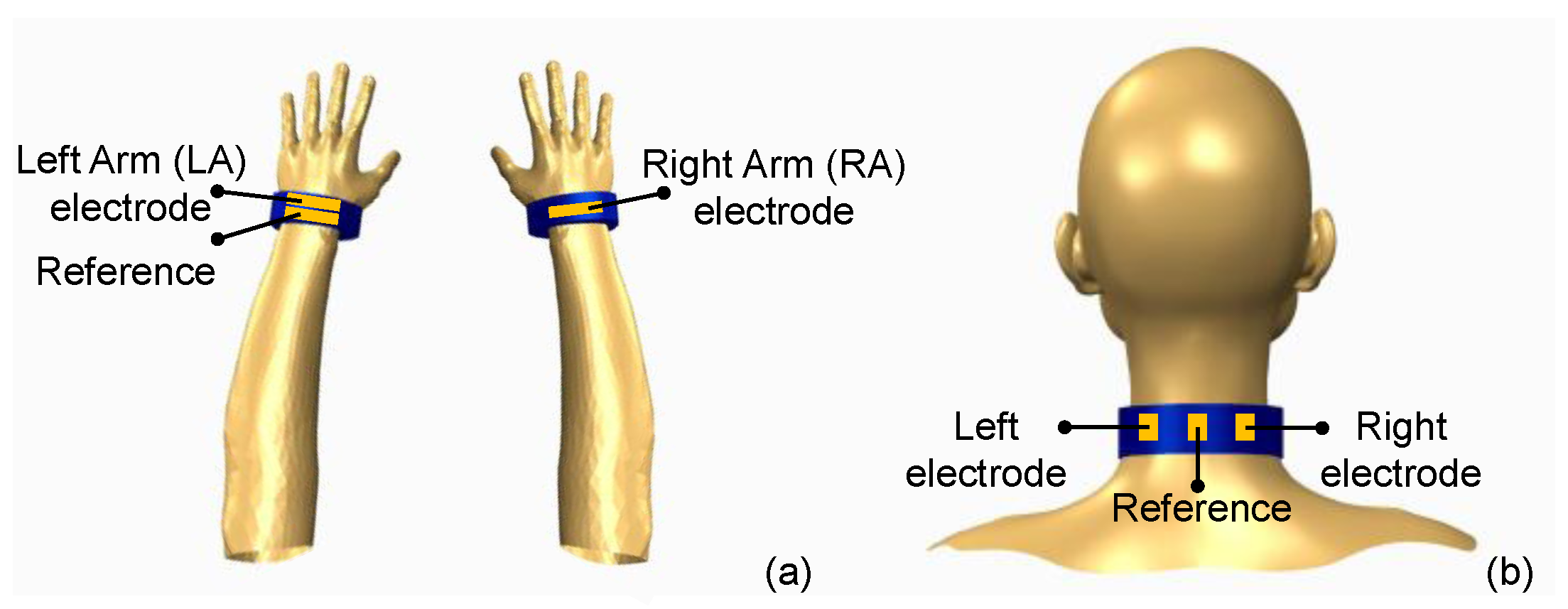
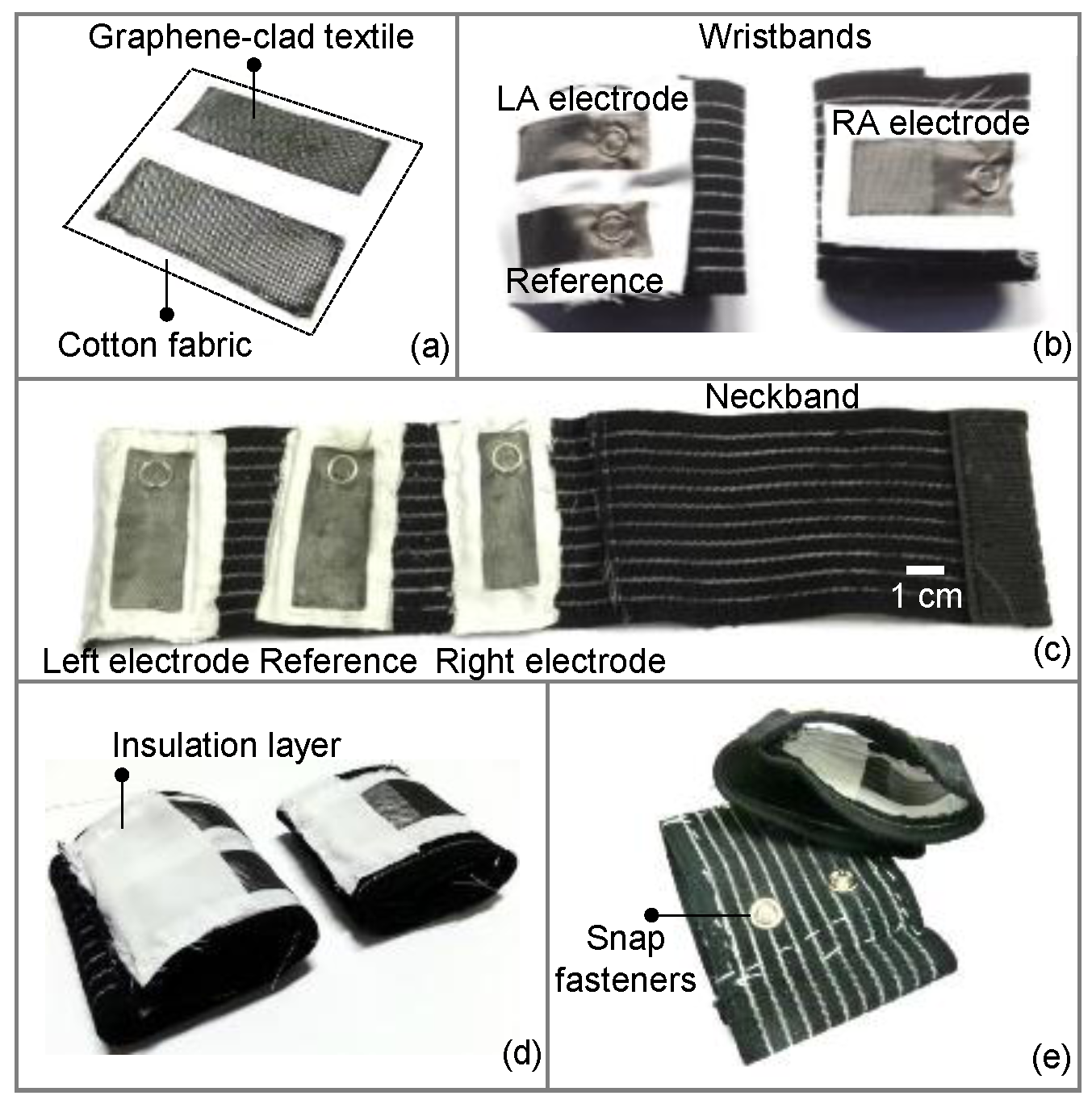
2.1. Preparation and Integration of Graphene-Clad Textiles into Wearable Garments
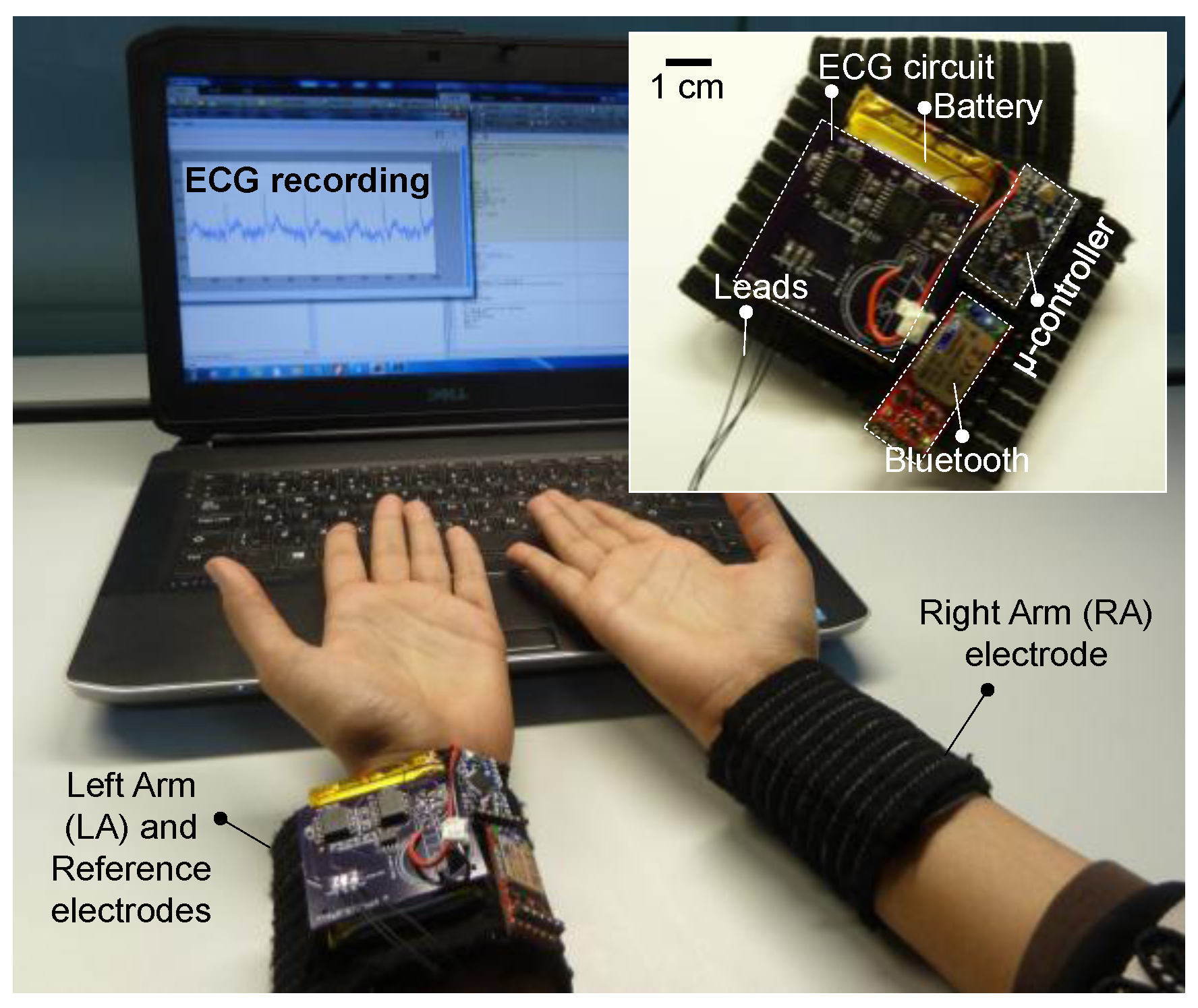
2.2. Performance Evaluation
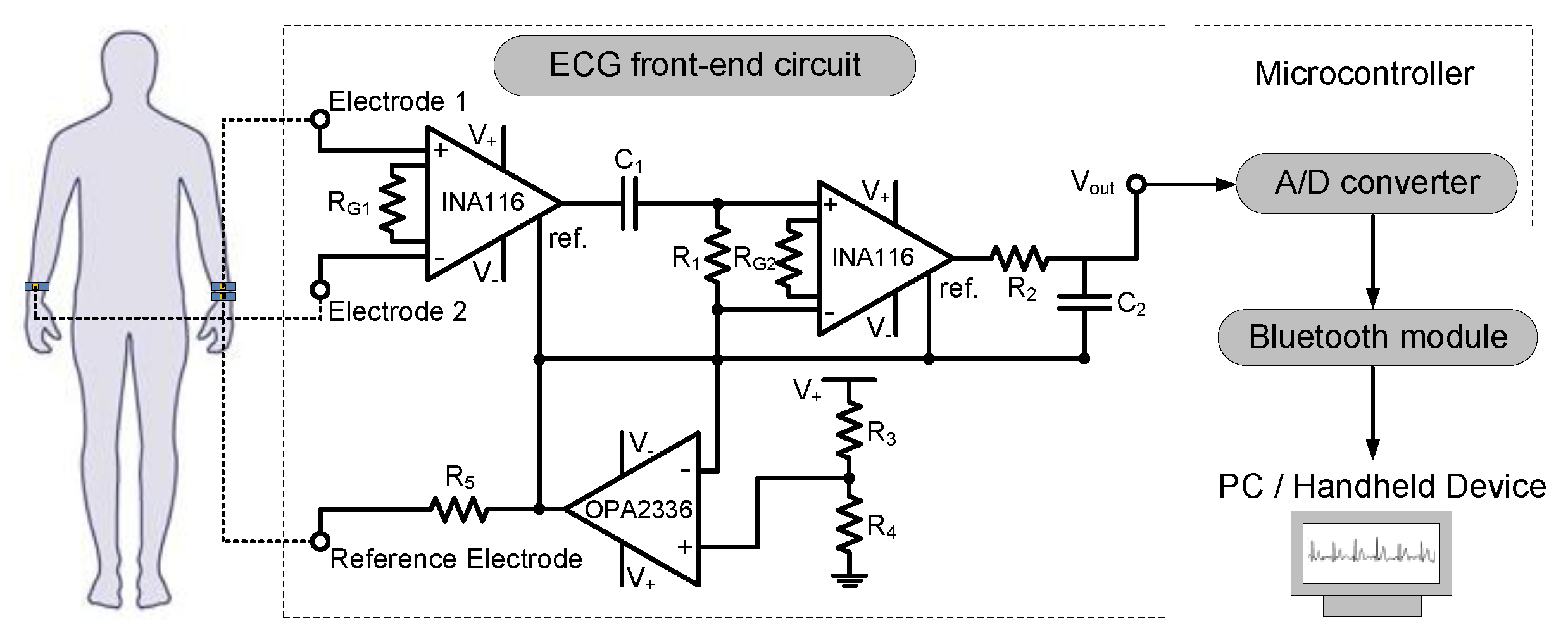
2.3. System-Level Design
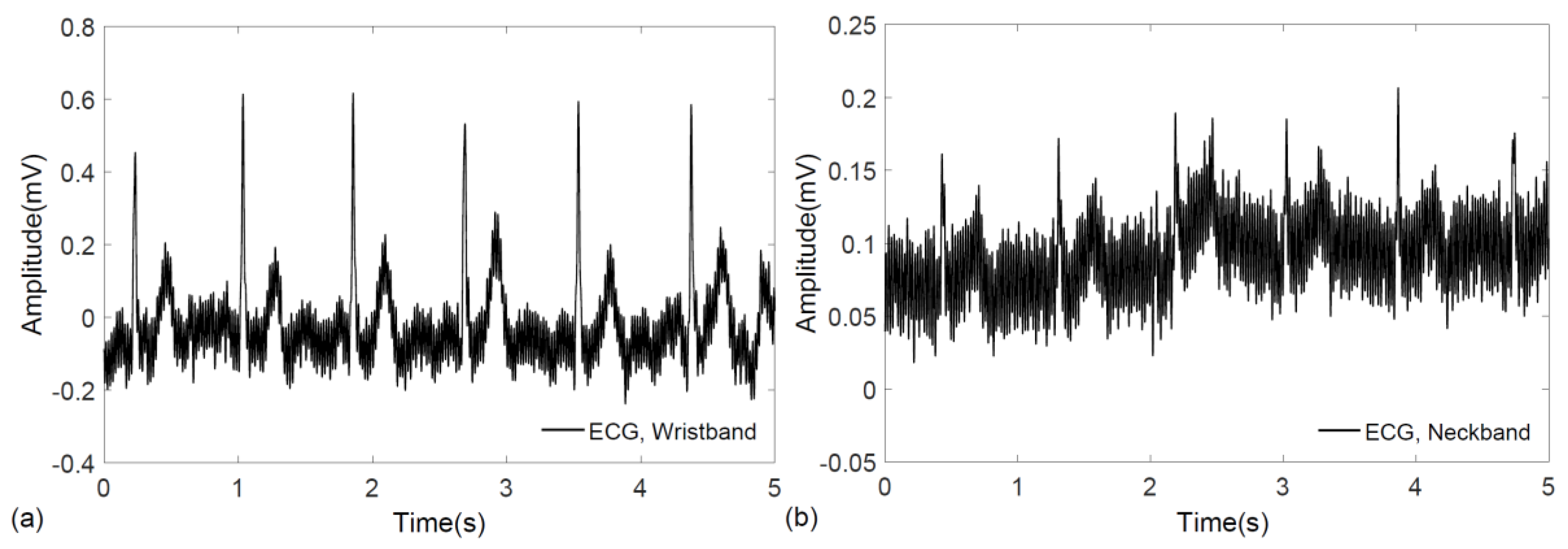
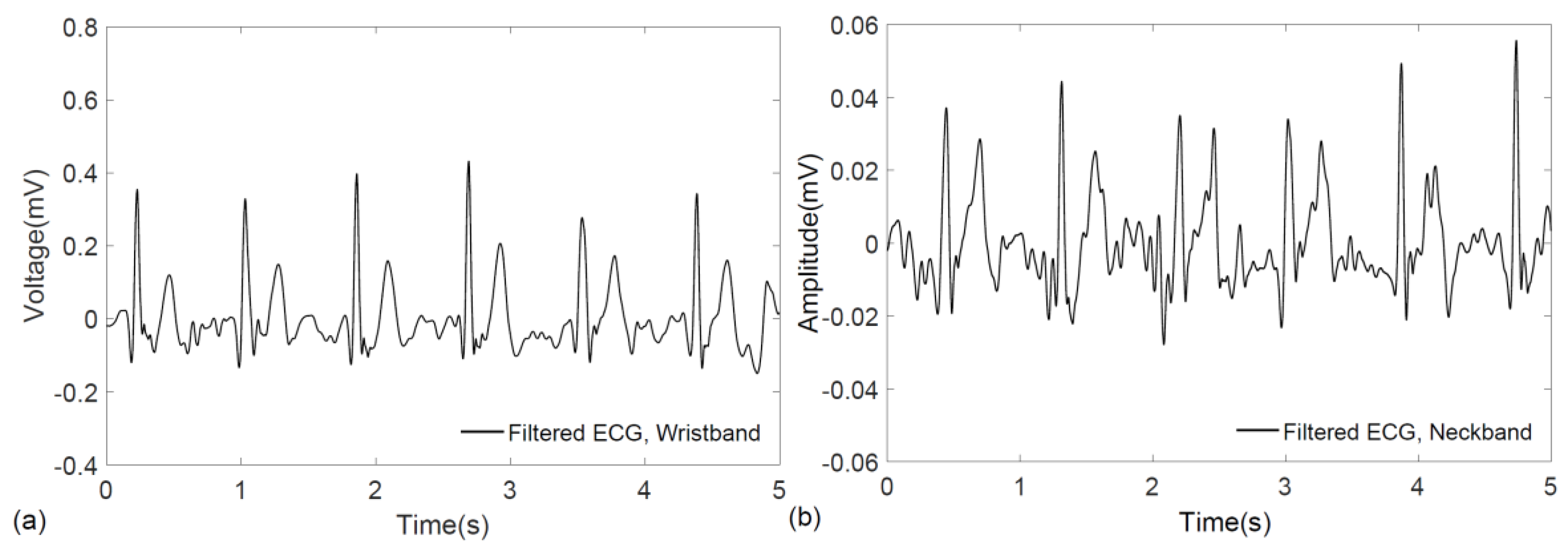
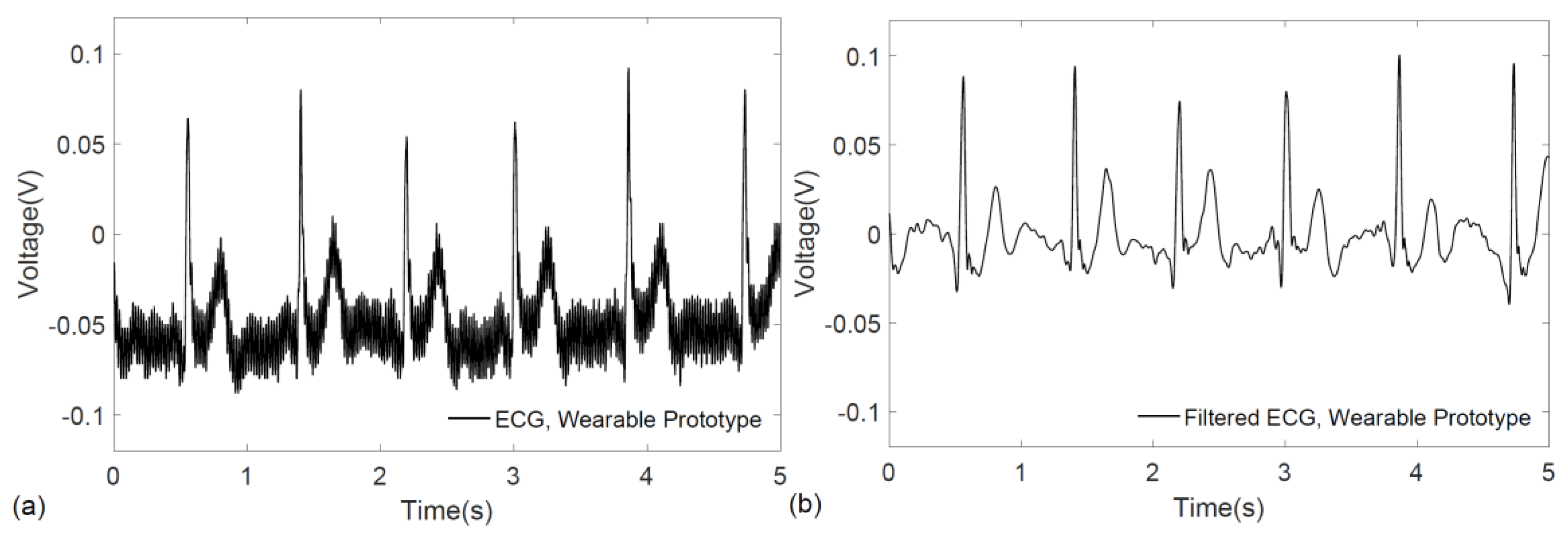
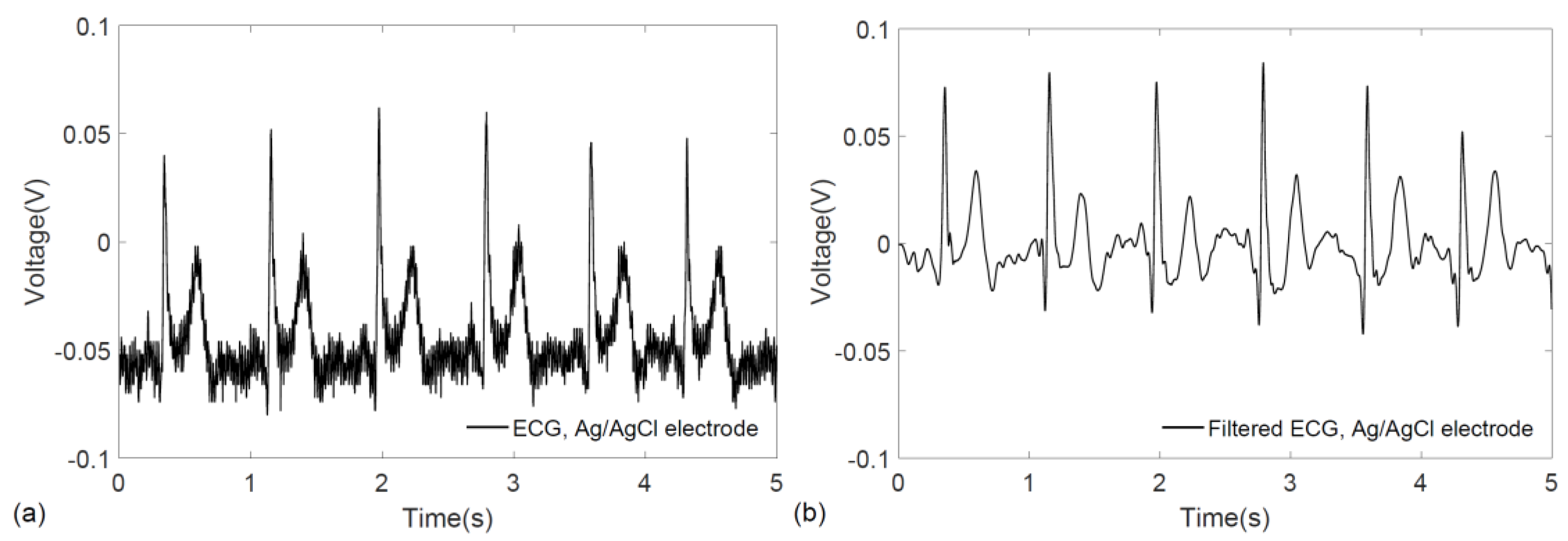
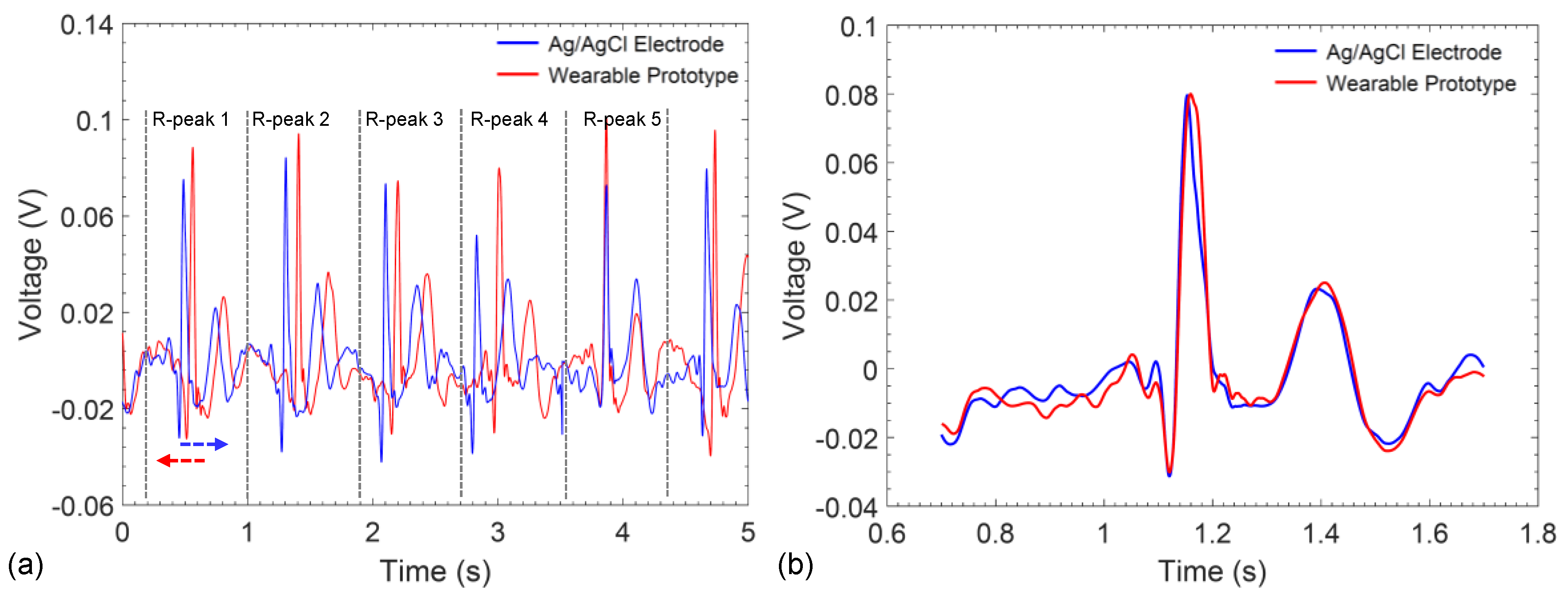
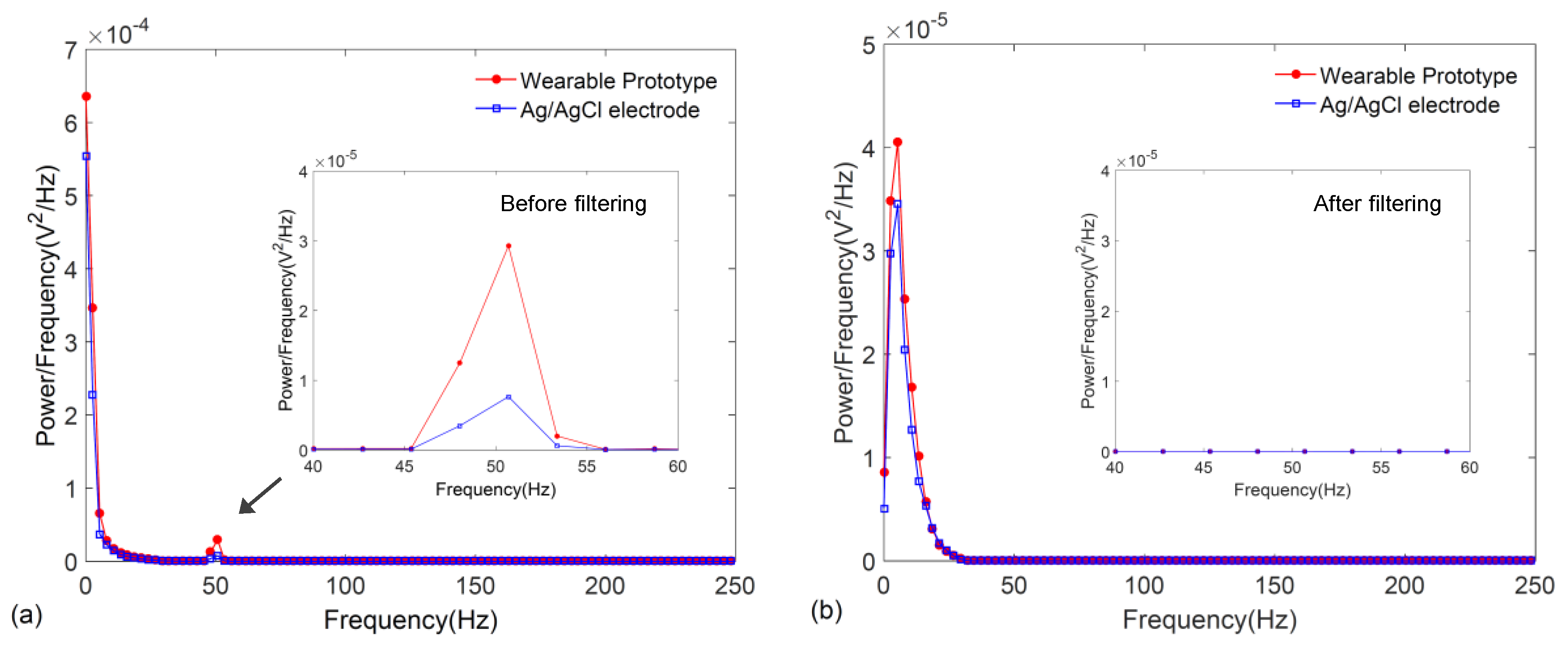
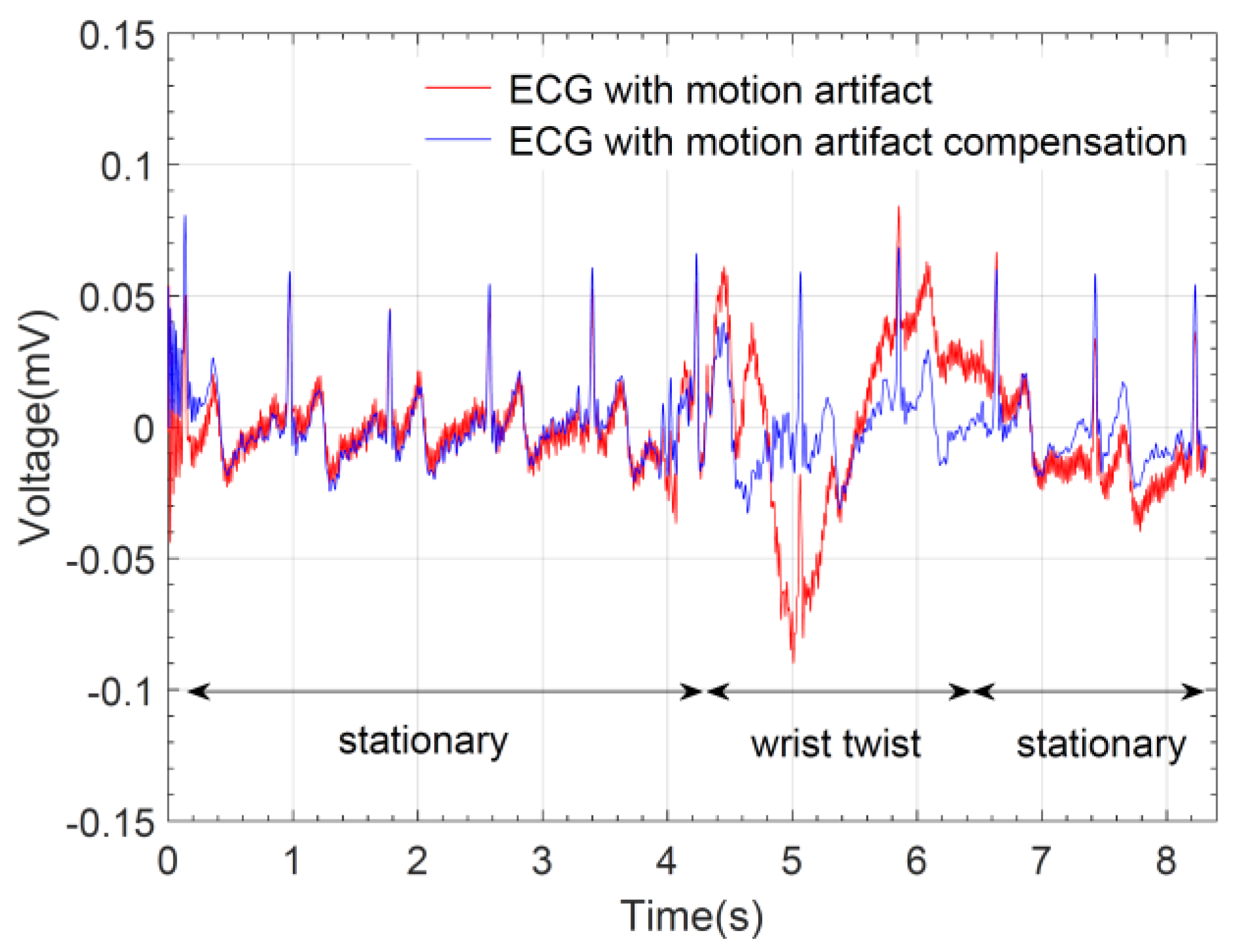
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jain, P.K.; Tiwari, A.K. Heart monitoring systems—A review. Comput. Biol. Med. 2014, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Järvelin, M.-R.; Doshi, R.N.; Shinbane, J.S.; Carlson, S.K.; Grazette, L.P.; Chang, P.M.; Sangha, R.S.; Huikuri, H.V.; Peters, N.S. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front. Physiol. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marozas, V.; Petrenas, A.; Daukantas, S.; Lukosevicius, A. A comparison of conductive textile-based and silver/silver chloride gel electrodes in exercise electrocardiogram recordings. J. Electrocardiol. 2011, 44, 189–194. [Google Scholar] [CrossRef] [PubMed]
- McAdams, E. Biomedical electrodes for biopotential monitoring and electrostimulation. In Bio-Medical CMOS ICs, 1st ed.; Yoo, H.-J., van Hoof, C., Eds.; Springer: New York, NY, USA, 2011; pp. 31–124. [Google Scholar]
- Xu, P.J.; Zhang, H.; Tao, X.M. Textile-structured electrodes for electrocardiogram. Text. Prog. 2008, 40, 183–213. [Google Scholar] [CrossRef]
- Ueno, A.; Akabane, Y.; Kato, T. Capacitive Sensing of Electrocardiographic Potential Through Cloth From the Dorsal Surface of the Body in a Supine Position: A Preliminary Study. IEEE Trans. Biomed. Eng. 2007, 54, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M.; Tay, F.E.H.; Guo, D.G.; Xu, L.; Yap, K.L. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators A Phys. 2009, 151, 17–22. [Google Scholar] [CrossRef]
- Yang, C.-S.; Yang, B.; Zhu, H.-Y.; Liu, J.-Q.; Peng, H.-L.; Wang, L.-F. MEMS-based flexible capacitive electrode for ECG measurement. Electron. Lett. 2013, 49, 739–740. [Google Scholar]
- Kim, H.-L.; Kim, Y.-H.; Kim, Y.-J. Miniature electrocardiography sensor using a flexible printed circuit and MEMS technology. In Proceedings of the IEEE International Conference on Multisensor Fusion and Integration for Intelligent Systems, Seoul, South Korea, 20–22 August 2008; pp. 545–550. [Google Scholar]
- Chen, C.-Y.; Chang, C.-L.; Chien, T.-F.; Luo, C.-H. Flexible PDMS electrode for one-point wearable wireless bio-potential acquisition. Sens. Actuators A Phys. 2013, 203, 20–28. [Google Scholar] [CrossRef]
- Chi, Y.M.; Cauwenberghs, G. Micropower non-contact EEG electrode with active common-mode noise suppression and input capacitance cancellation. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Minneapolis, MN, USA, 3–6 Septmber 2009; pp. 4218–4221. [Google Scholar]
- Fuhrhop, S.; Lamparth, S.; Heuer, S. A textile integrated long-term ECG monitor with capacitively coupled electrodes. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Beijing, China, 26–28 November 2009; pp. 21–24. [Google Scholar]
- Sardini, E.; Serpelloni, M. Instrumented wearable belt for wireless health monitoring. Procedia Eng. 2010, 5, 580–583. [Google Scholar] [CrossRef]
- Tseng, K.C.; Lin, B.; Liao, L.; Wang, Y.; Wang, Y. Development of a Wearable Mobile Electrocardiogram Monitoring System by Using Novel Dry Foam Electrodes. IEEE Syst. J. 2014, 8, 900–906. [Google Scholar] [CrossRef]
- Lee, Y.D.; Chung, W.Y. Wireless sensor network based wearable smart shirt for ubiquitous health and activity monitoring. Sens. Actuators B Chem. 2009, 140, 390–395. [Google Scholar] [CrossRef]
- Lee, S.M.; Sim, K.S.; Kim, K.K.; Lim, Y.G.; Park, K.S. Thin and flexible active electrodes with shield for capacitive electrocardiogram measurement. Med. Biol. Eng. Comput. 2010, 48, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.; Abächerli, R.; Meyer, V.; Rossi, R. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Torah, R.; Beeby, S.; Tudor, J. The development of screen printed conductive networks on textiles for biopotential monitoring applications. Sens. Actuators A Phys. 2014, 206, 35–41. [Google Scholar] [CrossRef]
- Merritt, C.R.; Nagle, H.T.; Grant, E. Fabric-based active electrode design and fabrication for health monitoring clothing. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Rattfält, L.; Lindén, M.; Hult, P.; Berglin, L.; Ask, P. Electrical characteristics of conductive yarns and textile electrodes for medical applications. Med. Biol. Eng. Comput. 2007, 45, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Nakashima, H.; Torimitsu, K. Conductive polymer combined silk fiber bundle for bioelectrical signal recording. PLoS ONE 2012, 7, e33689. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.L.; Gonçalves, L.M.; Carvalho, H. Deposition of conductive materials on textile and polymeric flexible substrates. J. Mater. Sci. Mater. Electron. 2012, 24, 635–643. [Google Scholar] [CrossRef]
- Oh, T.I.; Yoon, S.; Kim, T.E.; Wi, H.; Kim, K.J.; Woo, E.J.; Sadleir, R.J. Nanofiber web textile dry electrodes for long-term biopotential recording. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 204–211. [Google Scholar] [PubMed]
- Yapici, M.K.; Alkhidir, T.; Samad, Y.A.; Liao, K. Graphene-clad textile electrodes for electrocardiogram monitoring. Sens. Actuators B Chem. 2015, 221, 1469–1474. [Google Scholar] [CrossRef]
- Sörnmo, L.; Laguna, P. The electrocardiogram—A brief background. In Bioelectrical Signal Processing in Cardiac and Neurological Applications, 1st ed.; Sörnmo, L., Laguna, P., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 411–452. [Google Scholar]
- Banerjee, S.; Gupta, R.; Mitra, M. Delineation of ECG characteristic features using multiresolution wavelet analysis method. Measurement 2012, 45, 474–487. [Google Scholar] [CrossRef]
- Gargiulo, G.; Bifulco, P.; Cesarelli, M.; Romano, M.; Calvo, R.A.; Jin, C. An ultra-high input impedance ECG amplifier for long-term monitoring of athletes. Med. Dev. Evid. Res. 2010, 3, 1–9. [Google Scholar] [CrossRef]
- Hann, M.W. Ultra Low Power, 18 bit Precision ECG Data Acquisition System; Texas Instruments Incorporated: Dallas, TX, USA, 2013. [Google Scholar]
- Nemati, E.; Deen, M.J.; Mondal, T. A wireless wearable ECG sensor for long-term applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Spinelli, E.M.; Martinez, N.H.; Mayosky, M.A. A single supply biopotential amplifier. Med. Eng. Phys. 2001, 23, 235–238. [Google Scholar] [CrossRef]
- Alkhidir, T.; Sluzek, A.; Yapici, M.K. Simple Method for Adaptive Filtering of Motion Artifacts in E-Textile Wearable ECG Sensors. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3807–3810. [Google Scholar]
- Cömert, A.; Honkala, M.; Hyttinen, J. Effect of pressure and padding on motion artifact of textile electrodes. Biomed. Eng. Online 2013, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Manivannan, N.; Strudwick, A.; Balachandran, W. Graphene-enabled electrodes for electrocardiogram monitoring. Nanomaterials 2016, 6, 156. [Google Scholar] [CrossRef] [PubMed]
| Parts | Specifications |
|---|---|
| Bluethooth module | Power consumption: 26 µA sleep, 3 mA connected, 30 mA transmit |
| (BlueSMiRF Silver) | Baud rate: 57,600 |
| Bandwidth: 0.2–44 Hz | |
| Gain: 68 dB | |
| ECG front-end circuit | Power supply: 3.3–5 V |
| RG1–RG2, R1, R2, R3–R5: 1 kΩ, 100 kΩ, 39 kΩ, 100 kΩ | |
| C1, C2: 6.8 µF, 0.1 µF | |
| A/D converter | Resolution: 10 bit |
| (Arduino Pro Mini) | Power supply: 3.3–5 V |
| Ag/AgCl | Wearable Prototype | ||||
|---|---|---|---|---|---|
| R-peak 1 | R-peak 2 | R-peak 3 | R-peak 4 | R-peak 5 | |
| R-peak 1 | 81.9% | 94.4% | 95.0% | 92.3% | 84.5% |
| R-peak 2 | 83.5% | 87.4% | 81.0% | 97.0% | 85.5% |
| R-peak 3 | 93.3% | 89.9% | 82.1% | 91.7% | 92.9% |
| R-peak 4 | 89.3% | 92.8% | 89.4% | 81.7% | 91.8% |
| R-peak 5 | 89.0% | 89.9% | 84.3% | 81.3% | 84.4% |
| Average: | 88.3% | Maximum: | 97.0% | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yapici, M.K.; Alkhidir, T.E. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors 2017, 17, 875. https://doi.org/10.3390/s17040875
Yapici MK, Alkhidir TE. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors. 2017; 17(4):875. https://doi.org/10.3390/s17040875
Chicago/Turabian StyleYapici, Murat Kaya, and Tamador Elboshra Alkhidir. 2017. "Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors" Sensors 17, no. 4: 875. https://doi.org/10.3390/s17040875
APA StyleYapici, M. K., & Alkhidir, T. E. (2017). Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors, 17(4), 875. https://doi.org/10.3390/s17040875







