Fabrication of a Miniature Multi-Parameter Sensor Chip for Water Quality Assessment
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Instruments
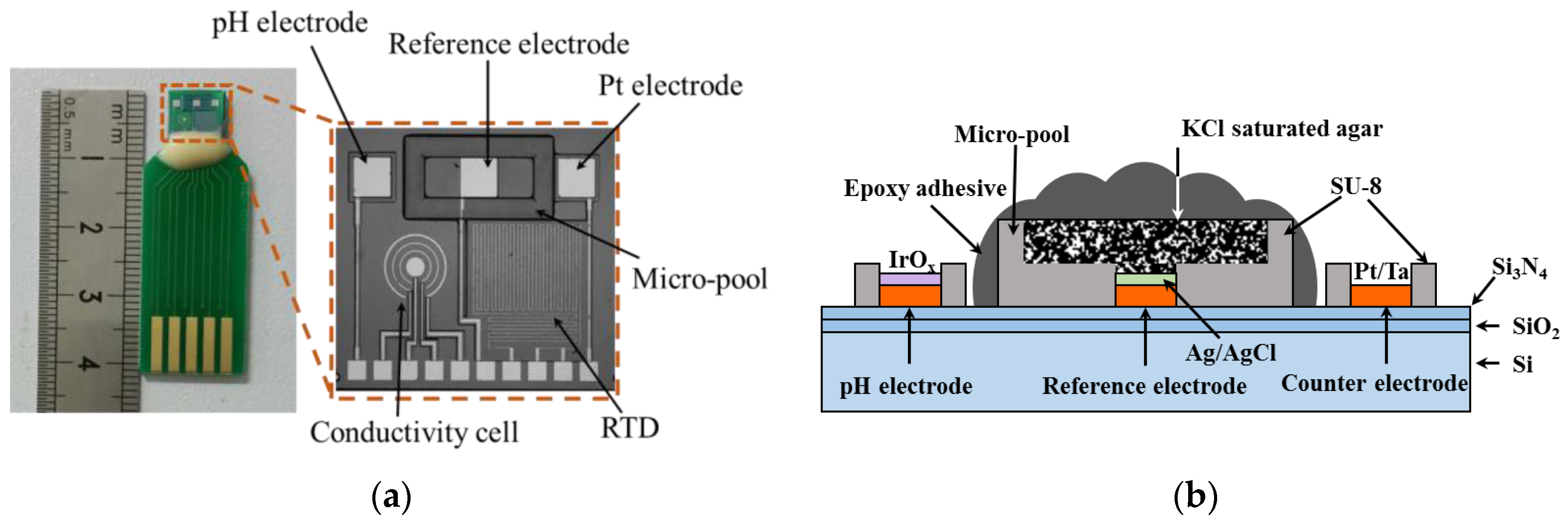
2.2. Design
2.3. Fabrication of Multi-Parameter Sensor Chip
2.4. Fabrication of the Solid State Reference Electrode
2.5. Preparation of pH Sensing Electrode
2.6. Measurement Procedure
3. Results and Discussion
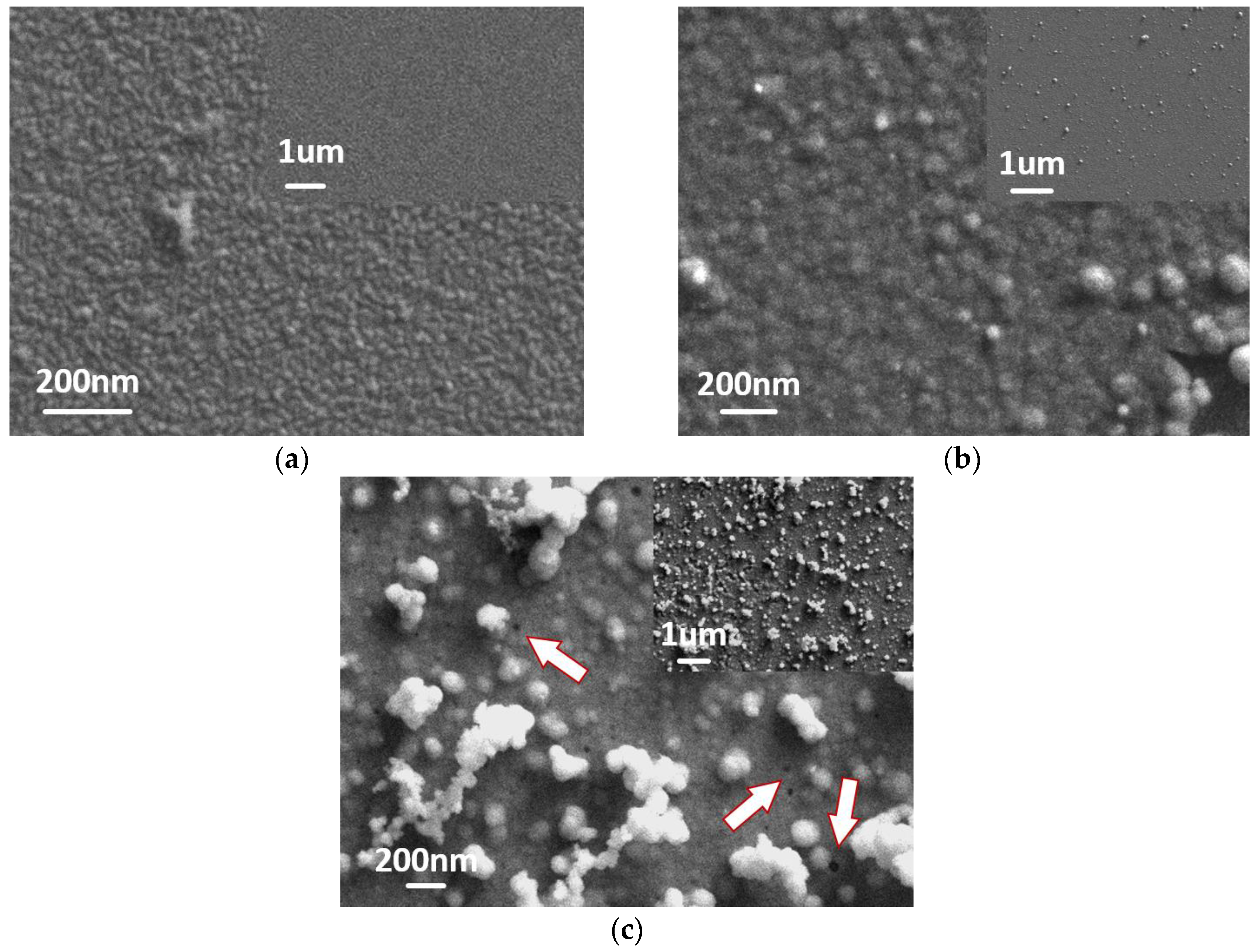
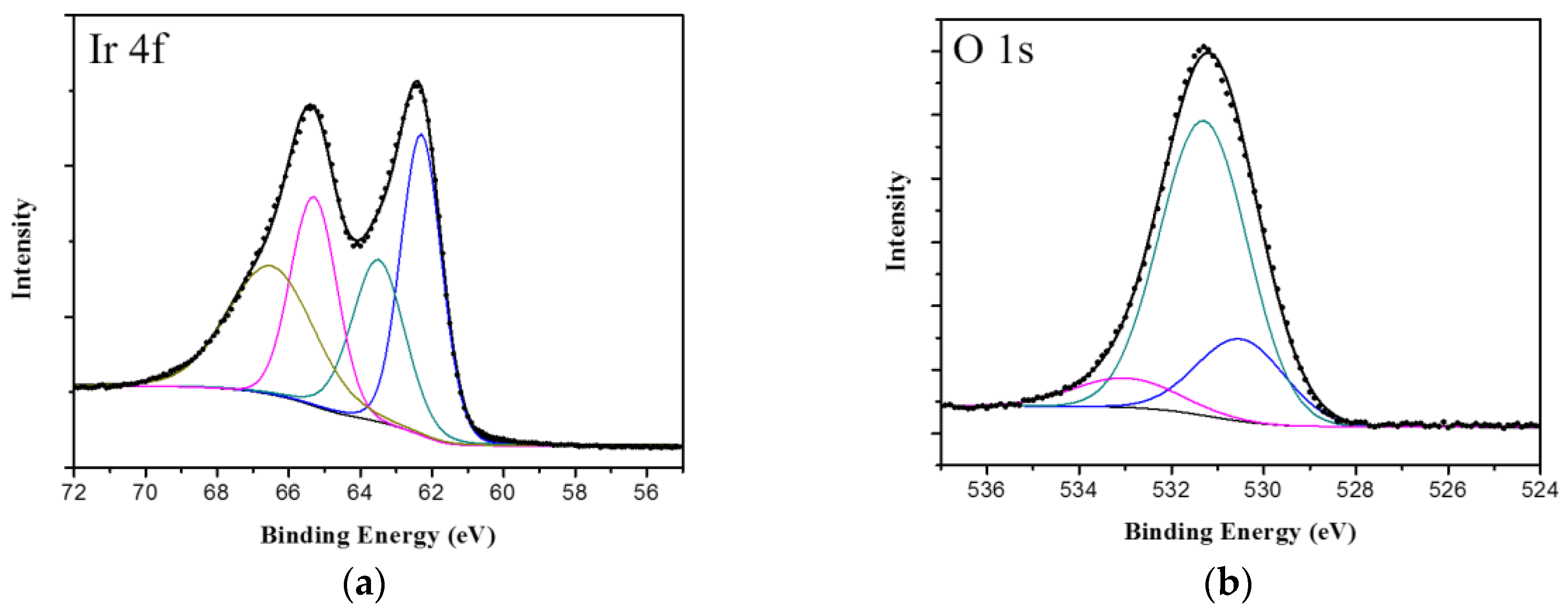
3.1. Electrodeposition of IrOx
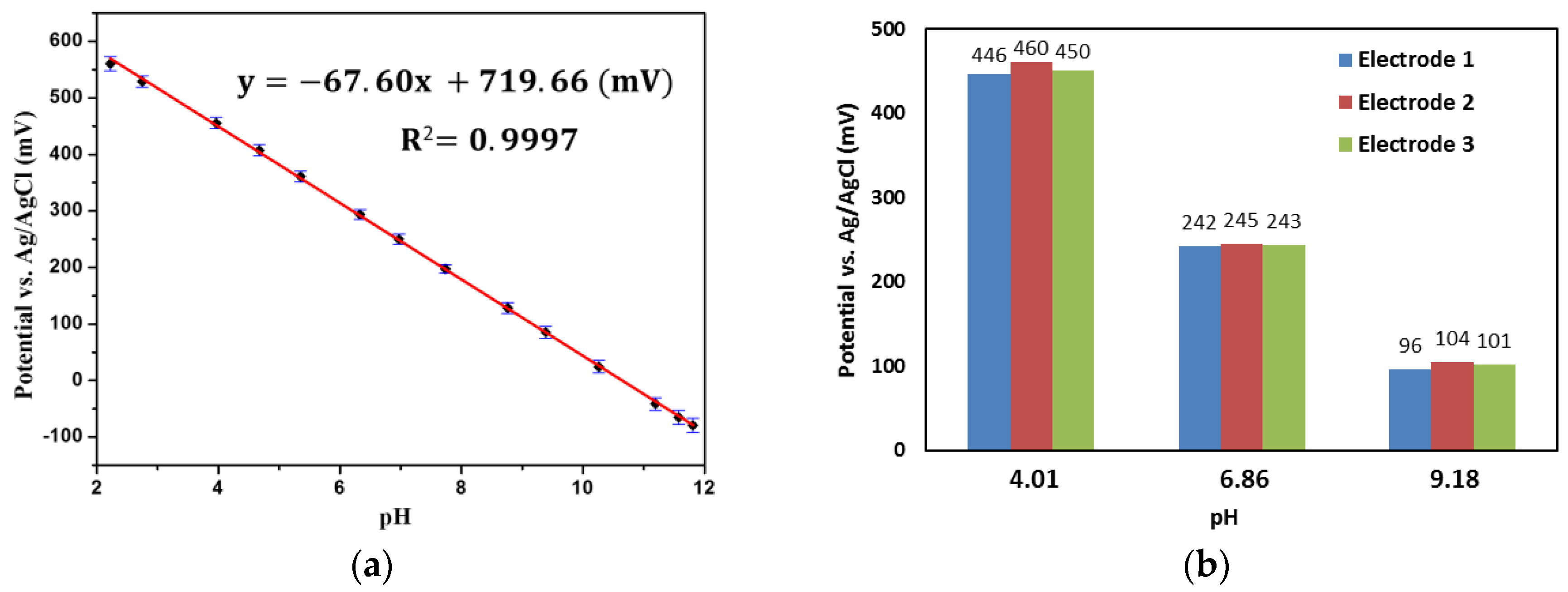
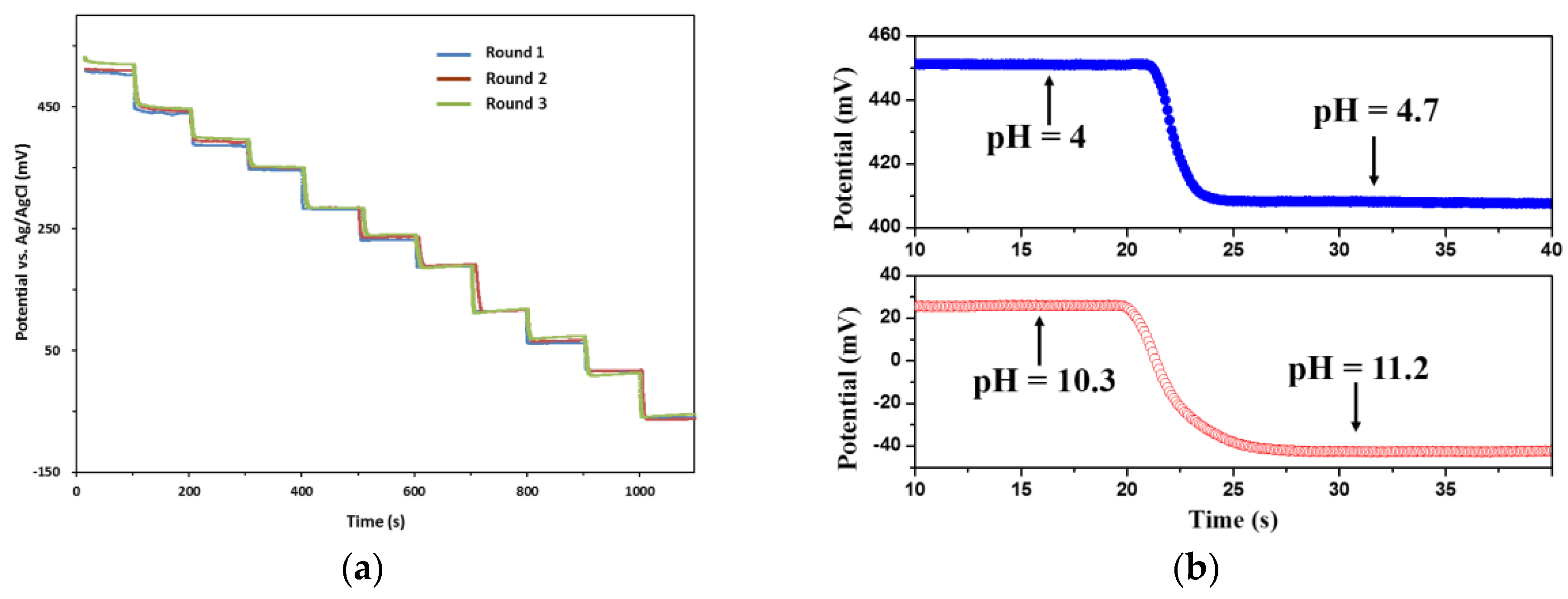
3.2. pH Measurement
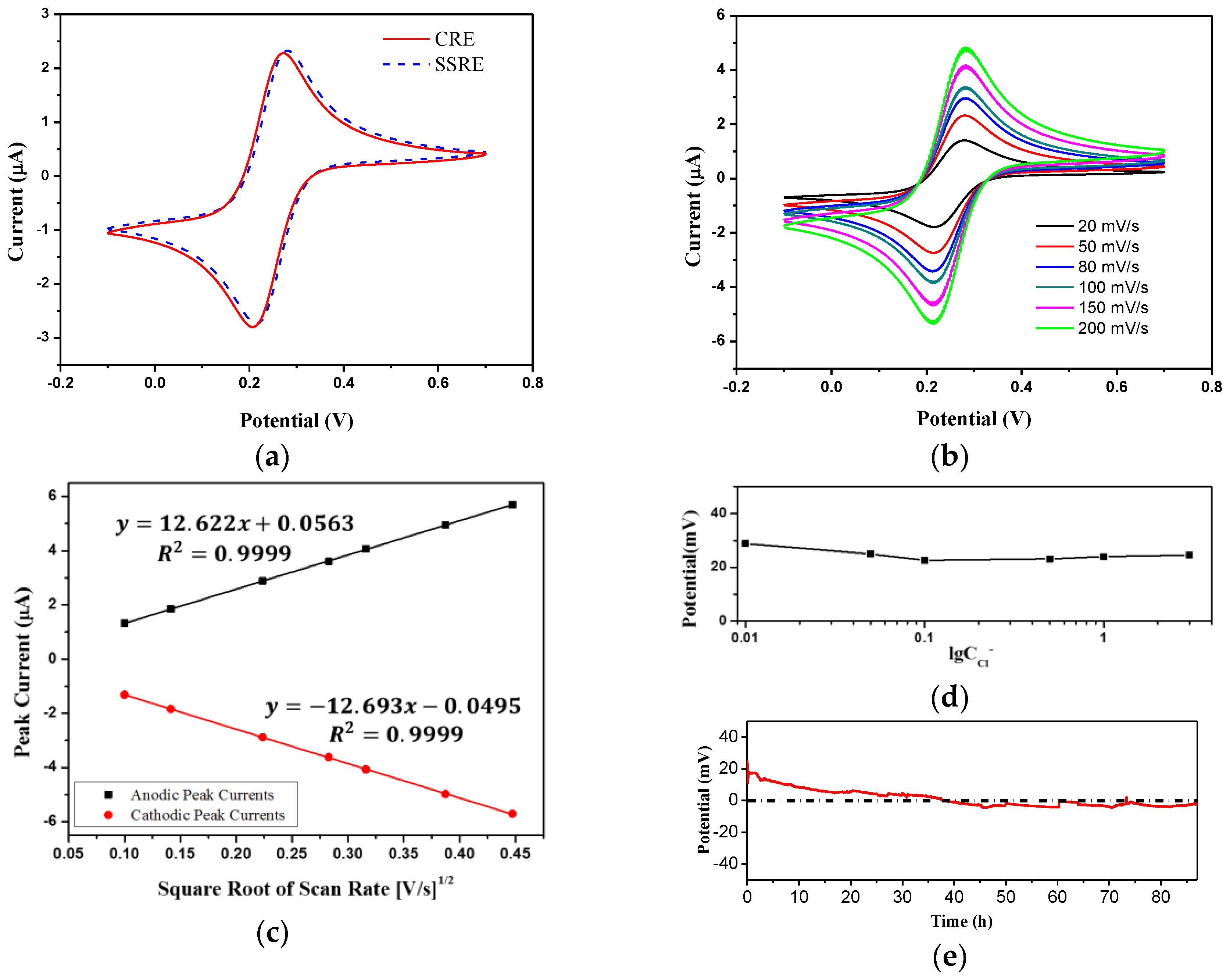
3.3. Characteristics of the Solid State Reference Electrode
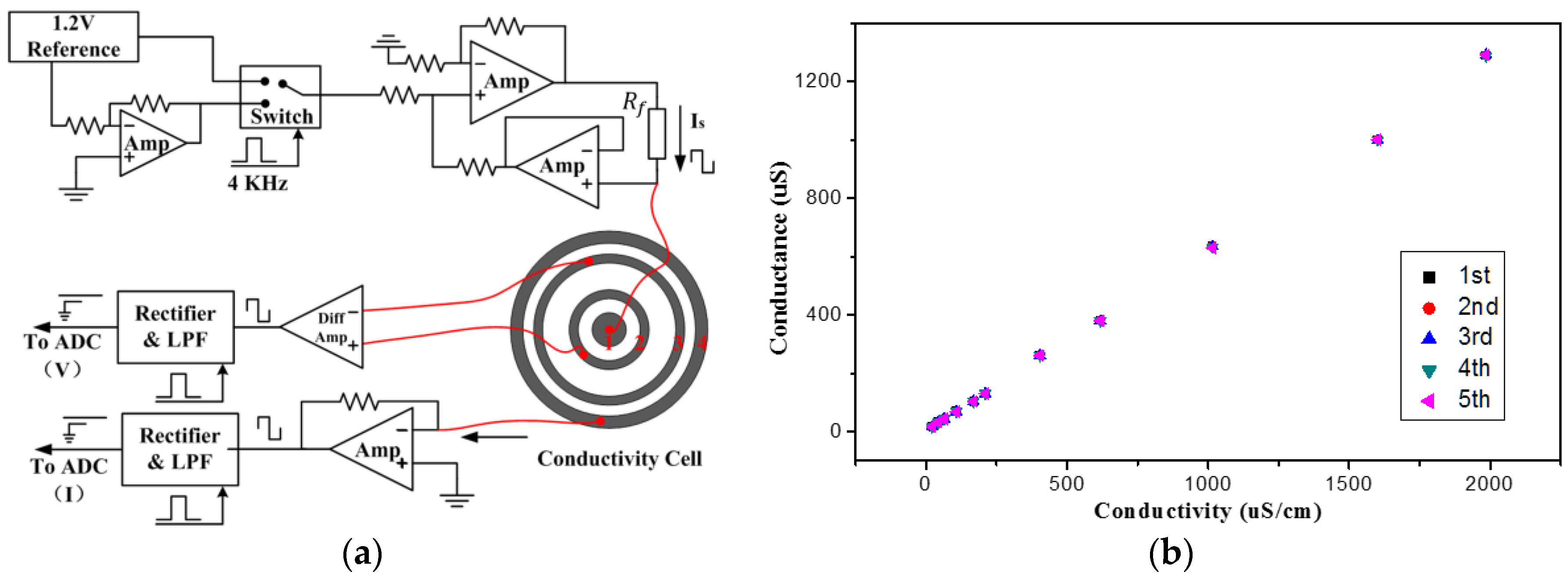
3.4. Conductivity Measurement
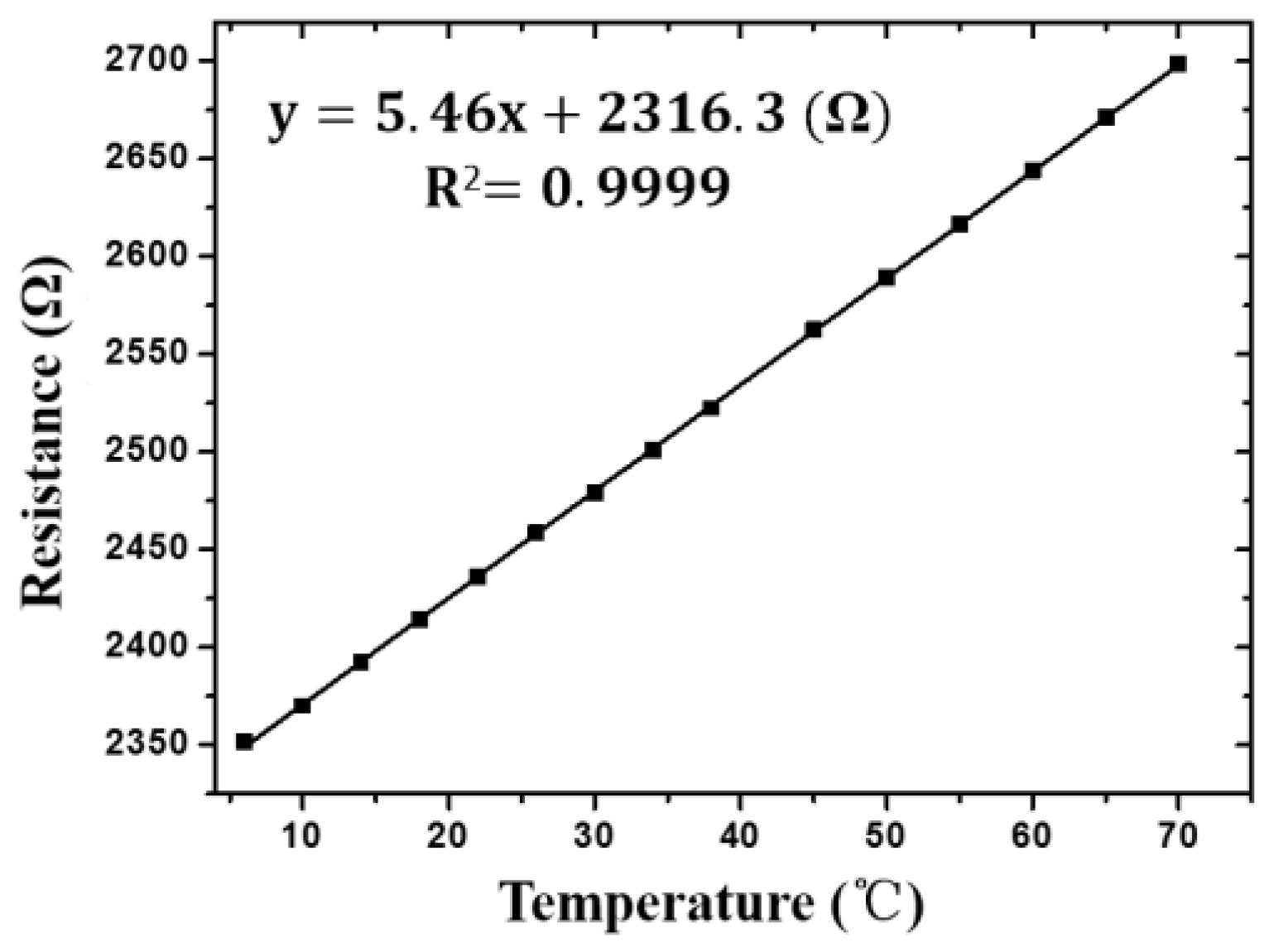
3.5. Temperature Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banna, M.H.; Najjaran, H.; Sadiq, R.; Imran, S.A.; Rodriguez, M.J.; Hoorfar, M. Miniaturized water quality monitoring pH and conductivity sensors. Sens. Actuators B Chem. 2014, 193, 434–441. [Google Scholar] [CrossRef]
- Yosemitech. Yosemitech Homepage: Y4000. Available online: http://www.yosemitech.com/product-20 (accessed on 9 November 2016).
- Hsu, L.; Selvaganapathy, P.R.; Brash, J.; Fang, Q.; Xu, C.Q.; Deen, M.J.; Chen, H. Development of a low-cost hemin-based dissolved oxygen sensor with anti-biofouling coating for water monitoring. IEEE Sens. J. 2014, 14, 3400–3407. [Google Scholar] [CrossRef]
- Hsu, L.H.H.; Hoque, E.; Kruse, P.; Ravi Selvaganapathy, P. A carbon nanotube based resettable sensor for measuring free chlorine in drinking water. Appl. Phys. Lett. 2015, 106, 063102. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Brien, D.O.; Best, M.; O’Brien, D.; Best, M. Water quality assessment by an integrated multi-sensor based on semiconductor RuO2 nanostructures. Meas. Sci. Technol. 2009, 20, 095201. [Google Scholar] [CrossRef]
- Guijarro, C.; Fuchs, K.; Bohrn, U.; Evamaria, S.; Stefan, W. Simultaneous detection of multiple bioactive pollutants using a multiparametric biochip for water quality monitoring. Biosens. Bioelectron. 2015, 72, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bian, C.; Tong, J.; Sun, J.; Xia, S. Acid/L-cysteine/gold nanoparticle modified microelectrode for simultaneous detection of copper and lead. Thin Solid Films 2012, 520, 6658–6663. [Google Scholar] [CrossRef]
- Organization, World Health. Guidelines for Drinking-Water Quality: Fourth Edition. Available online: http://www.who.int/water_sanitation_health/publications/dwqguidelines-4/en (accessed on 9 November 2016).
- Qin, Y.; Kwon, H.-J.; Howlader, M.M.R.; Deen, M.J. Microfabricated electrochemical pH and free chlorine sensors for water quality monitoring: Recent advances and research challenges. RSC Adv. 2015, 5, 69086–69109. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.H.; Dong, X.; Yang, J.; Long, H.Y.; So, P.L.; Chan, C.C. Miniature pH Optical Fiber Sensor Based on Fabry–Perot Interferometer. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 331–335. [Google Scholar] [CrossRef]
- Bahari, N.; Zain, A.M.; Abdullah, A.Z.; Bien, D.; Sheng, C.; Othman, M. Study on pH sensing properties of RF magnetron sputtered tantalum pentoxide (Ta2O5) thin film. In Proceedings of the 2010 IEEE International Conference on Semiconductor Electronics (ICSE), Malacca, Malaysia, 28–30 June 2010; pp. 76–78.
- Zhao, R.; Xu, M.; Wang, J.; Chen, G. A pH sensor based on the TiO2 nanotube array modified Ti electrode. Electrochim. Acta 2010, 55, 5647–5651. [Google Scholar] [CrossRef]
- Gerlach, G.; Guenther, M.; Sorber, J.; Suchaneck, G.; Arndt, K.F.; Richter, A. Chemical and pH sensors based on the swelling behavior of hydrogels. Sens. Actuators B Chem. 2005, 111–112, 555–561. [Google Scholar] [CrossRef]
- Sardarinejad, A.; Maurya, D.K.; Alameh, K. Physical The effects of sensing electrode thickness on ruthenium oxide thin-film pH sensor. Sens. Actuators A. Phys. 2014, 214, 15–19. [Google Scholar] [CrossRef]
- Uria, N.; Abramova, N.; Bratov, A.; Muñoz-Pascuall, F.X.; Baldrich, E. Miniaturized metal oxide pH sensors for bacteria detection. Talanta 2016, 147, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Chou, J.; Sun, T.; Hsiung, S. Development of the real-time pH sensing system for array sensors. Sens. Actuators B Chem. 2005, 108, 870–876. [Google Scholar] [CrossRef]
- Kuo, L.M.; Chou, Y.C.; Chen, K.N.; Lu, C.C.; Chao, S. A precise pH microsensor using RF-sputtering IrO2 and Ta2O5 films on Pt-electrode. Sens. Actuators B Chem. 2014, 193, 687–691. [Google Scholar] [CrossRef]
- Huang, X.R.; Ren, Q.Q.; Yuan, X.J.; Wen, W.; Chen, W.; Zhan, D.P. Iridium oxide based coaxial pH ultramicroelectrode. Electrochem. Commun. 2014, 40, 35–37. [Google Scholar] [CrossRef]
- Wang, M.; Yao, S.; Madou, M. A long-term stable iridium oxide pH electrode. Sens. Actuators B Chem. 2002, 81, 313–315. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Huang, W.D.; Rao, S.; Cao, H.; Tata, U.; Chiao, M.; Chiao, J.C. Sol-gel iridium oxide-based pH sensor array on flexible polyimide substrate. IEEE Sens. J. 2013, 13, 3857–3864. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Rao, S.; Seo, Y.; Schadt, K.; Hao, Y.; Chiao, J.; Member, S. Micro pH Sensors Based on Iridium Oxide Nanotubes. IEEE Trans. Nanotechnol. 2014, 13, 945–953. [Google Scholar] [CrossRef]
- Steegstra, P.; Ahlberg, E. Influence of oxidation state on the pH dependence of hydrous iridium oxide films. Electrochim. Acta 2012, 76, 26–33. [Google Scholar] [CrossRef]
- Kim, T.Y.; Yang, S. Fabrication method and characterization of electrodeposited and heat-treated iridium oxide films for pH sensing. Sens. Actuators B Chem. 2014, 196, 31–38. [Google Scholar] [CrossRef]
- Yamanaka, K. Anodically electrodeposited iridium oxide films (AEIROF’s) from alkaline solutions for electrochromic display devices. Jpn. J. Appl. Phys. 1989, 28, 632–637. [Google Scholar] [CrossRef]
- Bitziou, E.; O’Hare, D.; Patel, B.A. Simultaneous detection of pH changes and histamine release from oxyntic glands in isolated stomach. Anal. Chem. 2008, 80, 8733–8740. [Google Scholar] [CrossRef] [PubMed]
- Nadappuram, B.P.; Mckelvey, K.; Al Botros, R.; Colburn, A.W.; Unwin, P.R. Fabrication and Characterization of Dual Function Nanoscale pH-Scanning Ion Conductance Microscopy (SICM) Probes for High Resolution pH Mapping. Anal. Chem. 2013, 85, 8070–8074. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, A.N.; Zhang, T.C. Fabrication of Anodically Electrodeposited Iridium Oxide Film pH Microelectrodes for Microenvironmental Studies. Anal. Chem. 2002, 74, 5726–5733. [Google Scholar] [CrossRef] [PubMed]
- Steegstra, P.; Ahlberg, E. Involvement of nanoparticles in the electrodeposition of hydrous iridium oxide films. Electrochim. Acta 2012, 68, 206–213. [Google Scholar] [CrossRef]
- Elsen, H.A.; Monson, C.F.; Majda, M. Effects of Electrodeposition Conditions and Protocol on the Properties of Iridium Oxide pH Sensor Electrodes. J. Electrochem. Soc. 2009, 156, F1–F6. [Google Scholar] [CrossRef]
- Zhao, Y.; Vargas-barbosa, N.M.; Hernandez-pagan, E.A.; Mallouk, T.E. Anodic Deposition of Colloidal Iridium Oxide Thin Films from Hexahydroxyiridate(IV) Solutions. Small 2011, 7, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hernandez-Pagan, E.A.; Vargas-Barbosa, N.M.; Dysart, J.L.; Mallouk, T.E. A High Yield Synthesis of Ligand-Free Iridium Oxide Nanoparticles with High Electrocatalytic Activity. J. Phys. Chem. Lett. 2011, 2, 402–406. [Google Scholar] [CrossRef]
- Khalil, M.; Wang, S.; Yu, J.; Lee, R.L.; Liu, N. Electrodeposition of Iridium Oxide Nanoparticles for pH Sensing Electrodes. J. Electrochem. Soc. 2016, 163, B485–B490. [Google Scholar] [CrossRef]
- Shitanda, I.; Kiryu, H.; Itagaki, M. Improvement in the long-term stability of screen-printed planar type solid-state Ag/AgCl reference electrode by introducing poly (dimethylsiloxane) liquid junction. Electrochim. Acta 2011, 58, 528–531. [Google Scholar] [CrossRef]
- Kim, S.K.; Lim, H.; Chung, T.D.; Kim, H.C. A miniaturized electrochemical system with a novel polyelectrolyte reference electrode and its application to thin layer electroanalysis. Sens. Actuators B Chem. 2006, 115, 212–219. [Google Scholar] [CrossRef]
- Huang, I.Y.; Huang, R.S. Fabrication and characterization of a new planar solid-state reference electrode for ISFET sensors. Thin Solid Films 2002, 406, 255–261. [Google Scholar] [CrossRef]
- Adachi, T.; Suzuki, H. Dynamic feedback regulation of the potential of a microfabricated liquid-junction Ag/AgCl reference electrode. Sens. Actuators B Chem. 2011, 156, 228–235. [Google Scholar] [CrossRef]
- Li, W.; Liu, F.Y.; Wang, K.Y.; Wen, Z.Y. The Fabrication and Experiment of a Four-Electrode Conductivity Sensor for Fresh Water. Key Eng. Mater. 2011, 483, 341–344. [Google Scholar] [CrossRef]
- Ramos, P.M.; Pereira, J.M.D.; Member, S.; Ramos, H.M.G. A Four Terminal Water Quality Monitoring Conductivity Sensor. IEEE Trans. Instrum. Meas. 2008, 57, 577–583. [Google Scholar] [CrossRef]
- Kim, M.; Choi, W.; Lim, H.; Yang, S. Integrated micro fluidic-based sensor module for real-time measurement of temperature, conductivity, and salinity to monitor reverse osmosis. DES 2013, 317, 166–174. [Google Scholar] [CrossRef]
- He, D.; Shannon, M.A.; Miller, N.R. Micromachined Silicon Electrolytic Conductivity Probes with Integrated Temperature Sensor. IEEE Sens. J. 2005, 5, 1185–1196. [Google Scholar]
- Hyldgård, A.; Ólafsdóttir, Í.; Olesen, M.; Hedegaard, T.; Hansen, O.; Thomsen, E. V FISH & CHIPS: Four Electrode Conductivity/Salinity Sensor on a Silicon Multi-sensor Chip for Fisheries Research. In Proceedings of the IEEE SENSORS, Irvine, CA, USA, 30 October–3 November 2005.
- Broadbent, H.A.; Ivanov, S.Z.; Fries, D.P. Fabrication of a LCP-based conductivity cell and resistive temperature device via PCB MEMS technology. J. Micromech. Microeng. 2007, 17, 722–729. [Google Scholar] [CrossRef]
- Broadbent, H.A.; Ivanov, S.Z.; Fries, D.P. A miniature, low cost CTD system for coastal salinity measurements. Meas. Sci. Technol. 2007, 18, 3295–3302. [Google Scholar] [CrossRef]
- Negi, S.; Bhandari, R.; Solzbacher, F. Morphology and Electrochemical Properties of Activated and Sputtered Iridium Oxide Films for Functional Electrostimulation. J. Sens. Technol. 2012, 2, 138–147. [Google Scholar] [CrossRef]
- Lee, B.-S.; Ahn, S.H.; Park, H.-Y.; Choi, I.; Yoo, S.J.; Kim, H.-J.; Henkensmeier, D.; Kim, J.Y.; Park, S.; Nam, S.W.; et al. Development of electrodeposited IrO2 electrodes as anodes in polymer electrolyte membrane water electrolysis. Appl. Catal. B Environ. 2015, 179, 285–291. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Mara, M.W.; Kushner-lenho, M.N.; Schley, N.D.; Konezny, S.J.; Rivalta, I.; Negre, C.F.A.; Snoeberger, R.C.; Kokhan, O.; Huang, J.; et al. Characterization of an Amorphous Iridium Water-Oxidation Catalyst Electrodeposited from Organometallic Precursors. Inorg. Chem. 2013, 52, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
- Zhuiykov, S. Morphology of Pt-doped nanofabricated RuO2 sensing electrodes and their properties in water quality monitoring sensors. Sens. Actuators B Chem. 2009, 136, 248–256. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M.; Toniolo, R. Anodic electrodeposition of iridium oxide particles on glassy carbon surfaces and their electrochemical/SEM/XPS characterization. J. Electroanal. Chem. 2015, 736, 147–152. [Google Scholar] [CrossRef]
- Roushani, M.; Farokhi, S. Amperometric Detection of Isoprenaline Based on Glassy Carbon Electrode Modified by Iridium Oxide Nanoparticles. J. Braz. Chem. Soc. 2015, 26, 1034–1042. [Google Scholar] [CrossRef]
- Yao, S.; Wang, M.; Madou, M. A pH Electrode Based on Melt-Oxidized Iridium Oxide. J. Electrochem. Soc. 2001, 148, H29–H36. [Google Scholar] [CrossRef]
- Huang, W.D.; Cao, H.; Deb, S.; Chiao, M.; Chiao, J.C. A flexible pH sensor based on the iridium oxide sensing film. Sens. Actuators A Phys. 2011, 169, 1–11. [Google Scholar] [CrossRef]
- Kim, T.Y.; Hong, S.A.; Yang, S. A solid-state thin-film Ag/AgCl reference electrode coated with graphene oxide and its use in a pH sensor. Sensors 2015, 15, 6469–6482. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Park, S.; Boo, H.; Kim, C.; Dong, T. Nanoporous platinum solid-state reference electrode with layer-by-layer polyelectrolyte junction for pH sensing chip. Lab Chip 2011, 11, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Cvejin, K.; Kulawik, J.; Zaraska, K.; Szwagierczak, D.; Socha, R.P. Fabrication of thick film sensitive RuO2-TiO2 and Ag/AgCl/KCl reference electrodes and their application for pH measurements. Sens. Actuators B Chem. 2014, 204, 57–67. [Google Scholar] [CrossRef]
- Broadbent, H.A.; Ketterl, T.P.; Reid, C.S. A miniature rigid/flex salinity measurement device fabricated using printed circuit processing techniques. J. Micromech. Microeng. 2010, 20, 085008. [Google Scholar] [CrossRef]
- Hyldgård, A.; Birkelund, K.; Janting, J.; Thomsen, E.V. Direct media exposure of MEMS multi-sensor systems using a potted-tube packaging concept. Sens. Actuators A Phys. 2008, 142, 398–404. [Google Scholar] [CrossRef]
- Standards Press of China. Test Solutions of Electrolytic Conductivity Analyzer–Preparation Method of Sodium Chloride Solutions (GB/T 27503-2011); Standards Press of China: Beijing, China, 2011; pp. 1–10. [Google Scholar]
| Electrode Type | Fabrication Techenique | Test Solution | Test Mode | Stability | Literature |
|---|---|---|---|---|---|
| Ag/AgCl/GO | Sputtering, chemical chlorination, and drop casting | 3 M KCl | Intermittent (2 or 3 days’ interval) | 26 days | 2015 [52] |
| LBL-PE/npPt | Electrodeposition, photo polymerization | 0.1 M PBS | Continuous | 50 h | 2011 [53] |
| Ag/AgCl/KCl-glass | Screen printing | Deionized water | Continuous | 4 h | 2014 [54] |
| Ag/AgCl/KCl-agar/epoxy | Drop casting | Deionized water | Continuous | 56 h | This work |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Bian, C.; Tong, J.; Xia, S. Fabrication of a Miniature Multi-Parameter Sensor Chip for Water Quality Assessment. Sensors 2017, 17, 157. https://doi.org/10.3390/s17010157
Zhou B, Bian C, Tong J, Xia S. Fabrication of a Miniature Multi-Parameter Sensor Chip for Water Quality Assessment. Sensors. 2017; 17(1):157. https://doi.org/10.3390/s17010157
Chicago/Turabian StyleZhou, Bo, Chao Bian, Jianhua Tong, and Shanhong Xia. 2017. "Fabrication of a Miniature Multi-Parameter Sensor Chip for Water Quality Assessment" Sensors 17, no. 1: 157. https://doi.org/10.3390/s17010157
APA StyleZhou, B., Bian, C., Tong, J., & Xia, S. (2017). Fabrication of a Miniature Multi-Parameter Sensor Chip for Water Quality Assessment. Sensors, 17(1), 157. https://doi.org/10.3390/s17010157






