Off-Nadir Hyperspectral Sensing for Estimation of Vertical Profile of Leaf Chlorophyll Content within Wheat Canopies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Multi-Angle Hyperspectral Reflectance Measurement
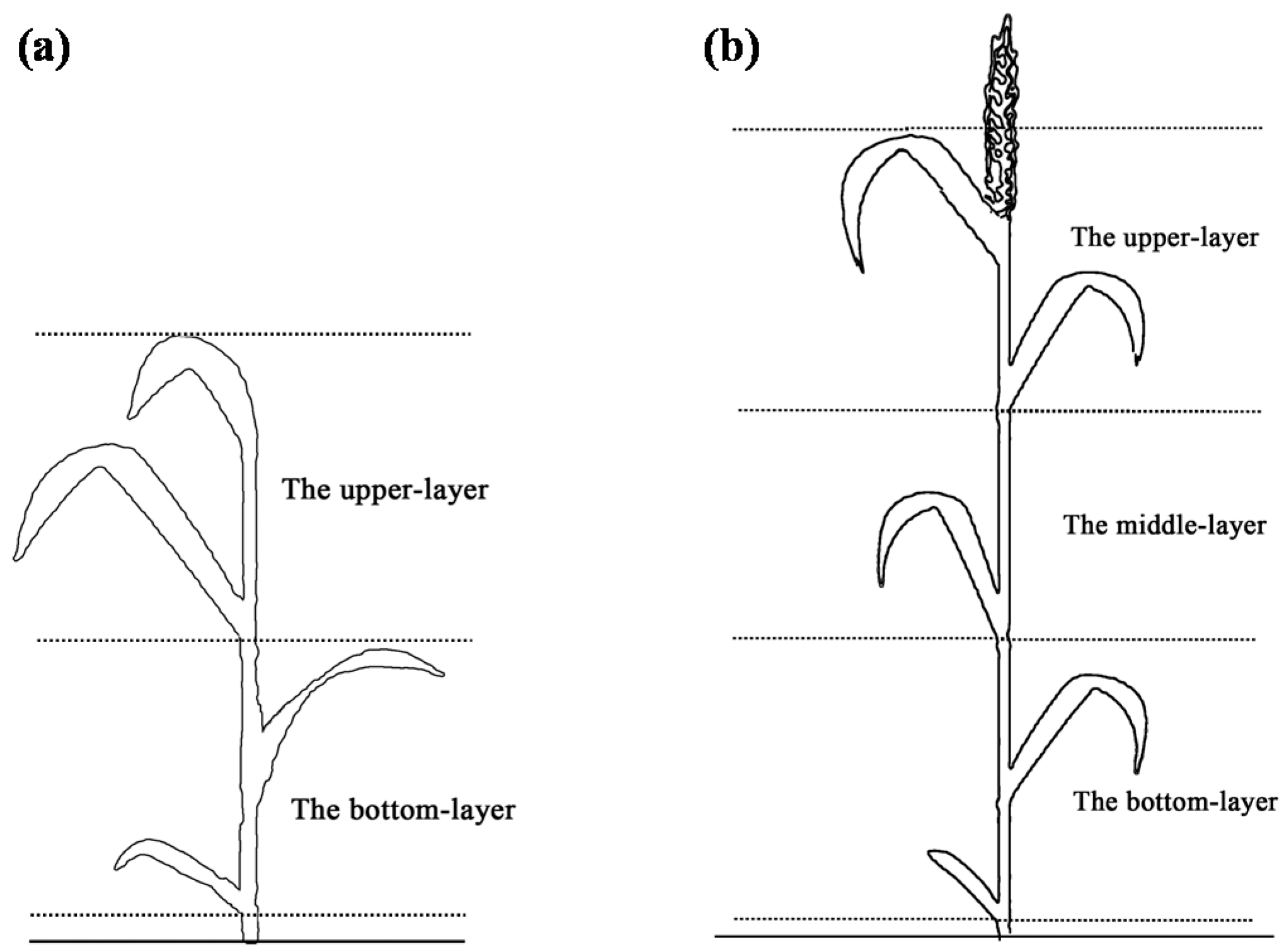
2.3. Leaf Chlorophyll Content Vertical Distribution Measurement
2.4. Vegetation Indices and Data Analysis
2.4.1. Published Vegetation Indices
2.4.2. Computing Two-Band and Three-Band Spectral Indices
2.4.3. Model Calibration and Validation
3. Results
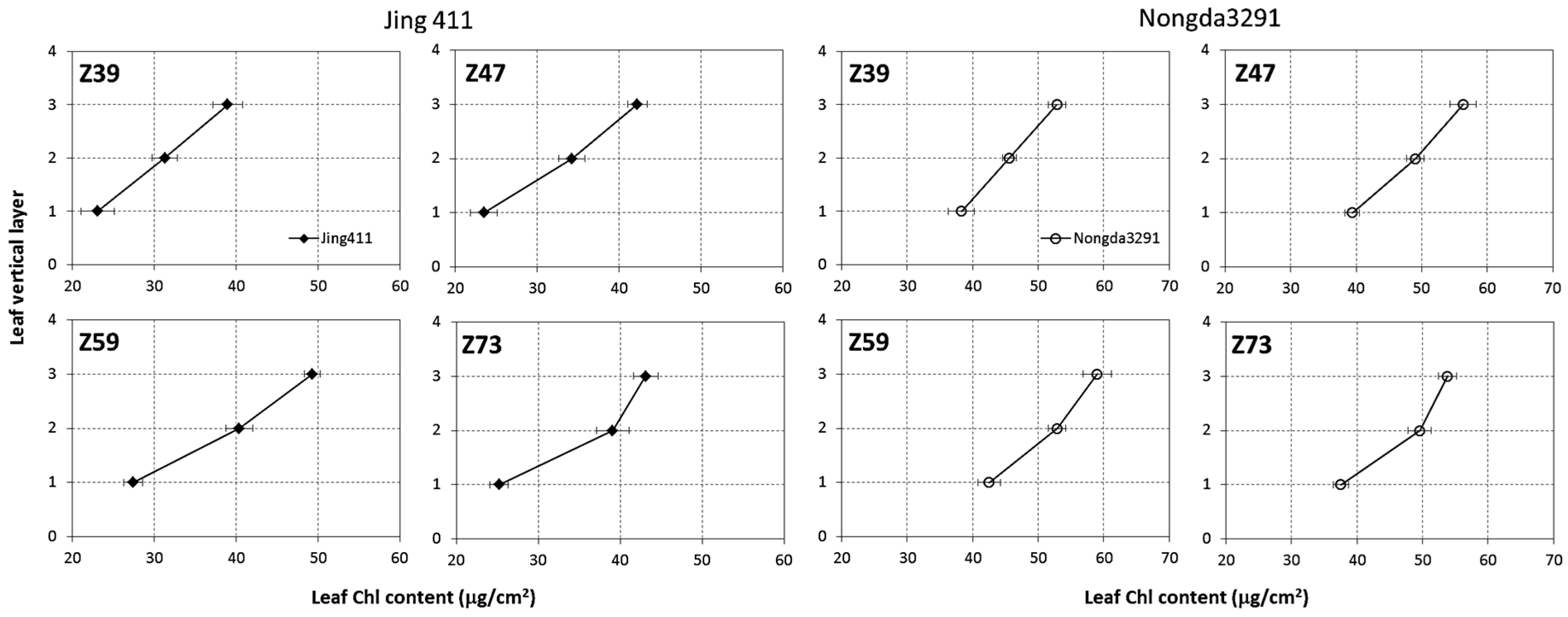
3.1. Vertical Distribution of Leaf Chlorophyll Content within Wheat Canopy
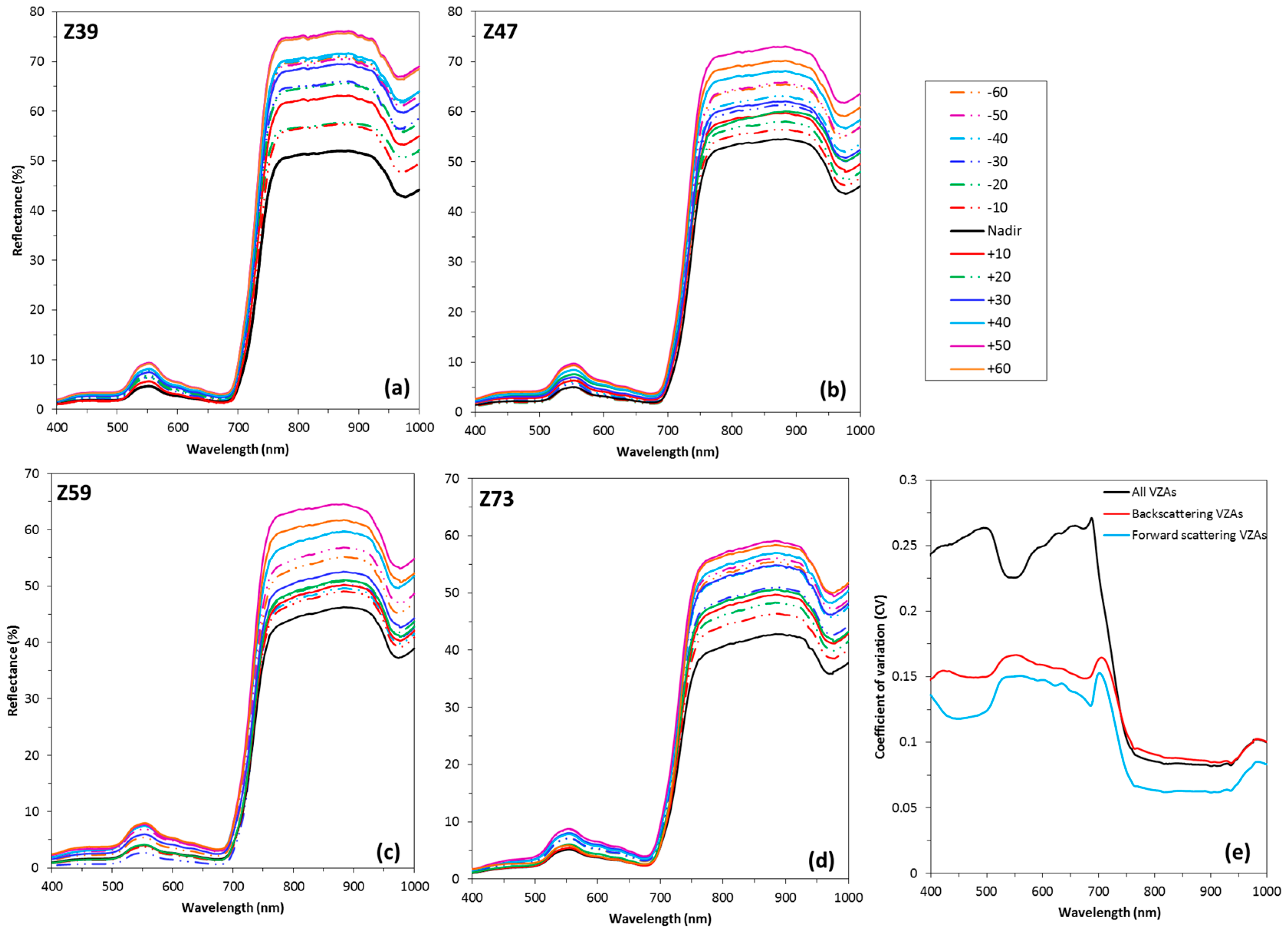
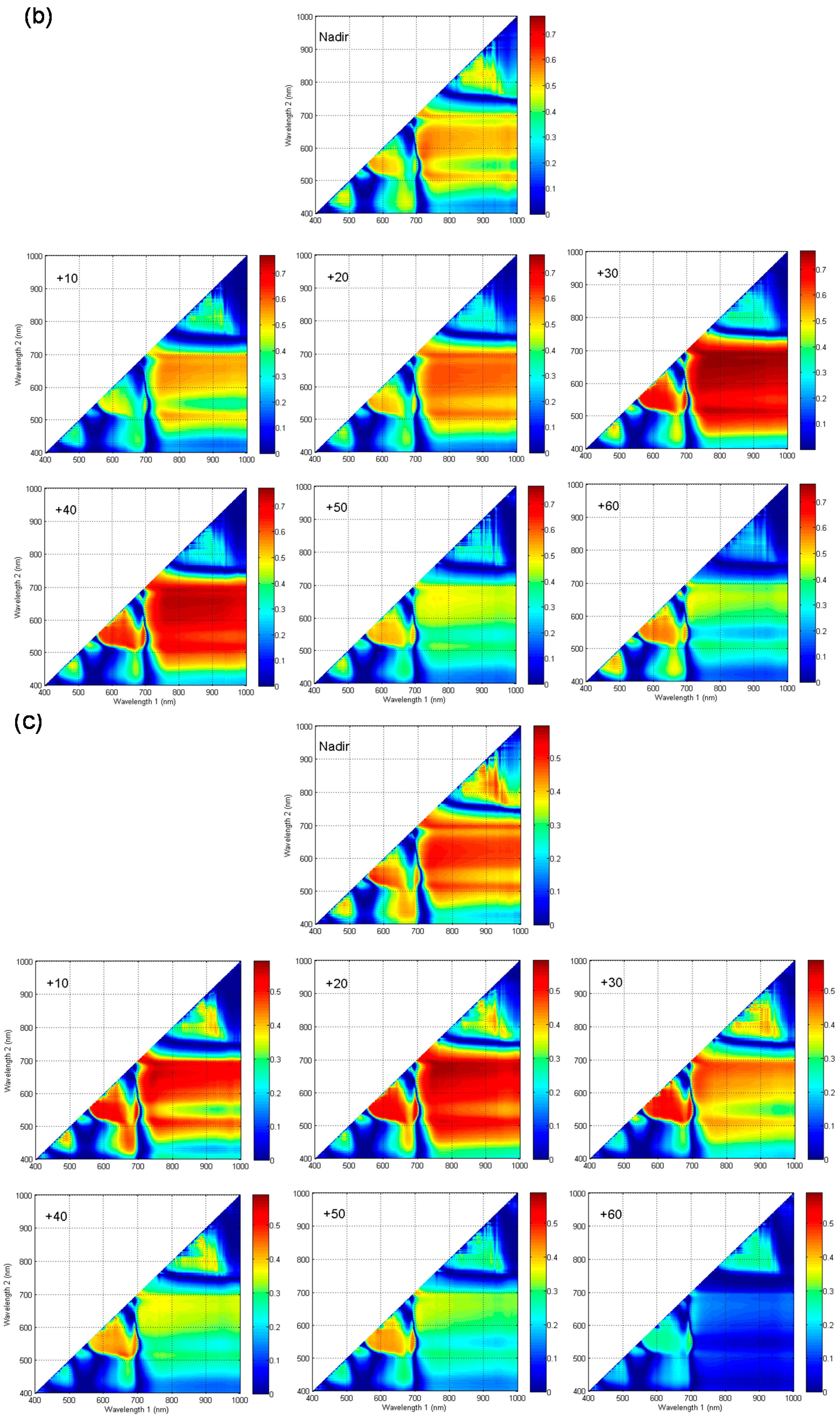
3.2. Response Characteristics of Spectral Reflectance among Different VZAs
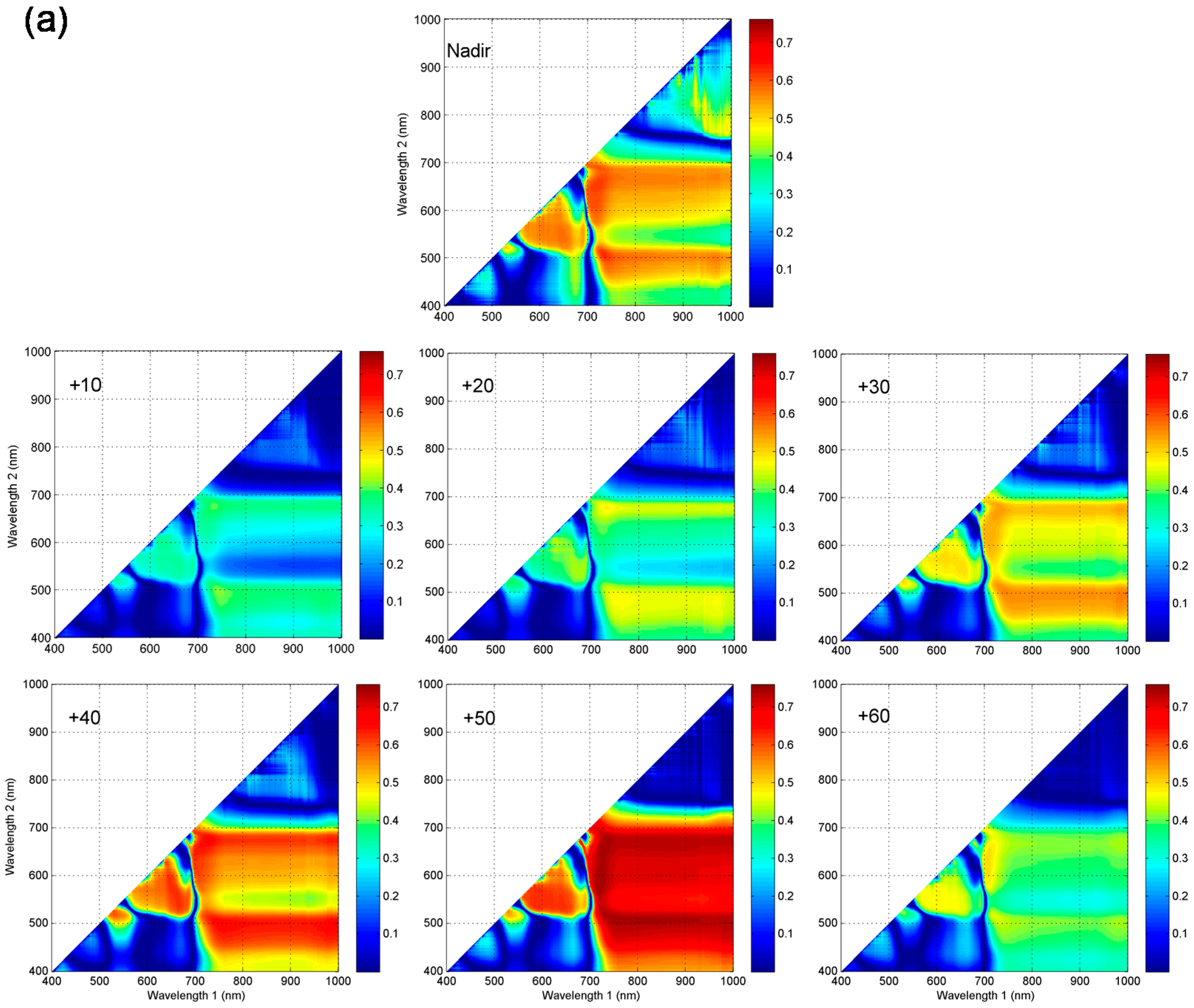
3.3. Sensitivity Analyses of Published Spectral Indices Derived from VZAs Data to Leaf Chlorophyll Content in Vertical Layers
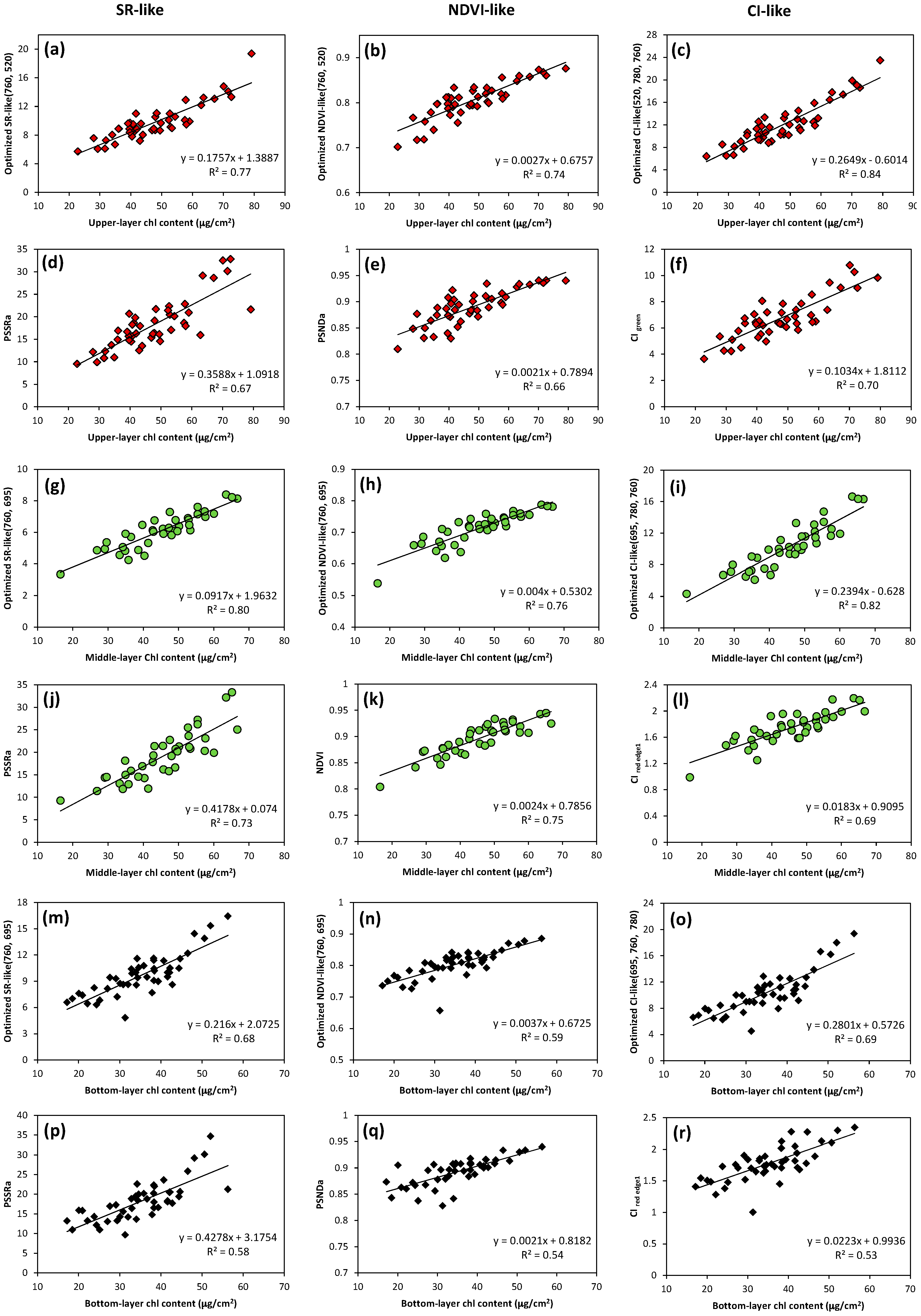
3.4. Optimization of Two-Band and Three-Band Indices for Estimation of Leaf Chlorophyll Content in Vertical Layers
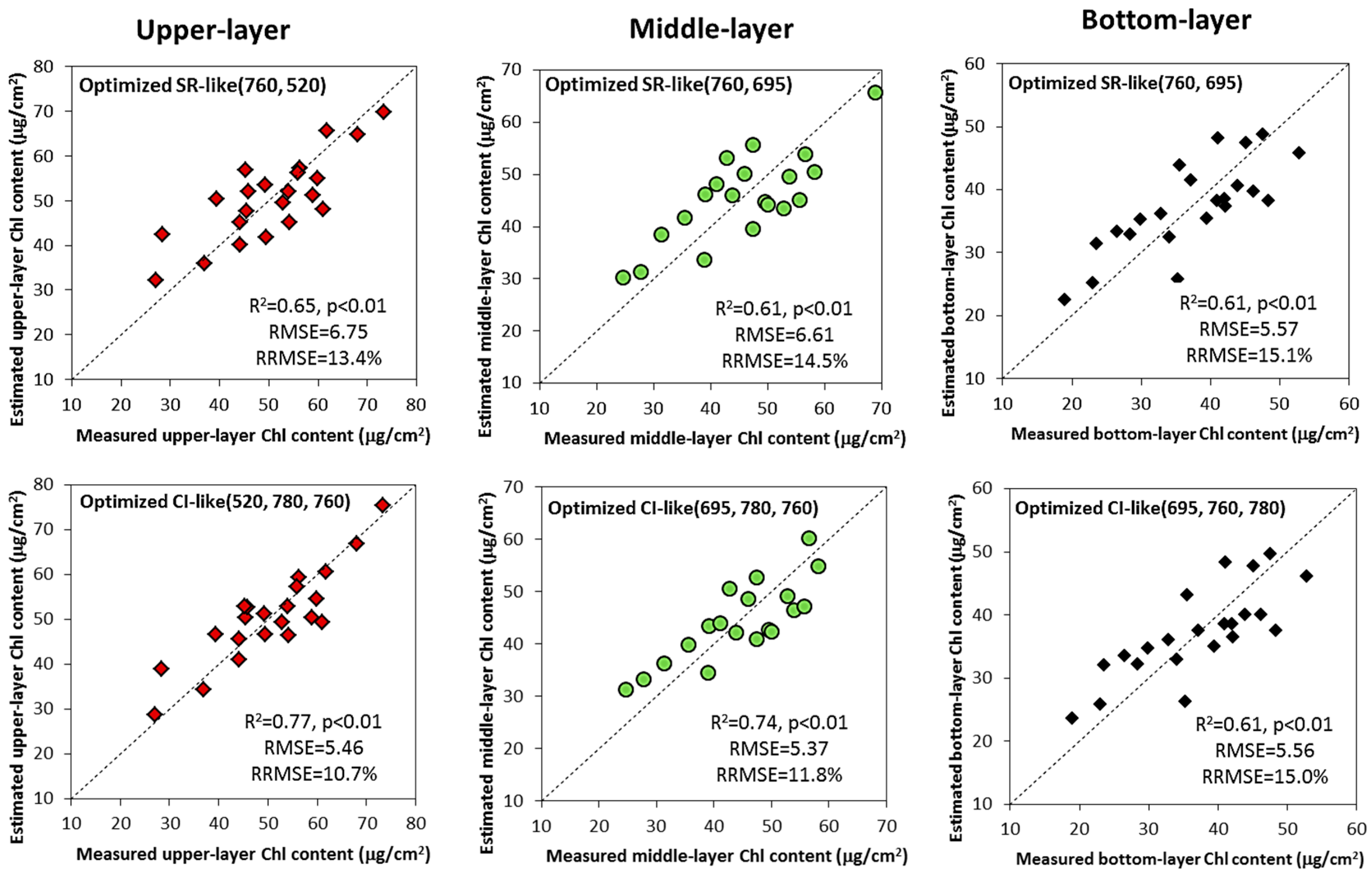
3.5. Validation of Vertical Leaf Chlorophyll Content Estimation Models
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Ciganda, V.; Gitelson, A.; Schepers, J. Vertical profile and temporal variation of chlorophyll in maize canopy: Quantitative “crop vigor” indicator by means of reflectance-based techniques. Agron. J. 2008, 100, 1409–1417. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived from hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS)—An algorithm for the remote estimation of the concentrations of chlorophyll-a, chlorophyll-b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Winterhalter, L.; Mistele, B.; Schmidhalter, U. Assessing the vertical footprint of reflectance measurements to characterize nitrogen uptake and biomass distribution in maize canopies. Field Crops Res. 2012, 129, 14–20. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yang, G.; Feng, H. Variations in crop variables within wheat canopies and responses of canopy spectral characteristics and derived vegetation indices to different vertical leaf layers and spikes. Remote Sens. Environ. 2015, 169, 358–374. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Huang, W.; Yang, G. Non-uniform vertical nitrogen distribution within plant canopy and its estimation by remote sensing: A review. Field Crops Res. 2013, 142, 75–84. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Macler, B.A.; Plummer, S.E.; Peterson, D.L. Reflectance spectroscopy of fresh whole leaves for the estimation of chemical concentration. Remote Sens. Environ. 1992, 39, 153–166. [Google Scholar] [CrossRef]
- Wu, C.Y.; Niu, Z.; Tang, Q.; Huang, W.J. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Clevers, J.; Kooistra, L. Using hyperspectral remote sensing data for retrieving canopy chlorophyll and nitrogen content. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Feret, J.-B.; Francois, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.R.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. Prospect-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Casa, R.; Castaldi, F.; Pascucci, S.; Pignatti, S. Chlorophyll estimation in field crops: An assessment of handheld leaf meters and spectral reflectance measurements. J. Agric. Sci. 2015, 153, 876–890. [Google Scholar] [CrossRef]
- Atzberger, C.; Guerif, M.; Baret, F.; Werner, W. Comparative analysis of three chemometric techniques for the spectroradiometric assessment of canopy chlorophyll content in winter wheat. Comput. Electron. Agric. 2010, 73, 165–173. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green lai of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C.; Corsi, F.; Cho, M. LAI and chlorophyll estimation for a heterogeneous grassland using hyperspectral measurements. ISPRS J. Photogramm. Remote Sens. 2008, 63, 409–426. [Google Scholar] [CrossRef]
- Lee, K.S.; Cohen, W.B.; Kennedy, R.E.; Maiersperger, T.K.; Gower, S.T. Hyperspectral versus multispectral data for estimating leaf area index in four different biomes. Remote Sens. Environ. 2004, 91, 508–520. [Google Scholar] [CrossRef]
- Wang, W.; Yao, X.; Yao, X.; Tian, Y.; Liu, X.; Ni, J.; Cao, W.; Zhu, Y. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crops Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.C.; Chen, X.P.; Schmidhalter, U. Optimising three-band spectral indices to assess aerial n concentration, n uptake and aboveground biomass of winter wheat remotely in china and germany. ISPRS J. Photogramm. Remote Sens. 2014, 92, 112–123. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P.H. Canopy vertical heterogeneity plays a critical role in reflectance simulation. Agric. For. Meteorol. 2013, 169, 111–121. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Z.; Huang, L.; Lamb, D.W.; Ma, Z.; Zhang, J.; Wang, J.; Zhao, C. Estimation of vertical distribution of chlorophyll concentration by bi-directional canopy reflectance spectra in winter wheat. Precis. Agric. 2011, 12, 165–178. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.J.; Lao, C.L.; Zhang, L.D.; Luo, C.B.; Tao, W.; Liu, L.Y.; Song, X.Y.; Ma, Z.H. Inversion of winter wheat foliage vertical distribution based on canopy reflected spectrum by partial least squares regression method. Spectrosc. Spectr. Anal. 2007, 27, 1319–1322. [Google Scholar]
- Liao, Q.H.; Zhang, D.Y.; Wang, J.H.; Yang, G.J.; Yang, H.; Craig, C.; Wong, Z.J.; Wang, D.C. Assessment of chlorophyll content using a new vegetation index based on multi-angular hyperspectral image data. Spectrosc. Spectr. Anal. 2014, 34, 1599–1604. [Google Scholar]
- Gnyp, M.L.; Panitzki, M.; Reusch, S. Proximal nitrogen sensing by off-nadir and nadir measurements in winter wheat canopy. In Precision Agriculture ’15; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015. [Google Scholar]
- Horler, D.N.H.; Dockray, M.; Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Zhao, C.J.; Li, H.L.; Li, P.S.; Yang, G.J.; Gu, X.H.; Lan, Y.B. Effect of vertical distribution of crop structure and biochemical parameters of winter wheat on canopy reflectance characteristics and spectral indices. IEEE Geosci. Remote Sens. Mag. 2017, 55, 236–247. [Google Scholar] [CrossRef]
- Stagakis, S.; Markos, N.; Sykioti, O.; Kyparissis, A. Monitoring canopy biophysical and biochemical parameters in ecosystem scale using satellite hyperspectral imagery: An application on a phlomis fruticosa mediterranean ecosystem using multiangular chris/proba observations. Remote Sens. Environ. 2010, 114, 977–994. [Google Scholar] [CrossRef]
- Sykioti, O.; Paronis, D.; Stagakis, S.; Kyparissis, A. Band depth analysis of chris/proba data for the study of a mediterranean natural ecosystem. Correlations with leaf optical properties and ecophysiological parameters. Remote Sens. Environ. 2011, 115, 752–766. [Google Scholar] [CrossRef]
- Wu, C.Y.; Niu, Z.; Wang, J.D.; Gao, S.A.; Huang, W.J. Predicting leaf area index in wheat using angular vegetation indices derived from in situ canopy measurements. Can. J. Remote Sens. 2010, 36, 301–312. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjon, A.; Lopez-Lozano, R.; Miller, J.R.; Martin, P.; Cachorro, V.; Gonzalez, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.J. Red and photographic infrared line combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra-experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The meris terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Vina, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semiempirical indices to assess carotenoids chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Richter, K.; Atzberger, C.; Hank, T.B.; Mauser, W. Derivation of biophysical variables from earth observation data: Validation and statistical measures. J. Appl. Remote Sens. 2012, 6. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; Wang, D.; Zhuang, Q.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Xia, X. Developing High Yielding Wheat Varieties from Core Parent “Jing 411”. Crops 2009, 4, 1–5. [Google Scholar]
- Wang, Z.J.; Wang, J.H.; Zhao, C.J.; Zhao, M.; Huang, W.J.; Wang, C.Z. Vertical distribution of nitrogen in different layers of leaf and stem and their relationship with grain quality of winter wheat. J. Plant Nutr. 2005, 28, 73–91. [Google Scholar] [CrossRef]
- Verrelst, J.; Schaepman, M.E.; Koetz, B.; Kneubuehler, M. Angular sensitivity analysis of vegetation indices derived from chris/proba data. Remote Sens. Environ. 2008, 112, 2341–2353. [Google Scholar] [CrossRef]
- Hasegawa, K.; Matsuyama, H.; Tsuzuki, H.; Sweda, T. Improving the estimation of leaf area index by using remotely sensed ndvi with brdf signatures. Remote Sens. Environ. 2010, 114, 514–519. [Google Scholar] [CrossRef]
- He, L.; Zhang, H.Y.; Zhang, Y.S.; Song, X.; Feng, W.; Kang, G.Z.; Wang, C.Y.; Guo, T.C. Estimating canopy leaf nitrogen concentration in winter wheat based on multi-angular hyperspectral remote sensing. Eur. J. Agron. 2016, 73, 170–185. [Google Scholar] [CrossRef]
- Sandmeier, S.; Muller, C.; Hosgood, B.; Andreoli, G. Physical mechanisms in hyperspectral brdf data of grass and watercress. Remote Sens. Environ. 1998, 66, 222–233. [Google Scholar] [CrossRef]
- WalterShea, E.A.; Privette, J.; Cornell, D.; Mesarch, M.A.; Hays, C.J. Relations between directional spectral vegetation indices and leaf area and absorbed radiation in alfalfa. Remote Sens. Environ. 1997, 61, 162–177. [Google Scholar] [CrossRef]
- Galvao, L.S.; Roberts, D.A.; Formaggio, A.R.; Numata, I.; Breunig, F.M. View angle effects on the discrimination of soybean varieties and on the relationships between vegetation indices and yield using off-nadir hyperion data. Remote Sens. Environ. 2009, 113, 846–856. [Google Scholar] [CrossRef]
- Song, X.; Xu, D.; He, L.; Feng, W.; Wang, Y.; Wang, Z.; Coburn, C.A.; Guo, T. Using multi-angle hyperspectral data to monitor canopy leaf nitrogen content of wheat. Precis. Agric. 2016, 17, 721–736. [Google Scholar] [CrossRef]
- Garcia-Haro, F.J.; Sommer, S. A fast canopy reflectance model to simulate realistic remote sensing scenarios. Remote Sens. Environ. 2002, 81, 205–227. [Google Scholar] [CrossRef]
- Casa, R.; Jones, H.G. Lai retrieval from multiangular image classification and inversion of a ray tracing model. Remote Sens. Environ. 2005, 98, 414–428. [Google Scholar] [CrossRef]
- Xiao, C.; Li, S.; Wang, K.; Lu, Y.; Bai, J.; Xie, R.; Gao, S.; Li, X.; Tan, H. Response of canopy direction reflectance spectrum for the wheat vertical leaf distribution. Sci. Agric. Sin. 2008, 41, 2271–2278. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from eos-modis. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]

| Spectral Indices | Formula | Reference |
|---|---|---|
| Two-band spectral indices | ||
| PSSRa (Pigment specific simple ratio) | [31] | |
| PSSRb (Pigment specific simple ratio) | [31] | |
| PSNDa (Pigment specific normalized difference) | [31] | |
| PSNDb (Pigment specific normalized difference) | [31] | |
| GI (Green index) | [32] | |
| PRI (Photochemical reflectance index) | [33] | |
| NDVI (Normalized difference vegetation index) | [34] | |
| NDVI2 (Normalized difference vegetation index) | [35] | |
| Three-band spectral indices | ||
| MCARI (Modified chlorophyll absorption ratio index) | [36] | |
| TCARI (Transformed chlorophyll absorption ratio index) | [1] | |
| MTCI (MERIS Terrestrial Chlorophyll index) | [37] | |
| CIgreen (Chlorophyll index at green band) | [38,39] | |
| CIred edeg1 (Chlorophyll index at red edge band) | [38,39] | |
| CIred edge2 (Chlorophyll index at red edge band) | [38,39] | |
| SIPI (Structure-insensitive pigment index) | [40] | |
| −60 | −50 | −40 | −30 | −20 | −10 | Nadir | +10 | +20 | +30 | +40 | +50 | +60 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two-band indices | |||||||||||||
| PSSRa | 0.15 | 0.14 | 0.03 | 0.03 | 0.06 | 0.14 | 0.60 | 0.27 | 0.32 | 0.28 | 0.51 | 0.67 | 0.27 |
| PSSRb | 0.19 | 0.16 | 0.07 | 0.04 | 0.09 | 0.16 | 0.62 | 0.34 | 0.39 | 0.31 | 0.46 | 0.67 | 0.27 |
| PSNDa | 0.17 | 0.11 | 0.05 | 0.02 | 0.09 | 0.12 | 0.56 | 0.36 | 0.45 | 0.33 | 0.61 | 0.66 | 0.41 |
| PSNDb | 0.16 | 0.10 | 0.02 | 0.01 | 0.04 | 0.08 | 0.54 | 0.27 | 0.33 | 0.27 | 0.46 | 0.63 | 0.32 |
| GI | 0.19 | 0.19 | 0.16 | 0.11 | 0.19 | 0.20 | 0.51 | 0.26 | 0.33 | 0.27 | 0.46 | 0.41 | 0.32 |
| PRI | 0.23 | 0.18 | 0.07 | 0.07 | 0.08 | 0.10 | 0.42 | 0.09 | 0.11 | 0.14 | 0.20 | 0.37 | 0.29 |
| NDVI | 0.16 | 0.11 | 0.04 | 0.01 | 0.06 | 0.10 | 0.57 | 0.33 | 0.41 | 0.31 | 0.53 | 0.65 | 0.35 |
| NDVI2 | 0.10 | 0.06 | 0.00 | 0.00 | 0.01 | 0.06 | 0.43 | 0.17 | 0.26 | 0.22 | 0.35 | 0.56 | 0.24 |
| Three-band indices | |||||||||||||
| MCARI | 0.02 | 0.02 | 0.09 | 0.10 | 0.13 | 0.08 | 0.04 | 0.06 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 |
| TCARI | 0.00 | 0.00 | 0.04 | 0.08 | 0.10 | 0.05 | 0.00 | 0.02 | 0.00 | 0.00 | 0.04 | 0.12 | 0.07 |
| MTCI | 0.07 | 0.03 | 0.00 | 0.00 | 0.00 | 0.03 | 0.36 | 0.03 | 0.11 | 0.12 | 0.17 | 0.42 | 0.15 |
| CIgreen | 0.09 | 0.04 | 0.00 | 0.00 | 0.00 | 0.05 | 0.62 | 0.12 | 0.26 | 0.21 | 0.35 | 0.70 | 0.19 |
| CIred edeg1 | 0.09 | 0.04 | 0.01 | 0.00 | 0.00 | 0.05 | 0.49 | 0.08 | 0.21 | 0.19 | 0.28 | 0.64 | 0.18 |
| CIred edge2 | 0.07 | 0.02 | 0.00 | 0.00 | 0.00 | 0.03 | 0.30 | 0.03 | 0.14 | 0.12 | 0.19 | 0.43 | 0.14 |
| SIPI | 0.12 | 0.12 | 0.05 | 0.04 | 0.12 | 0.09 | 0.39 | 0.18 | 0.18 | 0.15 | 0.18 | 0.24 | 0.19 |
| −60 | −50 | −40 | −30 | −20 | −10 | Nadir | +10 | +20 | +30 | +40 | +50 | +60 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two-band indices | |||||||||||||
| PSSRa | 0.08 | 0.18 | 0.15 | 0.22 | 0.34 | 0.40 | 0.52 | 0.47 | 0.51 | 0.73 | 0.57 | 0.34 | 0.14 |
| PSSRb | 0.10 | 0.21 | 0.19 | 0.22 | 0.34 | 0.35 | 0.48 | 0.49 | 0.53 | 0.70 | 0.55 | 0.32 | 0.14 |
| PSNDa | 0.23 | 0.22 | 0.18 | 0.20 | 0.36 | 0.37 | 0.49 | 0.55 | 0.58 | 0.72 | 0.62 | 0.46 | 0.41 |
| PSNDb | 0.19 | 0.19 | 0.17 | 0.22 | 0.33 | 0.39 | 0.57 | 0.50 | 0.56 | 0.70 | 0.61 | 0.41 | 0.38 |
| GI | 0.29 | 0.29 | 0.26 | 0.26 | 0.31 | 0.32 | 0.35 | 0.22 | 0.25 | 0.49 | 0.37 | 0.31 | 0.32 |
| PRI | 0.40 | 0.29 | 0.32 | 0.32 | 0.38 | 0.40 | 0.48 | 0.32 | 0.39 | 0.56 | 0.42 | 0.42 | 0.41 |
| NDVI | 0.22 | 0.22 | 0.18 | 0.20 | 0.35 | 0.36 | 0.50 | 0.50 | 0.54 | 0.75 | 0.61 | 0.41 | 0.38 |
| NDVI2 | 0.07 | 0.10 | 0.09 | 0.14 | 0.23 | 0.28 | 0.49 | 0.43 | 0.53 | 0.70 | 0.58 | 0.36 | 0.26 |
| Three-band indices | |||||||||||||
| MCARI | 0.09 | 0.07 | 0.07 | 0.11 | 0.12 | 0.04 | 0.03 | 0.02 | 0.01 | 0.00 | 0.01 | 0.00 | 0.02 |
| TCARI | 0.03 | 0.01 | 0.02 | 0.04 | 0.05 | 0.01 | 0.03 | 0.00 | 0.04 | 0.01 | 0.07 | 0.03 | 0.07 |
| MTCI | 0.01 | 0.03 | 0.02 | 0.06 | 0.10 | 0.24 | 0.30 | 0.20 | 0.39 | 0.47 | 0.42 | 0.22 | 0.09 |
| CIgreen | 0.02 | 0.05 | 0.02 | 0.07 | 0.15 | 0.30 | 0.37 | 0.29 | 0.43 | 0.60 | 0.51 | 0.23 | 0.08 |
| CIred edeg1 | 0.03 | 0.08 | 0.05 | 0.11 | 0.18 | 0.30 | 0.40 | 0.32 | 0.50 | 0.69 | 0.55 | 0.29 | 0.13 |
| CIred edge2 | 0.01 | 0.04 | 0.02 | 0.06 | 0.11 | 0.21 | 0.29 | 0.16 | 0.38 | 0.43 | 0.41 | 0.20 | 0.08 |
| SIPI | 0.36 | 0.24 | 0.31 | 0.21 | 0.40 | 0.39 | 0.40 | 0.42 | 0.43 | 0.49 | 0.45 | 0.37 | 0.40 |
| −60 | −50 | −40 | −30 | −20 | −10 | Nadir | +10 | +20 | +30 | +40 | +50 | +60 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two-band indices | |||||||||||||
| PSSRa | 0.04 | 0.14 | 0.19 | 0.24 | 0.32 | 0.35 | 0.50 | 0.53 | 0.58 | 0.47 | 0.29 | 0.24 | 0.01 |
| PSSRb | 0.06 | 0.20 | 0.28 | 0.26 | 0.33 | 0.32 | 0.46 | 0.53 | 0.56 | 0.49 | 0.29 | 0.22 | 0.01 |
| PSNDa | 0.08 | 0.09 | 0.19 | 0.17 | 0.27 | 0.31 | 0.43 | 0.54 | 0.54 | 0.47 | 0.36 | 0.33 | 0.13 |
| PSNDb | 0.05 | 0.05 | 0.12 | 0.15 | 0.22 | 0.29 | 0.50 | 0.46 | 0.51 | 0.40 | 0.26 | 0.26 | 0.09 |
| GI | 0.21 | 0.37 | 0.37 | 0.31 | 0.27 | 0.25 | 0.28 | 0.28 | 0.31 | 0.39 | 0.25 | 0.24 | 0.14 |
| PRI | 0.17 | 0.18 | 0.21 | 0.27 | 0.29 | 0.33 | 0.49 | 0.31 | 0.41 | 0.36 | 0.25 | 0.28 | 0.14 |
| NDVI | 0.06 | 0.08 | 0.17 | 0.15 | 0.23 | 0.27 | 0.44 | 0.47 | 0.53 | 0.43 | 0.27 | 0.26 | 0.10 |
| NDVI2 | 0.01 | 0.01 | 0.04 | 0.09 | 0.17 | 0.29 | 0.47 | 0.50 | 0.52 | 0.35 | 0.24 | 0.21 | 0.04 |
| Three-band indices | |||||||||||||
| MCARI | 0.14 | 0.21 | 0.17 | 0.17 | 0.11 | 0.02 | 0.00 | 0.05 | 0.00 | 0.00 | 0.03 | 0.02 | 0.04 |
| TCARI | 0.08 | 0.10 | 0.06 | 0.08 | 0.04 | 0.00 | 0.01 | 0.01 | 0.00 | 0.02 | 0.01 | 0.00 | 0.02 |
| MTCI | 0.00 | 0.00 | 0.01 | 0.04 | 0.11 | 0.26 | 0.33 | 0.28 | 0.39 | 0.32 | 0.16 | 0.13 | 0.00 |
| CIgreen | 0.00 | 0.01 | 0.03 | 0.07 | 0.15 | 0.30 | 0.35 | 0.39 | 0.48 | 0.34 | 0.20 | 0.14 | 0.00 |
| CIred edge1 | 0.00 | 0.01 | 0.04 | 0.08 | 0.17 | 0.31 | 0.38 | 0.45 | 0.53 | 0.40 | 0.24 | 0.18 | 0.01 |
| CIred edge2 | 0.00 | 0.00 | 0.02 | 0.04 | 0.11 | 0.24 | 0.30 | 0.33 | 0.39 | 0.31 | 0.15 | 0.11 | 0.00 |
| SIPI | 0.10 | 0.08 | 0.12 | 0.11 | 0.21 | 0.24 | 0.37 | 0.43 | 0.37 | 0.36 | 0.23 | 0.23 | 0.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, W.; Huang, W.; Zhou, X.; Ye, H.; Dong, Y.; Casa, R. Off-Nadir Hyperspectral Sensing for Estimation of Vertical Profile of Leaf Chlorophyll Content within Wheat Canopies. Sensors 2017, 17, 2711. https://doi.org/10.3390/s17122711
Kong W, Huang W, Zhou X, Ye H, Dong Y, Casa R. Off-Nadir Hyperspectral Sensing for Estimation of Vertical Profile of Leaf Chlorophyll Content within Wheat Canopies. Sensors. 2017; 17(12):2711. https://doi.org/10.3390/s17122711
Chicago/Turabian StyleKong, Weiping, Wenjiang Huang, Xianfeng Zhou, Huichun Ye, Yingying Dong, and Raffaele Casa. 2017. "Off-Nadir Hyperspectral Sensing for Estimation of Vertical Profile of Leaf Chlorophyll Content within Wheat Canopies" Sensors 17, no. 12: 2711. https://doi.org/10.3390/s17122711
APA StyleKong, W., Huang, W., Zhou, X., Ye, H., Dong, Y., & Casa, R. (2017). Off-Nadir Hyperspectral Sensing for Estimation of Vertical Profile of Leaf Chlorophyll Content within Wheat Canopies. Sensors, 17(12), 2711. https://doi.org/10.3390/s17122711







