A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater
Abstract
1. Introduction
2. Materials and Methods
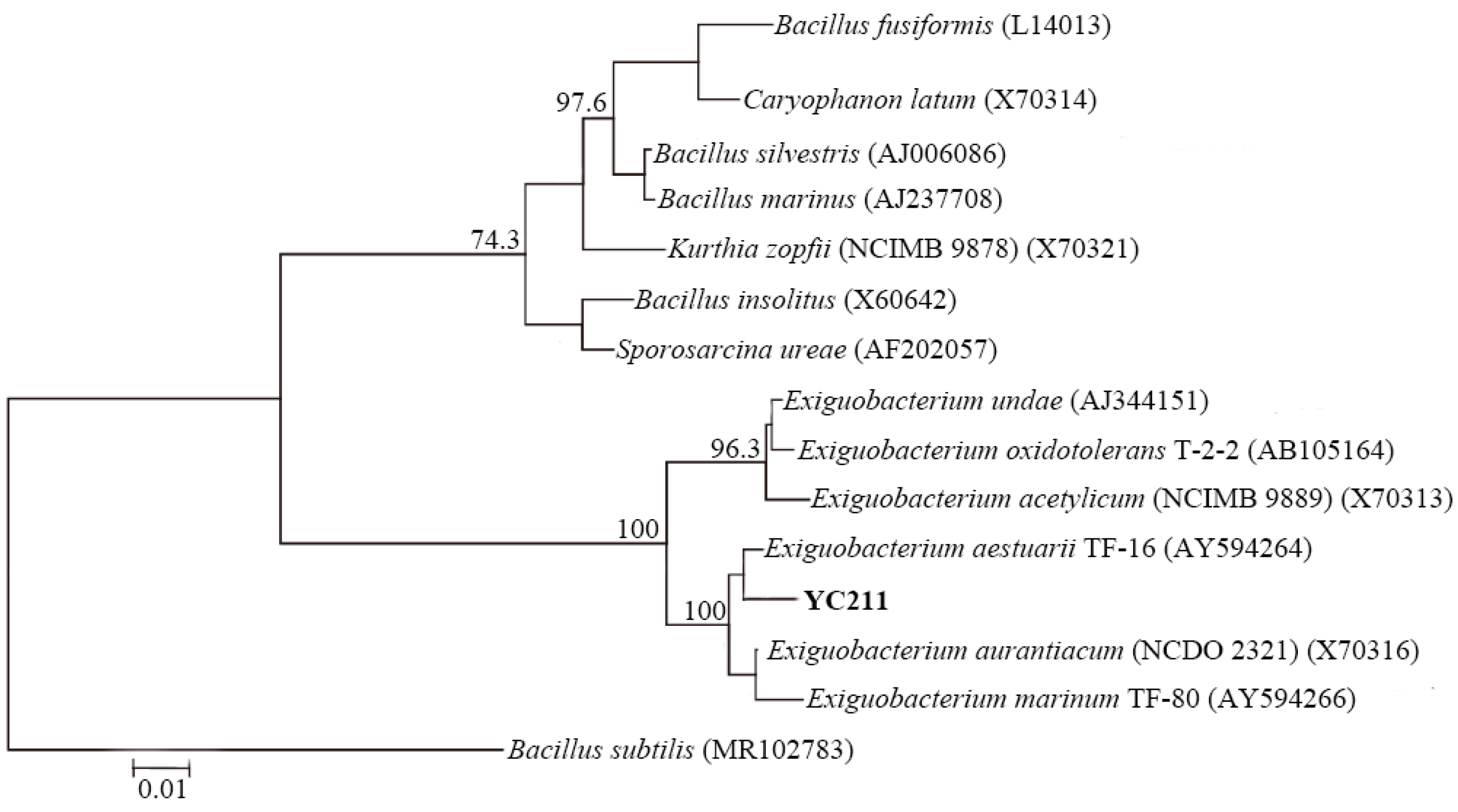
2.1. Bacterial Strains, Cultivation, and Identification
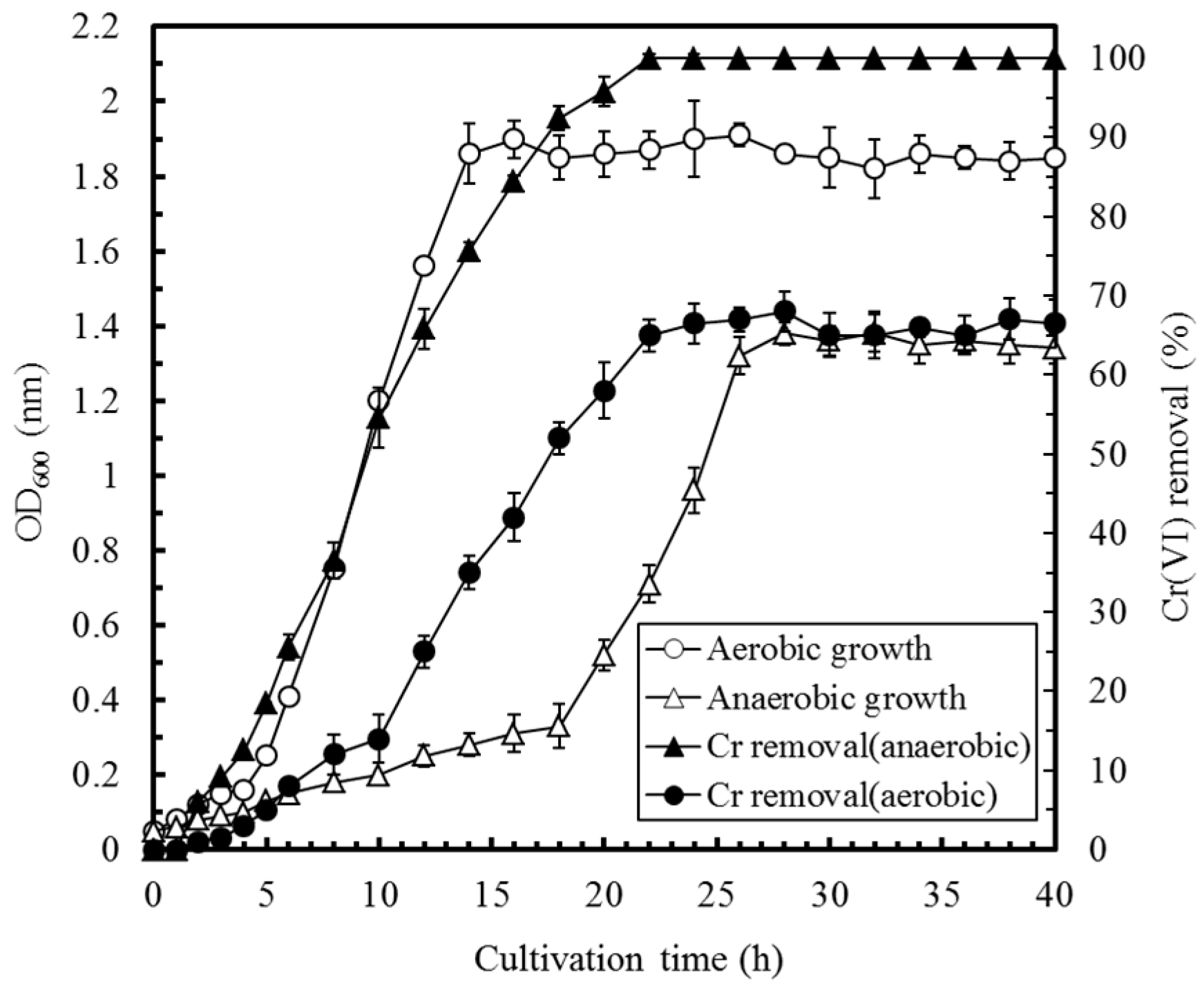
2.2. Bacterial Growth and Cr(VI) Removal
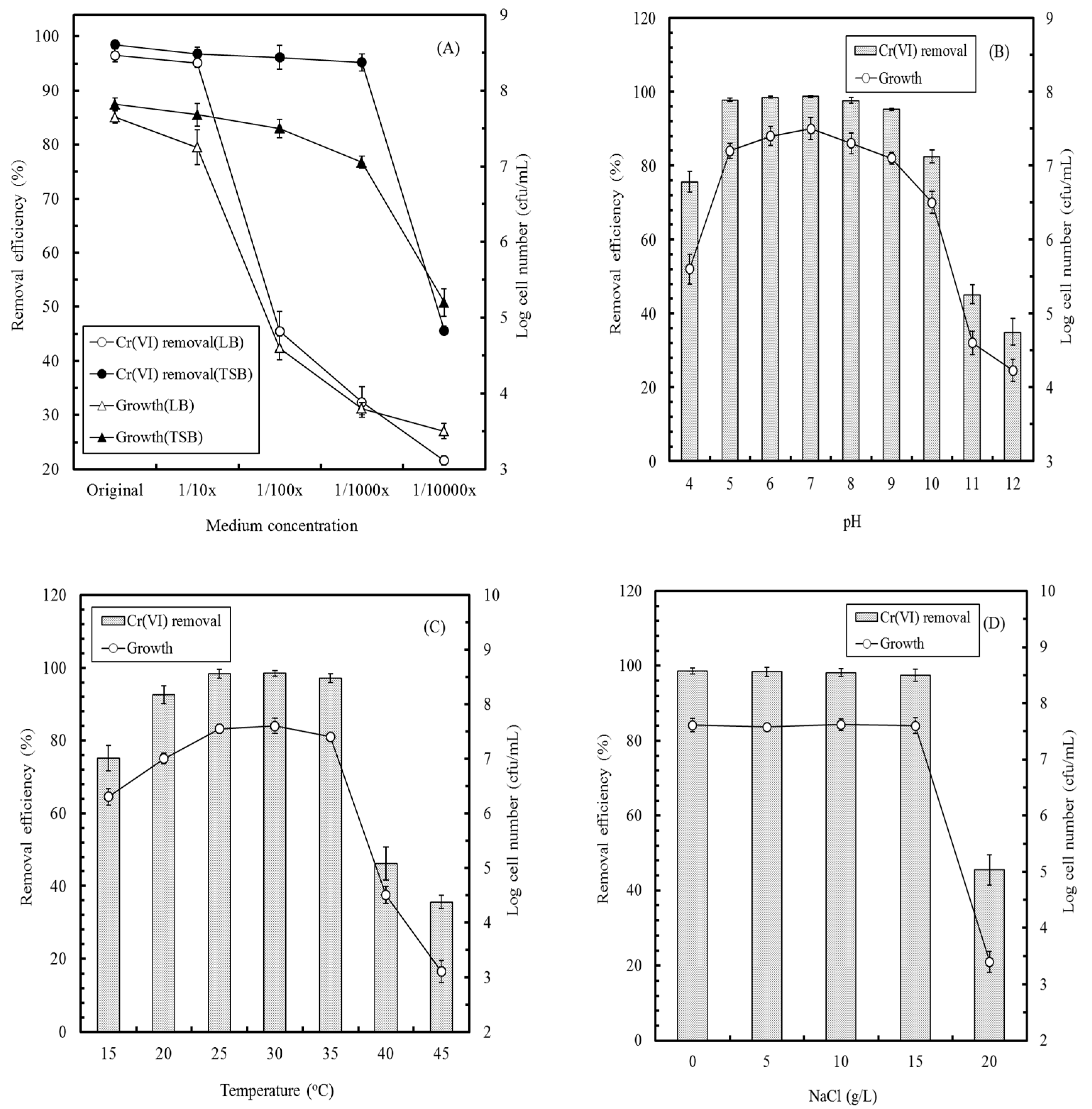
2.3. Factors Affecting Cr(VI) Removal by the YC211 Strain
2.4. Construction of the MFC-Based Biosensor and Its Operation
2.5. Cr(VI) Measurement in Artificial and Actual Electroplating Wastewater
2.6. Analysis
3. Results and Discussion
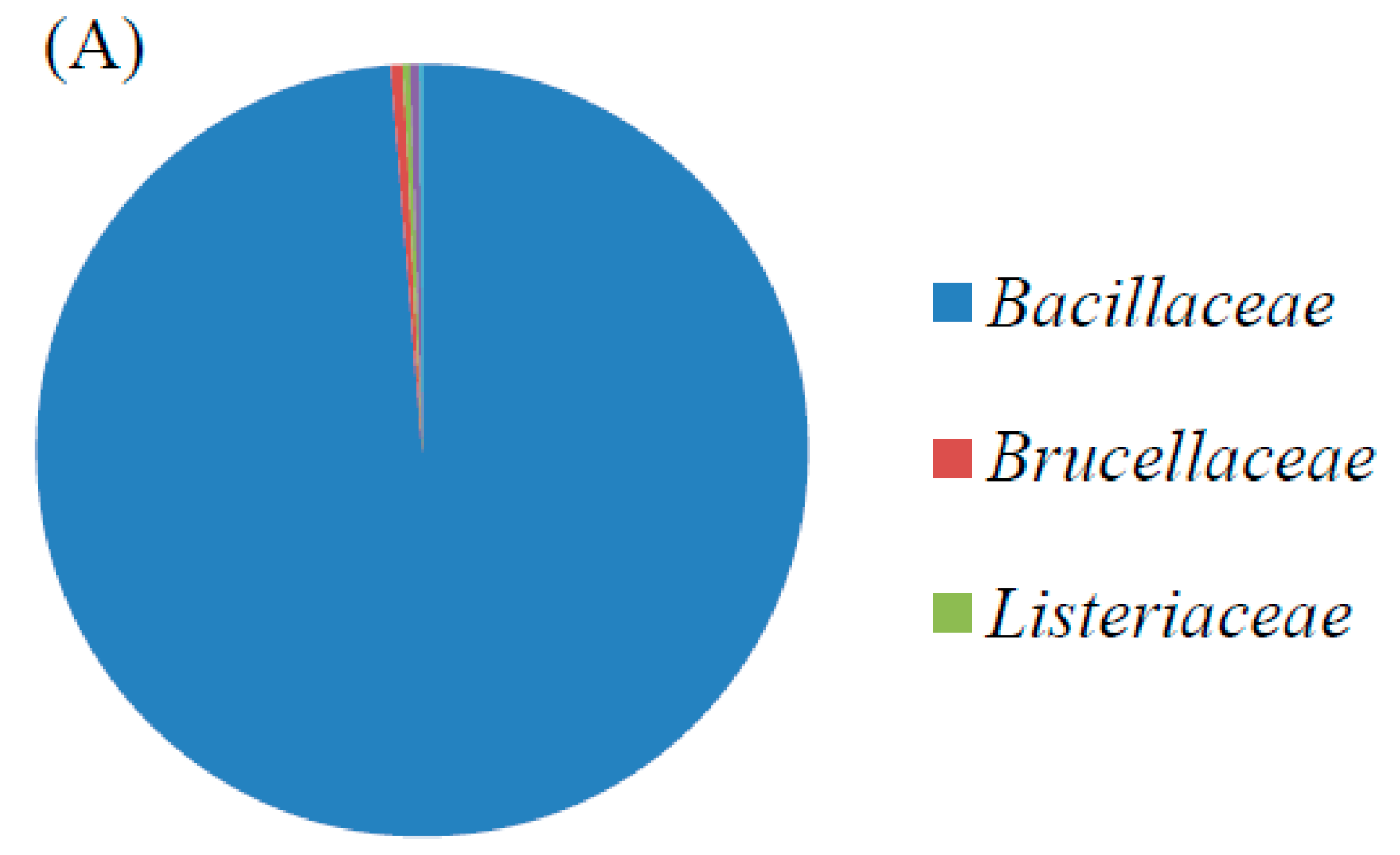
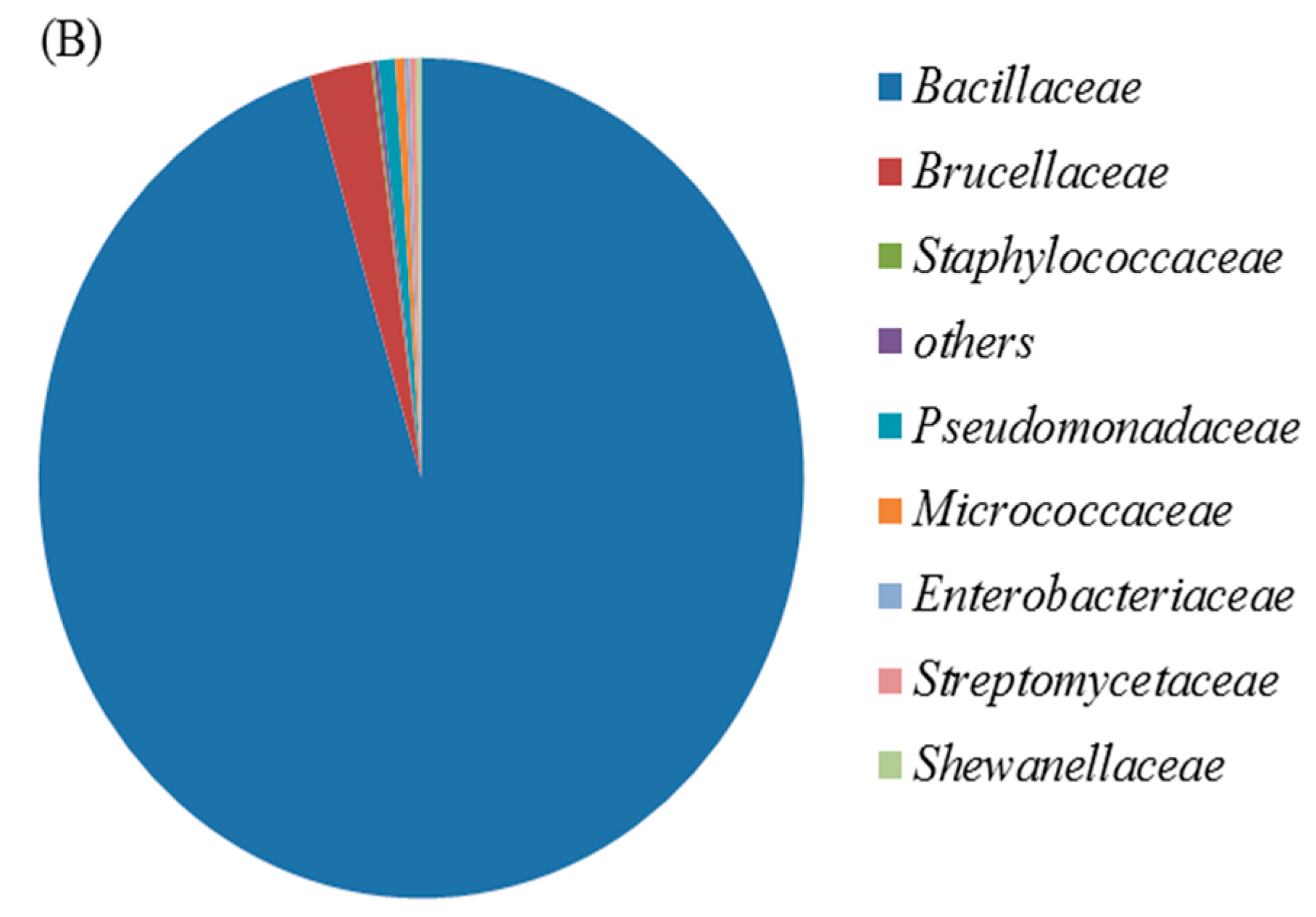
3.1. Identification and Characterization of the Cr(VI)-Reducing Bacterium
3.2. Factors Affecting Cr(VI) Removal by E. aestuarii YC211
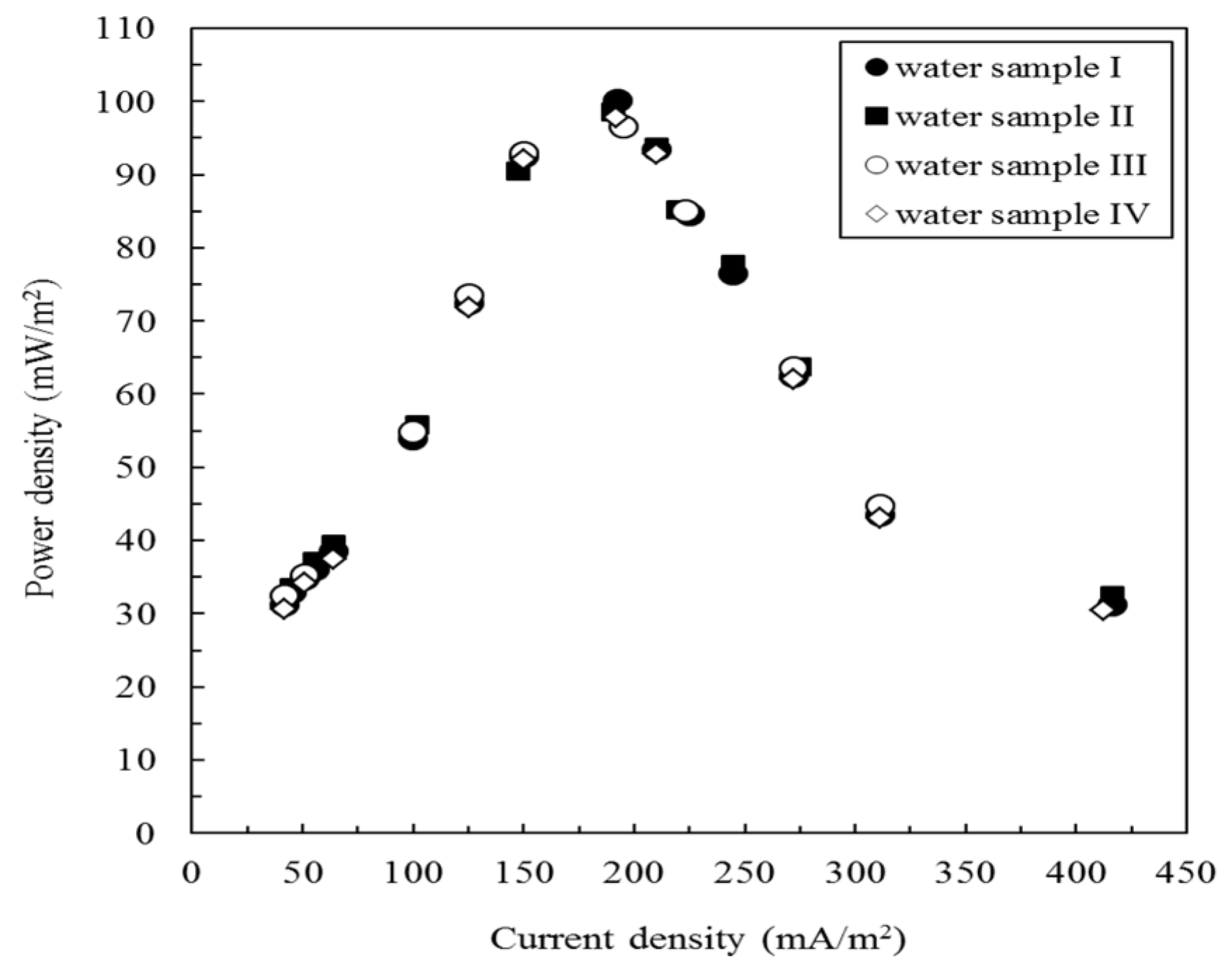
3.3. Effect of Coexisting Ions on the MFC Performance
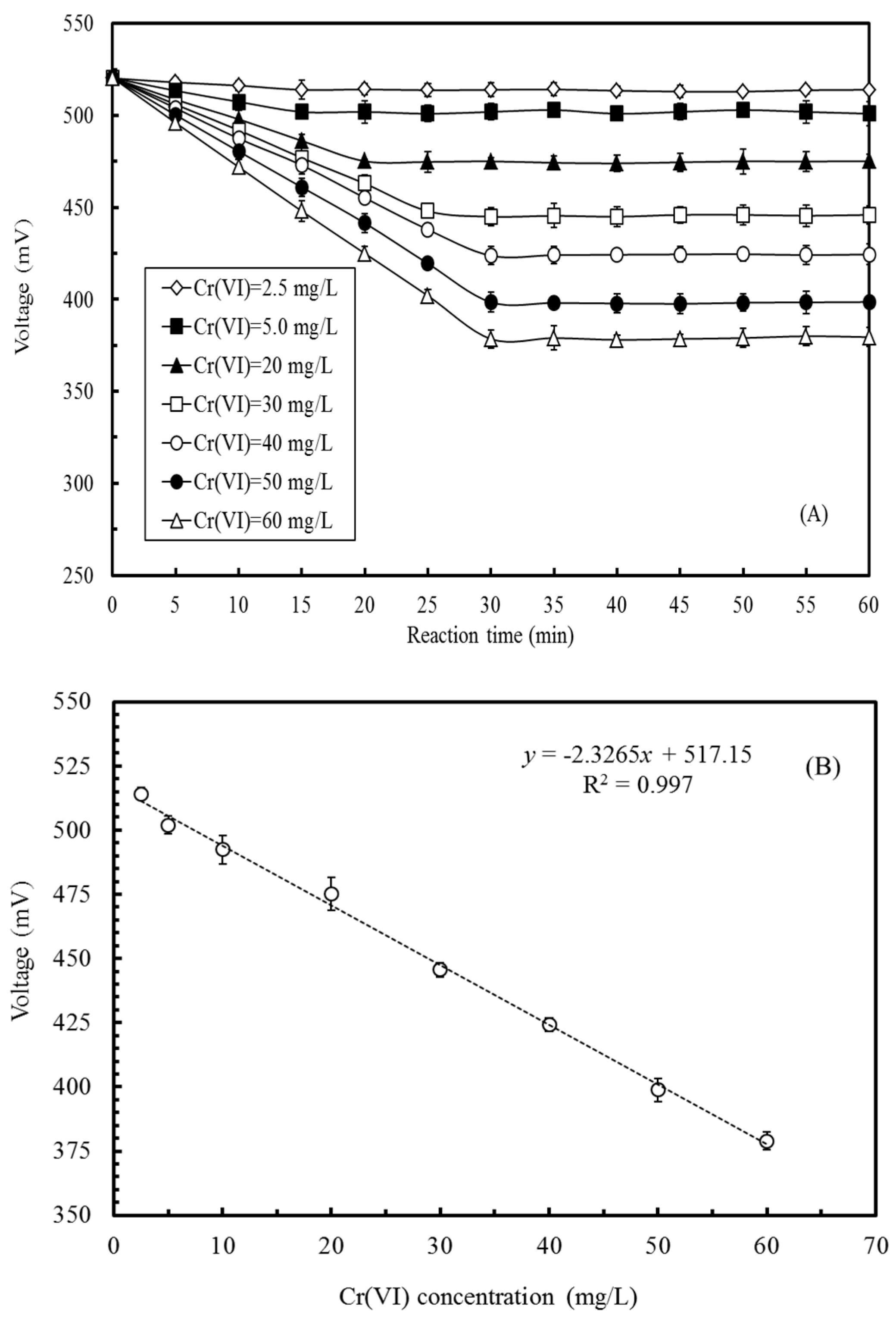
3.4. Effects of Cr(VI) Concentration on the Voltage Output of the MFC
3.5. Cr(VI) Measurement in Artificial and Actual Electroplating Wastewater
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bohrn, U.; Mucha, A.; Werner, C.F.; Trattner, B.; Backer, M.; Krumbe, C.; Schienle, M.; Stutz, E.; Schmitt-Landsiedel, D.; Fleischer, M.; et al. A critical comparison of cell-based sensor systems for the detection of Cr(VI) in aquatic environment. Sens. Actuators B Chem. 2013, 182, 58–65. [Google Scholar] [CrossRef]
- Branco, R.; Cristovao, A.; Morais, P.V. Highly sensitive, highly specific whole-cell bioreporters for the detection of chromate in environmental samples. PLoS ONE 2013, 8, e54005. [Google Scholar] [CrossRef] [PubMed]
- Samborska, A.; Stepniewska, Z.; Stepniewski, W. Influence of different oxidation states of chromium (VI, III) on soil urease activity. Geoderma 2004, 122, 317–322. [Google Scholar] [CrossRef]
- Coelho, C.; Branco, R.; Natal-da-Luz, T.; Sousa, J.P.; Morais, P.V. Evaluation of bacterial biosensors to determine chromate bioavailability and to assess ecotoxicity of soils. Chemosphere 2015, 128, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, F.A.; Poliandri, A.H.B.; Machiavelli, L.I.; Cabilla, J.P.; Duvilanski, B.H. In vivo and in vitro effects of chromium VI on anterior pituitary hormone release and cell viability. Toxicol. Appl. Pharmacol. 2007, 218, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Diaz, M.I.; Diaz-Perez, C.; Vargas, E.; Riveros-Rosas, H.; Campos-Garcia, J.; Cervantes, C. Mechanisms of bacterial resistance to chromium compounds. Biometals 2008, 21, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Cheng, C.Y.; Liu, M.H.; Chen, T.Y.; Hsieh, M.C.; Chung, Y.C. Utility of Ochrobactrum anthropi YC152 in a microbial fuel cell as an early warning device for hexavalent chromium determination. Sensors 2016, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Bagheri, H. Determination of Cr(VI) with selective sensing of Cr(VI) anions by a PVC-membrane electrode based on quinaldine red. J. Chin. Chem. Soc. 2009, 56, 289–295. [Google Scholar] [CrossRef]
- Michel, C.; Ouerd, A.; Battaglia-Brunet, F.; Guigues, N.; Grasa, J.P.; Bruschi, M.; Ignatiadis, I. Cr(VI) quantification using an amperometric enzyme-based sensor: Interference and physical and chemical factors controlling the biosensor response in ground waters. Biosens. Bioelectron. 2006, 22, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Nepomuscene, N.J.; Daniel, D.; Krastanov, A. Biosensor to detect chromium in wastewater. Biotechnol. Biotechnol. Equip. 2007, 21, 377–381. [Google Scholar] [CrossRef]
- Gurung, A.; Oh, S.; Kim, K.D.; Shin, B. Semi-continuous detection of toxic hexavalent chromium using a sulfur-oxidizing bacteria biosensor. J. Environ. Manag. 2012, 106, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, B.; Dong, Q.; Lei, Y.; Li, Y.; Ren, J.; McCutcheon, J.; Li, B. Flat microliter membrane-based microbial fuel cell as “on-line sticker sensor” for self-supported in situ monitoring of wastewater shocks. Bioresour. Technol. 2015, 197, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Cheng, C.Y.; Liu, M.H.; Chung, Y.C. Effects of operating parameters on measurements of biochemical oxygen demand using a mediatorless microbial fuel cell biosensor. Sensors 2016, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi1, V.; Verma, P. Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioprocess. 2016, 3, 38. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Jia, H.; Zhang, H. Bioelectricity generation in an integrated system combining microbial fuel cell and tubular membrane reactor: Effects of operation parameters performing amicrobial fuel cell-based biosensor for tubular membrane bioreactor. Bioresour. Technol. 2014, 170, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Biosens. Bioelectron. 2012, 38, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Angelidaki, I.; Zhang, Y. Microbial electrochemical monitoring of volatile fatty acids during anaerobic digestion. Environ. Sci. Technol. 2016, 50, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, X.; Zhao, N.; Angelidaki, I.; Zhang, Y. Bio-electrolytic sensor for rapid monitoring of volatile fatty acids in anaerobic digestion process. Water Res. 2017, 111, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lei, Y.; Li, B. A batch-mode cube microbial fuel cell based “shock” biosensor for wastewater quality monitoring. Biosens. Bioelectron. 2014, 62, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Paul, A.K. Hexavalent chromium reduction by aerobic heterotrophic bacteria indigenous to chromite mine overburden. Braz. J. Microbiol. 2013, 44, 307–315. [Google Scholar] [PubMed]
- Cheung, K.H.; Gu, J.D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar] [CrossRef]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An overview of electron acceptors in microbial fuel cell. Front. Microbiol. 2017, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Corby-Harris, V.; Snyder, L.A.; Schwan, M.R.; Maes, P.; McFrederick, Q.S.; Anderson, K.E. Origin and effect of alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl. Environ. Microbiol. 2014, 80, 7460–7472. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Cheng, C.Y.; Chen, C.K.; Hsieh, M.C.; Lin, S.T.; Ho, K.Y.; Li, J.W.; Lin, C.P.; Chung, Y.C. Hexavalent chromium removal and bioelectricity generation by Ochrobactrum sp. YC211 under different oxygen conditions. J. Environ. Sci. Health Part A 2014, 51, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Naz, I.; Hodgson, D.; Smith, A.; Marchesi, J.; Ahmed, S.; Avignone-Rossa, C.; Saroj, D.P. Effect of the chemical composition of filter media on the microbial community in wastewater biofilms at different temperatures. RSC Adv. 2016, 6, 104345–104353. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Lee, M.H.; Jung, S.Y.; Song, J.J.; Oh, T.K.; Yoon, J.H. Exiguobacterium aestuarii sp. nov. and Exiguobacterium marinum sp. nov., isolated from a tidal flat of the Yellow Sea in Korea. Int. J. Syst. Evol. Microbiol. 2005, 55, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Pattanapipitpaisal, P.; Mabbett, A.N.; Finlay, J.A.; Beswick, A.J.; Paterson-Beedle, M.; Essa, A.; Wright, J.; Tolley, M.R.; Badar, U.; Ahmed, N.; et al. Reduction of Cr(VI) and bioaccumulation of chromium by Gram positive and Gram negative microorganisms not previously exposed to Cr-stress. Environ. Technol. 2002, 23, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Chatterjee, C.; Gupta, B. Isolation and characterization of heavy metal tolerant Gram-positive bacteria with bioremedial properties from municipal waste rich soil of Kestopur canal (Kolkata), West Bengal, India. Biologia 2012, 67, 827–836. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J.Q.; Wu, X.L.; Tang, Y.Q.; Chen, F.M. Characteristics of removal of Cr(VI) by Rhodococcus sp. Chr-9 and Exiguobacterium sp. Chr-43. Chin. J. Appl. Environ. Biol. 2012, 18, 971–977. [Google Scholar] [CrossRef]
- Okeke, B.C. Bioremoval of hexavalent chromium from water by a salt tolerant bacterium, Exiguobacterium sp. GS1. J. Ind. Microbiol. Biotechnol. 2008, 35, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Vyrides, I.; Stuckey, D.C. Chromium removal mechanisms and bacterial community in an integrated membrane bioreactor system. Environ. Eng. Sci. 2011, 28, 661–670. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bernarda, A.; Huang, C.Y.; Lee, D.J.; Chang, J.S. Micro-sized microbial fuel cell: A mini-review. Bioresour. Technol. 2011, 102, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Joutey, N.T.; Sayel, H.; Bahafid, W.; El Ghachtouli, N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015, 233, 45–69. [Google Scholar] [PubMed]
- Xafenias, N.; Zhang, Y.; Banks, C.J. Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ. Sci. Technol. 2013, 47, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
| Artificial Wastewater | Electroplating Wastewater | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard Cr(VI) Concentration of (mg/L) | ||||||||||
| 7.5 | 15 | 25 | 35 | 45 | 55 | A | B | C | D | |
| MFC biosensor | 8.1 ± 0.25 | 13.7 ± 0.67 | 25.8 ± 1.35 | 36.3 ± 2.05 | 47.1 ± 2.80 | 52.6 ± 1.55 | 32.5 ± 2.16 | 50.8 ± 1.81 | 278 ± 9.43 | 516 ± 13.52 |
| Colorimetric method | 7.4 ± 0.15 | 15.1 ± 0.75 | 24.1 ± 1.02 | 36.2 ± 2.12 | 46.4 ± 1.81 | 56.2 ± 2.07 | 31.8 ± 2.37 | 53.6 ± 2.62 | 296 ± 12.27 | 543 ± 15.18 |
| Deviation (%) 1 | 8.0 | −8.7 | 3.2 | 3.7 | 4.7 | −4.4 | – | – | – | – |
| Deviation (%) 2 | −1.3 | 0.7 | −3.6 | 3.4 | 3.1 | 2.2 | – | – | – | – |
| Deviation (%) 3 | 9.5 | −9.3 | 7.1 | 0.3 | 1.5 | −6.4 | 2.2 | −5.2 | −6.1 | −5.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-C.; Tsai, T.-H.; Liu, M.-H.; Kuo, J.-L.; Chang, Y.-C.; Chung, Y.-C. A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater. Sensors 2017, 17, 2461. https://doi.org/10.3390/s17112461
Wu L-C, Tsai T-H, Liu M-H, Kuo J-L, Chang Y-C, Chung Y-C. A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater. Sensors. 2017; 17(11):2461. https://doi.org/10.3390/s17112461
Chicago/Turabian StyleWu, Li-Chun, Teh-Hua Tsai, Man-Hai Liu, Jui-Ling Kuo, Yung-Chu Chang, and Ying-Chien Chung. 2017. "A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater" Sensors 17, no. 11: 2461. https://doi.org/10.3390/s17112461
APA StyleWu, L.-C., Tsai, T.-H., Liu, M.-H., Kuo, J.-L., Chang, Y.-C., & Chung, Y.-C. (2017). A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater. Sensors, 17(11), 2461. https://doi.org/10.3390/s17112461






