Gas Sensors Based on Polymer Field-Effect Transistors
Abstract
:1. Introduction
2. Polymer Field-Effect Transistor (PFET) Sensors with Semiconducting Polymers as the Sensing Layer
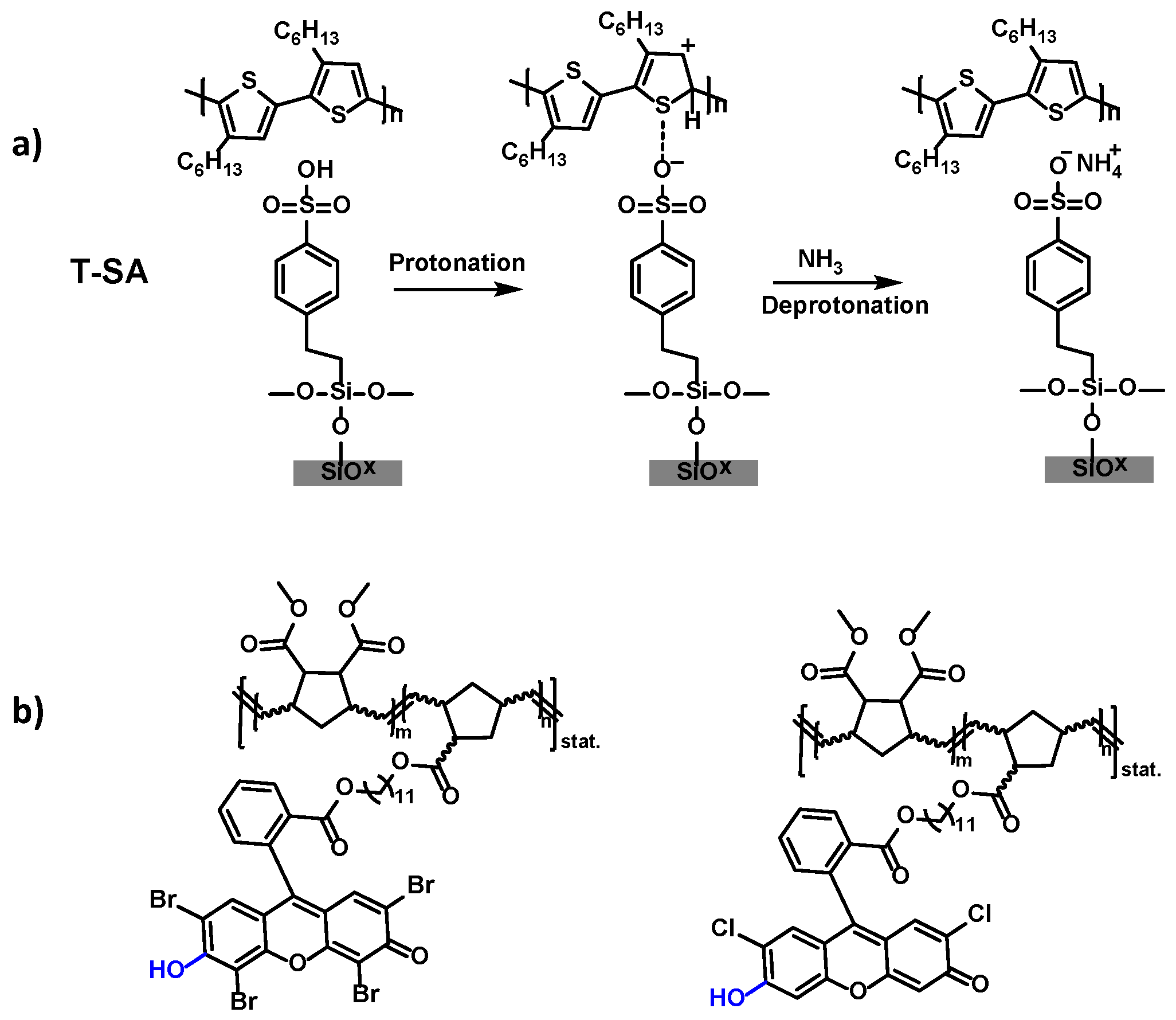
2.1. NH3 and Amine Gas Sensors
2.2. NO2 Gas Sensor
2.3. Alcohol Sensor
2.4. Other Gas Sensors
3. PFET Sensors with Dielectric Layer as Sensing Layer
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mabeck, J.T.; Malliaras, G.G. Chemical and biological sensors based on organic thin-film transistors. Anal. Bioanal. Chem. 2006, 384, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Locklin, J.; Bao, Z.N. Effect of morphology on organic thin film transistor sensors. Anal. Bioanal. Chem. 2006, 384, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fine, D.; Sharma, D.; Torsi, L.; Dodabalapur, A. Nanoscale organic and polymeric field-effect transistors as chemical sensors. Anal. Bioanal. Chem. 2006, 384, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Bartic, C.; Borghs, G. Organic thin-film transistors as transducers for (bio) analytical applications. Anal. Bioanal. Chem. 2006, 384, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Sokolov, A.N.; Bao, Z.N. Material and device considerations for organic thin-film transistor sensors. J. Mater. Chem. 2009, 19, 3351–3363. [Google Scholar] [CrossRef]
- Vishinkin, R.; Haick, H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015, 11, 6142–6164. [Google Scholar] [CrossRef] [PubMed]
- Torsi, L.; Dodabalapur, A.; Sabbatini, L.; Zambonin, P.G. Multi-parameter gas sensors based on organic thin-film-transistors. Sens. Actuators B Chem. 2000, 67, 312–316. [Google Scholar] [CrossRef]
- Bayn, A.; Feng, X.; Müllen, K.; Haick, H. Field effect transistors based on polycyclic aromatic hydrocarbons for the detection and classification of volatile organic compounds. ACS Appl. Mater. Interfaces 2013, 5, 3431–3440. [Google Scholar] [CrossRef] [PubMed]
- Pfister, G. Hopping transport in a molecularly doped organic polymer. Phys. Rev. B 1977, 16, 3676–3687. [Google Scholar] [CrossRef]
- Tsao, H.N.; Müllen, K. Improving polymer transistor performance via morphology control. Chem. Soc. Rev. 2010, 39, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Assadi, A.; Gustafsson, G.; Willander, M.; Svensson, C.; Inganäs, O. Determination of field-effect mobility of poly(3-hexylthiophene) upon exposure to NH3 gas. Synth. Met. 1990, 37, 123–130. [Google Scholar] [CrossRef]
- Zan, H.W.; Li, C.H.; Yu, C.K.; Meng, H.F. Sensitive gas sensor embedded in a vertical polymer space-charge-limited transistor. Appl. Phys. Lett. 2012, 101, 023303. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, A.K.; Joshi, L.; Chakrabarti, P.; Takashima, W.; Kaneto, K.; Prakash, R. Poly-3-hexylthiophene based organic field-effect transistor: Detection of low concentration of ammonia. Sens. Actuator B Chem. 2012, 171, 962–968. [Google Scholar] [CrossRef]
- Cavallari, M.R.; Izquierdo, J.E.E.; Braga, G.S.; Dirani, E.A.T.; Pereira-da-Silva, M.A.; Rodriguez, E.F.G.; Fonseca, F.J. Enhanced sensitivity of gas sensor based on poly(3-hexylthiophene) thin-film transistors for disease diagnosis and environment monitoring. Sensors 2015, 15, 9592–9609. [Google Scholar] [CrossRef] [PubMed]
- Klug, A.; Denk, M.; Bauer, T.; Sandholzer, M.; Scherf, U.; Slugovc, C.; List, E.J.W. Organic field-effect transistor based sensors with sensitive gate dielectrics used for low-concentration ammonia detection. Org. Electron. 2013, 14, 500–504. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.Z.; Du, H.F.; Zhou, Y.; Xie, F.B.; Jiang, Y.D.; Tai, H.L. The fabrication and optimization of thin-film transistors based on poly(3-hexylthiophene) films for nitrogen dioxide detection. IEEE Sens. J. 2016, 16, 1865–1871. [Google Scholar] [CrossRef]
- Lienerth, P.; Fall, S.; Leveque, P.; Soysal, U.; Heiser, T. Improving the selectivity to polar vapors of OFET-based sensors by using the transfer characteristics hysteresis response. Sens. Actuator B Chem. 2016, 225, 90–95. [Google Scholar] [CrossRef]
- Sandberg, H.G.O.; Backlund, T.G.; Osterbacka, R.; Jussila, S.; Makela, T.; Stubb, H. Applications of an all-polymer solution-processed high-performance, transistor. Synth. Met. 2005, 155, 662–665. [Google Scholar] [CrossRef]
- Pacher, P.; Lex, A.; Proschek, V.; Etschmaier, H.; Tchernychova, E.; Sezen, M.; Scherf, U.; Grogger, W.; Trimmel, G.; Slugovc, C.; et al. Chemical control of local doping in organic thin-film transistors: From depletion to enhancement. Adv. Mater. 2008, 20, 3143–3148. [Google Scholar] [CrossRef]
- Ryu, G.S.; Park, K.H.; Park, W.T.; Kim, Y.H.; Noh, Y.Y. High-performance diketopyrrolopyrrole-based organic field-effect transistors for flexible gas sensors. Org. Electron. 2015, 23, 76–81. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.X.; Luo, H.W.; Yao, J.J.; Liu, Z.T.; Zhang, D.Q. Highly sensitive thin-film field-effect transistor sensor for ammonia with the DPP-bithiophene conjugated polymer entailing thermally cleavable tert-butoxy groups in the side chains. ACS Appl. Mater. Interfaces 2016, 8, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Zhuang, X.M.; Shi, W.; Yang, X.; Li, L.; Yu, J.S. Poly(3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuator B Chem. 2016, 225, 10–15. [Google Scholar] [CrossRef]
- Besar, K.; Yang, S.; Guo, X.G.; Huang, W.; Rule, A.M.; Breysse, P.N.; Kymissis, I.J.; Katz, H.E. Printable ammonia sensor based on organic field effect transistor. Org. Electron. 2014, 15, 3221–3230. [Google Scholar] [CrossRef]
- Chen, D.J.; Lei, S.; Chen, Y.Q. A single polyaniline nanofiber field effect transistor and its gas sensing mechanisms. Sensors 2011, 11, 6509–6516. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Cho, J.; Sim, K.M.; Ha, J.U.; Chung, D.S. Morphology-driven high-performance polymer transistor-based ammonia gas sensor. ACS Appl. Mater. Interfaces 2016, 8, 6570–6576. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Swensen, J.S. Dual-transduction-mode sensing approach for chemical detection. Sens. Actuator B Chem. 2012, 174, 366–372. [Google Scholar] [CrossRef]
- Liao, F.; Yin, S.; Toney, M.F.; Subramanian, V. Physical discrimination of amine vapor mixtures using polythiophene gas sensor arrays. Sens. Actuator B Chem. 2010, 150, 254–263. [Google Scholar] [CrossRef]
- Das, A.; Dost, R.; Richardson, T.; Grell, M.; Morrison, J.J.; Turner, M.L. A nitrogen dioxide sensor based on an organic transistor constructed from amorphous semiconducting polymers. Adv. Mater. 2007, 19, 4018–4023. [Google Scholar] [CrossRef]
- Cheon, K.H.; Cho, J.; Kim, Y.H.; Chung, D.S. Thin film transistor gas sensors incorporating high-mobility diketopyrrolopyrole-based polymeric semiconductor doped with graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 14004–14010. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; An, T.K.; Kim, J.; Hwang, J.; Park, S.; Nam, S.; Cha, H.; Park, W.J.; Baik, J.M.; Park, C.E. A composite of a graphene oxide derivative as a novel sensing layer in an organic field-effect transistor. J. Mater. Chem. C 2014, 2, 4539–4544. [Google Scholar] [CrossRef]
- Torsi, L.; Tanese, M.C.; Cioffi, N.; Gallazzi, M.C.; Sabbatini, L.; Zambonin, P.G.; Raos, G.; Meille, S.V.; Giangregorio, M.M. Side-chain role in chemically sensing conducting polymer field-effect transistors. J. Phys. Chem. B 2003, 107, 7589–7594. [Google Scholar] [CrossRef]
- Torsi, L.; Tafuri, A.; Cioffi, N.; Gallazzi, M.C.; Sassella, A.; Sabbatini, L.; Zambonin, P.G. Regioregular polythiophene field-effect transistors employed as chemical sensors. Sens. Actuator B Chem. 2003, 93, 257–262. [Google Scholar] [CrossRef]
- Lv, A.; Wang, M.; Wang, Y.; Bo, Z.; Chi, L. Investigation into the sensing process of high-performance H2S sensors based on polymer transistors. Chem. Eur. J. 2016, 22, 3654–3659. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Dost, R.; Richardson, T.H.; Grell, M.; Wedge, D.C.; Kell, D.B.; Morrison, J.J.; Turner, M.L. Low cost, portable, fast multiparameter data acquisition system for organic transistor odour sensors. Sens. Actuator B Chem. 2009, 137, 586–591. [Google Scholar] [CrossRef]
- Dudhe, R.S.; Sinha, J.; Kumar, A.; Rao, V.R. Polymer composite-based OFET sensor with improved sensitivity towards nitro based explosive vapors. Sens. Actuator B Chem. 2010, 148, 158–165. [Google Scholar] [CrossRef]
- Wang, F.; Gu, H.; Swager, T.M. Carbon nanotube/polythiophene chemiresistive sensors for chemical warfare agents. J. Am. Chem. Soc. 2008, 130, 5392–5393. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, Y.; Muro, K.; Yoshino, K. Gas-sensitive and temperature-dependent schottky-gated field-effect transistors utilizing poly(3-alkylthiophene)s. Synth. Met. 1993, 57, 4111–4116. [Google Scholar] [CrossRef]
- Wang, B.; Huynh, T.P.; Wu, W.W.; Hayek, N.; Do, T.T.; Cancilla, J.C.; Torrecilla, J.S.; Nahid, M.M.; Colwell, J.M.; Gazit, O.M.; et al. A highly sensitive diketopyrrolopyrrole-based ambipolar transistor for selective detection and discrimination of xylene isomers. Adv. Mater. 2016, 28, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.B.; Liu, V.; Subramanian, V.; Sivula, K.; Luscombe, C.; Murphy, A.; Liu, J.S.; Frechet, J.M.J. Printable polythiophene gas sensor array for low-cost electronic noses. J. Appl. Phys. 2006, 100, 014506. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Janata, J. Conductometric sensors. In Principles of Chemical Sensors, 2nd ed.; Springer: Boston, MA, USA, 2009; pp. 241–266. [Google Scholar]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Eom, K.H.; Hyun, K.H.; Lin, S.; Kim, J.W. The meat freshness monitoring system using the smart RFID tag. Int. J. Distrib. Sens. Netw. 2014, 2014, 591812. [Google Scholar] [CrossRef]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food-existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Toney, M.F.; Subramanian, V. Thickness changes in polythiophene gas sensors exposed to vapor. Sens. Actuator B Chem. 2010, 148, 74–80. [Google Scholar] [CrossRef]
- Ram, M.K.; Yavuz, O.; Aldissi, M. NO2 gas sensing based on ordered ultrathin films of conducting polymer and its nanocomposite. Synth. Met. 2005, 151, 77–84. [Google Scholar] [CrossRef]
- Chandrashekar, T.K.; Krishnan, V. Donor properties of metallomacrocyclic tetrapyrrole pigments with sym-trinitrobenzene. Inorg. Chem. 1981, 20, 2782–2786. [Google Scholar] [CrossRef]
- Huynh, T.P.; Sharma, P.S.; Sosnowska, M.; D’Souza, F.; Kutner, W. Functionalized polythiophenes: Recognition materials for chemosensors and biosensors of superior sensitivity, selectivity, and detectability. Prog. Polym. Sci. 2015, 47, 1–25. [Google Scholar] [CrossRef]
- Feng, L.R.; Tang, W.; Zhao, J.Q.; Yang, R.Z.; Hu, W.; Li, Q.F.; Wang, R.L.; Guo, X.J. Unencapsulated air-stable organic field effect transistor by all solution processes for low power vapor sensing. Sci. Rep. 2016, 6, 20671. [Google Scholar] [CrossRef] [PubMed]
- Tanese, M.C.; Fine, D.; Dodabalapur, A.; Torsi, L. Organic thin-film transistor sensors: Interface dependent and gate bias enhanced responses. Microelectr. J. 2006, 37, 837–840. [Google Scholar] [CrossRef]
- Tanese, M.C.; Fine, D.; Dodabalapur, A.; Torsi, L. Interface and gate bias dependence responses of sensing organic thin-film transistors. Biosens. Bioelectron. 2005, 21, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Covington, J.A.; Gardner, J.W.; Bartlett, P.N.; Toh, C.S. Conductive polymer gate fet devices for vapour sensing. IEE Proc.-Circuit Device Syst. 2004, 151, 326–334. [Google Scholar] [CrossRef]
- Potje-Kamloth, K. Chemical gas sensors based on organic semiconductor polypyrrole. Crit. Rev. Anal. Chem. 2002, 32, 121–140. [Google Scholar] [CrossRef]
- Meijerink, M.G.H.; Koudelka-Hep, M.; de Rooij, N.F.; Strike, D.J.; Hendrikse, J.; Olthuis, W.; Bergveld, P. Gas-dependent field effect transistor with an electrodeposited conducting polymer gate contact. Electrochem. Solid State Lett. 1999, 2, 138–139. [Google Scholar] [CrossRef]
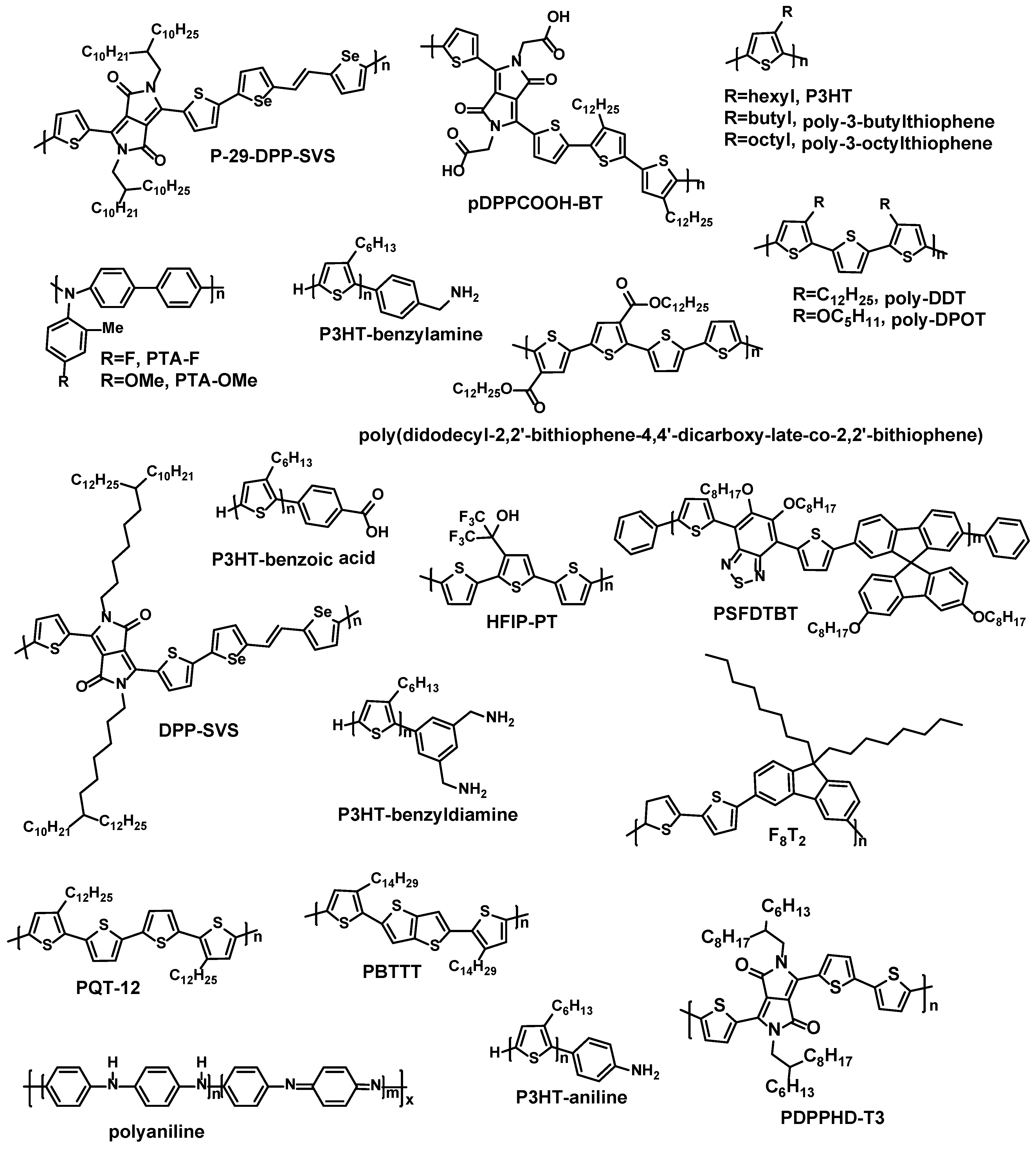
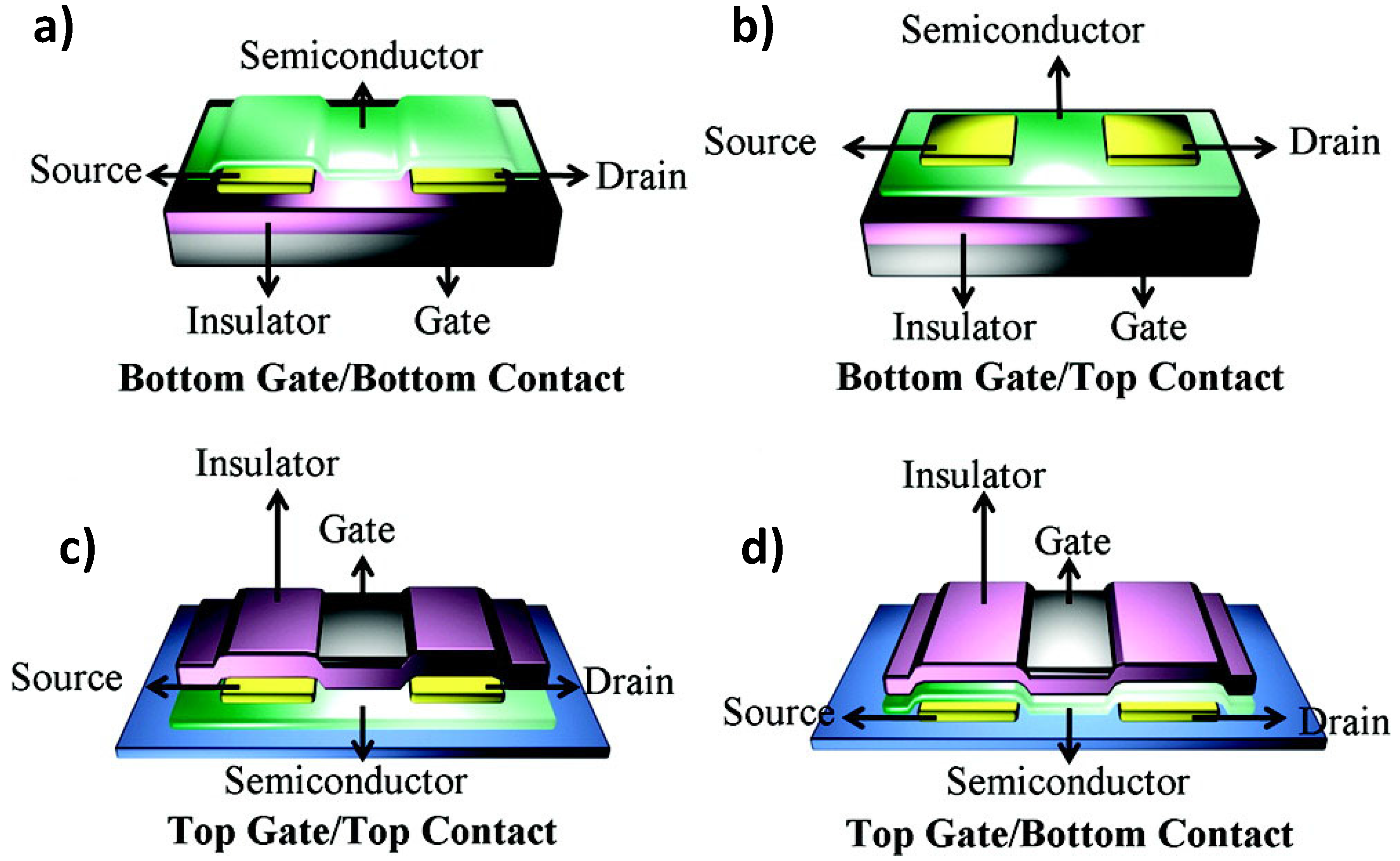

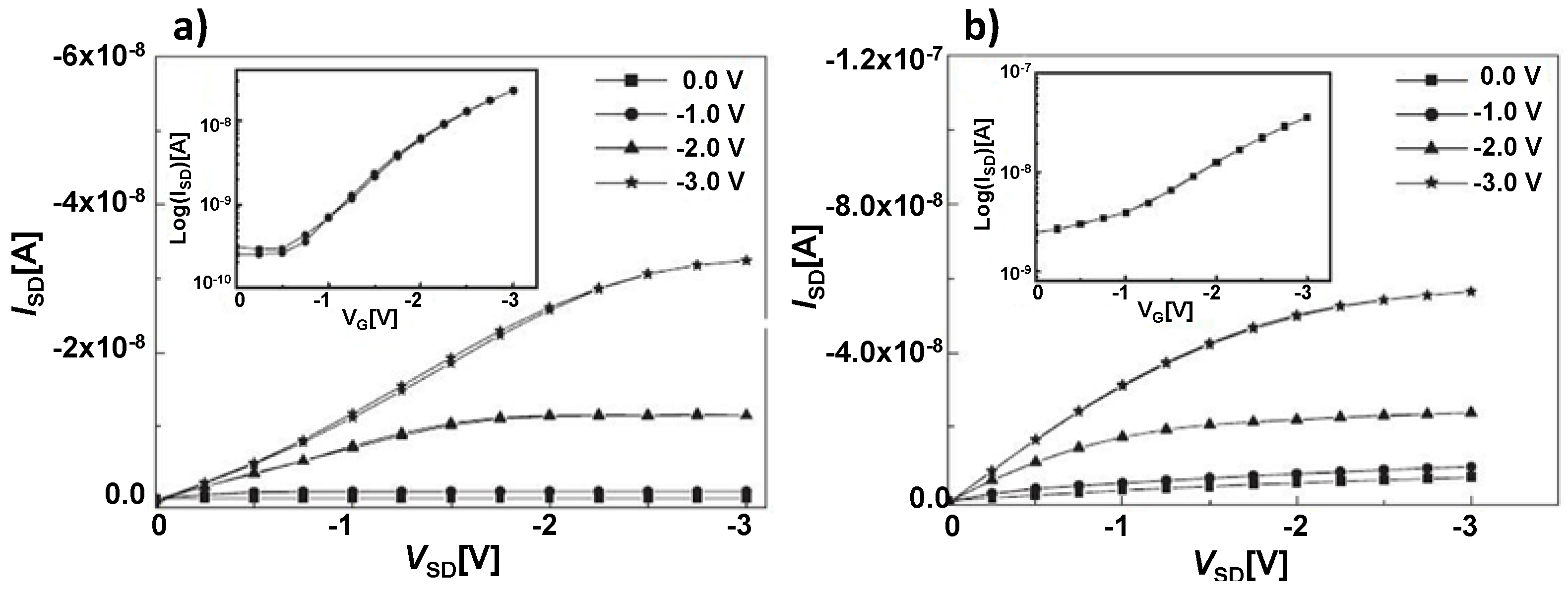
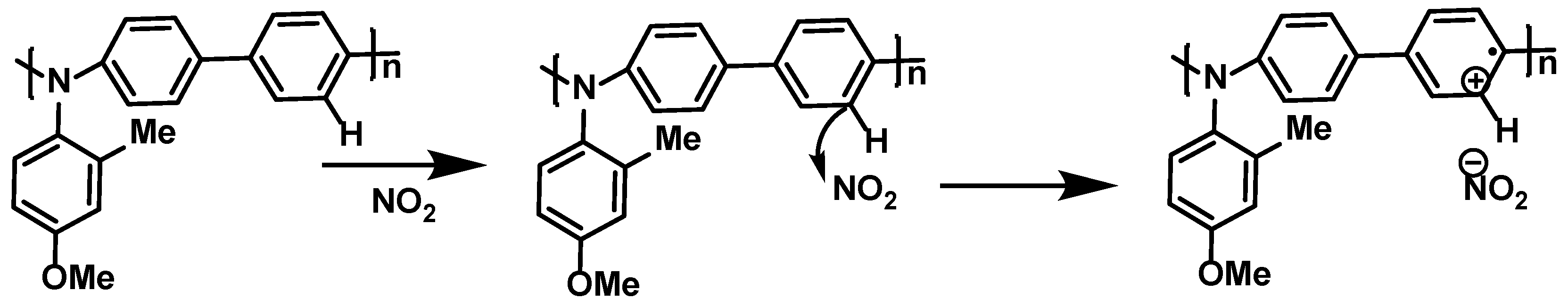

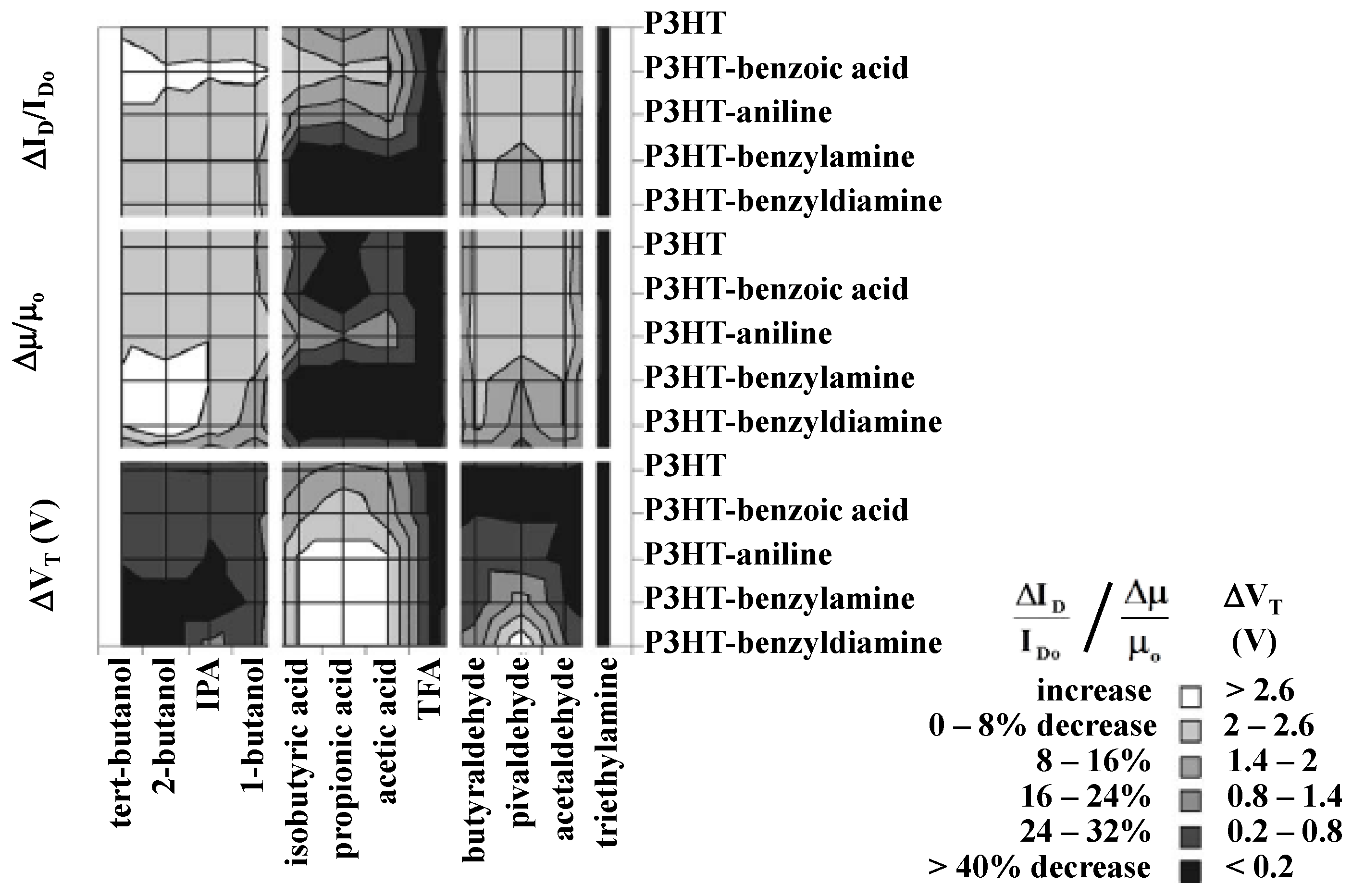
| Materials | Analytes | /ppm | Device | –VDS/V | –VGS/V | Response Time | Reference |
|---|---|---|---|---|---|---|---|
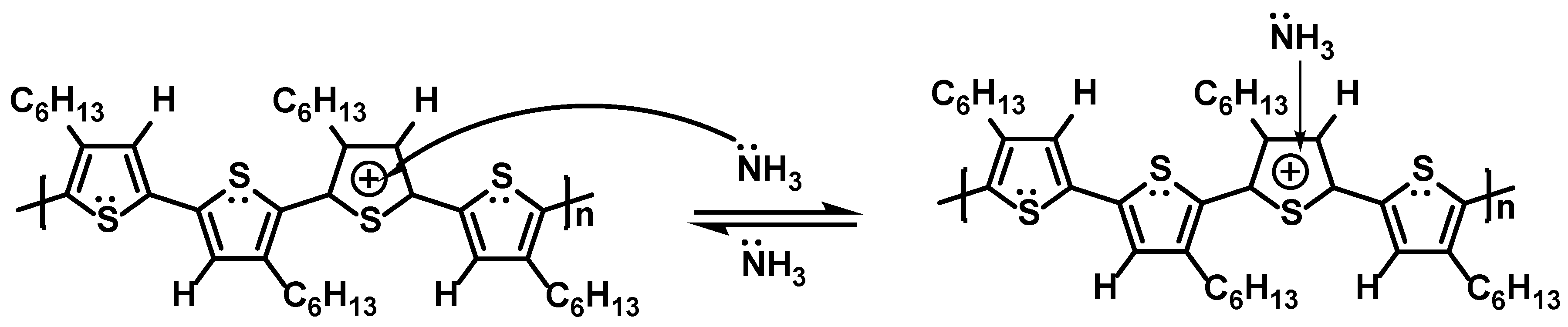
| P3HT | NH3 | 100% | BGBC | 4 | 4 | 10 min | [11] |
| NH3 | 0.03–1 | Vertical | 1 | 1 | - | [12] | |
| NH3 | 0.1–25 | BGBC | 60 | 30 | 5–25 s | [13] | |
| NH3 | 16–198 | BGBC | 1 | 1 | 52 s (99 ppm) | [14] | |
| NH3 | 100 | BGBC | 100/10 | 85 | 600 s | [15] | |
| NO2 | 0.038–25 | BGBC | 50 | 50 | 5 min | [16] | |
| ethanol acetone | 500–7000 | BGBC | 60 | 60 | 8–15 min | [17] | |
| ethanol methanol | SVP 3 | TGTC | 2 | 0.8 | - | [18] | |
| NH3 diethylamine triethylamine | - | BGTC | 45 | 60 | 0.5–2 h | [19] | |
| P-29-DPP-SVS | NH3 | 29–1000 | BGTC | 5 | 5 | 5 min | [20] |
| pDPPCOOH-BT | NH3 | 0.01–1000 | BGBC | 60 | 80 | 50 s | [21] |
| 1,4-diaminobutane, Et3N, piperidine | 10 ppm | BGBC | 60 | 80 | 50 s | [21] | |
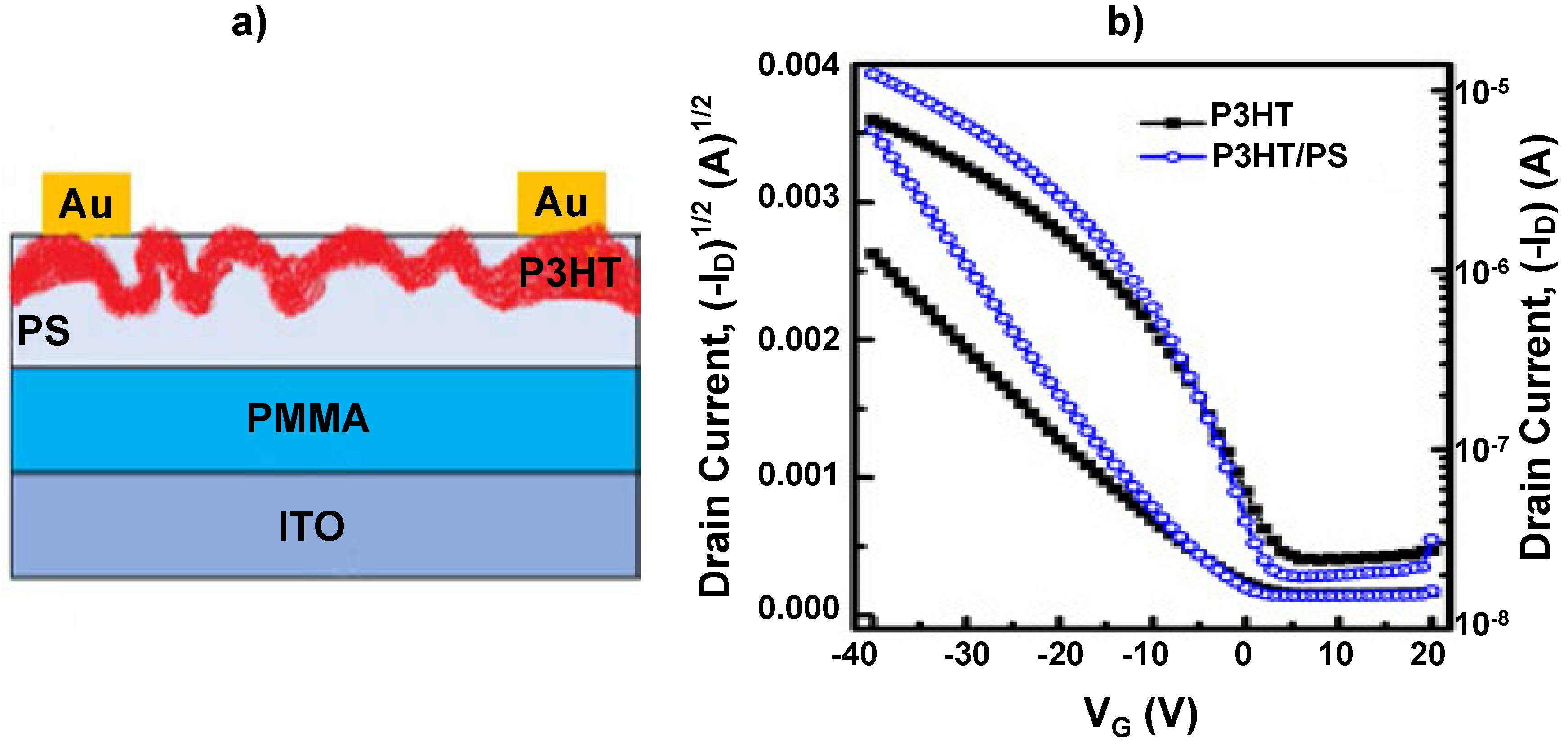
| P3HT/PS | NH3 | 5–50 | BGTC | 40 | 40 | 5–10 min | [22] |
| PQT-12 | NH3 | 0.1–15 | BGBC | 90 | corona charge | ≥5 min | [23] |
| polyaniline | NH3 | 1–20 | BGBC | 8 | 10 | 10 s | [24] |
| PBTTT | NH3 | 10–100 | BGTC | 30 | 30 | 40 s | [25] |
| DMNB | 6.5 | BGTC | 100 | 53 | 10 min–20 h | [26] | |
| Array sensor of Poly-3-butylthiophene Poly-3-octylthiophene | octylamine butylamine | 8.9 | BGBC | 20 | 20 | 900 s | [27] |
| hexylamine triethylamine | 30 | BGBC | 20 | 20 | 900 | [27] | |
| PTA-F | NO2 | 0.01–50 | BGTC | 3 | 3 | 20 s | [28] |
| PTA-OMe | |||||||
| DPP-SVS/GO 1 | ethanol | SVP 3 | BGTC | 30 | 30 | 20 s | [29] |
| F8T2/GO | ethanol acetone | SVP 3 | BGTC | 80 | 80 | 10 min | [30] |
| Poly-DPOT | Ethanol 1-hexanol | 700 | BGBC | 100 | 40 | 2 min | [31,32] |
| Poly-DDT | |||||||
| PSFDTBT | H2S | 0.001–1 | BGTC | 30 | 30 | 5–15 s | [33] |
| PTA-OMe | MeOH | 103–106 | BGTC | 3 | 3 | 2–3 min | [34] |
| EtOH | |||||||
| PrOH | |||||||
| IPA | |||||||
| P3HT/CuTPP/ADB | RDX | <0.01 | BGBC | 40 | 20 | 20 min | [35] |
| TNT | |||||||
| DNB | |||||||
| HFIP-PT/SWCNT 2 | DMMP | SVP 3 | BGBC | 0.001 | 5 | 10 min | [36] |
| poly-butylthiophene | CHCl3 | SVP 3 | BGTC | 10 | 3 | - | [37] |
| PDPPHD-T3 | o-xylene | 40-320 | BGBC | 70 | 60 | 12 s | [38] |
| m-xylene | |||||||
| p-xylene | |||||||
| Sensor array of P3HT P3HT-benzoic acid P3HT-aniline P3HT-benzylamine P3HT-benzyldiamine | tert-butanol | 40 | BGBC | 20 | 20 | 2 min | [39] |
| 2-butanol | |||||||
| isopropanol | |||||||
| 1-butanol | |||||||
| isobutyric acid | |||||||
| propionic acid | |||||||
| acetic acid | |||||||
| TFA | |||||||
| butyraldehyde | |||||||
| pivaldehyde | |||||||
| acetaldehyde | |||||||
| triethylamine |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, A.; Pan, Y.; Chi, L. Gas Sensors Based on Polymer Field-Effect Transistors. Sensors 2017, 17, 213. https://doi.org/10.3390/s17010213
Lv A, Pan Y, Chi L. Gas Sensors Based on Polymer Field-Effect Transistors. Sensors. 2017; 17(1):213. https://doi.org/10.3390/s17010213
Chicago/Turabian StyleLv, Aifeng, Yong Pan, and Lifeng Chi. 2017. "Gas Sensors Based on Polymer Field-Effect Transistors" Sensors 17, no. 1: 213. https://doi.org/10.3390/s17010213
APA StyleLv, A., Pan, Y., & Chi, L. (2017). Gas Sensors Based on Polymer Field-Effect Transistors. Sensors, 17(1), 213. https://doi.org/10.3390/s17010213





