Plasmonic Optical Fiber-Grating Immunosensing: A Review
Abstract
:1. Introduction
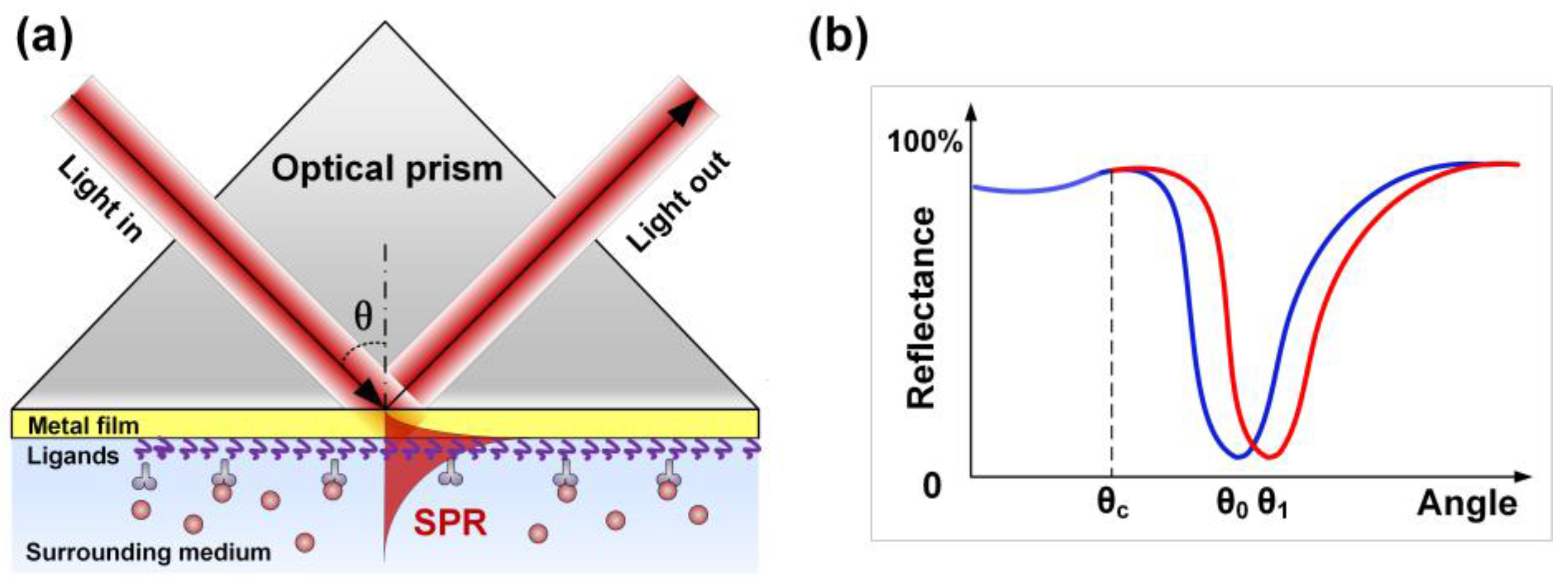
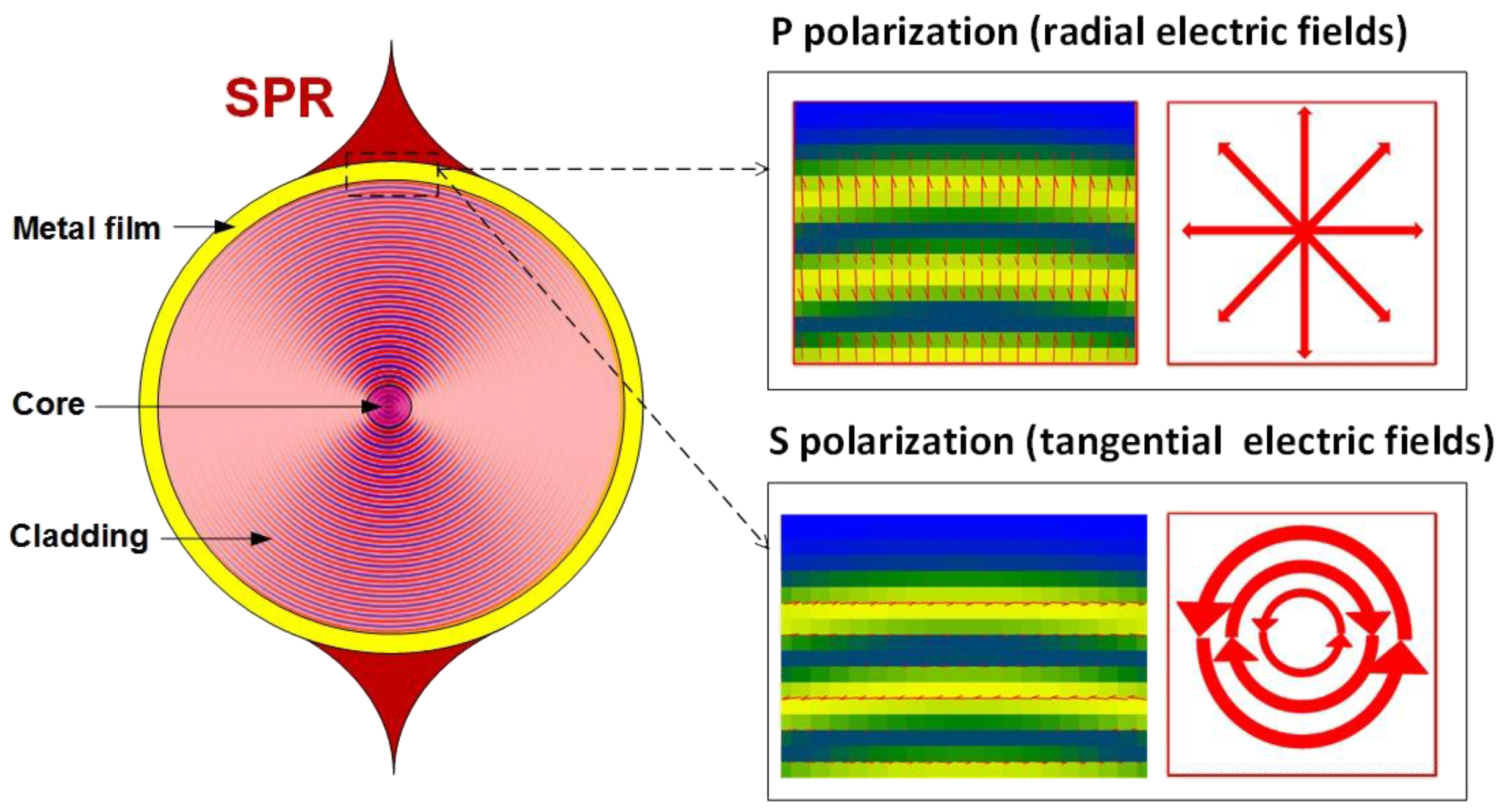
2. Review of Grating Configurations Used for SPR Excitation
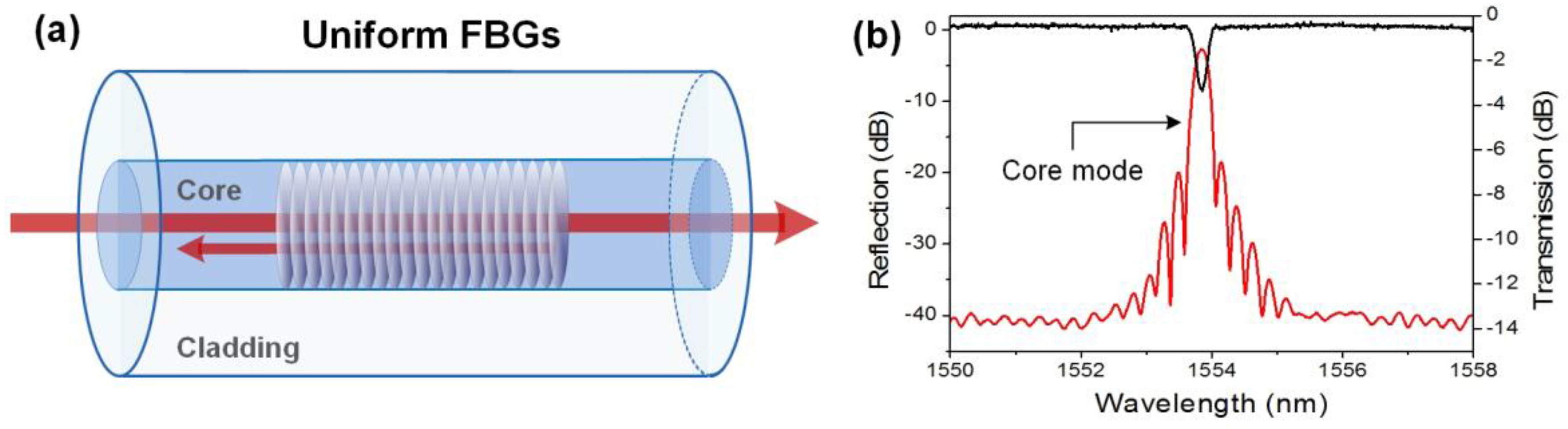
2.1. Unclad Uniform Fiber Bragg Gratings
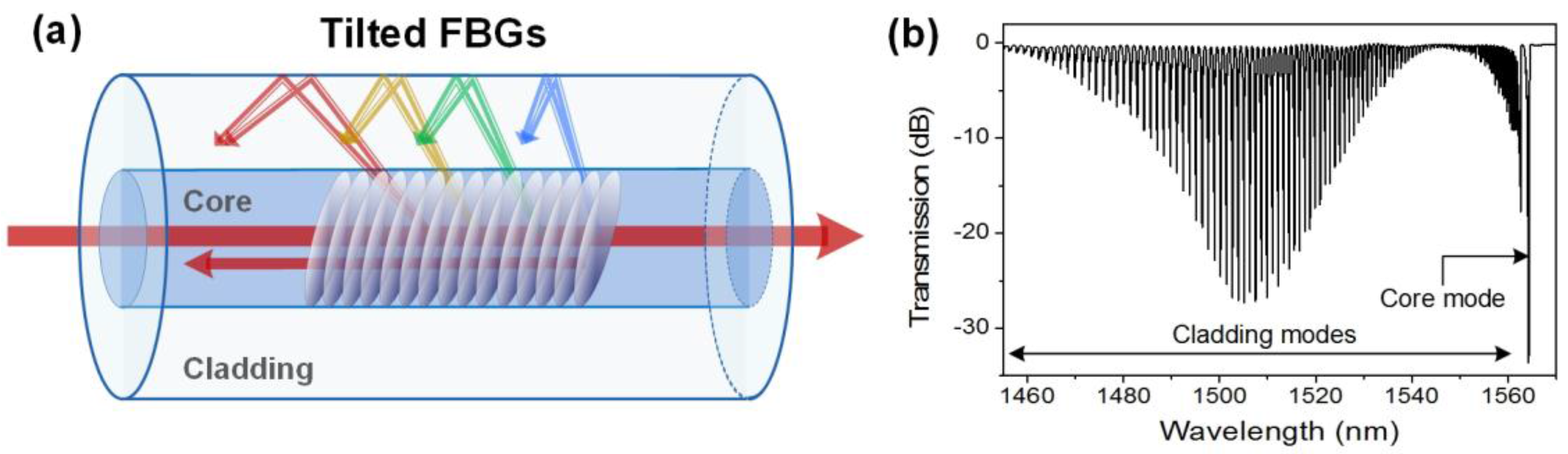
2.2. Tilted-Fiber Bragg Gratings
2.3. Excessively Tilted Fiber Gratings
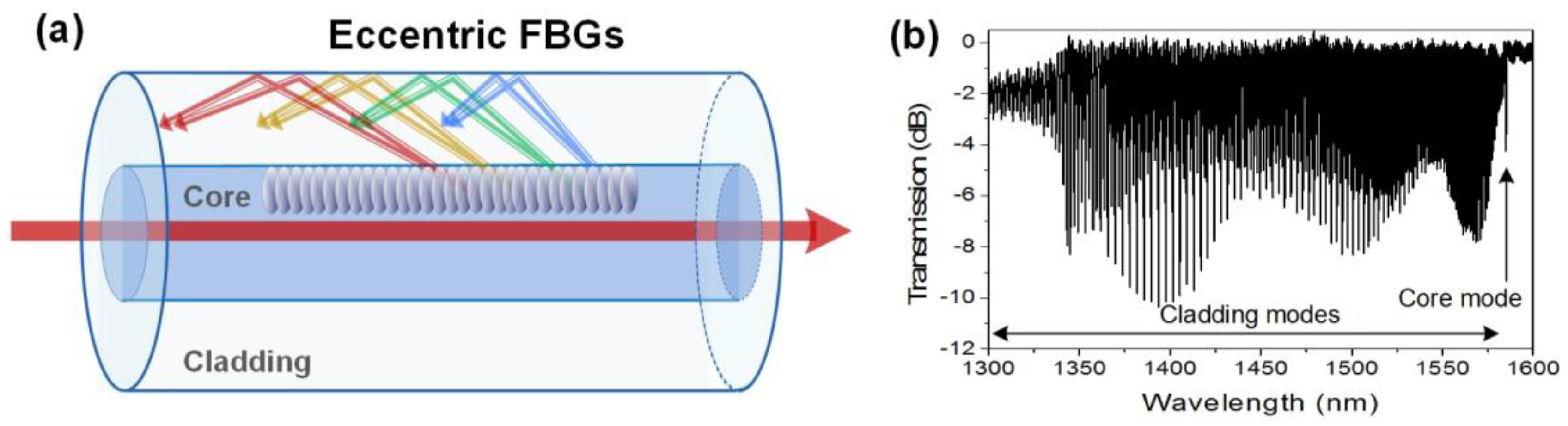
2.4. Eccentric Fiber Bragg Gratings
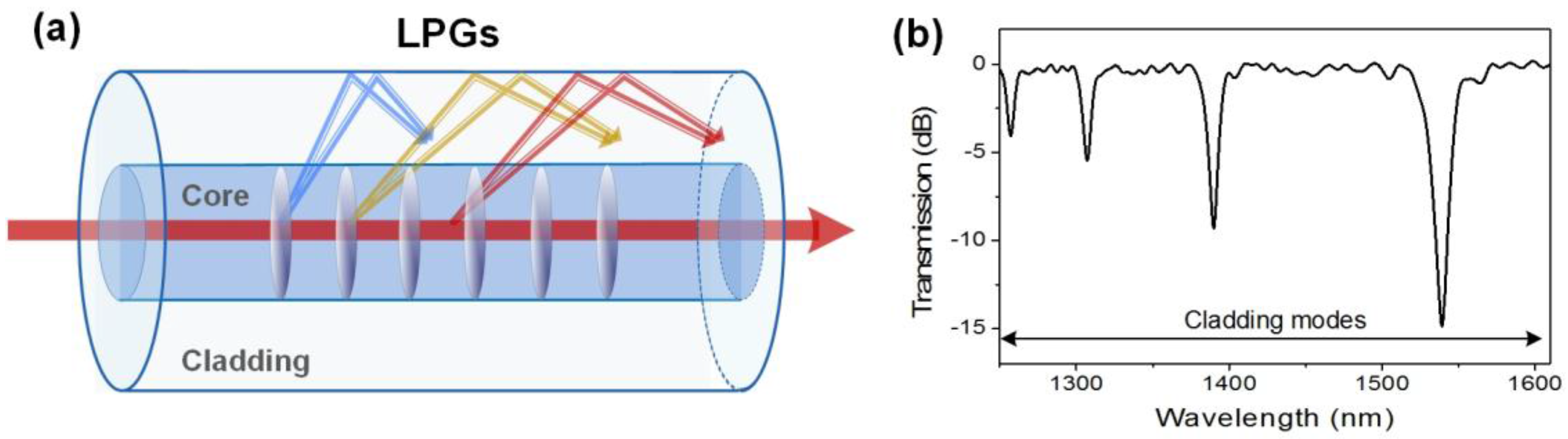
2.5. Long-Period Fiber Gratings
2.6. Additional Considerations
3. Interactions with Metals and Surface Biochemical Functionalization
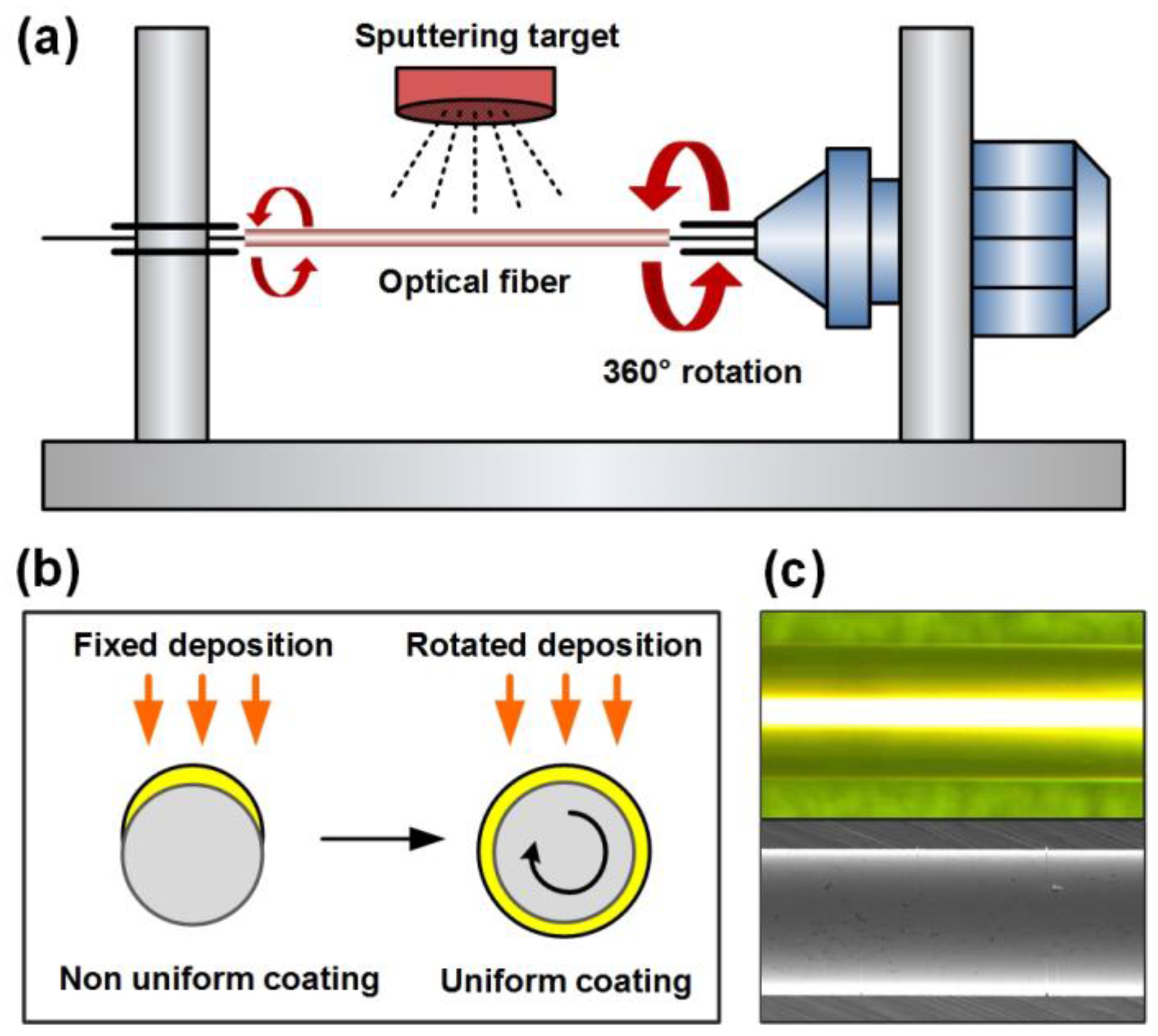
3.1. Metal Layer Deposition
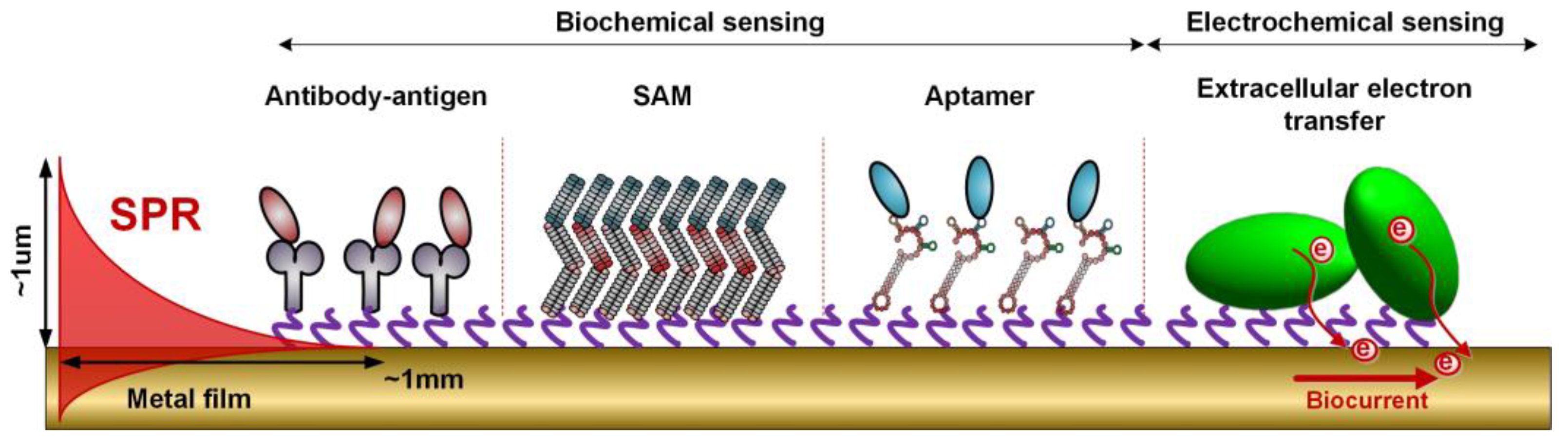
3.2. Surface Biofunctionalization
4. Interrogation of Plasmonic Fiber-Grating (Bio)chemical Sensors
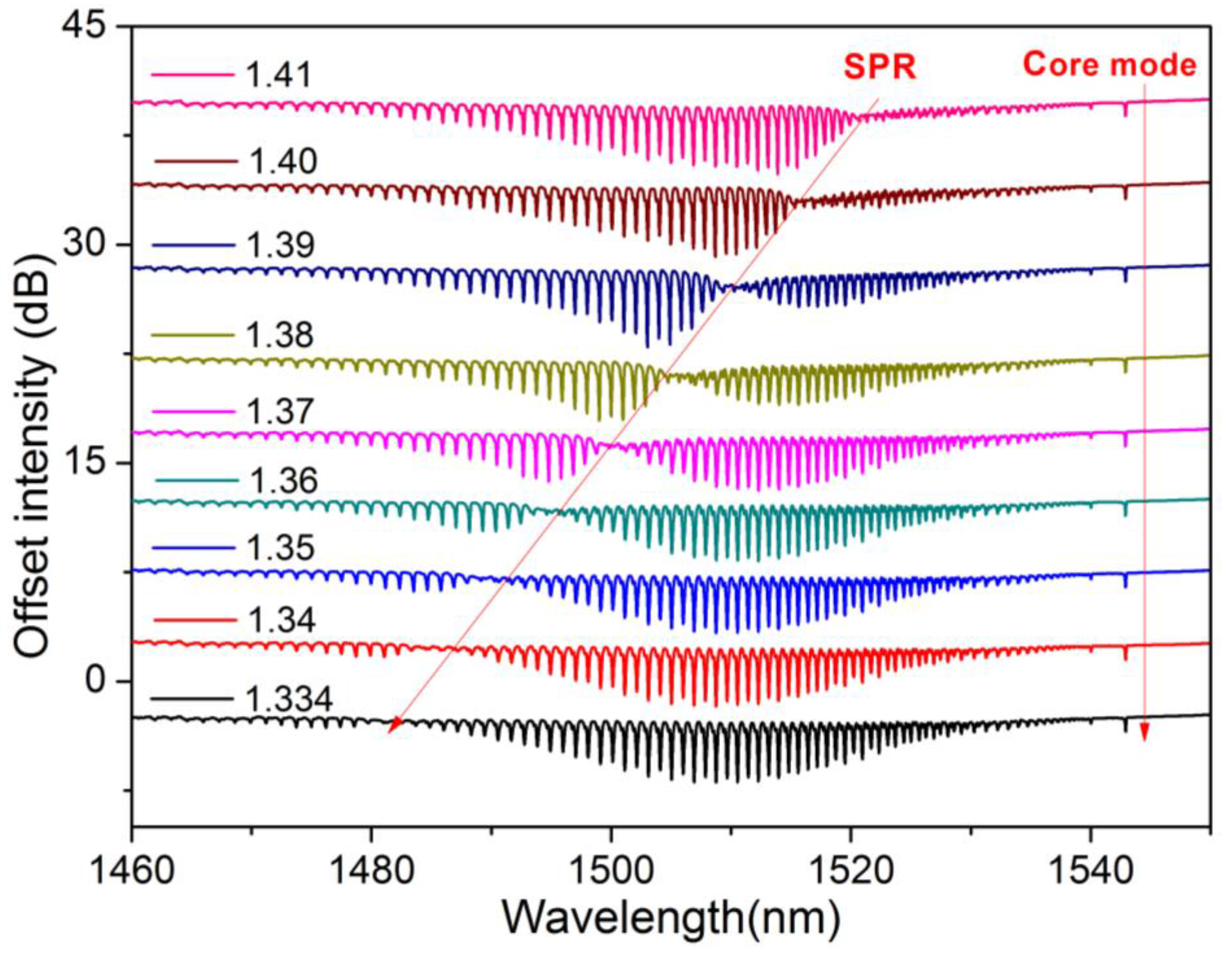
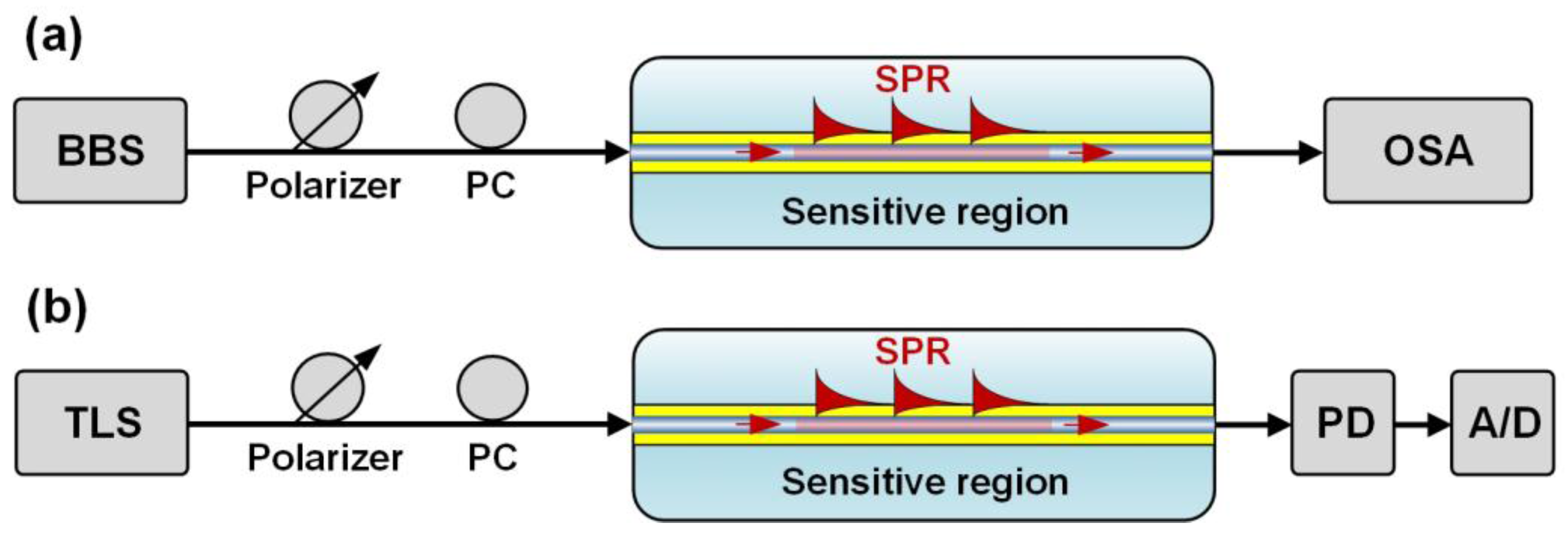
4.1. Spectrometer-Based Interrogation
4.2. Intensity or Optical Power-Based Interrogation
4.3. Other Interrogation Techniques
5. Protein and Cell Detection and Quantification
5.1. Overview of Plasmonic Fiber-Grating Immunosensors
5.2. Detection of Cancer Biomarkers
- (1)
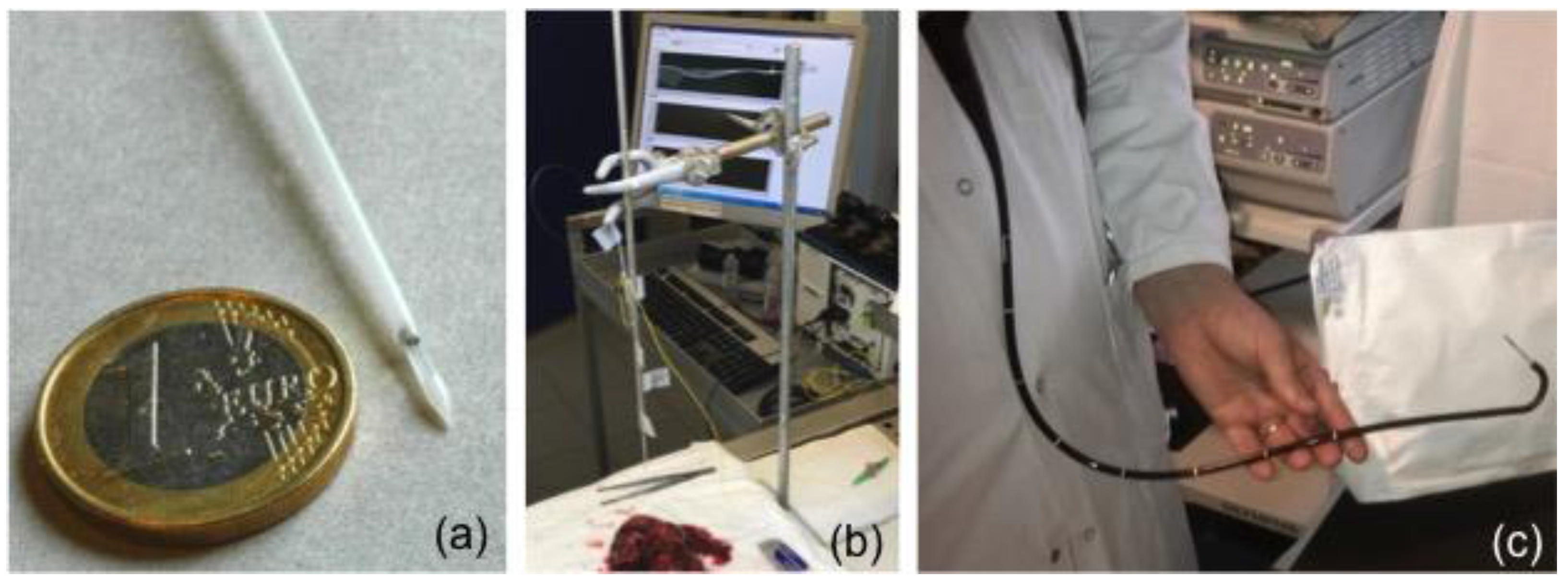
- Mirror deposition on the fiber cross-section to operate in reflection mode. Optical fibers containing gratings are cleaved beyond the grating location to use them in reflection, through the use of a silver mirror deposited on the cleaved fiber end face. This can be as simple as using a silver glue.
- (2)
- Grating insertion into a specially-designed protective packaging allowing it to be used in various media. In [102], a packaging was made of a hollow cylindrical needle manufactured in polyoxymethylene C2521 Hostaform. As depicted in Figure 12, this packaging provides a window to expose the sensor location to the surrounding medium. It has been tailored for possible insertion in the operating channel of an endoscope.
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kretschmann, E.; Raether, H. Radiative decay of non radiative surface plasmon excited by light. Z. Naturforsch. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Hecht, B.; Bielefeldt, H.; Novotny, L.; Inouye, Y.; Pohl, D.W. Local excitation, scattering, and interference of surface plasmons. Phys. Rev. Lett. 1996, 77, 1889–1892. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.F. Bending effects in optical fibers. J. Lightwave Technol. 1984, LT-2, 617–628. [Google Scholar] [CrossRef]
- Gupta, B.D.; Dodeja, H.; Tomar, A.K. Fibre-optic evanescent field absorption sensor based on a U-shaped probe. Opt. Quantum Electron. 1996, 28, 1629–1639. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-optic sensors based on surface plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Gupta, B.D.; Verma, R.K. Surface Plasmon resonance-based fiber optic sensors: Principle, probe designs and some applications. J. Sens. 2009, 2009, 979761. [Google Scholar] [CrossRef]
- Pollet, J.; Delport, F.; Janssen, K.P.F.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA-protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Brenci, M.; Chiavaioli, F.; Gianetti, A.; Trono, C. Optical fiber gratings as tools for chemical and biochemical sensing. Anal. Bioanal. Chem. 2012, 402, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Guo, T.; Albert, J. Review of recent plasmonic fiber optic biochemical sensors: Improving the limit of detection. Anal. Bioanal. Chem. 2015, 407, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, R.C.; Yee, S.S. A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Actuators B 1993, 12, 213–220. [Google Scholar] [CrossRef]
- Othonos, A.; Kalli, K. Fiber Bragg Gratings: Fundamentals and Applications in Telecommunications and Sensing; Artech House: London, UK, 1999. [Google Scholar]
- Erdogan, T. Fiber grating spectra. J. Lightwave Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef]
- Carver, G.E.; Farkas, D.L.; Porque, J.; Feder, K.S.; Westbrook, P.S. Visible wavelength fiber Bragg grating arrays for high speed biomedical spectral sensing. In Proceedings of the Advanced Photonics & Renewable Energy Congress, Karlsruhe, Germany, 21–24 June 2010. OSA Technical Digest; Paper BthB5. [Google Scholar]
- Becker, M.; Elsmann, T.; Schwuchow, A.; Rothhardt, M.; Dochow, S.; Bartelt, H. Fiber Bragg gratings in the visible spectral range with ultraviolet femtosecond laser inscription. IEEE Photonics Technol. Lett. 2014, 26, 1653–1656. [Google Scholar] [CrossRef]
- Malo, B.; Hill, K.O.; Bilodeau, F.; Johnson, D.C.; Albert, J. Point-by-point fabrication of micro-Bragg gratings in photosensitive fiber using single excimer pulse refractive-index modification techniques. Electron. Lett. 1993, 29, 1668–1669. [Google Scholar] [CrossRef]
- Marshall, G.D.; Williams, R.J.; Jovanovic, N.; Steel, M.J.; Withford, M.J. Point-by-point written fiber-Bragg gratings and their application in complex grating designs. Opt. Express 2010, 18, 19844–19859. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.O.; Malo, B.; Bilodeau, F.; Johnson, D.C.; Albert, J. Bragg grating fabricated in monomode photosensitive optical fiber by UV exposure through a phase mask. Appl. Phys. Lett. 1993, 62, 1035–1037. [Google Scholar] [CrossRef]
- Meltz, G.; Morey, W.W.; Glen, W.H. Formation of Bragg gratings in optical fibers by a transverse holographic method. Opt. Lett. 1989, 14, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Limberger, H.G.; Fonjallaz, P.Y.; Lambelet, P.; Salathe, R.P.; Zimmer, C.; Gilgen, H.H. Optical low-coherence reflectometry (OLCR) characterization of efficient Bragg gratings in optical fiber. SPIE Proc. 1993, 2044, 272–283. [Google Scholar]
- Hill, K.; Meltz, G. Fiber Bragg grating technology: Fundamentals and overview. J. Lightwave Technol. 1997, 15, 1263–1276. [Google Scholar] [CrossRef]
- Lyons, E.R.; Lee, H.P. Demonstration of an etched cladding fiber Bragg grating filter with reduced tuning force requirement. IEEE Photonics Technol. Lett. 1999, 11, 1626–1628. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Cong, J.; Xu, J.; Chen, K. Demonstration of etched cladding fiber Bragg grating-based sensors with hydrogel coating. Sens. Actuators B Chem. 2003, 96, 468–472. [Google Scholar] [CrossRef]
- Iadicicco, A.; Cusano, A.; Cutolo, A.; Bernini, R.; Giordano, M. Thinned fiber Bragg gratings as high sensitivity refractive index sensor. IEEE Photonics Technol. Lett. 2004, 16, 1149–1151. [Google Scholar] [CrossRef]
- Zhou, K.M.; Chen, X.F.; Zhang, L.; Bennion, I. Implementation of optical chemsensors based on HF-etched fiber Bragg grating structures. Meas. Sci. Technol. 2006, 17, 1140–1145. [Google Scholar] [CrossRef]
- Chen, N.; Yun, B.; Wang, Y.; Cui, Y. Theoretical and experimental study on etched fiber Bragg grating cladding mode resonances for ambient refractive index sensing. J. Opt. Soc. Am. B 2007, 24, 439–445. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, Z.M.; Shao, L.Y.; Cen, K.F.; Sheng, D.R.; Chen, J.; Zhou, H. Design and characteristics of refractive index sensor based on thinned and microstructure fiber Bragg grating. Appl. Opt. 2008, 47, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Kim, I.S.; Lee, C.H.; Lee, J. A temperature-insensitive cladding-etched fiber Bragg grating using a liquid mixture with a negative thermo-optic coefficient. Sensors 2012, 12, 7886–7892. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.B.; Zhao, M.F.; Zhou, X.J.; Shi, S.H.; Han, X.; Wang, Y. Etched fiber Bragg grating for refractive index distribution measurement. Optik 2013, 124, 2777–2780. [Google Scholar] [CrossRef]
- Schroeder, K.; Ecke, W.; Mueller, R.; Willsch, R.; Andreev, A. A fibre Bragg grating refractometer. Meas. Sci. Technol. 2001, 12, 757. [Google Scholar] [CrossRef]
- Tien, C.L.; Chen, H.W.; Liu, W.F.; Jyu, S.S.; Lin, S.W.; Lin, Y.S. Hydrogen sensor based on side-polished fiber Bragg gratings coated with thin palladium film. Thin Solid Films 2008, 516, 5360–5363. [Google Scholar] [CrossRef]
- Yang, M.; Dai, J.; Li, X.; Wang, J. Side-polished fiber Bragg grating refractive index sensor with TbFeCo magnetoptic thin film. J. Appl. Phys. 2010, 108, 033102. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Chen, Y.; Cao, K.; Liao, H.; Zhang, P. Side-polished fiber Bragg grating hydrogen sensor with WO3-Pd composite film as sensing materials. Opt. Express 2011, 19, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wang, Q.; Xu, L.; Liu, S.; He, J.; Zhao, J.; Li, Z.; Wang, Y. D-shaped fiber grating refractive index sensor induced by an ultrashort pulse laser. Appl. Opt. 2016, 55, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Si, G.Y.; Luan, F.J.; Xu, K.; Qi, Y.W.; Li, H.N. Recent research progress of optical fiber sensors based on D-shaped structure. Opt. Laser Technol. 2017, 90, 149–157. [Google Scholar] [CrossRef]
- Erdogan, T.; Sipe, J.E. Tilted fiber phase gratings. J. Opt. Soc. Am. A 1996, 13, 296–313. [Google Scholar] [CrossRef]
- Laffont, G.; Ferdinand, P. Tilted short-period fiber Bragg grating induced coupling to cladding modes for accurate refractometry. Meas. Sci. Technol. 2001, 12, 765–772. [Google Scholar] [CrossRef]
- Caucheteur, C.; Mégret, P. Demodulation technique for weakly tilted fiber Bragg grating refractometer. IEEE Photonics Technol. Lett. 2005, 17, 2703–2705. [Google Scholar] [CrossRef]
- Chan, C.F.; Chen, C.; Jafari, A.; Laronche, A.; Thomson, D.J.; Albert, J. Optical fiber refractometer using narrowband cladding-mode resonance shifts. Appl. Opt. 2007, 46, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Albert, J. Stain-optic coefficients of individual cladding modes of single mode fibre: Theory and experiment. Electron. Lett. 2006, 42, 1027–1028. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Liu, F.; Guan, B.O.; Albert, J. Ultrasensitive plasmonic sensing in air using optical fibre spectral combs. Nat. Commun. 2016, 7, 13371. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Zhang, X.; Guo, T.; Guan, B.O. Wide range refractive index measurement using a multi-angle tilted fiber Bragg grating. IEEE Photonics Technol. Lett. 2017, 29, 719–722. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, L.; Chen, X.; Bennion, I. Low thermal sensitivity grating devices based on ex-45° tilting structure capable of forward-propagating cladding modes coupling. J. Lightwave Technol. 2006, 24, 5087–5094. [Google Scholar] [CrossRef]
- Suo, R.; Chen, X.; Zhou, K.; Zhang, L.; Bennion, I. In-fibre directional transverse loading sensor based on excessively tilted fibre Bragg gratings. Meas. Sci. Technol. 2009, 20, 034015. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, L.; Chen, X.; Bennion, I. Optic sensors of high refractive-index responsivity and low thermal cross sensitivity that use fiber Bragg gratings of >80° tilted structures. Opt. Lett. 2006, 31, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yin, G.; Zhou, K.; Wang, C.; Gan, X.; Zhao, J.; Zhang, L. Graphene-induced unique polarization tuning properties of excessively tilted fiber grating. Opt. Lett. 2016, 41, 5450–5453. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, H.; Wang, C.; Sun, Z.; Yin, G.; Zhou, K.; Wang, Y.; Zhao, W.; Zhang, L. Theoretical and experimental analysis of excessively tilted fiber Bragg gratings. Opt. Express 2016, 24, 12107–12115. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Jovanovic, N.; Becker, R.G.; Marshall, G.D.; Withford, M.J.; Tünnermann, A.; Nolte, S.; Steel, M.J. Cladding mode coupling in highly localized fiber Bragg gratings: Modal properties and transmission spectra. Opt. Express 2011, 19, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Jovanovic, N.; Krämer, R.G.; Marshall, G.D.; Withford, M.J.; Tünnermann, A.; Nolte, S.; Steel, M.J. Cladding mode coupling in highly localized fiber Bragg gratings II: Complete vectorial analysis. Opt. Express 2012, 20, 21434–21449. [Google Scholar] [CrossRef] [PubMed]
- Chah, K.; Voisin, V.; Kinet, D.; Caucheteur, C. Surface plasmon resonance in eccentric femtosecond-laser-induced fiber Bragg gratings. Opt. Lett. 2014, 39, 6887–6890. [Google Scholar] [CrossRef] [PubMed]
- Chah, K.; Kinet, D.; Caucheteur, C. Negative axial strain sensitivity in gold-coated eccentric fiber Bragg gratings. Sci. Rep. 2016, 6, 38042. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Qiao, X.; Albert, J. Off-axis ultraviolet-written fiber Bragg gratings for directional bending measurements. IEEE J. Lightwave Technol. 2017, 35, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Vengsarkar, A.M.; Lemaire, P.J.; Judkins, J.B.; Bhatia, V.; Erdogan, T.; Sipe, J.E. Long-period fiber gratings as band-rejection filters. J. Lightwave Technol. 1996, 14, 58–65. [Google Scholar] [CrossRef]
- Bhatia, V.; Vengsarkar, A.M. Optical fiber long-period grating sensors. Opt. Lett. 1996, 21, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Heather, J.P.; Alan, D.K.; Frank, B. Analysis of the response of long period fiber gratings to external index of refraction. J. Lightwave Technol. 1998, 16, 1606–1612. [Google Scholar]
- Chong, J.H.; Shum, P.; Haryono, H.; Yohana, A.; Rao, M.K.; Lu, C.; Zhu, Y. Measurements of refractive index sensitivity using long-period grating refractometer. Opt. Commun. 2004, 229, 65–69. [Google Scholar] [CrossRef]
- Tsuda, H.; Urabe, K. Characterization of long-period grating refractive index sensors and their applications. Sensors 2009, 9, 4559–4571. [Google Scholar] [CrossRef] [PubMed]
- Patrick, H.J.; Chang, C.; Vohar, S.T. Long period fibre gratings for structural bend sensing. Electron. Lett. 1998, 24, 1773–1775. [Google Scholar] [CrossRef]
- Cheng, S.F.; Chau, L.K. Colloidal gold-modified optical fiber for chemical and biochemical sensing. Anal. Chem. 2003, 75, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, O.; de la Pena, F.; Leary, R.K.; Holland, D.J.; Ducati, C.; Midgley, P.A. Three-dimensional imaging of localized surface plasmon resonances of metal nanoparticles. Nature 2013, 502, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.H.; Chen, H.J.; Kou, X.S.; Yeung, M.H.; Wang, J.F. Optical fiber-excited surface plasmon resonance spectroscopy of single and ensemble gold nanorods. J. Phys. Chem. C 2008, 112, 8105–8109. [Google Scholar] [CrossRef]
- Cao, J.; Tu, M.H.; Sun, T.; Grattan, K.T.V. Wavelength-based localized surface plasmon resonance optical fiber biosensor. Sens. Actuators B Chem. 2013, 181, 611–619. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Gouveia, C.A.J.; Jorge, P.A.S.; Baldini, F. Towards a uniform metrological assessment of grating-based optical fiber sensors: From refractometers to biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, J.; Ji, Q.; Zhang, Y.; Ruan, X.; Dai, Y.; Cai, Z. Turning the resonance of the excessively tilted LPFG assisted surface plasmon polaritons: Optimum design rules for ultra-sensitive refractometric sensor. IEEE Photonics J. 2017. to appear. [Google Scholar]
- Offermans, P.; Shaafsma, M.C.; Rodriguez, S.R.K.; Zhang, Y.; Crego-Calama, M.; Brongersma, S.H.; Rivas, J.G. Universal scaling of the figure of merit of plasmonic sensors. ACS Nano 2011, 5, 5151–5157. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, P.; Carotenuto, B.; Pisco, M.; Ricciardi, A.; Quero, G.; Consales, M.; Crescitelli, A.; Esposito, E.; Cusano, A. Lab on fiber technology for biological sensing applications. Laser Photonics Rev. 2016, 10, 922–961. [Google Scholar] [CrossRef]
- Lepinay, S.; Staff, A.; Ianoul, A.; Albert, J. Improved detection limits of protein optical fiber biosensors coated with gold nanoparticles. Biosensens. Bioelectron. 2014, 52, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.; Lin, Y.; Wei, J.; Bono, T.; Lindquist, R.G. An enhanced LSPR fiber-optic nanoprobe for ultrasensitive detection of protein biomarkers. Biosens. Bioelectron. 2014, 61, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, L.; Wang, A.; Huang, R.; Ding, L.; Su, R.; Qi, W.; He, Z. Bioinspired fabrication of optical fiber SPR sensors for immunoassays using polydopamine-accelerated electroless plating. J. Mater. Chem. C 2016, 4, 7554–7562. [Google Scholar] [CrossRef]
- Feng, D.; Zhou, W.; Qiao, X.; Albert, J. High resolution fiber optic surface plasmon resonance sensors with single-sided gold coatings. Opt. Express 2016, 24, 16454–16464. [Google Scholar] [CrossRef] [PubMed]
- Audino, R.; Destefanis, G.; Gorgellino, F.; Pollino, E.; Tamagno, S. Interface behaviour evaluation in Au/Cr, Au/Ti and Au/Pd/Ti thin films by means of resistivity and stylus measurements. Thin Solid Films 1975, 36, 343–347. [Google Scholar] [CrossRef]
- Guzman, L.; Miotello, A.; Checchetto, R.; Adami, M. Ion beam-induced enhanced adhesion of gold films deposited on glass. Surf. Coat. Technol. 2002, 159, 558–562. [Google Scholar] [CrossRef]
- Chen, W.; Tseng, Y.; Hsieh, S.; Liu, W. Silanization of solid surfaces via mercaptopropylsilatrane: A new approach of constructing gold colloid monolayers. R. Soc. Chem. Adv. 2014, 4, 46527–46535. [Google Scholar] [CrossRef]
- Švorčík, V.; Siegel, J.; Šutta, P.; Mistrík, J.; Janíček, P.; Worsch, P.; Kolská, Z. Annealing of gold nanostructures sputtered on glass substrate. Appl. Phys. A 2011, 102, 605–610. [Google Scholar] [CrossRef]
- Antohe, I.; Schouteden, K.; Goos, P.; Delport, F.; Spasic, D.; Lammertyn, J. Thermal annealing of gold coated fiber optic surfaces for improved plasmonic biosensing. Sens. Actuators B Chem. 2016, 229, 678–685. [Google Scholar] [CrossRef]
- Naik, G.V.; Kim, J.; Boltasseva, A. Oxides and nitrides as alternative plasmonic materials in the optical range. Opt. Express 2011, 1, 1090–1099. [Google Scholar] [CrossRef]
- Daems, D.; Peeters, B.; Delport, F.; Remans, T.; Lammertyn, J.; Spasic, D. Identification and quantification of celery allergens using fiber optic surface plasmon resonance PCR. Sensors 2017, 17, 1754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, R.; Zhou, X.; Shi, H. Free-energy-driven lock/open assembly-based optical DNA sensor for cancer-related microRNA detection with a shortened time-to-result. Appl. Mater. Interfaces 2017, 9, 25789–25795. [Google Scholar] [CrossRef] [PubMed]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Ta, D.T.; Guedens, W.; Vranken, T.; Vanschoenbeek, K.; Redeker, E.S.; Michiels, L.; Adriaensens, P. Enhanced biosensor platforms for detecting the atherosclerotic biomarker VCAM1 based on bioconjugation with uniformly oriented VCAM1-targeting nanobodies. Biosensors 2016, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, P.K. Non-antibody protein-based biosensors. Essays Biochem. 2016, 60, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, X.; Lu, Y.; Shan, D.; Xu, B.; He, M.; Shi, H.; Qian, Y. Facile screening of potential xenoestrogens by an estrogen receptor-based reusable optical biosensor. Biosens. Bioelectron. 2017, 97, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Wink, T.; Van Zuilen, S.J.; Bult, A.; Van Bennkom, W.P. Self-assembled monolayers for biosensors. Analyst 1997, 122, 43–50. [Google Scholar] [CrossRef]
- Klantsataya, E.; Jia, P.; Ebendorff-Heidepriem, H.; Monro, T.M.; François, A. Plasmonic fiber optic refractometric sensors: From conventional architectures to recent design trends. Sensors 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Voisin, V.; Albert, J. Polarized spectral combs probe optical fiber surface plasmons. Opt. Express 2013, 21, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Baiad, M.D.; Gagné, M.; Madore, W.J.; De Montigny, E.; Godbout, N.; Boudoux, C.; Kashyap, R. Surface plasmon resonance sensor interrogation with a double-clad fiber coupler and cladding modes excited by a tilted fiber Bragg grating. Opt. Lett. 2013, 38, 4911–4914. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, C.; Voisin, V.; Malachovská, V.; Dubois, V.; Mégret, P.; Wattiez, R.; Caucheteur, C. Small biomolecule immunosensing with plasmonic optical fiber grating sensor. Biosens. Bioelectron. 2016, 77, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ran, Y.; Wang, G. Label-free detection of cancer biomarkers using an in-line taper fiber-optic interferometer and a fiber Bragg grating. Sensors 2017, 17, 2559. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yang, M.; Yu, X.; Lu, H. Optical hydrogen sensor based on etched fiber Bragg grating sputtered with Pd/Ag composite film. Opt. Fiber Technol. 2013, 19, 26–30. [Google Scholar] [CrossRef]
- Sridevi, S.; Vasu, K.S.; Jayaraman, N.; Asokan, S.; Sood, A.K. Optical bio-sensing devices based on etched fiber Bragg gratings coated with carbon nanotubes and grapheme oxide along with a specific dendrimer. Sens. Actuators B Chem. 2014, 195, 150–155. [Google Scholar]
- Alwis, L.; Bremer, K.; Sun, T.; Grattan, K.T. Analysis of the characteristics of PVA-coated LPG-based sensors to coating thickness and changes in the external refractive index. IEEE Sens. J. 2013, 13, 1117–1124. [Google Scholar] [CrossRef]
- Wei, W.; Nong, J.; Zhang, G.; Tang, L.; Jiang, X.; Chen, N.; Luo, S.; Lan, G.; Zhu, Y. Graphene-based long-period fiber grating surface plasmon resonance sensor for high-sensitivity gas sensing. Sensors 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Schuster, T.; Herschel, R.; Neumann, N.; Schäffer, C.G. Miniaturized long-period fiber grating assisted surface plasmon resonance sensor. J. Lightwave Technol. 2012, 30, 1003–1008. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Duan, Z.; Liu, Z.; Liu, Z.; Wu, Z.; Gu, Y.; Sun, C.; Peng, W. A novel low-power-consumption all-fiber-optic anemometer with simple system design. Sensors 2017, 17, 2107. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.D.; Wu, C.W.; Chiang, C.C. Tilted fiber Bragg grating sensor with graphene oxide coating for humidity sensing. Sensors 2017, 17, 2129. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Xu, Y.; Wu, S.; Zhao, M.; Jiang, P.; Shi, S.; Zhang, Z.; Wang, Y.; Wang, L.; Liu, Y. A novel immunosensor based on excessively tilted fiber Bragg grating coated with gold nanospheres improves the detection limit of Newcastle disease virus. Biosens. Bioelectron. 2017, 100, 169. [Google Scholar] [CrossRef] [PubMed]
- Guo, T. Fiber grating assisted surface plasmon resonance for biochemical and electrochemical sensing. IEEE J. Lightwave Technol. 2017, 35, 3223–3333. [Google Scholar] [CrossRef]
- Guo, T.; Liu, F.; Guan, B.-O.; Albert, J. Tilted fiber grating mechanical and biochemical sensors. Opt. Laser Technol. 2016, 78, 19–33. [Google Scholar] [CrossRef]
- Burgmeier, J.; Feizpour, A.; Schade, W.; Reinhard, B.M. Plasmonic nanoshell functionalized etched fiber Bragg gratings for highly sensitive refractive index measurements. Opt. Lett. 2015, 40, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Hervás, J.; Tosi, D.; García-Miquel, H.; Barrera, D.; Fernández-Pousa, C.R.; Sales, S. KLT based interrogation technique for FBG multiplexed sensor tracking. IEEE J. Lightwave Technol. 2017, 35, 3387–3392. [Google Scholar] [CrossRef]
- Ribaut, C.; Loyez, M.; Larrieu, J.C.; Chevineau, S.; Lambert, P.; Remmelink, M.; Wattiez, R.; Caucheteur, C. Cancer biomarker sensing using packaged plasmonic optical fiber gratings: Towards in vivo diagnosis. Biosens. Bioelectron. 2017, 92, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chen, X.; Liu, F.; Guan, B.O.; Guo, T. Plasmonic fiber-optic refractometers based on a high Q-factor amplitude interrogation. IEEE Sens. J. 2016, 16, 5974–5978. [Google Scholar] [CrossRef]
- Xiong, Y.; Ren, N.; Wu, M.; Liang, H.; Han, J.; Yang, W. Sensitivity-enhanced FBG demodulation system with multi-sideband filtering method. Opt. Commun. 2017, 382, 246–252. [Google Scholar] [CrossRef]
- Zheng, J.; Dong, X.; Ji, J.; Su, H.; Shum, P.P. Power-referenced refractometer with tilted fiber Bragg grating cascaded by chirped grating. Opt. Commun. 2014, 312, 106–109. [Google Scholar] [CrossRef]
- Coelho, L.C.C.; de Almeida, J.M.; Moayyed, H.; Santos, J.L.; Viegas, D. Multiplexing of surface plasmon resonance sensing devices on etched single-mode fiber. J. Lightwave Technol. 2015, 33, 432–438. [Google Scholar] [CrossRef]
- González-Vila, Á.; Kinet, D.; Mégret, M.; Caucheteur, C. Narrowband interrogation of plasmonic optical fiber biosensors based on spectral combs. Opt. Laser Technol. 2017, 96, 141–146. [Google Scholar] [CrossRef]
- Bremer, K.; Roth, B. Fibre optic surface plasmon resonance sensor system designed for smartphones. Opt. Express 2015, 23, 17179–17184. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Guo, T.; Albert, J. Polarization-assited fiber Bragg grating sensors: Tutorial and review. J. Lightwave Technol. 2017, 35, 3311–3322. [Google Scholar] [CrossRef]
- Moayyed, H.; Leite, I.T.; Coelho, L.; Santos, J.L.; Viegas, D. Analysis of a plasmonic based optical fiber optrode with phase interrogation. Photonics Sens. 2016, 6, 221–233. [Google Scholar] [CrossRef]
- Caucheteur, C.; Shevchenko, Y.; Shao, L.Y.; Wuilpart, M.; Albert, J. High resolution interrogation of tilted fiber grating SPR sensors from polarization properties measurement. Opt. Express 2011, 19, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Bialiayeu, A.; Caucheteur, C.; Ahamad, N.; Ianoul, A.; Albert, J. Self-optimized metal coatings for fiber plasmonic by electroless deposition. Opt. Express 2011, 19, 18742–18753. [Google Scholar] [CrossRef] [PubMed]
- Renoirt, J.M.; Debliquy, M.; Albert, J.; Ianoul, A.; Caucheteur, C. Surface plasmon resonances in oriented silver nanowire coatings on optical fibers. J. Phys. Chem. C 2014, 118, 11035–11042. [Google Scholar] [CrossRef]
- Bette, S.; Caucheteur, C.; Wuilpart, M.; Mégret, P. Theoretical and experimental study of differential group delay and polarization dependent loss of Bragg gratings written in birefringent fiber. Opt. Commun. 2007, 269, 331–337. [Google Scholar] [CrossRef]
- DeLisa, M.P.; Zhang, Z.; Shiloach, M.; Pilevar, S.; Davis, C.C.; Sirkis, J.S.; Bently, W.E. Evanescent wave long-period fiber Bragg grating as an immobilized antibody sensor. Anal. Chem. 2000, 72, 2895–2900. [Google Scholar] [CrossRef] [PubMed]
- Chryssis, A.N.; Saini, S.S.; Lee, S.M.; Yi, H.; Bentley, W.E.; Dagenais, M. Detecting hybridization of DNA by highly sensitive evanescent field etched core fiber Bragg grating sensors. IEEE J. Sel. Top. Quantum Electron. 2005, 11, 864–872. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Pilla, P.; Trono, C.; Baldini, F.; Chiavaioli, F.; Giordano, M.; Cusano, A. Giant sensitivity of long period gratings in transition mode near the dispersion tuning point: An integrated design approach. Opt. Lett. 2012, 37, 4152–4154. [Google Scholar] [CrossRef] [PubMed]
- Chiavaioli, F.; Baldini, F.; Trono, C. Manufacturing and spectral features of different types of long period fiber gratings: Phase-shifted, turn-around point, internally tilted, and pseudo-random. Fibers 2017, 5, 29. [Google Scholar] [CrossRef]
- Quero, G.; Consales, M.; Severino, R.; Vaiano, P.; Boniello, A.; Sandomenico, A.; Ruvo, M.; Borriello, A.; Diodato, L.; Zuppolini, S.; et al. Long period fiber grating nano-optrode for cancer biomarker detection. Biosens. Bioelectron. 2016, 80, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.; Hernandez, F.U.; James, S.W.; Morgan, S.P.; Clark, M.; Tatam, R.P.; Korposh, S. Highly sensitive optical fibre long period grating biosensor anchored with silica core gold shell nanoparticles. Biosens. Bioelectron. 2016, 75, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Cheng, S.F.; Hsu, W.T.; Chiang, T.Y.; Chau, L.K. Fiber-optic biochemical sensing with a colloidal gold-modified long period fiber grating. Sens. Actuators B Chem. 2006, 119, 105–109. [Google Scholar] [CrossRef]
- Shevchenko, Y.; Francis, T.J.; Blair, D.A.D.; Walsh, R.; DeRosa, M.C.; Albert, J. In situ biosensing with a surface plasmon resonance fiber grating aptasensor. Anal. Chem. 2011, 83, 7027–7034. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Lepinay, S.; Caucheteur, C.; Derosa, M.C. High resolution grating-assisted surface plasmon resonance fiber optic aptasensor. Methods 2013, 63, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Voisin, V.; Pilate, J.; Damman, P.; Mégret, M.; Caucheteur, C. Highly sensitive detection of molecular interactions with plasmonic optical fiber grating sensors. Biosens. Bioelectron. 2014, 51, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, Y.; Camci-Unal, G.; Cuttica, D.F.; Dokmeci, M.R.; Albert, J.; Khademhosseini, A. Surface plasmon resonance fiber sensor for real-time and label-free monitoring of cellular behavior. Biosens. Bioelectron. 2014, 56, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Qian, S.; Liu, Z.; Wang, Q.; Gu, Y.; Wu, Z.; Jing, Z.; Sun, C.; Peng, W. A novel fiber optic surface plasmon resonance biosensors with special boronic acid derivative to detect glycoprotein. Sensors 2017, 17, 2259. [Google Scholar] [CrossRef] [PubMed]
- Malachovska, V.; Ribaut, C.; Voisin, V.; Surin, M.; Leclère, P.; Wattiez, R.; Caucheteur, C. Fiber-optic SPR immunosensors tailored to target epithelial cells through membrane receptors. Anal. Chem. 2015, 87, 5957–5965. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Liu, F.; Liang, X.; Qiu, X.; Huang, Y.; Xie, C.; Xu, P.; Mao, W.; Guan, B.O.; Albert, J. Highly sensitive detection of urinary protein variations using tilted fiber grating sensors with plasmonic nanocoatings. Biosens. Bioelectron. 2016, 78, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Guo, T.; Xie, C.; Xu, P.; Lao, J.; Zhang, X.; Xu, J.; Chen, X.; Huang, Y.; Liang, X.; Mao, W.; Guan, B.O. Specific detection of aquaporin-2 using plasmonic tilted fiber grating sensors. J. Lightwave Technol. 2017, 35, 3360–3365. [Google Scholar] [CrossRef]
- Candiani, A.; Bertucci, A.; Giannetti, S.; Konstantaki, M.; Manicardi, A.; Pissadakis, S.; Cucinotta, A.; Corradini, R.; Selleri, S. Label-free DNA biosensor based on peptide nucleic acid-functionalized microstructured optical fiber-Bragg grating. J. Biomed. Opt. 2013, 18, 057004. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, F.; Geernaert, T.; Baghdasaryan, T.; Thienpont, H. Challenges in the fabrication of fibre Bragg gratings in silica and polymer microstructured optical fibres. Laser Photonics Rev. 2014, 8, 27–52. [Google Scholar] [CrossRef]
- Hu, X.; Mégret, P.; Caucheteur, C. Surface plasmon excitation at near-infrared wavelengths in polymer optical fibers. Opt. Lett. 2015, 40, 3998–4001. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, A.; Polis, M.; Theodosiou, A.; Koutsides, C.; Kalli, K. Femtosecond laser inscribed Bragg gratings in low loss cytop polymer optical fiber. IEEE Photonics Technol. Lett. 2015, 27, 693–696. [Google Scholar] [CrossRef]
- Tow, K.H.; Chow, D.M.; Vollrath, F.; Dicaire, I.; Gheysens, T.; Thévenaz, L. Exploring the use of native spider silk as an optical fibre for chemical sensing. J. Lightwave Technol. 2017. to appear. [Google Scholar]
- Trevisanutto, J.O.; Linhananta, A.; Das, G. Plasmonic structure: Fiber grating formed by gold nanorods on a tapered fiber. Opt. Lett. 2016, 41, 5789–5792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Liu, Z.; Duan, Z.; Cui, W.; Han, J.; Gu, Y.; Wu, Z.; Jing, Z.; Sun, C.; et al. Fiber-optics anemometer based on single-walled carbon nanotube coated tilted fiber Bragg grating. Opt. Express 2017, 25, 24521–24530. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, B.; Zhang, A.; Rao, Y.J.; Wang, Z.; Cheng, Y.; Gong, Y.; Zhang, W.; Chen, Y.; Chiang, K.S. Graphene-coated microfiber Bragg grating for high sensitivity gas sensing. Opt. Lett. 2014, 39, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Arasu, P.T.; Noor, A.S.M.; Shabaneh, A.A.; Yaacob, M.H.; Lim, H.H.; Mahdi, M.A. Fiber Bragg grating assisted surface plasmon resonance sensor with graphene oxide sensing layer. Opt. Commun. 2016, 380, 260–266. [Google Scholar] [CrossRef]
- Grigorenko, A.N.; Polini, M.; Novoselov, K.S. Graphene plasmonics. Nat. Photonics 2012, 6, 749–758. [Google Scholar] [CrossRef]
- Gang, X.; Wang, Y.; Zhang, F.; Zhao, C.; Jiang, B.; Fang, L.; Li, D.; Wu, H.; Ren, Z.; Zhao, J. Graphene-controlled fiber Bragg grating and enabled optical bistability. Opt. Lett. 2016, 41, 603–606. [Google Scholar]

| Grating Architecture | Functional Materials | Analyte and Sensor Performances | Ref. |
|---|---|---|---|
| LPFG | SiO2:Au NPs modified with biotin | Streptavidin detection Sensitivity: 6.88 nm/(ng/mm2) | [121] |
| LPFG | Self-assembled Au colloids + dinitrophenyl compound (DNP) | Detection of anti-DNP LOD: 950 pM | [122] |
| TFBG | Au layer + thiol-modified aptamers | Thrombin detection in buffer and serum solutions LOD: 22 nM | [123,124] |
| TFBG | Au layer + self-assembled monolayer (SAM) + anti-transferrins | Transferrin detection LOD: 10−6 g/mL | [125] |
| TFBG | Au layer + fibronectin | Analysis of cellular behavior under different stimuli | [126] |
| TFBG | APTMS, glutaraldehyde and cysteamine thin films + Au nanocages/nanospheres | Biotin detection LOD: 11 pM (nanospheres)–8 pM (nanocages) | [68] |
| TFBG | Au layer + boronic acid | Glycoprotein detection LOD: 2 × 10−5 g/mL | [127] |
| TFBG | Au layer + SAM + anti-cytokeratins + bovine serum albumin (BSA) | Detection of cytokeratins 7 and 17 for lung cancer diagnosis LOD: 1 pM | [88,102] |
| TFBG | Au layer + SAM + EGFR (epidermal growth factor receptor) antibodies | Detection of epithelial cells through their EGFR LOD: 3 × 106 cells/mL | [128] |
| TFBG | Au layer with different thicknesses | Detection of proteinuria in rat urine LOD: 1.5 × 10−3 mg/mL | [129] |
| TFBG | Au layer + SAM + aquaporin-2 antibodies | Detection of aquaporin-2 for nephrotic syndrome analysis LOD: 1.5 ng/mL | [130] |
| ETFG | Au NPs + cysteamine + activated staphylococcal protein A | Detection of Newcastle disease virus LOD: 25 pg/mL in a 200 µL volume | [97] |
| FBG | Oligonucleotide-functionalized Au NPs | DNA target sequences | [131] |
| Stage | Generic Process | Practical Implementation in [88,102] |
|---|---|---|
| 1. Grating manufacturing and optimization | – Local (mechanical or chemical) stripping of the polymer jacket of a photosensitive or hydrogen-loaded standard single mode fiber. – Use of dedicated laser and technique to photo-inscribe a grating in the stripped region. – Thermal annealing (in the case of hydrogen-loaded fibers) to stabilize the grating spectrum. | – 1 cm long 7° TFBGs in hydrogen-loaded standard telecommunication-grade single-mode optical fibers. – Use of a frequency-doubled argon laser emitting 60 mW at 244 nm and the phase mask technique. – Thermal annealing at 100 °C during 24 h. |
| 2. Metal deposition and optimization | – Surface fiber cleaning with ethanol and/or piranha solution to remove contaminants. – Metal deposition using one of the techniques described in Section 3.1 (+use of a buffer layer or thermal annealing to improve gold adherence). | – ~50 nm gold coating deposited around the TFBGs using a sputtering process (thickness measured with a built-in Quartz microbalance). – Two depositions with 90° rotation between each. – Thermal annealing during 2 h at 200 °C. – Washing using absolute ethanol and dried under N2. |
| 3. Biochemical functionalization | – Metal surface cleaning, usually with absolute ethanol. – Covalent immobilization of bioreceptors. This step strongly depends on the targets, as described in Section 3.2. – Deposition of blocking agents (most often bovine serum albumin (BSA) or milk caseins) to avoid unspecific interactions. – Rinsing to remove all non-linked molecules. | – Surface cleaning with absolute ethanol. – SAM of S2PEG6COOH alkanethiols on the gold surface. – Surface activation using NHS/EDC process. – Anchoring of anti-CK17 antibodies by immersion in a pH-controlled solution. – Deposition of BSA (5 % w/v in phosphate-buffered saline (PBS)). – Rinsing with PBS. |
| 4. Interrogation and data-processing | – Splicing of the grating to fiber pigtails. – Connection to a measurement set-up including polarization control to record the reflected/transmitted amplitude spectrum (remote computer control for real-time operation). – Data-processing based on the tracking of the SPR mode as a function of time. | – Use of a MicronOptics FBG interrogator and a polarization controller, allowing to record spectral measurements at 10 Hz rate with 1 pm wavelength resolution). – Insertion of the sensors in various complex media (PBS + serum, gel matrices and fresh biopsied lung tissues). – Record of the amplitude spectrum and computation of the wavelength shift and amplitude variation of the most sensitive cladding mode resonance in the P-polarized spectrum. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, T.; González-Vila, Á.; Loyez, M.; Caucheteur, C. Plasmonic Optical Fiber-Grating Immunosensing: A Review. Sensors 2017, 17, 2732. https://doi.org/10.3390/s17122732
Guo T, González-Vila Á, Loyez M, Caucheteur C. Plasmonic Optical Fiber-Grating Immunosensing: A Review. Sensors. 2017; 17(12):2732. https://doi.org/10.3390/s17122732
Chicago/Turabian StyleGuo, Tuan, Álvaro González-Vila, Médéric Loyez, and Christophe Caucheteur. 2017. "Plasmonic Optical Fiber-Grating Immunosensing: A Review" Sensors 17, no. 12: 2732. https://doi.org/10.3390/s17122732
APA StyleGuo, T., González-Vila, Á., Loyez, M., & Caucheteur, C. (2017). Plasmonic Optical Fiber-Grating Immunosensing: A Review. Sensors, 17(12), 2732. https://doi.org/10.3390/s17122732







