Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers †
Abstract
1. Introduction
2. SAW Overview
2.1. SAW Design and Operation
2.2. Piezoelectric Materials and Their Cutting Angles
2.3. Temperature Dependicies
2.4. Operation in Liquid Mediums
2.5. Resonating Frequency and Senstivity Relationship
2.6. SAW RFID-Tags
3. Chemical Recognition Layers
3.1. Host-Guest Strategies
3.2. Metal Oxide Nanofilms and Composites
3.3. Carbon-Based Nanomaterials
3.4. Functional Polymeric Layers
3.5. Biological Receptors
4. Challenges and Opportunities
4.1. Device-Related Issues
4.2. Interfacial Receptors
4.3. Emerging Trends and Opportunities
5. Summary and Outlook
Author Contributions
Conflicts of Interest
References
- Pohl, A. A review of wireless SAW sensors. IEEE Trans. Ultrason. Ferroelectr. 2000, 47, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Reindl, L.; Scholl, G.; Ostertag, T.; Ruppel, C.; Bulst, W.-E.; Seifert, F. In SAW devices as wireless passive sensors. In Proceedings of the Ultrasonics Symposium, San Antonio, TX, USA, 3–6 November 1996. [Google Scholar]
- Buff, W.; Plath, F.; Schmeckebier, O.; Rusko, M.; Vandahl, T.; Luck, H.; Moller, F.; Malocha, D. Remote sensor system using passive SAW sensors. In Proceedings of the Ultrasonics Symposium, Cannes, France, 31 October–3 November 1994; pp. 585–588. [Google Scholar]
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw. Theory 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Lee, B. Review of the present status of optical fiber sensors. Opt. Fiber Technol. 2003, 9, 57–79. [Google Scholar] [CrossRef]
- Caruso, M.J. Applications of magnetic sensors for low cost compass systems. In Proceedings of the Position Location and Navigation Symposium, San Diego, CA, USA, 13–16 March 2000; pp. 177–184. [Google Scholar]
- Lenz, J.; Edelstein, S. Magnetic sensors and their applications. IEEE Sens. J. 2006, 6, 631–649. [Google Scholar] [CrossRef]
- Ryu, S.; Yoo, I.; Song, S.; Yoon, B.; Kim, J.-M. A thermoresponsive fluorogenic conjugated polymer for a temperature sensor in microfluidic devices. J. Am. Chem. Soc. 2009, 131, 3800–3801. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, C.; Wang, D.; Liu, S. Fret-derived ratiometric fluorescent K+ sensors fabricated from thermoresponsive poly (n-isopropylacrylamide) microgels labeled with crown ether moieties. J. Phys. Chem. B 2010, 114, 12213–12220. [Google Scholar] [CrossRef] [PubMed]
- Casalinuovo, I.; Pierro, D.; Bruno, E.; Francesco, P.; Coletta, M. Experimental use of a new surface acoustic wave sensor for the rapid identification of bacteria and yeasts. Lett. App. Microbiol. 2006, 42, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Curie, J.; Curie, P. Development by pressure of polar electricity in hemihedral crystals with inclined faces. Bull. Soc. Min. Fr. 1880, 3, 90–102. [Google Scholar]
- Curie, J.; Curie, P. Contractions et dilatations produites par des tensions électriques dans les cristaux hémièdres à faces inclinées. Compt. Rend. 1881, 93, 1137–1140. (In French) [Google Scholar]
- Lippmann, G. Principe de la conservation de l’électricité, ou second principe de la théorie des phénomènes électriques. J. Phys. Théor. Appl. 1881, 10, 381–394. (In French) [Google Scholar] [CrossRef]
- White, R.; Voltmer, F. Direct piezoelectric coupling to surface elastic waves. Appl. Phys. Lett. 1965, 7, 314–316. [Google Scholar] [CrossRef]
- Ash, E. Surface wave grating reflectors and resonators. In Proceedings of the G-MTT 1970 International Microwave Symposium, Newport Beach, CA, USA, 11–14 May 1970; pp. 385–386. [Google Scholar]
- Staples, E.; Schoenwald, J.; Rosenfeld, R.; Hartmann, C. Uhf surface acoustic wave resonators. In Proceedings of the Ultrasonics Symposium, Milwaukee, WI, USA, 11–14 November 1974; pp. 245–252. [Google Scholar]
- Lieberzeit, P.; Greibl, W.; Jenik, M.; Dickert, F.L.; Fischerauer, G.; Bulst, W.-E. Cavities generated by self-organised monolayers as sensitive coatings for surface acoustic wave resonators. Anal. Bioanal. Chem. 2007, 387, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Wei, C.; Dai, L.; Roy, A.; Tolle, T.B. Multifunctional chemical vapor sensors of aligned carbon nanotube and polymer composites. J. Am. Chem. Soc. 2006, 128, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, S.; Piletsky, S.A.; Turner, A.P. Application of natural receptors in sensors and assays. Anal. Chem. 2002, 74, 3942–3951. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, C.; Verardi, P.; Verona, E.; D’Amico, A.; Di Natale, C.; Saggio, G.; Serafini, M.; Paolesse, R.; Huq, S. Advances in SAW-based gas sensors. Smart Mater. Struct. 1997, 6, 689. [Google Scholar] [CrossRef]
- Afzal, A.; Iqbal, N.; Mujahid, A.; Schirhagl, R. Advanced vapor recognition materials for selective and fast responsive surface acoustic wave sensors: A review. Anal. Chim. Acta 2013, 787, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Dickert, F.L. SAW and functional polymers. In Gas Sensing Fundamentals; Springer: Berlin, Germany, 2013; pp. 213–245. [Google Scholar]
- Calabrese, G.S.; Wohltjen, H.; Roy, M.K. Surface acoustic wave devices as chemical sensors in liquids. Evidence disputing the importance of rayleigh wave propagation. Anal. Chem. 1987, 59, 833–837. [Google Scholar] [CrossRef]
- Da Cunha, M.P.; Malocha, D.C.; Puccio, D.W.; Thiele, J.; Pollard, T.B. Lgx pure shear horizontal SAW for liquid sensor applications. IEEE Sens. J. 2003, 3, 554–561. [Google Scholar] [CrossRef]
- Sehra, G.; Cole, M.; Gardner, J.W. Miniature taste sensing system based on dual sh-SAW sensor device: An electronic tongue. Sens. Actuators B Chem. 2004, 103, 233–239. [Google Scholar] [CrossRef]
- Rayleigh, L. On waves propagated along the plane surface of an elastic solid. Proc. Lond. Math. Soc. 1885, 1, 4–11. [Google Scholar] [CrossRef]
- Lam, C.; Wang, C.Y.; Wang, S. A review of the recent development of temperature stable cuts of quartz for SAW applications. In Proceedings of the Fourth International Symposium on Acoustic Wave Devices for Future Mobile Communication Systems, Chiba, Japan, 3–5 March 2010; pp. 3–5. [Google Scholar]
- Bu, G.; Ciplys, D.; Shur, M.; Schowalter, L.J.; Schujman, S.; Gaska, R. Surface acoustic wave velocity in single-crystal aln substrates. IEEE Trans. Ultrason. Ferroelectr. 2006, 53, 251–254. [Google Scholar] [CrossRef]
- Neumeister, J.; Thum, R.; Lüder, E. A SAW delay-line oscillator as a high-resolution temperature sensor. Sens. Actuators A Phys. 1990, 22, 670–672. [Google Scholar] [CrossRef]
- Binder, A.; Fachberger, R. Wireless SAW temperature sensor system for high-speed high-voltage motors. IEEE Sens. J. 2011, 11, 966–970. [Google Scholar] [CrossRef]
- Fachberger, R.; Erlacher, A. Monitoring of the temperature inside a lining of a metallurgical vessel using a SAW temperature sensor. Proced. Chem. 2009, 1, 1239–1242. [Google Scholar] [CrossRef]
- Hamidon, M.N.; Skarda, V.; White, N.; Krispel, F.; Krempl, P.; Binhack, M.; Buff, W. Fabrication of high temperature surface acoustic wave devices for sensor applications. Sens. Actuators A Phys. 2005, 123, 403–407. [Google Scholar] [CrossRef]
- Bo, L.; Xiao, C.; Hualin, C.; Mohammad, M.A.; Xiangguang, T.; Luqi, T.; Yi, Y.; Tianling, R. Surface acoustic wave devices for sensor applications. J. Semicond. 2016, 37, 021001. [Google Scholar]
- Hornsteiner, J.; Born, E.; Fischerauer, G.; Riha, E. Surface acoustic wave sensors for high-temperature applications. In Proceedings of the 1998 IEEE International Frequency Control Symposium, Pasadena, CA, USA, 29 May 1998; pp. 615–620. [Google Scholar]
- Bu, G.; Ciplys, D.; Shur, M.; Schowalter, L.; Schujman, S.; Gaska, R. Temperature coefficient of SAW frequency in single crystal bulk AlN. Electron. Lett. 2003, 39, 755–757. [Google Scholar] [CrossRef]
- Naumenko, N.; Solie, L. Optimal cuts of Langasite, La3Ga5SiO14 for SAW devices. IEEE Trans. Ultrason. Ferroelectr. 2001, 48, 530–537. [Google Scholar] [CrossRef]
- Li, Z.; Jones, Y.; Hossenlopp, J.; Cernosek, R.; Josse, F. Analysis of liquid-phase chemical detection using guided shear horizontal-surface acoustic wave sensors. Anal. Chem. 2005, 77, 4595–4603. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Saitoh, A.; Horikoshi, Y. Measurement of acoustic properties of liquid using liquid flow Sh-SAW sensor system. Sens. Actuators B Chem. 2001, 76, 69–73. [Google Scholar] [CrossRef]
- Martin, F.; Newton, M.I.; McHale, G.; Melzak, K.A.; Gizeli, E. Pulse mode shear horizontal-surface acoustic wave (Sh-SAW) system for liquid based sensing applications. Biosens. Bioelectron. 2004, 19, 627–632. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Glanzing, G.; Leidl, A.; Lieberzeit, P.A.; Dickert, F.L. Imprinted sol–gel materials for monitoring degradation products in automotive oils by shear transverse wave. Anal. Chim. Acta 2010, 675, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Dickert, F.L.; Forth, P.; Bulst, W.-E.; Fischerauer, G.; Knauer, U. SAW devices-sensitivity enhancement in going from 80 Mhz to 1 Ghz. Sens. Actuators B Chem. 1998, 46, 120–125. [Google Scholar] [CrossRef]
- Plessky, V.P.; Reindl, L.M. Review on SAW rfid tags. IEEE Trans. Ultrason. Ferroelectr. 2010, 57. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.; Posnicek, T.; Kellner, K. Position estimation of RFID-based sensors using SAW compressive receivers. Sens. Actuators A Phys. 2016, 244, 277–284. [Google Scholar] [CrossRef]
- Dickert, F.L.; Bertlein, G.; Mages, G.; Bulst, W.E. Triphenyl methane dyes as sensor materials for solvent detection with surface-acoustic-wave devices. Adv. Mater. 1990, 2, 420–422. [Google Scholar] [CrossRef]
- Minot, B.; Frenois, C.; Besnard, S.; Bordet, J.; Martins, N.; Pereira, F. Sensitive materials for chemical agents vapor detection using SAW sensors. Proced. Eng. 2016, 168, 423–427. [Google Scholar] [CrossRef]
- Chen, C.; Yang, M.; Chen, J.; Liang, C. The viscoelastic contribution to polymer-coated SAW sensor for TNT vapor detection. Optik 2016, 127, 3638–3642. [Google Scholar] [CrossRef]
- Gan, H.; Man, Y.C.; Tan, C.; NorAini, I.; Nazimah, S. Characterisation of vegetable oils by surface acoustic wave sensing electronic nose. Food Chem. 2005, 89, 507–518. [Google Scholar] [CrossRef]
- Lammertyn, J.; Veraverbeke, E.A.; Irudayaraj, J. Znose™ technology for the classification of honey based on rapid aroma profiling. Sens. Actuators B Chem. 2004, 98, 54–62. [Google Scholar] [CrossRef]
- Dickert, F.L.; Haunschild, A. Sensor materials for solvent vapor detection—Donor–acceptor and host–guest interactions. Adv. Mater. 1993, 5, 887–895. [Google Scholar] [CrossRef]
- Dickert, F.L.; Bauer, P.A. The detection of halogenated hydrocarbons via host–guest chemistry—A mass-sensitive sensor study with QMB-and SAW-devices. Adv. Mater. 1991, 3, 436–438. [Google Scholar] [CrossRef]
- Dickert, F.L.; Sikorski, R. Supramolecular strategies in chemical sensing. Mater. Sci. Eng. C 1999, 10, 39–46. [Google Scholar] [CrossRef]
- Li, D.; Ma, M. Surface acoustic wave microsensors based on cyclodextrin coatings. Sens. Actuators B Chem. 2000, 69, 75–84. [Google Scholar] [CrossRef]
- Dickert, F.; Bruckdorfer, T.; Feigl, H.; Haunschild, A.; Kuschow, V.; Obermeier, E.; Bulst, W.-E.; Knauer, U.; Meges, G. Supramolecular detection of solvent vapours with qmb and SAW devices. Sens. Actuators B Chem. 1993, 13, 297–301. [Google Scholar] [CrossRef]
- Dickert, F.L.; Haunschild, A.; Reif, M.; Bulst, W.E. Sensors for organic solvent detection in the PPM range based on dioxo [6.1. 6.1] paracyclophanes—from molecular modeling to analyte recognition. Adv. Mater. 1993, 5, 277–279. [Google Scholar] [CrossRef]
- Dickert, F.L.; Reif, M.; Reif, H. Molecular modelling of host-guest interactions for mass sensitive chemical sensors. Fresen. J. Anal. Chem. 1995, 352, 620–624. [Google Scholar] [CrossRef]
- Dickert, F.L.; Reif, M.; Sikorski, R. Shaping tetraazaparacyclophanes for selective solvent vapour detection: Structural variations in coating design. J. Incl. Phenom. Macrocycl. 2001, 40, 45–49. [Google Scholar] [CrossRef]
- Dickert, F.L.; Schuster, O. Mass sensitive detection of solvent vapors with calix [n] arenes—conformational adaptation to the analyte. Adv. Mater. 1993, 5, 826–829. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Greibl, W.; Stathopulos, H.; Dickert, F.L.; Fischerauer, G.; Bulst, W.-E. Covalently anchored supramolecular monolayers on quartz surfaces for use in SAW sensors. Sens. Actuators B Chem. 2006, 113, 677–683. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ao, D.; Li, W.; Zu, X.; Li, S.; Fu, Y.Q. NH3 sensing property and mechanisms of quartz surface acoustic wave sensors deposited with SiO2, TiO2, and SiO2-TiO2 composite films. Sens. Actuators B Chem. 2018, 254, 1165–1173. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. ZnO/ST-quartz SAW resonator: An efficient NO2 gas sensor. Sens. Actuators B Chem. 2017, 252, 840–845. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Raj, V.B.; Nimal, A.; Parmar, Y.; Sharma, M.; Sreenivas, K.; Gupta, V. Cross-sensitivity and selectivity studies on zno surface acoustic wave ammonia sensor. Sens. Actuators B Chem. 2010, 147, 517–524. [Google Scholar] [CrossRef]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Tomar, M.; Sharma, M.U.; Gupta, V. Effect of metal oxide sensing layers on the distinct detection of ammonia using surface acoustic wave (SAW) sensors. Sens. Actuators B Chem. 2013, 187, 563–573. [Google Scholar] [CrossRef]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Sharma, M.U.; Tomar, M.; Gupta, V. Distinct detection of liquor ammonia by ZNO/SAW sensor: Study of complete sensing mechanism. Sens. Actuators B Chem. 2017, 238, 83–90. [Google Scholar] [CrossRef]
- Hong, H.-S.; Chung, G.-S. Surface acoustic wave humidity sensor based on polycrystalline AlN thin film coated with sol–gel derived nanocrystalline zinc oxide film. Sens. Actuators B Chem. 2010, 148, 347–352. [Google Scholar] [CrossRef]
- Phan, D.-T.; Chung, G.-S. Surface acoustic wave hydrogen sensors based on ZnO nanoparticles incorporated with a Pt catalyst. Sens. Actuators B Chem. 2012, 161, 341–348. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Kong, F.; Wang, S.; Zhu, B.; Guo, X.; Zhang, J.; Wang, Y.; Wu, S. Au-doped wo 3-based sensor for NO2 detection at low operating temperature. Sens. Actuators B Chem. 2008, 134, 133–139. [Google Scholar] [CrossRef]
- Kabir, K.M.M.; Lay, B.; Kandjani, A.E.; Sabri, Y.M.; Ippolito, S.J.; Bhargava, S.K. A nanoengineered surface acoustic wave device for analysis of mercury in gas phase. Sens. Actuators B Chem. 2016, 234, 562–572. [Google Scholar] [CrossRef]
- Jakubik, W. Investigations of thin film structures of WO3 and WO3 with Pd for hydrogen detection in a surface acoustic wave sensor system. Thin Solid Films 2007, 515, 8345–8350. [Google Scholar] [CrossRef]
- Jakubik, W.; Urbańczyk, M.; Maciak, E. SAW hydrogen gas sensor based on WO3 and Pd nanostructures. Proced. Chem. 2009, 1, 200–203. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Dreschel, W.R.; Ong, K.G.; Grimes, C.A. Ammonia detection using nanoporous alumina resistive and surface acoustic wave sensors. Sens. Actuators B Chem. 2003, 94, 27–35. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, C.; Ju, Y.; Xu, H.; Qiu, Y. A novel NO2 gas sensor using dual track SAW device. Sens. Actuators A Phys. 2010, 159, 168–173. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A.; Mujahid, A. Layer-by-layer assembly of low-temperature-imprinted poly(methacrylic acid)/gold nanoparticle hybrids for gaseous formaldehyde mass sensing. RSC Adv. 2014, 4, 43121–43130. [Google Scholar] [CrossRef]
- Afzal, A.; Feroz, S.; Iqbal, N.; Mujahid, A.; Rehman, A. A collaborative effect of imprinted polymers and au nanoparticles on bioanalogous detection of organic vapors. Sens. Actuators B Chem. 2016, 231, 431–439. [Google Scholar] [CrossRef]
- Sadek, A.; Wlodarski, W.; Shin, K.; Kaner, R.B.; Kalantar-Zadeh, K. A layered surface acoustic wave gas sensor based on a polyaniline/In2O3 nanofibre composite. Nanotechnology 2006, 17, 4488. [Google Scholar] [CrossRef]
- Dewan, N.; Singh, S.; Sreenivas, K.; Gupta, V. Influence of temperature stability on the sensing properties of SAW NOx sensor. Sens. Actuators B Chem. 2007, 124, 329–335. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Armentano, I.; Kenny, J.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm no 2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.; Morozov, S.; Hill, E.; Blake, P.; Katsnelson, M.; Novoselov, K. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, E.; Scorsone, E.; Bergonzo, P. Modified diamond nanoparticles as sensitive coatings for chemical SAW sensors. Proced. Chem. 2009, 1, 943–946. [Google Scholar] [CrossRef]
- Chevallier, E.; Scorsone, E.; Bergonzo, P. New sensitive coating based on modified diamond nanoparticles for chemical SAW sensors. Sens. Actuators B Chem. 2011, 154, 238–244. [Google Scholar] [CrossRef]
- Penza, M.; Antolini, F.; Antisari, M.V. Carbon nanotubes as SAW chemical sensors materials. Sens. Actuators B Chem. 2004, 100, 47–59. [Google Scholar] [CrossRef]
- Penza, M.; Tagliente, M.; Aversa, P.; Cassano, G. Organic-vapor detection using carbon-nanotubes nanocomposite microacoustic sensors. Chem. Phys Lett. 2005, 409, 349–354. [Google Scholar] [CrossRef]
- Penza, M.; Antolini, F.; Vittori-Antisari, M. Carbon nanotubes-based surface acoustic waves oscillating sensor for vapour detection. Thin Solid Films 2005, 472, 246–252. [Google Scholar] [CrossRef]
- Penza, M.; Aversa, P.; Cassano, G.; Wlodarski, W.; Kalantar-Zadeh, K. Layered SAW gas sensor with single-walled carbon nanotube-based nanocomposite coating. Sens. Actuators B Chem. 2007, 127, 168–178. [Google Scholar] [CrossRef]
- Penza, M.; Tagliente, M.; Aversa, P.; Cassano, G.; Capodieci, L. Single-walled carbon nanotubes nanocomposite microacoustic organic vapor sensors. Mater. Sci. Eng. C 2006, 26, 1165–1170. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Balashov, S.M.; Balachova, O.V.; Braga, A.V.U.; Filho, A.P.; Moshkalev, S. Influence of the deposition parameters of graphene oxide nanofilms on the kinetic characteristics of the SAW humidity sensor. Sens. Actuators B Chem. 2015, 217, 88–91. [Google Scholar] [CrossRef]
- Le, X.; Wang, X.; Pang, J.; Liu, Y.; Fang, B.; Xu, Z.; Gao, C.; Xu, Y.; Xie, J. A high performance humidity sensor based on surface acoustic wave and graphene oxide on AlN/Si layered structure. Sens. Actuators B Chem. 2018, 255, 2454–2461. [Google Scholar] [CrossRef]
- Ricco, A.; Martin, S.; Zipperian, T. Surface acoustic wave gas sensor based on film conductivity changes. Sens. Actuators 1985, 8, 319–333. [Google Scholar] [CrossRef]
- Arsat, R.; Breedon, M.; Shafiei, M.; Spizziri, P.; Gilje, S.; Kaner, R.; Kalantar-zadeh, K.; Wlodarski, W. Graphene-like nano-sheets for surface acoustic wave gas sensor applications. Chem. Phys. Lett. 2009, 467, 344–347. [Google Scholar] [CrossRef]
- Xuan, W.; He, M.; Meng, N.; He, X.; Wang, W.; Chen, J.; Shi, T.; Hasan, T.; Xu, Z.; Xu, Y. Fast response and high sensitivity ZnO/glass surface acoustic wave humidity sensors using graphene oxide sensing layer. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Liron, Z.; Kaushansky, N.; Frishman, G.; Kaplan, D.; Greenblatt, J. The polymer-coated SAW sensor as a gravimetric sensor. Anal. Chem. 1997, 69, 2848–2854. [Google Scholar] [CrossRef]
- Levit, N.; Pestov, D.; Tepper, G. High surface area polymer coatings for SAW-based chemical sensor applications. Sens. Actuators B Chem. 2002, 82, 241–249. [Google Scholar] [CrossRef]
- Joo, B.-S.; Huh, J.-S.; Lee, D.-D. Fabrication of polymer SAW sensor array to classify chemical warfare agents. Sens. Actuators B Chem. 2007, 121, 47–53. [Google Scholar] [CrossRef]
- Grate, J.W. Hydrogen-bond acidic polymers for chemical vapor sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, X.; Long, Y.; Tang, X.; Chen, Z.; Jiang, Y. Real-time detection of styrene using SAW sensors based on hexafluoroisopropanol group functionalized hydrogen-bond acidic polymers. Sens. Actuators B Chem. 2015, 206, 252–257. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G.; Sergi, A.; Lo Sterzo, C.; Russo, M.V. Saw chemical sensing using poly-ynes and organometallic polymer films. Sens. Actuators B Chem. 2001, 81, 88–98. [Google Scholar] [CrossRef]
- Barié, N.; Bücking, M.; Rapp, M. A novel electronic nose based on miniaturized SAW sensor arrays coupled with spme enhanced headspace-analysis and its use for rapid determination of volatile organic compounds in food quality monitoring. Sens. Actuators B Chem. 2006, 114, 482–488. [Google Scholar] [CrossRef]
- Dickert, F.L.; Hayden, O. Molecular imprinting in chemical sensing. TrAC Trends Anal. Chem. 1999, 18, 192–199. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Hu, J.; Kaganove, S.N.; Keinath, S.E.; Keeley, D.L.; Dvornic, P.R. Hydrogen-bond acidic hyperbranched polymers for surface acoustic wave (SAW) sensors. Chem. Mater. 2004, 16, 5357–5364. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C.; Keeley, D.L.; Voit, B.; Eichhorn, K.J.; Mikhaylova, Y. Hyperbranched polyesters with internal and exo-presented hydrogen-bond acidic sensor groups for surface acoustic wave sensors. J. Appl. Polym. Sci. 2008, 107, 1401–1406. [Google Scholar] [CrossRef]
- Milella, E.; Penza, M. SAW gas detection using langmuir–blodgett polypyrrole films. Thin Solid Films 1998, 327, 694–697. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Baker, C.O.; Powell, D.A.; Wlodarski, W.; Kaner, R.B.; Kalantar-zadeh, K. Polyaniline nanofiber based surface acoustic wave gas sensors—Effect of nanofiber diameter on H2 response. IEEE Sens. J. 2007, 7, 213–218. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Wlodarski, W.; Shin, K.; Kaner, R.B.; Kalantar-zadeh, K. A polyaniline/WO3 nanofiber composite-based ZnO/64° yx linbo3 SAW hydrogen gas sensor. Synth. Met. 2008, 158, 29–32. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.; Fontecha, J.; Horrillo, M.; Vera, C.; Obieta, I.; Bustero, I. New sensitive layers for surface acoustic wave gas sensors based on polymer and carbon nanotube composites. Sens. Actuators B Chem. 2012, 175, 67–72. [Google Scholar] [CrossRef]
- Grate, J.W.; Patrash, S.J.; Kaganove, S.N.; Wise, B.M. Hydrogen bond acidic polymers for surface acoustic wave vapor sensors and arrays. Anal. Chem. 1999, 71, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Ying, Z.; Jiang, Y.; Liu, Z.; Yang, T.; Xie, G. Synthesis and evaluation of a new polysiloxane as SAW sensor coatings for dmmp detection. Sens. Actuators B Chem. 2008, 134, 409–413. [Google Scholar] [CrossRef]
- Hotel, O.; Poli, J.-P.; Mer-Calfati, C.; Scorsone, E.; Saada, S. A review of algorithms for SAW sensors e-nose based volatile compound identification. Sens. Actuators B Chem. 2018, 255, 2472–2482. [Google Scholar] [CrossRef]
- Singh, H.; Raj, V.B.; Kumar, J.; Durani, F.; Mishra, M.; Nimal, A.T.; Sharma, M.U. SAW mono sensor for identification of harmful vapors using PCA and ANN. Process Saf. Environ. Prot. 2016, 102, 577–588. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Amin, I. Use of the SAW sensor electronic nose for detecting the adulteration of virgin coconut oil with rbd palm kernel olein. J. Am. Oil Chem. Soc. 2010, 87, 263–270. [Google Scholar] [CrossRef]
- Ricco, A.J.; Crooks, R.M.; Osbourn, G.C. Surface acoustic wave chemical sensor arrays: New chemically sensitive interfaces combined with novel cluster analysis to detect volatile organic compounds and mixtures. Acc. Chem. Res. 1998, 31, 289–296. [Google Scholar] [CrossRef]
- Penza, M.; Cassano, G. Application of principal component analysis and artificial neural networks to recognize the individual vocs of methanol/2-propanol in a binary mixture by SAW multi-sensor array. Sens. Actuators B Chem. 2003, 89, 269–284. [Google Scholar] [CrossRef]
- Santos, J.P.; Fernández, M.J.; Fontecha, J.L.; Lozano, J.; Aleixandre, M.; García, M.; Gutiérrez, J.; Horrillo, M.C. SAW sensor array for wine discrimination. Sens. Actuators B Chem. 2005, 107, 291–295. [Google Scholar] [CrossRef]
- Sunil, T.T.; Chaudhuri, S.; Mishra, V. Optimal selection of SAW sensors for e-nose applications. Sens. Actuators B Chem. 2015, 219, 238–244. [Google Scholar] [CrossRef]
- Lozano, J.; Fernández, M.; Fontecha, J.; Aleixandre, M.; Santos, J.; Sayago, I.; Arroyo, T.; Cabellos, J.; Gutiérrez, F.; Horrillo, M. Wine classification with a zinc oxide SAW sensor array. Sens. Actuators B Chem. 2006, 120, 166–171. [Google Scholar] [CrossRef]
- Biswas, S.; Heindselmen, K.; Wohltjen, H.; Staff, C. Differentiation of vegetable oils and determination of sunflower oil oxidation using a surface acoustic wave sensing device. Food Control 2004, 15, 19–26. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Nagarajan, R.; Kumar, J.; Gu, Z.; Cho, J.; Kurup, P. Dynamic chemical vapor sensing with nanofibrous film based surface acoustic wave sensors. Sens. Actuators A Phys. 2011, 167, 8–13. [Google Scholar] [CrossRef]
- Mosbach, K. Molecular imprinting. Trend Biochem. Sci. 1994, 19, 9–14. [Google Scholar] [CrossRef]
- Hayden, O.; Mann, K.J.; Krassnig, S.; Dickert, F.L. Biomimetic abo blood-group typing. Angew. Chem. Int. Ed. 2006, 45, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Dickert, F.L.; Hayden, O. Imprinting with sensor development—On the way to synthetic antibodies. Fresen. J. Anal. Chem. 1999, 364, 506–511. [Google Scholar] [CrossRef]
- Dickert, F.L.; Forth, P.; Lieberzeit, P.; Tortschanoff, M. Molecular imprinting in chemical sensing—Detection of aromatic and halogenated hydrocarbons as well as polar solvent vapors. Fresen. J. Anal. Chem. 1998, 360, 759–762. [Google Scholar] [CrossRef]
- Dickert, F.L.; Tortschanoff, M.; Bulst, W.E.; Fischerauer, G. Molecularly imprinted sensor layers for the detection of polycyclic aromatic hydrocarbons in water. Anal. Chem. 1999, 71, 4559–4563. [Google Scholar] [CrossRef]
- Wen, W.; Shitang, H.; Shunzhou, L.; Minghua, L.; Yong, P. Enhanced sensitivity of SAW gas sensor coated molecularly imprinted polymer incorporating high frequency stability oscillator. Sens. Actuators B Chem. 2007, 125, 422–427. [Google Scholar] [CrossRef]
- Cao, B.-Q.; Huang, Q.-B.; Pan, Y. Study on the surface acoustic wave sensor with self-assembly imprinted film of calixarene derivatives to detect organophosphorus compounds. Am. J. Anal. Chem. 2012, 3, 664. [Google Scholar] [CrossRef]
- Dickert, F.; Hayden, O. Bioimprinting of polymers and sol− gel phases. Selective detection of yeasts with imprinted polymers. Anal. Chem. 2002, 74, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, I.; Viespe, C.; Grigoriu, C. Nanocomposite sensitive polymeric films for SAW sensors deposited by the maple direct write technique. Sens. Actuators B Chem. 2011, 158, 418–422. [Google Scholar] [CrossRef]
- Sayago, I.; Fernández, M.J.; Fontecha, J.L.; Horrillo, M.C.; Vera, C.; Obieta, I.; Bustero, I. Surface acoustic wave gas sensors based on polyisobutylene and carbon nanotube composites. Sens. Actuators B Chem. 2011, 156, 1–5. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tan, C.Y.; Tan, S.Y.; Wong, M.S.F.; Chen, Y.F.; Zhang, L.; Yao, K.; Gan, S.K.E.; Verma, C.; Tan, Y.-J. SAW sensor for influenza a virus detection enabled with efficient surface functionalization. Sens. Actuators B Chem. 2015, 209, 78–84. [Google Scholar] [CrossRef]
- Schlensog, M.D.; Gronewold, T.M.; Tewes, M.; Famulok, M.; Quandt, E. A love-wave biosensor using nucleic acids as ligands. Sens. Actuators B Chem. 2004, 101, 308–315. [Google Scholar] [CrossRef]
- Bröker, P.; Lücke, K.; Perpeet, M.; Gronewold, T.M. A nanostructured SAW chip-based biosensor detecting cancer cells. Sens. Actuators B Chem. 2012, 165, 1–6. [Google Scholar] [CrossRef]
- Länge, K.; Bender, F.; Voigt, A.; Gao, H.; Rapp, M. A surface acoustic wave biosensor concept with low flow cell volumes for label-free detection. Anal. Chem. 2003, 75, 5561–5566. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-L.; Yang, Y.; Chen, X.; Mohammad, M.A.; Ye, T.-X.; Guo, C.-R.; Yi, L.-T.; Zhou, C.-J.; Liu, J.; Ren, T.-L. A third-order mode high frequency biosensor with atomic resolution. Biosens. Bioelectron. 2015, 71, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, S.; Cao, K.; Wang, P.; Su, Y.; Zhu, X.; Wan, Y. A microfluidic love-wave biosensing device for PSA detection based on an aptamer beacon probe. Sensors 2015, 15, 13839–13850. [Google Scholar] [CrossRef] [PubMed]
- Ramshani, Z.; Reddy, A.S.; Narakathu, B.B.; Wabeke, J.T.; Obare, S.O.; Atashbar, M.Z. Sh-SAW sensor based microfluidic system for the detection of heavy metal compounds in liquid environments. Sens. Actuators B Chem. 2015, 217, 72–77. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.; Nguyen, N.-T.; Walton, A.; Flewitt, A.; Zu, X.-T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef]
- Mitsakakis, K.; Tserepi, A.; Gizeli, E. SAW device integrated with microfluidics for array-type biosensing. Microelectron. Eng. 2009, 86, 1416–1418. [Google Scholar] [CrossRef]
- Matatagui, D.; Moynet, D.; Fernández, M.J.; Fontecha, J.; Esquivel, J.P.; Gràcia, I.; Cané, C.; Déjous, C.; Rebière, D.; Santos, J.P.; et al. Detection of bacteriophages in dynamic mode using a love-wave immunosensor with microfluidics technology. Sens. Actuators B Chem. 2013, 185, 218–224. [Google Scholar] [CrossRef]
- Vikström, A.; Voinova, M.V. Soft-film dynamics of SH-SAW sensors in viscous and viscoelastic fluids. Sens. Bio-Sens. Res. 2016, 11, 78–85. [Google Scholar] [CrossRef]
- Vikström, A.; Voinova, M.V. The dynamics of viscoelastic layered systems studied by surface acoustic wave (SAW) sensors operated in a liquid phase. Proced. Technol. 2017, 27, 102–103. [Google Scholar] [CrossRef]
- Salim, Z.T.; Hashim, U.; Arshad, M.K.M.; Fakhri, M.A.; Salim, E.T. Frequency-based detection of female aedes mosquito using surface acoustic wave technology: Early prevention of dengue fever. Microelectron. Eng. 2017, 179, 83–90. [Google Scholar] [CrossRef]
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas. Sci. Technol. 2005, 16, 1535. [Google Scholar] [CrossRef]
- Grate, J.W. Acoustic wave microsensor arrays for vapor sensing. Chem. Rev. 2000, 100, 2627–2648. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zeng, Y. Uncertainty-aware frequency estimation algorithm for passive wireless resonant SAW sensor measurement. Sens. Actuators A Phys. 2016, 237, 136–146. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Palfinger, C.; Dickert, F.L.; Fischerauer, G. SAW RFID-tags for mass-sensitive detection of humidity and vapors. Sensors 2009, 9, 9805–9815. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Greco, G.; Cecchini, M. A rayleigh surface acoustic wave (r-SAW) resonator biosensor based on positive and negative reflectors with sub-nanomolar limit of detection. Sens. Actuators B Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef]
- Sisman, A.; Gur, E.; Ozturk, S.; Enez, B.; Okur, B.; Toker, O. A low-cost biomarker-based SAW-biosensor design for early detection of prostate cancer. Proced. Technol. 2017, 27, 248–249. [Google Scholar] [CrossRef]
- Ritter, F.; Hedrich, J.; Deck, M.; Ludwig, F.; Shakirov, D.; Rapp, B.E.; Länge, K. Polymer structures on surface acoustic wave biosensors. Proced. Technol. 2017, 27, 35–36. [Google Scholar] [CrossRef]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Girasole, M.; Fosca, M.; Dinarelli, S.; Staiano, M.; Marzullo, V.M.; Capo, A.; et al. A shear horizontal surface acoustic wave biosensor for a rapid and specific detection of d-serine. Sens. Actuators B Chem. 2016, 226, 1–6. [Google Scholar] [CrossRef]
- Toma, K.; Miki, D.; Yoshimura, N.; Arakawa, T.; Yatsuda, H.; Mitsubayashi, K. A gold nanoparticle-assisted sensitive SAW (surface acoustic wave) immunosensor with a regeneratable surface for monitoring of dust mite allergens. Sens. Actuators B Chem. 2017, 249, 685–690. [Google Scholar] [CrossRef]
- Li, S.; Sankaranarayanan, S.K.; Fan, C.; Su, Y.; Bhethanabotla, V.R. Achieving lower insertion loss and higher sensitivity in a SAW biosensor via optimization of waveguide and microcavity structures. IEEE Sens. J. 2017, 17, 1608–1616. [Google Scholar] [CrossRef]
- Ten, S.; Hashim, U.; Gopinath, S.; Liu, W.; Foo, K.; Sam, S.; Rahman, S.; Voon, C.; Nordin, A. Highly sensitive escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017, 93, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Real Time Chemical Process Measurements Using a GC/SAW System. Available online: http://www.estcal.com/blog/real-time-chemical-process-measurements-using-gcsaw-system (accessed on 16 October 2017).
- Nimal, A.; Mittal, U.; Singh, M.; Khaneja, M.; Kannan, G.; Kapoor, J.; Dubey, V.; Gutch, P.; Lal, G.; Vyas, K. Development of handheld SAW vapor sensors for explosives and cw agents. Sens. Actuators B Chem. 2009, 135, 399–410. [Google Scholar] [CrossRef]
- Available online: http://www.cbrnetechindex.com/p/4386/Biosensor-Applications-Sweden-AB/Biosens (accessed on 16 October 2017).
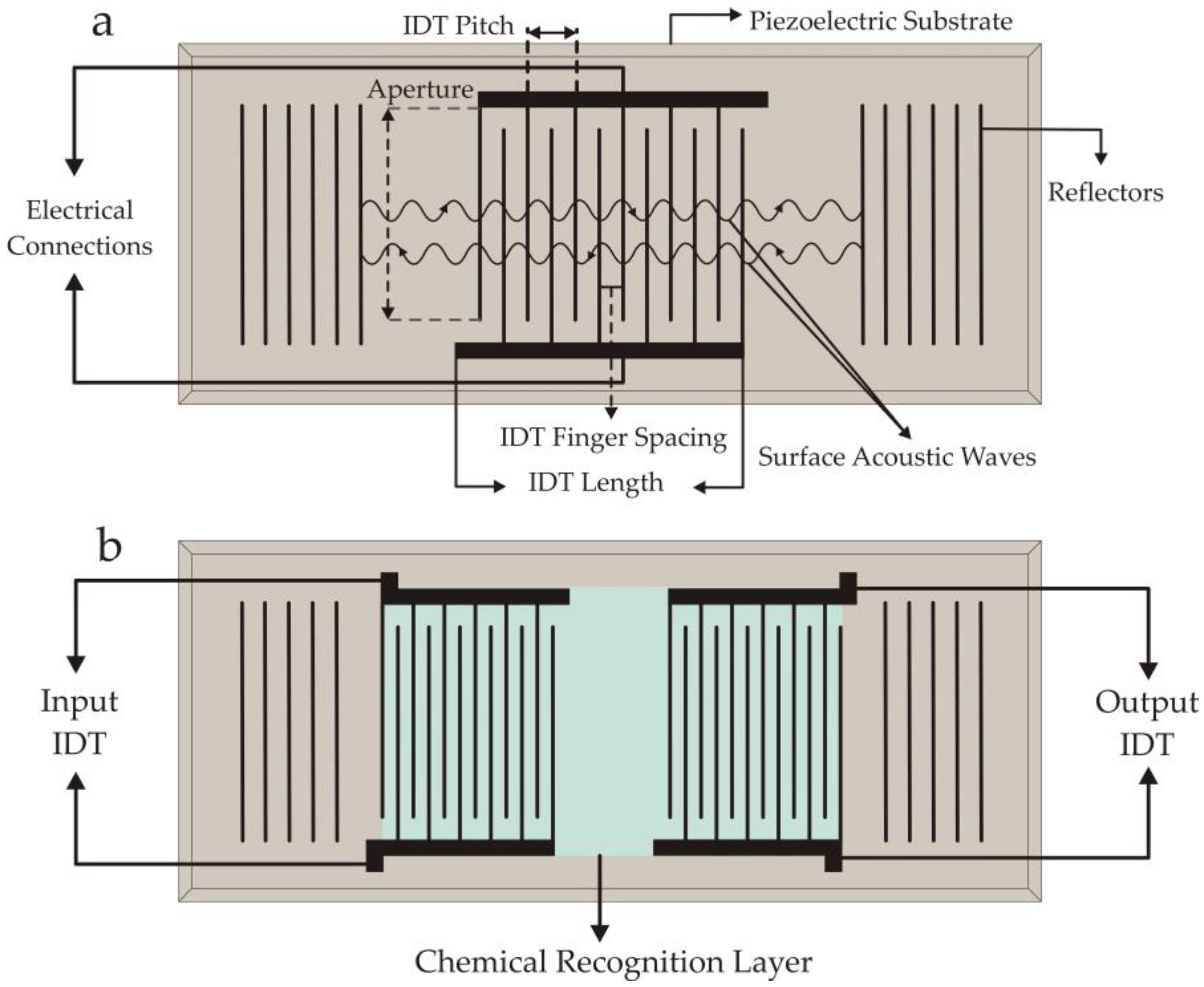
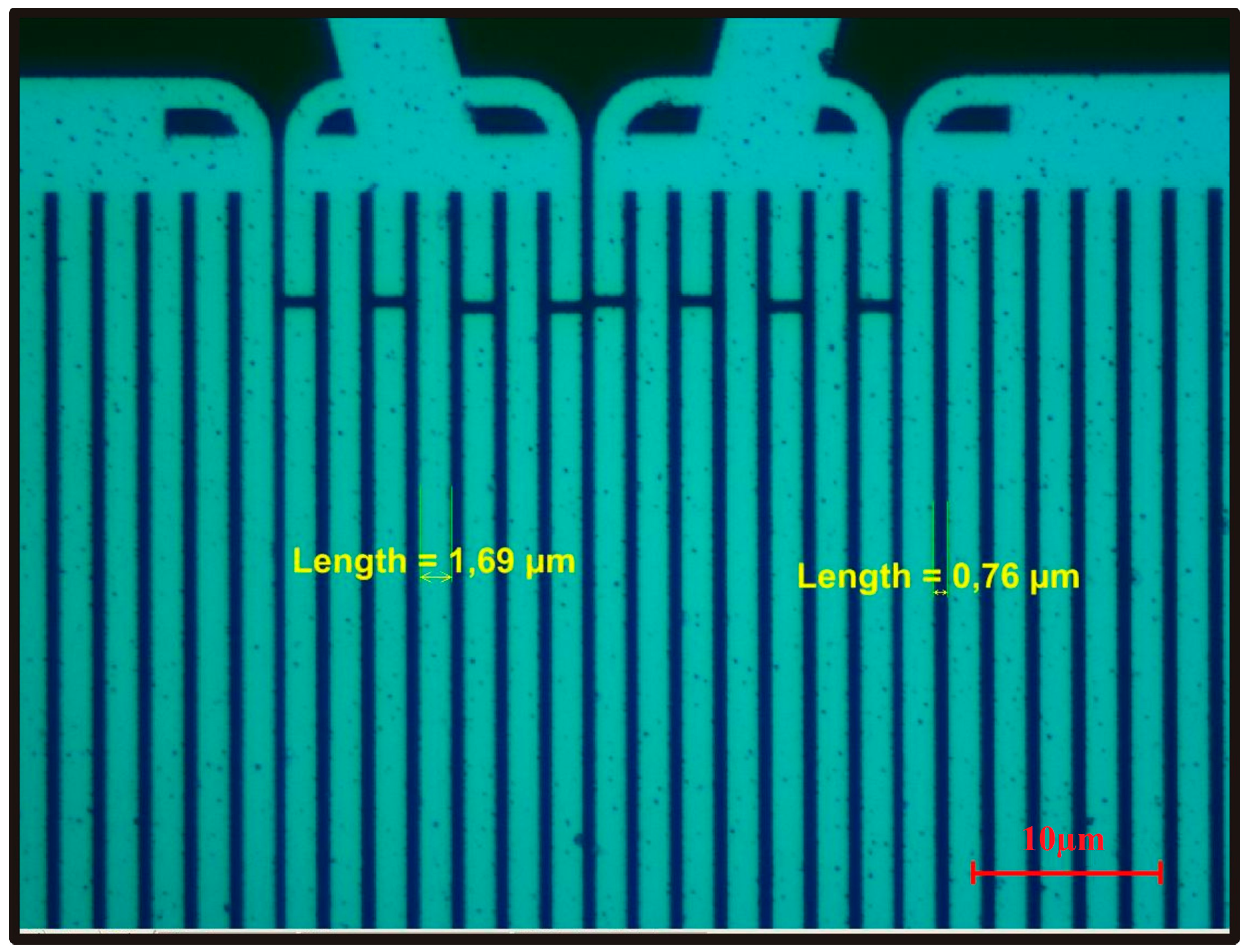

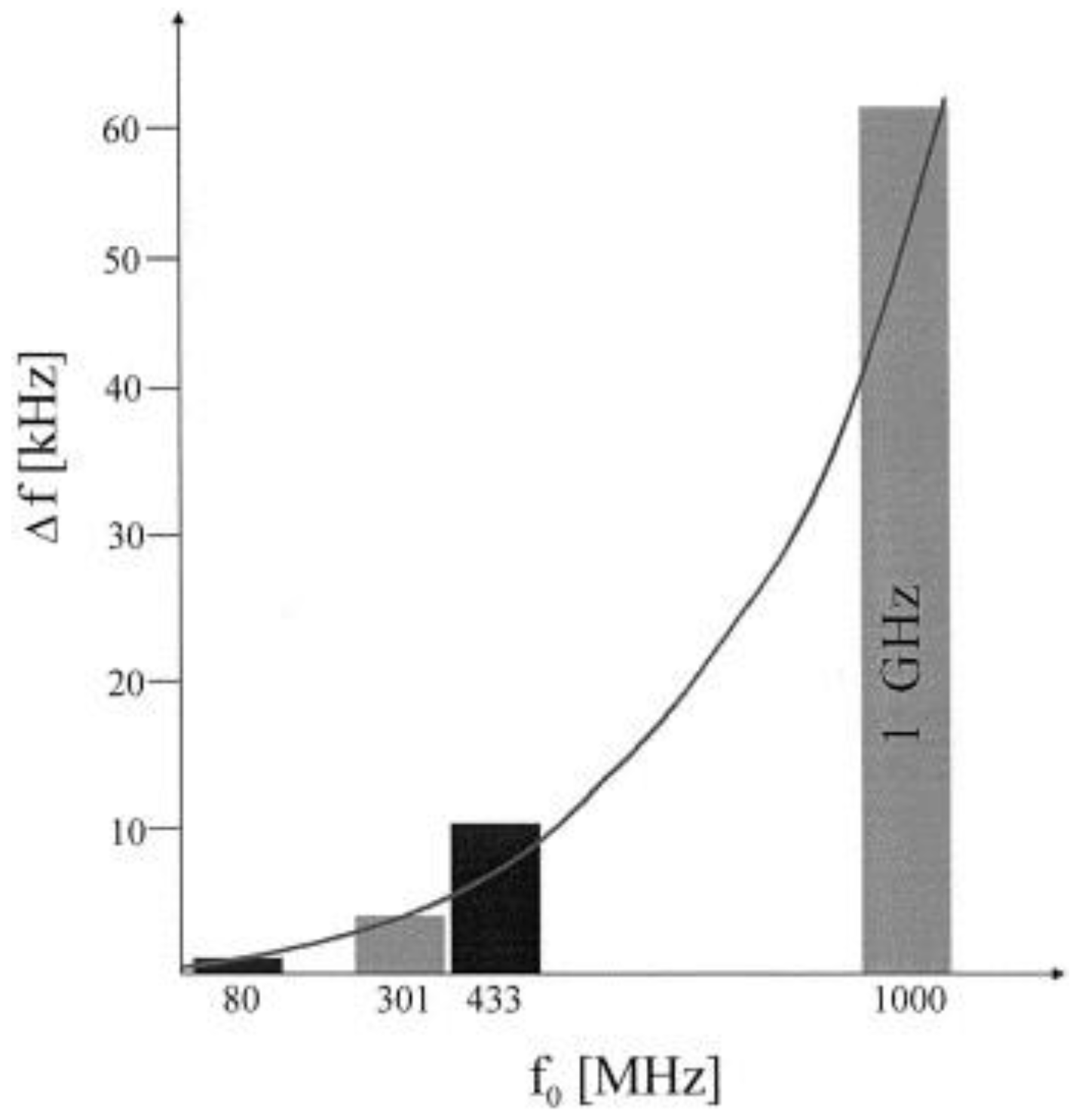

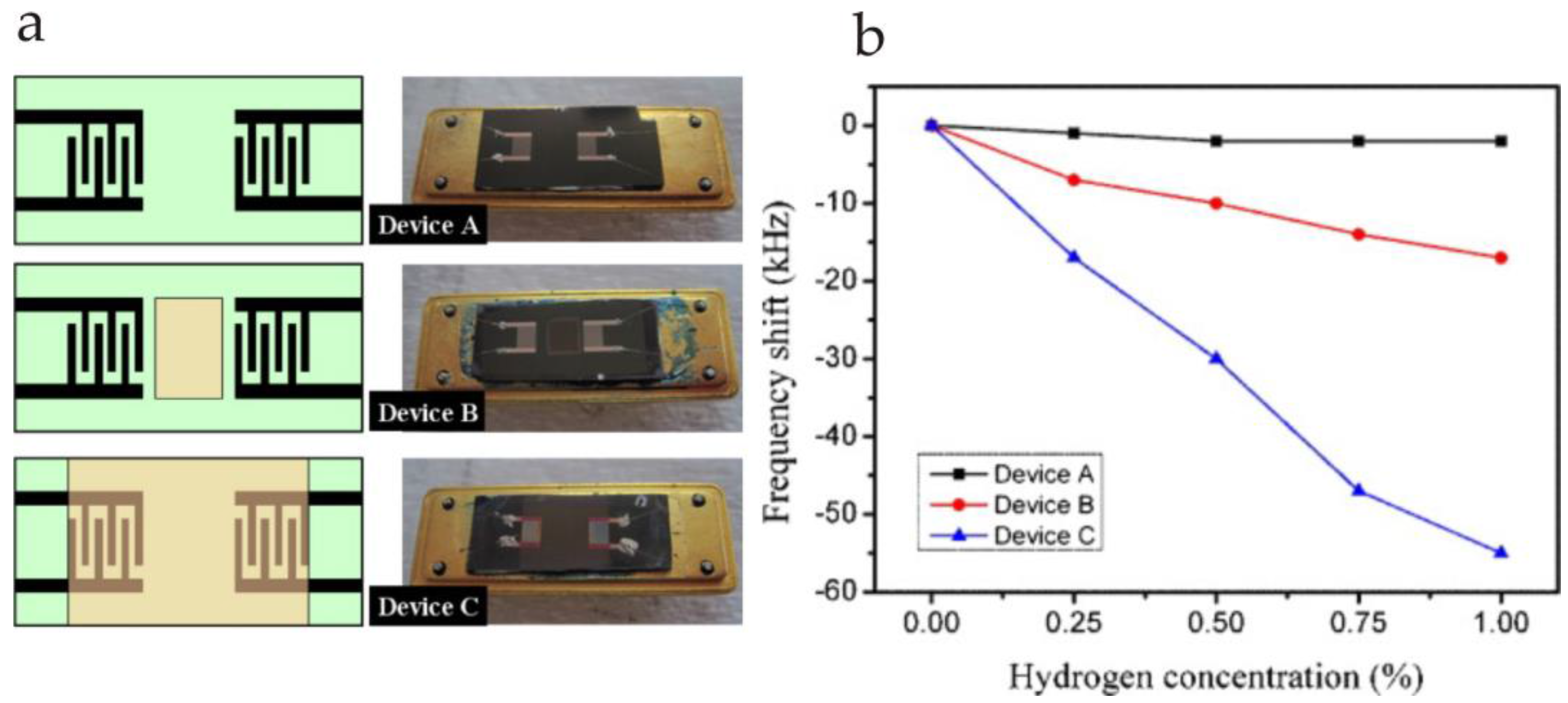
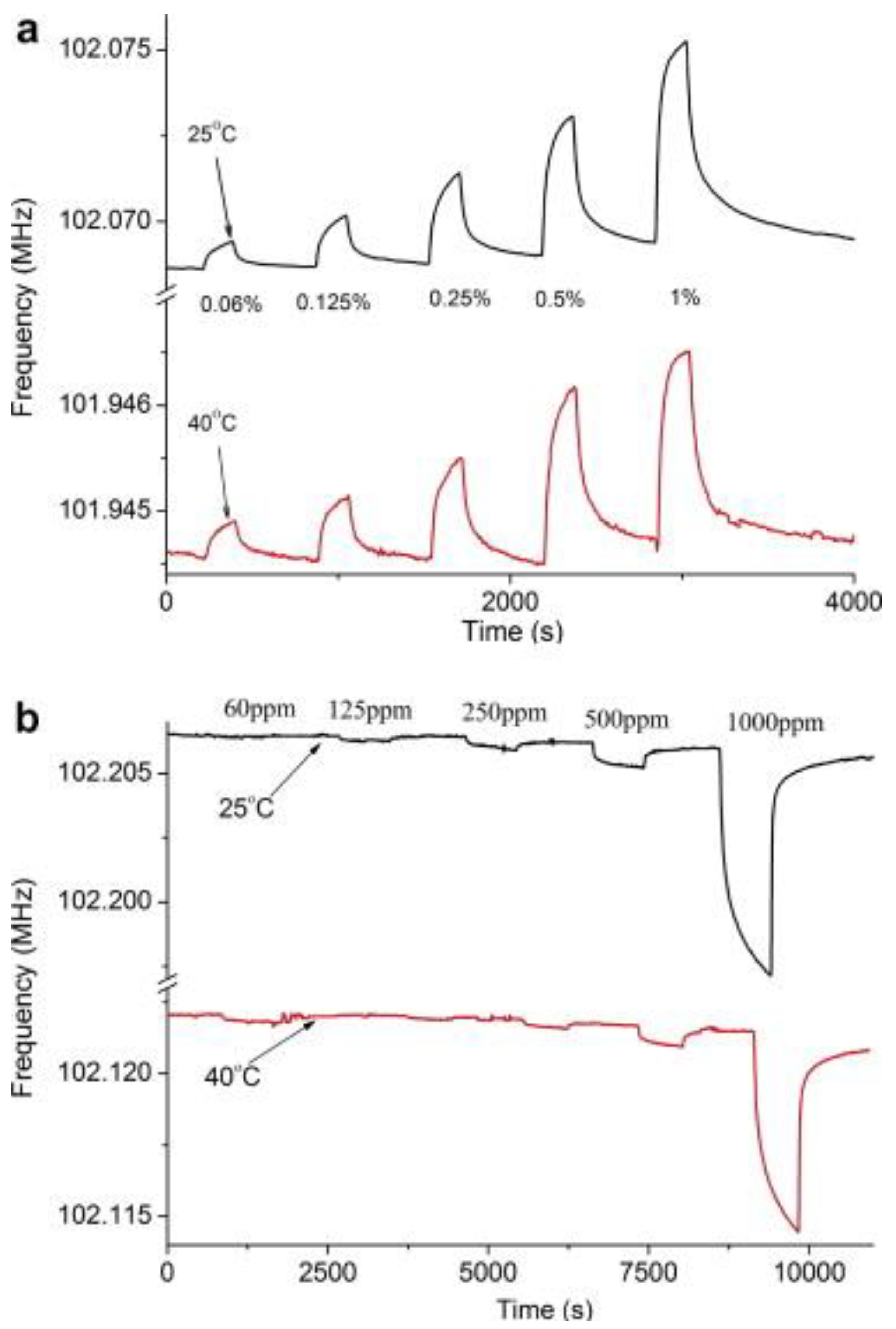


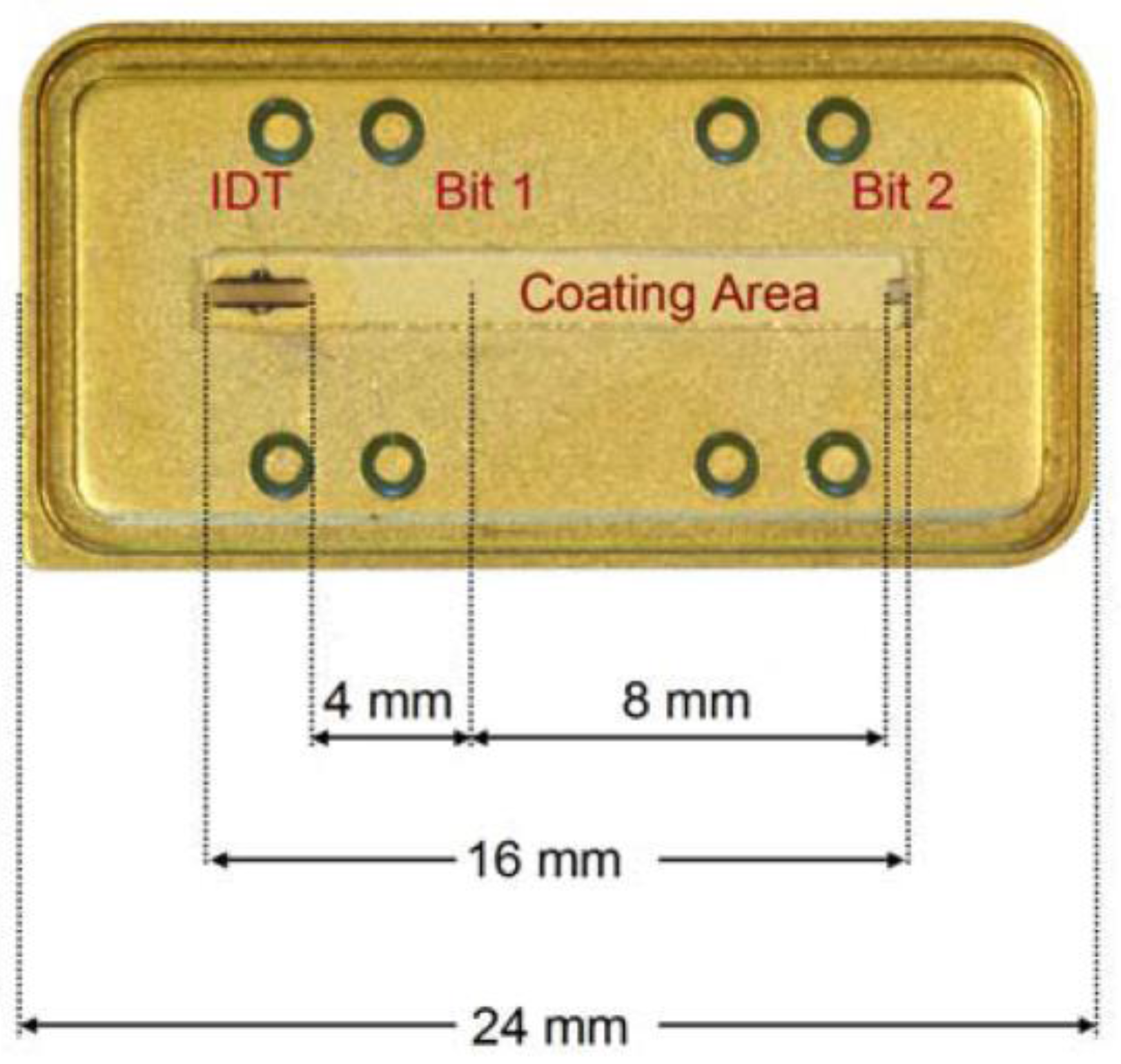
| Piezoelectric Material | Dielectric Constant | TCF a (ppm/°C) | Max. Working b Temperature | Velocity (m/s) | Comments |
|---|---|---|---|---|---|
| Quartz (ST-X) | 3.8 | 0 | 573 °C | 3159 | Common and inexpensive, Stable against temperature, Not suitable in aqueous phase. |
| LiTaO3 (X-112° Y) | 43 | 18 | ≈300 °C | 3300 | High frequency SAW devices, Suitable for liquids phase operation, Temperature drifts |
| LiTaO3 (36° Y-X) | 32 | 4160 | |||
| LiNbO3 (128° Y-X) | 85.2 | 75 | ≈300 °C | 3979 | High frequency SAW devices, Suitable for SH-SAW liquid phase, Larger TCF values |
| LiNbO3 (64° Y-X) | 80 | 4742 | |||
| LiNbO3 (Y-Z) | 94 | 3488 | |||
| AlN | 8.5 | 19 | ≥1000 °C | 5700 | High frequency SAW devices and used in form of thin layer on other substrates, High thermal stability |
| La3Ga5SiO14 | 18.23 | ≈0 | 1470 °C | 2734 | Enhanced piezoelectricity, High thermal stability Low insertion loss |
| Type of VOC | SWCNTs Dispersed into Ethanol | MWCNTs Dispersed into Ethanol | ||||||
| Sensitivity (∆f/f)/c (ppm/ppm) | Sensitivity ∆f/c (kHz/ppm) | LOD (ppm) | Absolute Sensitivity ∆f/c/∆fw (Hz/ppm/kHz) | Sensitivity (∆f/f)/c (ppm/ppm) | Sensitivity ∆f/c (kHz/ppm) | LOD (ppm) | Absolute Sensitivity ∆f/c/∆fw (Hz/ppm/kHz) | |
| Ethanol | 15.88 | 6.89 | 1.3 | 34.45 | 4.13 | 1.79 | 5.0 | 8.95 |
| Ethyl Acetate | 7.67 | 3.32 | 2.7 | 16.6 | 2.09 | 0.90 | 10.0 | 4.50 |
| Toluene | 7.32 | 3.17 | 2.8 | 15.85 | 2.33 | 1.01 | 9.0 | 5.05 |
| SWCNTs Dispersed into Toluene | MWCNTs Dispersed into Toluene | |||||||
| Sensitivity (∆f/f)/c (ppm/ppm) | Sensitivity ∆f/c (kHz/ppm) | LOD (ppm) | Absolute Sensitivity ∆f/c/∆fw (Hz/ppm/kHz) | Sensitivity (∆f/f)/c (ppm/ppm) | Sensitivity ∆f/c (kHz/ppm) | LOD (ppm) | Absolute Sensitivity ∆f/c/∆fw (Hz/ppm/kHz) | |
| Ethanol | 14.62 | 6.34 | 1.4 | 31.70 | 2.02 | 0.87 | 10.2 | 4.35 |
| Ethyl Acetate | 12.58 | 5.45 | 1.6 | 27.25 | 3.99 | 1.73 | 5.2 | 8.65 |
| Toluene | 17.22 | 7.47 | 1.2 | 37.35 | 4.67 | 2.02 | 4.4 | 10.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujahid, A.; Dickert, F.L. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. https://doi.org/10.3390/s17122716
Mujahid A, Dickert FL. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors. 2017; 17(12):2716. https://doi.org/10.3390/s17122716
Chicago/Turabian StyleMujahid, Adnan, and Franz L. Dickert. 2017. "Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers" Sensors 17, no. 12: 2716. https://doi.org/10.3390/s17122716
APA StyleMujahid, A., & Dickert, F. L. (2017). Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors, 17(12), 2716. https://doi.org/10.3390/s17122716






