Novel Isoprene Sensor for a Flu Virus Breath Monitor
Abstract
:1. Introduction
2. Results and Discussion
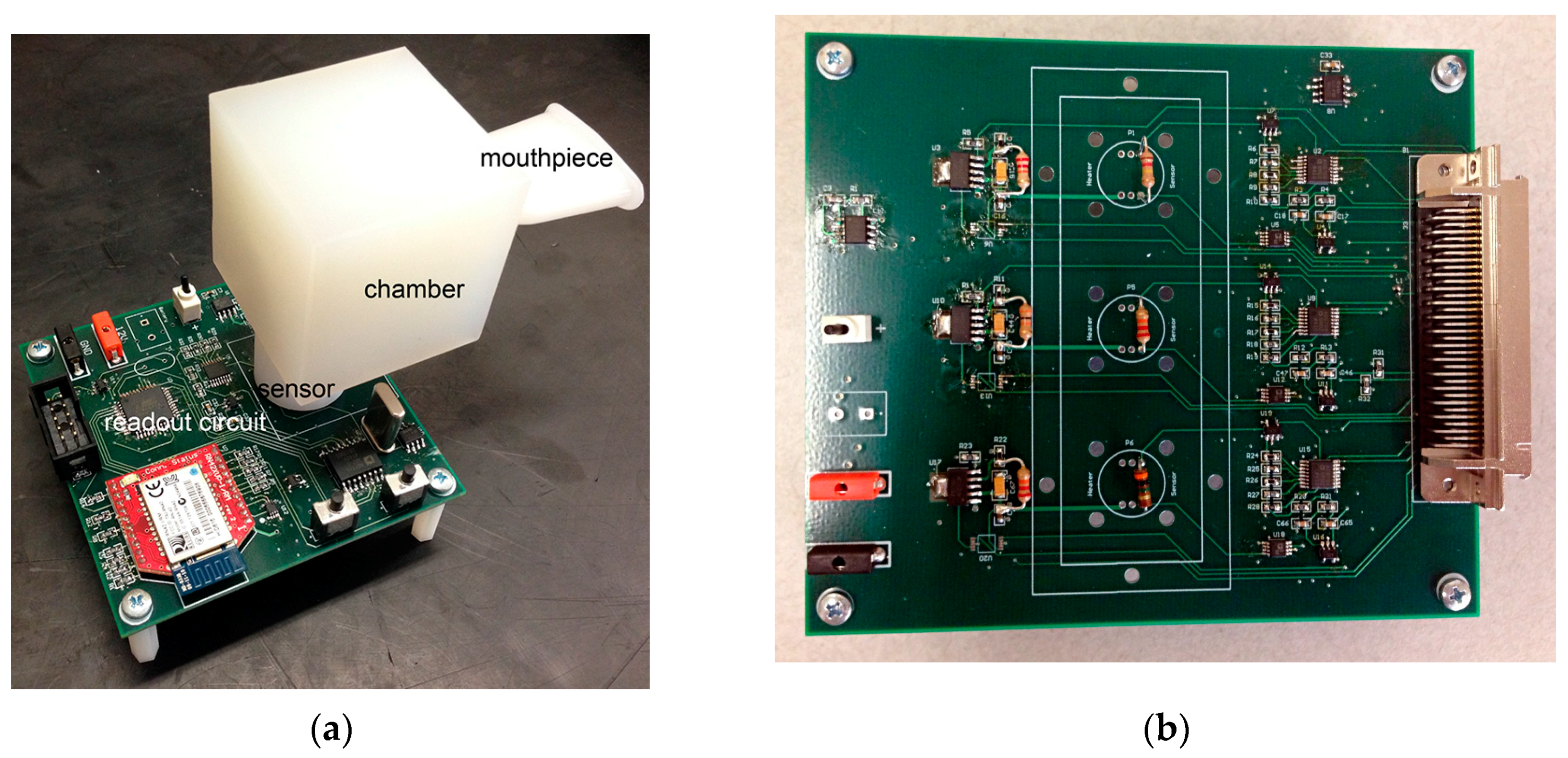
2.1. Description of the Breathalyzer Instrumentation
2.1.1. Three-Nanosensor Array Microsystem
2.1.2. Novel Isoprene Detector
2.1.3. The Breathalyzer Device
2.2. Proposed Use of the Breathalyzer in In-Vitro Studies
Limitations of In-Vitro Studies with a Live Attenuated Influenza Virus (FluMist®)
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- CDC: Flu Activity Expands. Available online: http://www.cdc.gov/flu/news/flu-activity-expands.htm (accessed on 2 January 2017).
- Weekly US Influenza Surveillance Report. Available online: http://www.cdc.gov/flu/weekly (accessed on 2 January 2017).
- Sanchez, A.; Lukwiya, M.; Bausch, D.; Mahanty, S.; Sanchez, A.J.; Wagoner, K.D.; Rollin, P.E. Analysis of Human Peripheral Blood Samples from Fatal and Nonfatal Cases of Ebola (Sudan) Hemorragic Fever: Cellular Responses, Virus Load, and Nitric Oxide Levels. J. Virol. 2004, 78, 10370–10377. [Google Scholar] [CrossRef] [PubMed]
- Mashir, A.; Paschke, K.M.; van Duin, D.; Shreshta, N.K.; Laskowski, D.; Storer, M.K.; Yen-Lieberman, B.; Gordon, S.; Aytekin, M.; Dweik, R.A. Effect of influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FENO) and other volatiles in exhaled breath. J. Breath Res. 2011, 5, 037107. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Cataneo, R.N.; Chaturvedi, A.; Danaher, P.J.; Devadiga, A.; Legendre, D.A.; Nail, K.L.; Schmitt, P.; Wai, J. Effect of influenza vaccination on oxidative stress products in breath. J. Breath Res. 2010, 4, 026001. [Google Scholar] [CrossRef] [PubMed]
- Schivo, M.; Aksenov, A.A.; Linderholm, A.L.; McCartney, M.M.; Simmons, J.; Harper, R.W.; Davis, C.E. Volatile emanations from in vitro airway cells infected with human rhinovirus. J. Breath Res. 2014, 8, 037110. [Google Scholar] [CrossRef] [PubMed]
- Gouma, P.; Prasad, A.; Stanacevic, M. A Selective Nanosensor Device for Exhaled Breath Analysis. J. Breath Res. 2011, 5, 037110. [Google Scholar] [CrossRef] [PubMed]
- Gouma, P.I.; Prasad, A.K.; Iyer, K.K. Selective Nanoprobes for ‘Signaling Gases’. Nanotechnology 2006, 17, S48–S53. [Google Scholar] [CrossRef] [PubMed]
- Gouma, P.; Kalyanasundaram, K.; Yun, X.; Stanacevic, M.; Wang, L. Chemical sensor and breath analyzer for ammonia detection in exhaled human breath. IEEE Sens. 2010, 10, 49–53. [Google Scholar] [CrossRef]
- Wang, L.; Kalyanasundaram, K.; Stanacevic, M.; Gouma, P. Nanosensor Device for Breath Acetone Detection. Sens. Lett. 2010, 8, 709–712. [Google Scholar] [CrossRef]
- Gouma, P. Nanoscale Polymorphic Oxides for Selective Chemosensors. Sci. Adv. Mater. 2011, 3, 787–793. [Google Scholar] [CrossRef]
- Gouma, P.I. Controlling Gas Selectivity through Polyorphic Selection for Metal Oxide Chemical Detectors. Chem. Sens. 2004, 20 (Suppl. B), 186–187. [Google Scholar]
- Gouma, P.; Stanacevic, M.; Simon, S. An overview of the translation of selective semiconducting gas sensors from first results to automotive exhaust gas monitors to a platform for breath-based diagnostics. Transl. Mater. Res. 2015, 2, 045001. [Google Scholar] [CrossRef]
- Gouma, P.I. Nanostructured Polymorphic Oxides for Advanced Chemosensors. Rev. Adv. Mater. Sci. 2003, 5, 123–138. [Google Scholar]
- Wang, L.; Gouma, P. Selective Microstructure Synthesis and Sensing Dependencies: A WO3 study. In Metal Oxide Nanomaterials for Chemical Sensors; Carpenter, M.A., Mathur, S., Kolmakov, A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Wang, L. Tailored Synthesis and Characterization of Selective Metabolite-Detecting Nanoprobes for Handheld Breath Analysis. Ph.D. Thesis, Stony Brook University, Stony Brook, NY, USA, December 2008. [Google Scholar]
- Gerand, B.; Nowogrocki, G.; Guenot, J.; Figlarz, M. Structural study of a new hexagonal form of tungsten trioxide. J. Solid State Chem. 1979, 29, 429–434. [Google Scholar] [CrossRef]
- Ohira, S.; Li, J.; Lonneman, W.A.; Dasgupta, P.K.; Toda, K. Can breath isoprene be measured by ozone chemiluminescence? Anal. Chem. 2007, 79, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Teleki, A.; Pratsinis, S.E.; Kalyanasundaram, K.; Gouma, P.I. Sensing of organic vapors by flame-made TiO2 nanoparticles. Sens. Actuators B 2006, 119, 683–690. [Google Scholar] [CrossRef]
- Gouma, P.; Sood, S.; Stanacevic, M.; Simon, S. Selective Chemosensing and diagnostic Breathalyzer. Proced. Eng. 2014, 87, 9–15. [Google Scholar] [CrossRef]
- Perrone, L.A.; Belser, A.J.; Wadford, D.A.; Katz, J.M.; Tumpey, T.M. Inducible Nitric Oxide Contributes to Viral Pathogenesis Following Highly Pathogenic Influenza Virus Infection in Mice. J. Infect. Dis. 2013, 207. [Google Scholar] [CrossRef] [PubMed]
- Askenov, A.A.; Sandrock, C.E.; Zhao, W.; Sankaran, S.; Schivo, M.; Harper, R.; Cardona, C.J.; Xing, Z.; Davis, C.E. Cellular Scent of Influenza Virus Infection. ChemBioChem 2014, 15, 1040–1048. [Google Scholar]

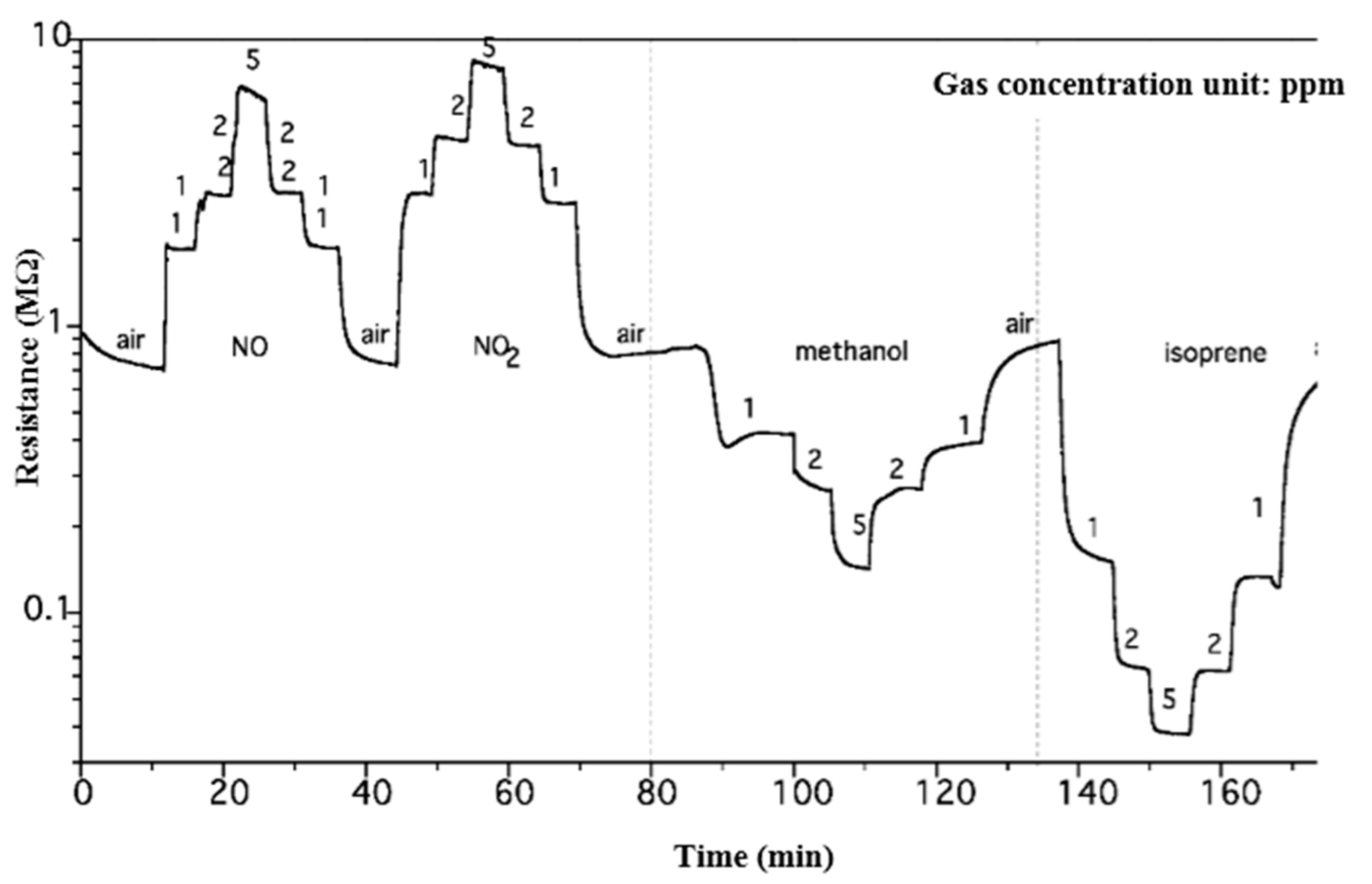
| Sensitivity | Response Time (s) | Recovery Time (s) | |
|---|---|---|---|
| NO | 2.66 | 24 | 98 |
| NO2 | 2.98 | 82 | 77 |
| methanol | 2 | 112 | 380 |
| isoprene | 7.34 | 65 | 145 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouma, P.-I.; Wang, L.; Simon, S.R.; Stanacevic, M. Novel Isoprene Sensor for a Flu Virus Breath Monitor. Sensors 2017, 17, 199. https://doi.org/10.3390/s17010199
Gouma P-I, Wang L, Simon SR, Stanacevic M. Novel Isoprene Sensor for a Flu Virus Breath Monitor. Sensors. 2017; 17(1):199. https://doi.org/10.3390/s17010199
Chicago/Turabian StyleGouma, Pelagia-Irene, Lisheng Wang, Sanford R. Simon, and Milutin Stanacevic. 2017. "Novel Isoprene Sensor for a Flu Virus Breath Monitor" Sensors 17, no. 1: 199. https://doi.org/10.3390/s17010199
APA StyleGouma, P.-I., Wang, L., Simon, S. R., & Stanacevic, M. (2017). Novel Isoprene Sensor for a Flu Virus Breath Monitor. Sensors, 17(1), 199. https://doi.org/10.3390/s17010199






