Electrodermal Activity Sensor for Classification of Calm/Distress Condition
Abstract
:1. Introduction
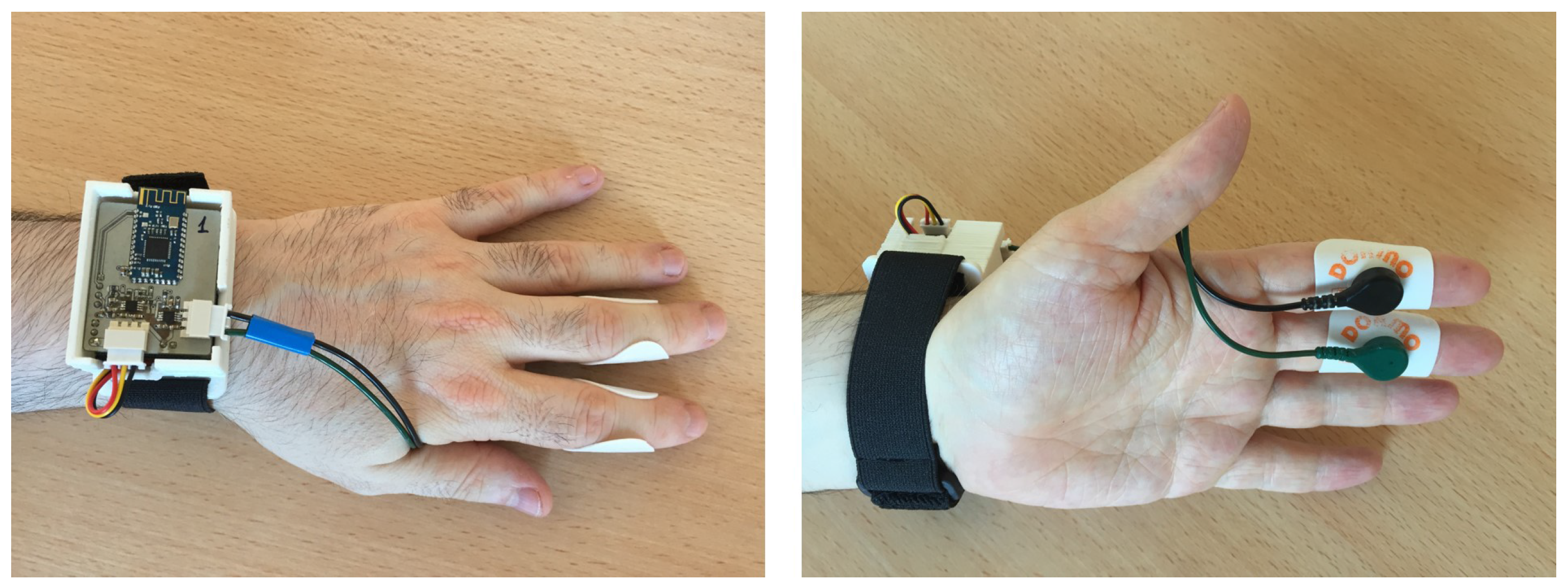
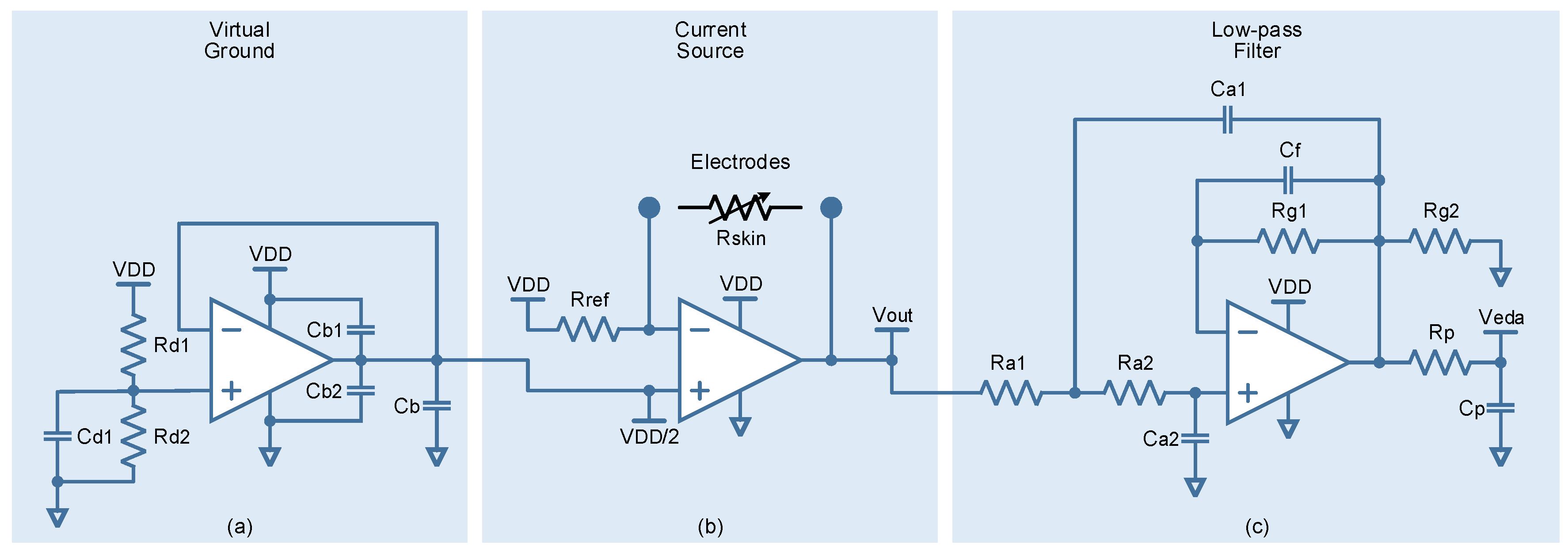
2. Signal Monitoring and Hardware Description
3. Experimental Protocol
3.1. Experimental Design
3.2. Study Population
4. Methodology
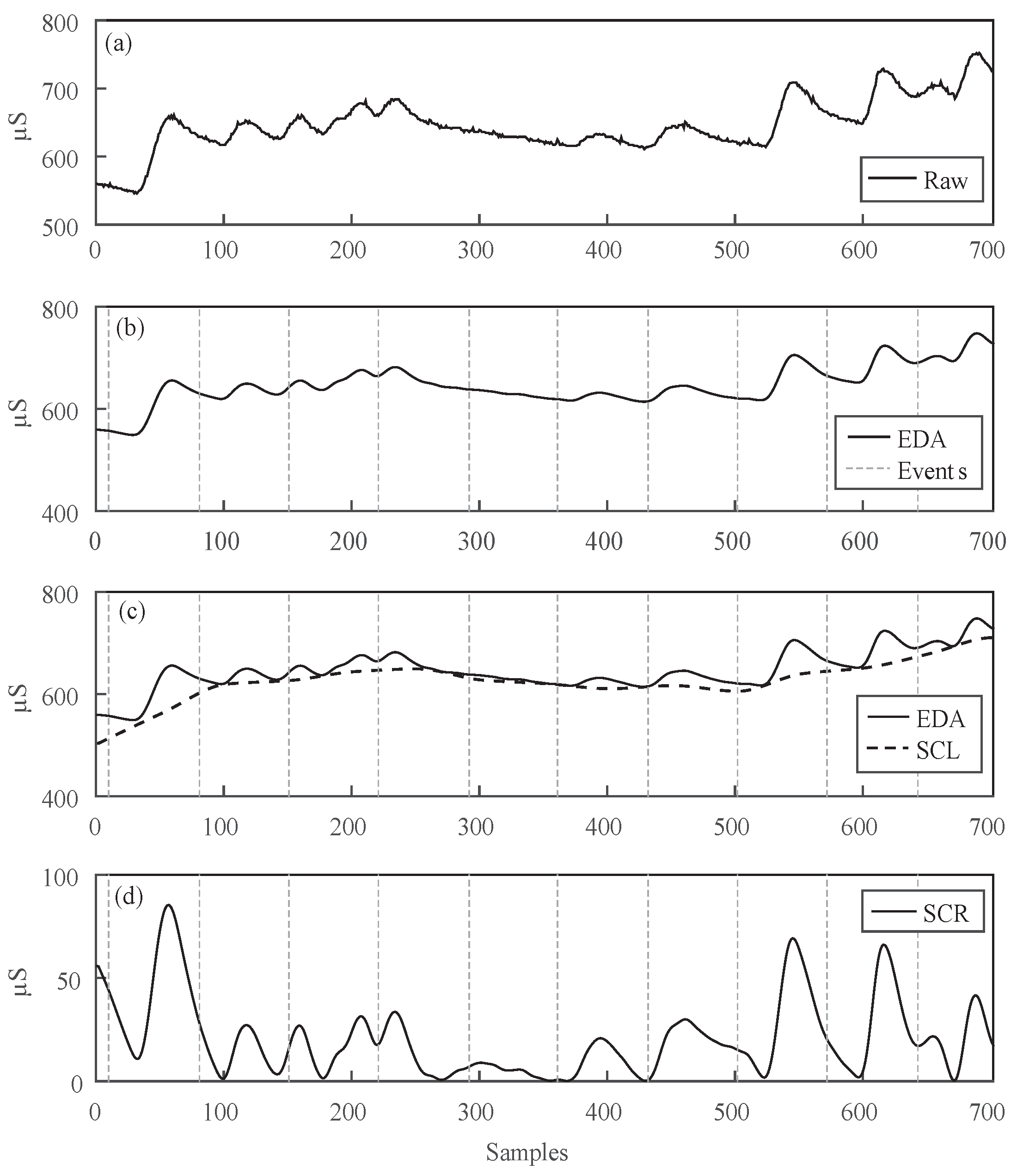
4.1. Signal Processing
4.2. Feature Sets
4.3. Statistical Analysis
5. Results
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EDA | electrodermal activity |
| IAPS | International Affective Picture System |
| SCL | skin conductance level |
| SCR | skin conductance response |
| SMNA | sudomotor nerve activity |
References
- Rebolledo-Mendez, G.; Reyes, A.; Paszkowicz, S.; Domingo, M.C.; Skrypchuk, L. Developing a body sensor network to detect emotions during driving. IEEE Trans. Intell. Transp. Syst. 2014, 15, 1850–1854. [Google Scholar] [CrossRef]
- Fernández-Caballero, A.; Latorre, J.M.; Pastor, J.M.; Fernández-Sotos, A. Improvement of the elderly quality of life and care through smart emotion regulation. In Ambient Assisted Living and Daily Activities; Pecchia, L., Chen, L., Nugent, C., Bravo, J., Eds.; Springer: New York, NY, USA, 2014; pp. 348–355. [Google Scholar]
- Fernández-Caballero, A.; Martínez-Rodrigo, A.; Pastor, J.M.; Castillo, J.C.; Lozano-Monasor, E.; López, M.T.; Zangróniz, R.; Latorre, J.M.; Fernández-Sotos, A. Smart environment architecture for emotion recognition and regulation. J. Biomed. Inf. 2016, 64, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mozos, O.M.; Sandulescu, V.; Andrews, S.; Ellis, D.; Bellotto, N.; Dobrescu, R.; Ferrandez, J.M. Stress detection using wearable physiological and sociometric sensors. Int. J. Neural Syst. 2017, 27, 1650041. [Google Scholar] [CrossRef] [PubMed]
- Kikhia, B.; Stavropoulos, T.G.; Andreadis, S.; Karvonen, N.; Kompatsiaris, I.; Sävenstedt, S.; Pijl, M.; Melander, C. Utilizing a wristband sensor to measure the stress level for people with dementia. Sensors 2016, 16, 1989. [Google Scholar] [CrossRef] [PubMed]
- Sioni, R.; Chittaro, L. Stress detection using physiological sensors. IEEE Comput. 2015, 48, 26–33. [Google Scholar] [CrossRef]
- Seoane, F.; Mohino-Herranz, I.; Ferreira, J.; Alvarez, L.; Buendia, R.; Ayllón, D.; Llerena, C.; Gil-Pita, R. Wearable biomedical measurement systems for assessment of mental stress of combatants in real time. Sensors 2014, 14, 7120–7141. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodrigo, A.; Zangróniz, R.; Pastor, J.M.; Latorre, J.M.; Fernández-Caballero, A. Emotion detection in ageing adults from physiological sensors. In Ambient Intelligence-Software and Applications; Mohamed, A., Novais, P., Pereira, A., Villarrubia, G., Fernández-Caballero, A., Eds.; Springer: New York, NY, USA, 2015; pp. 253–261. [Google Scholar]
- Castillo, J.C.; Castro-González, A.; Fernández-Caballero, A.; Latorre, J.M.; Pastor, J.M.; Fernández-Sotos, A.; Salichs, M.A. Software architecture for smart emotion recognition and regulation of the ageing adult. Cogn. Comput. 2016, 8, 357–367. [Google Scholar] [CrossRef]
- Healey, J.A.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef]
- Veltman, J.A.; Gaillard, A.W.K. Physiological indicies of workload in a simulated flight task. Biol. Psychol. 1996, 42, 323–342. [Google Scholar] [CrossRef]
- Koji, N.; Nozawa, A.; Ide, H. Evaluation of emotions by nasal skin temperature on auditory stimulus and olfactory stimulus. IEEJ Trans. Electron. Inf. Syst. 2004, 124, 1914–1915. [Google Scholar] [CrossRef]
- Hanson, M.A.; Powell, H.C., Jr.; Barth, A.T.; Ringgenberg, K.; Calhoun, B.H.; Aylor, J.H.; Lach, J. Body area sensors networks: Challenges and opportunities. Computer 2009, 42, 58–65. [Google Scholar] [CrossRef]
- Torniainen, J.; Cowley, B.; Henelius, A.; Lukander, K.; Pakarinen, S. Feasibility of an electrodermal activity ring prototype as a research tool. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 6433–6436. [Google Scholar]
- Suoja, K.; Liukkonen, J.; Jussila, J.; Salonius, H.; Venho, N.; Sillanpää, V.; Vuori, V.; Helander, N. Application for pre-processing and visualization of electrodermal activity wearable data. In Proceedings of the EMBEC & NBC 2017, Tampere, Finland, 11–15 June 2017; pp. 93–96. [Google Scholar]
- Van Dooren, M.; de Vries, J.J.G.; Janssen, J.H. Emotional sweating across the body: Comparing 16 different skin conductance measurement locations. Physiol. Behav. 2012, 106, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.E.; Schell, A.M.; Filion, D.L. The electrodermal system. In Handbook of Psychophysiology; Cacioppo, J., Tassinay, L.G., Berntson, G.G., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 159–181. [Google Scholar]
- Jänig, W.J. Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Boucsein, W. Electrodermal Activity; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Martínez-Rodrigo, A.; Zangróniz, R.; Pastor, J.M.; Fernández-Caballero, A. Arousal level classification in the ageing adult by measuring electrodermal skin conductivity. In Ambient Intelligence for Health; Bravo, J., Hervás, R., Villarreal, V., Eds.; Springer: New York, NY, USA, 2015; pp. 213–223. [Google Scholar]
- Lang, P.J. The emotion probe: Studies of motivation and attention. Am. Psychol. 1995, 50, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Valenza, G.; Lanata, A.; Scilingo, E.P. The role of nonlinear dynamics in affective valence and arousal recognition. IEEE Trans. Affect. Comput. 2012, 3, 237–249. [Google Scholar] [CrossRef]
- Macefield, V.G.; Wallin, B.G. The discharge behaviour of single sympathetic neurones supplying human sweat glands. J. Auton. Nerv. Syst. 1996, 61, 277–286. [Google Scholar] [CrossRef]
- Edelberg, R.; Greiner, T.; Burch, N.R. Some membrane properties of the effector in the galvanic skin response. J. Appl. Physiol. 1960, 15, 691–696. [Google Scholar] [PubMed]
- AnalogDevices, AD8603/AD8607/AD8609 Precision Micropower, Low Noise CMOS, Rail-to-Rail Input/Output Operational Amplifiers. 2008. Available online: http://www.analog.com/media/en/technical-documentation/data-sheets/AD8603_8607_8609.pdf (accessed on 8 June 2017).
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Technical Manuel and Affective Ratings; NIMH Center for the Study of Emotion and Attention: Gainesville, FL, USA, 1997. [Google Scholar]
- Morris, J.D. Observations: SAM: The self-assessment manikin; an efficient cross-cultural measurement of emotional response. J. Advert. Res. 1995, 35, 63–68. [Google Scholar]
- Russell, J.A. A circumplex model of affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Uhrig, M.K.; Trautmann, N.; Baumgärtner, U.; Treede, R.D.; Henrich, F.; Hiller, W.; Marschall, S. Emotion elicitation: A comparison of pictures and films. Front. Psychol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.A. Wearable and Automotive Systems for Affect Recognition from Physiology; Massachusetts Institute of Technology: Cambridge, MA, USA, 2000. [Google Scholar]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodrigo, A.; Alcaraz, R.; Rieta, J.J. Morphological variability of the p-wave for premature envision of paroxysmal atrial fibrillation events. Physiol. Meas. 2013, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koelstra, S.; Yazdani, A.; Soleymani, M.; ühl, C.M.; Lee, J.S.; Nijholt, A.; Pun, T.; Ebrahimi, T.; Patras, I. Single trial classification of EEG and peripheral physiological signals for recognition of emotions induced by music videos. In Brain Informatics; Zhong, N., Peng, H., Eds.; Springer: New York, NY, USA, 2010; pp. 89–100. [Google Scholar]
- Jung, Y.; Jianhua, H. A k-fold averaging cross-validation procedure. J. Nonparametr. Stat. 2015, 27, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiman, L. Classification and Regression Trees; Wadsworth International Group: Belmont, CA, USA, 1984. [Google Scholar]
- Salai, M.; Vassányi, I.; Kósa, I. Stress detection using low cost heart rate sensors. J. Healthc. Eng. 2016, 2016, 5136705. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Cowley, B.; Filetti, M.; Lukander, K.; Torniainen, J.; Henelius, A.; Ahonen, L.; Barral, O.; Kosunen, I.; Valtonen, T.; Huotilainen, M.; et al. The psychophysiology primer: A guide to methods and a broad review with a focus on human-computer interaction. Found. Trends Hum. Comput. Interact. 2015, 3–4, 151–308. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Syst. Man Cybern. B 2010, 40, 1–12. [Google Scholar]
- Poh, M.Z.; Swenson, N.C.; Picard, R.W. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans. Biomed. Eng. 2010, 57, 1243–1252. [Google Scholar] [PubMed]
- Kim, J.; Kwon, S.; Seo, S.; Park, K. Highly wearable galvanic skin response sensor using flexible and conductive polymer foam. In Proceedings of the 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; IEEE: Chicago, IL, USA, 2014; pp. 6631–6634. [Google Scholar]
- Guo, R.; Li, S.; He, L.; Gao, W.; Qi, H.; Owens, G. Pervasive and unobtrusive emotion sensing for human mental health. In Proceedings of the IEEE 7th International Conference on Pervasive Computing Technologies for Healthcare, Venice, Italy, 5–8 May 2013; pp. 436–439. [Google Scholar]
- Setz, C.; Arnrich, B.; Schumm, J.; Marca, R.L.; Troster, G.; Ehlert, U. Discriminating stress from cognitive load using a wearable EDA device. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, H.; Maslov, A.V.; Pechenizkiy, M. Stress detection from speech and galvanic skin response signals. In Proceedings of the IEEE 26th International Symposium on Computer-Based Medical Systems, Porto, Portugal, 20–22 June 2013; pp. 209–214. [Google Scholar]
- Subramanian, R.; Wache, J.; Abadi, M.; Vieriu, R.; Winkler, S.; Sebe, N. ASCERTAIN: Emotion and personality recognition using commercial sensors. IEEE Trans. Affect. Comput. 2016. [Google Scholar] [CrossRef]
- Sandulescu, V.; Andrews, S.; Ellis, D.; Bellotto, N.; Mozos, O.M. Stress detection using wearable physiological sensors. In Artificial Computation in Biology and Medicine; Ferrández, J.M., Álvarez-Sánchez, J., de la Paz López, F., Toledo-Moreo, F., Adeli, H., Eds.; Springer: New York, NY, USA, 2015; pp. 526–532. [Google Scholar]
- Sharma, N.; Gedeon, T. Hybrid genetic algorithms for stress recognition in reading. In Evolutionary Computation, Machine Learning and Data Mining in Bioinformatics; Marchiori, E., Moore, J.H., Rajapakse, J.C., Eds.; Springer: New York, NY, USA, 2013; pp. 117–128. [Google Scholar]
- Salafi, T.; Kah, J.C.Y. Design of unobtrusive wearable mental stress monitoring device using physiological sensor. In Proceedings of the 7th WACBE World Congress on Bioengineering, Singapore, 6–8 July 2015; Goh, J., Lim, C.T., Eds.; Springer: New York, NY, USA, 2015; pp. 11–14. [Google Scholar]
- Chen, C.-C.; Lin, S.-C.; Young, M.-S.; Yang, C.-L. Quantifying the accumulated stress level using a point-of-care test device. Biomed. Eng. 2014, 26, 1450053. [Google Scholar] [CrossRef]
| Analysis | Features |
|---|---|
| Temporal | MSC, SDSC, MASC, MISC, DRSC, FMSC, FDSC, SMSC, SDSC |
| Morphological | ALSC, INSC, APSC, RMSC, ILSC, ELSC, KUSC, SKSC, KUSC, MOSC |
| Frequency | F1SC, F2SC, F3SC |
| Feature | Calm Condition | Distress Condition | |
|---|---|---|---|
| Acronym | Mean ± Std | Mean ± Std | |
| MSC | 5.5339 ± 4.2228 | 13.0193 ± 8.6201 | 1.03 |
| SDSC | 4.4618 ± 4.8976 | 12.5249 ± 9.0340 | 1.33 |
| MASC | 28.6079 ± 27.44 | 69.4104 ± 48.0310 | 2.68 |
| DRSC | 28.5653 ± 27.4660 | 69.3719 ± 48.0145 | 2.67 |
| FDSC | 0.9932 ± 0.9665 | 2.2660 ± 1.6756 | 1.50 |
| ALSC | 1.4049 ± 99.6809 | 1.4153 ± 279.7989 | 0.0175 |
| INSC | 193.9833 ± 148.3517 | 457.2628 ± 304.9061 | 1.11 |
| APSC | 4.6324 ± 9.2181 | 23.8873 ± 36.4345 | 0.0026 |
| RMSC | 7.3106 ± 6.3476 | 18.0970 ± 12.4265 | 1.20 |
| ILSC | 5.5067 ± 4.1480 | 12.8120 ± 8.2006 | 7.31 |
| ELSC | 0.0065 ± 0.0129 | 0.0330 ± 0.0484 | 0.002 |
| SKSC | 1.8838 ± 1.1882 | 3.1146 ± 0.7159 | 0.0031 |
| MOSC | 2.2337 ± 5.0694 | 11.7973 ± 19.7930 | 0.0057 |
| F1SC | 2.9219 ± 5.4380 | 14.1513 ± 20.2989 | 0.0018 |
| F2SC | 0.1631 ± 0.2984 | 1.4143 ± 2.1767 | 8.99 |
| F3SC | 0.1391 ± 0.3231 | 1.2288 ± 2.3907 | 0.0076 |
| Feature | Learning | Test | ||||
|---|---|---|---|---|---|---|
| Acronym | Se (%) | Sp (%) | Ac (%) | Se (%) | Sp (%) | Ac (%) |
| MSC | 75.95 | 83.10 | 79.52 | 69.00 | 76.78 | 72.95 |
| SDSC | 85.06 | 78.95 | 82.43 | 78.57 | 76.07 | 77.38 |
| MASC | 81.45 | 76.35 | 78.91 | 74.07 | 72.28 | 73.34 |
| DRSC | 82.92 | 75.42 | 79.18 | 76.64 | 71.00 | 73.62 |
| FDSC | 74.58 | 80.43 | 77.50 | 69.21 | 74.85 | 71.81 |
| ALSC | 72.15 | 76.86 | 74.52 | 70.92 | 75.64 | 73.19 |
| APSC | 84.98 | 79.88 | 82.43 | 79.92 | 78.71 | 79.28 |
| RMSC | 85.92 | 78.92 | 82.42 | 81.57 | 78.85 | 79.94 |
| ILSC | 85.12 | 79.71 | 82.42 | 81.78 | 78.14 | 79.99 |
| ELSC | 71.78 | 73.14 | 72.25 | 63.00 | 75.35 | 69.16 |
| SKSC | 77.44 | 81.08 | 79.29 | 73.28 | 75.71 | 74.53 |
| MOSC | 86.19 | 78.75 | 82.50 | 75.28 | 75.21 | 75.18 |
| F1SC | 77.15 | 77.94 | 77.57 | 74.85 | 75.85 | 75.38 |
| F2SC | 85.29 | 85.93 | 85.61 | 84.92 | 78.50 | 81.61 |
| F3SC | 85.12 | 79.71 | 82.42 | 80.35 | 76.42 | 78.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangróniz, R.; Martínez-Rodrigo, A.; Pastor, J.M.; López, M.T.; Fernández-Caballero, A. Electrodermal Activity Sensor for Classification of Calm/Distress Condition. Sensors 2017, 17, 2324. https://doi.org/10.3390/s17102324
Zangróniz R, Martínez-Rodrigo A, Pastor JM, López MT, Fernández-Caballero A. Electrodermal Activity Sensor for Classification of Calm/Distress Condition. Sensors. 2017; 17(10):2324. https://doi.org/10.3390/s17102324
Chicago/Turabian StyleZangróniz, Roberto, Arturo Martínez-Rodrigo, José Manuel Pastor, María T. López, and Antonio Fernández-Caballero. 2017. "Electrodermal Activity Sensor for Classification of Calm/Distress Condition" Sensors 17, no. 10: 2324. https://doi.org/10.3390/s17102324
APA StyleZangróniz, R., Martínez-Rodrigo, A., Pastor, J. M., López, M. T., & Fernández-Caballero, A. (2017). Electrodermal Activity Sensor for Classification of Calm/Distress Condition. Sensors, 17(10), 2324. https://doi.org/10.3390/s17102324






